Abstract
Background
The National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS) has self-reported health measures available for both pediatric and adult populations, but no pediatric measures are available currently in the sleep domains.
Objective
The purpose of this observational study was to perform preliminary validation studies on age-appropriate, self-reported sleep measures in healthy adolescents.
Methods
This study examined 25 healthy adolescents’ self-reported daytime sleepiness, sleep disturbance, sleep-related impairment, and sleep patterns. Healthy adolescents completed a physical exam at the NIH Clinical Center (Bethesda, MD), had no chronic medical conditions, and were not taking any chronic medications. The Cleveland Adolescent Sleepiness Questionnaire (CASQ), PROMIS Sleep Disturbance (v. 1.0; 8a), and PROMIS Sleep-Related Impairment (v. 1.0; 8b) questionnaires were completed, and sleep patterns were assessed using actigraphy.
Results
Total scores on the three sleep questionnaires were correlated (all Spearman’s r > .70, p < .001). Total sleep time determined by actigraphy was negatively correlated with the CASQ (p ≤ .02), PROMIS Sleep Disturbance (p ≤ .02) and PROMIS Sleep-Related Impairment (p ≤ .02).
Discussion
The field of pediatric sleep is rapidly expanding, and researchers and clinicians will benefit from well-designed, psychometrically sound, sleep questionnaires. Findings suggest the potential research and clinical utility of adult versions of PROMIS sleep measures in adolescents. Future studies should include larger, more diverse samples, and explore additional psychometric properties of PROMIS sleep measures to provide age-appropriate, validated, and reliable measures of sleep in adolescents.
Keywords: actigraphy, adolescence, CASQ, psychometrics, self-report, sleep, PROMIS
Sleep health and sufficient sleep are particularly important during adolescence, when vital physical, cognitive, emotional, and social changes occur. Although sleep is a primary aspect of adolescent development, insufficient sleep among adolescents is common, growing progressively worse over the course of adolescence. Adolescents physiologically require about 9−10 hours of sleep; however, they sleep closer to seven hours a night, with circadian rhythm changes making it more difficult for adolescents to fall asleep and also harder to wake up in the morning (Reiter & Rosen, 2014; Wolfson & Carskadon, 1998). Sleep health has been added to the agenda for Healthy People 2020, with one of the objectives being to increase the proportion of adolescents who get a sufficient amount of sleep (HealthyPeople.gov, 2016).
Insufficient sleep can impact daytime function and leave adolescents feeling lethargic, sluggish, irritable, and moody, and can inhibit focus and concentration, slow response time, and decrease learning of cognitive tasks, leading to lower academic performance (Randazzo, Muehlbach, Schweitzer, & Walsh, 1998). Sleep deprivation in adolescents has been associated with obesity, depression, anxiety, delinquent behavior, and alcohol use, as well as suicidal behavior (Liu, 2004; National Heart, Lung, and Blood Institute, 2011; J. Owens, Adolescent Sleep Working Group, & Committee on Adolescence, 2014; Wolfson & Carskadon, 2003). In prospective cohort studies as well as simulated driving environments, poor sleep quality is associated with sleepiness at the wheel and significantly increases the risk of automobile accