Abstract
The cotyledons of jojoba (Simmondsia chinensis) seeds contained 50 to 60% of their weight as intracellular wax esters. During germination there was a gradual decrease in the wax content with a concomitant rise in soluble carbohydrates, suggesting that the wax played the role of a food reserve. Thin layer chromatography revealed that both the fatty alcohol and fatty acid were metabolized. The disappearance of wax was matched with an increase of catalase, a marker enzyme of the gluconeogenic process in other fatty seedlings. Subcellular organelles were isolated by sucrose gradient centrifugation from the cotyledons at the peak stage of germination. The enzymes of the β oxidation of fatty acid and of the glyoxylate cycle were localized in the glyoxysomes but not in the mitochondria. The glyoxysomes had specific activities of individual enzymes similar to those of the castor bean glyoxysomes. An active alkaline lipase was detected in the wax bodies at the peak stage of germination but not in the ungerminated seeds. No lipase was detected in glyoxysomes or mitochondria. After the wax in the wax bodies had been extracted with diethyl ether, the organelle membrane was isolated and it still retained the alkaline lipase. The gluconeogenesis from wax in the jojoba seedling appears to be similar, but with modification, to that from triglyceride in other fatty seedlings.
Full text
PDF
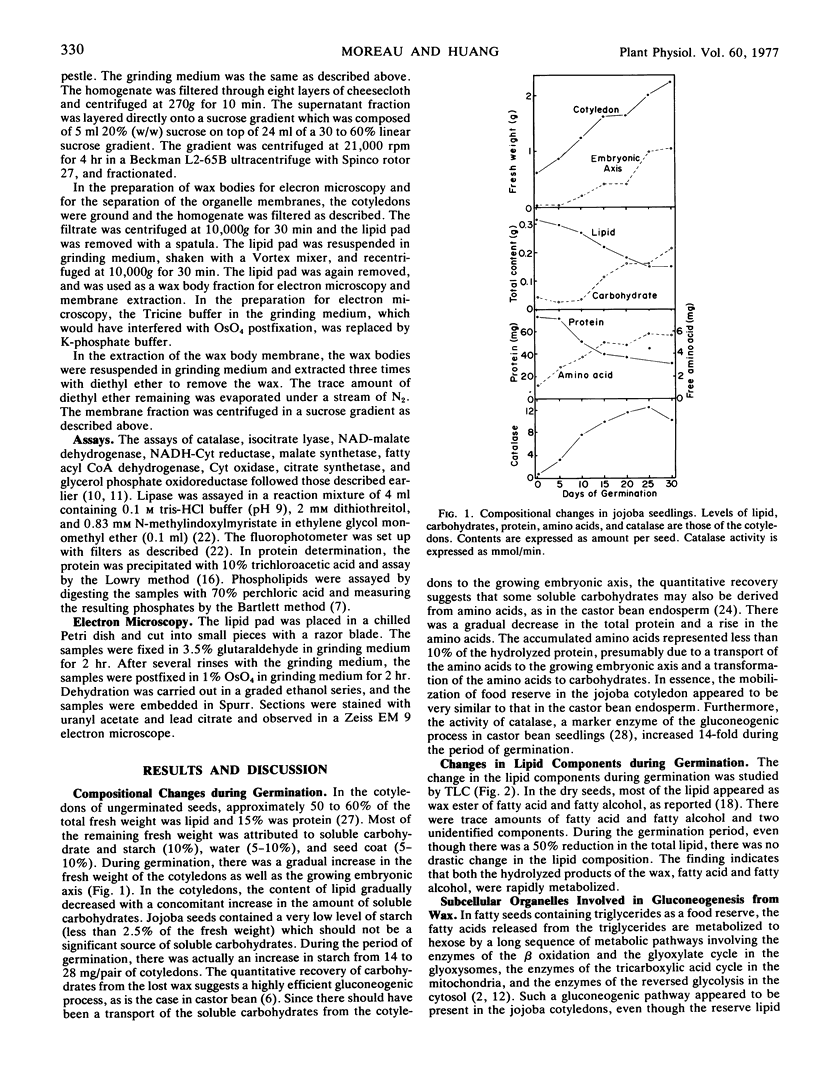
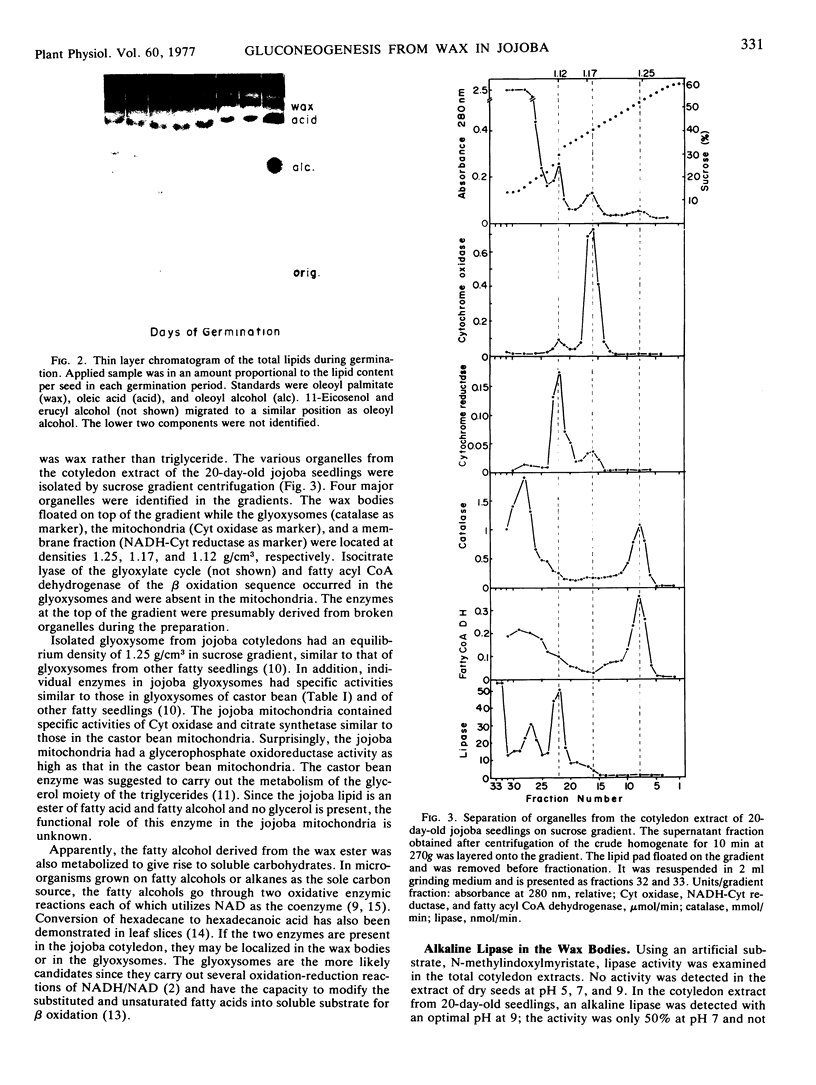
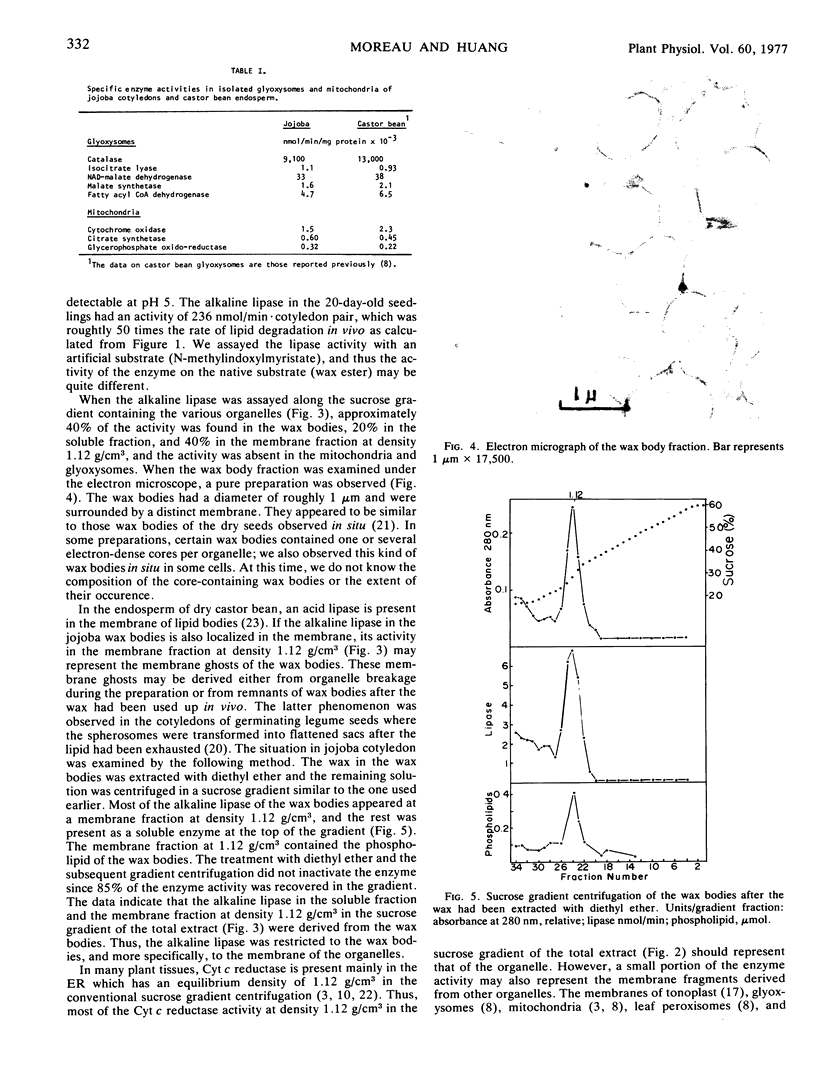

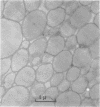
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Ching T. M. Compositional changes of douglas fir seeds during germination. Plant Physiol. 1966 Oct;41(8):1313–1319. doi: 10.1104/pp.41.8.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Baumgartner B., Harris N. Regulation of reserve protein metabolism in the cotyledons of mung bean seedlings. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3168–3172. doi: 10.1073/pnas.73.9.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P., Tolbert N. E., Schnarrenberger C. A comparison of microbody membranes with microsomes and mitochondria from plant and animal tissue. Arch Biochem Biophys. 1972 Sep;152(1):199–215. doi: 10.1016/0003-9861(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Huang A. H. Comparative studies of glyoxysomes from various Fatty seedlings. Plant Physiol. 1975 May;55(5):870–874. doi: 10.1104/pp.55.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H. Enzymes of glycerol metabolism in the storage tissues of Fatty seedlings. Plant Physiol. 1975 Mar;55(3):555–558. doi: 10.1104/pp.55.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton D., Stumpf P. K. Fat Metabolism in Higher Plants. XXXVII. Characterization of the beta-Oxidation Systems From Maturing and Germinating Castor Bean Seeds. Plant Physiol. 1969 Apr;44(4):508–516. doi: 10.1104/pp.44.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton D., Stumpf P. K. Fat metabolism in higher plants. LXII. The pathway of ricinoleic acid catabolism in the germinating castor bean (Ricinus communis L.) and pea (Pisum sativum L.). Arch Biochem Biophys. 1971 Jan;142(1):48–60. doi: 10.1016/0003-9861(71)90258-x. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Oxidation of paraffins by plant tissues. Plant Physiol. 1969 Feb;44(2):315–317. doi: 10.1104/pp.44.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lebeault J. M., Roche B., Duvnjak Z., Azoulay E. Alcool-et aldéhyde-déshydrogénases particulaires de Candida tropicalis cultivé sur hydrocarbures. Biochim Biophys Acta. 1970 Dec 16;220(3):373–385. doi: 10.1016/0005-2744(70)90269-x. [DOI] [PubMed] [Google Scholar]
- Mollenhauer H. H., Totten C. Studies on seeds. II. Origin and degradation of lipid vesicles in pea and bean cotyledons. J Cell Biol. 1971 Feb;48(2):395–405. doi: 10.1083/jcb.48.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Beevers H. Lipase Activities in Castor Bean Endosperm during Germination. Plant Physiol. 1974 Jul;54(1):23–28. doi: 10.1104/pp.54.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory R. L., Yatsu L. Y., Kircher H. W. Association of lipase activity with the spherosomes of Ricinus communis. Arch Biochem Biophys. 1968 Feb;123(2):255–264. doi: 10.1016/0003-9861(68)90132-x. [DOI] [PubMed] [Google Scholar]
- Stewart C. R., Beevers H. Gluconeogenesis from amino acids in germinating castor bean endosperm and its role in transport to the embryo. Plant Physiol. 1967 Nov;42(11):1587–1595. doi: 10.1104/pp.42.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEMM E. W., WILLIS A. J. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954 Jul;57(3):508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle R. J., Huang A. H. Development and properties of fructose 1,6-bisphosphatase in the endosperm of castor-bean seedlings. Biochem J. 1976 Mar 15;154(3):647–652. doi: 10.1042/bj1540647. [DOI] [PMC free article] [PubMed] [Google Scholar]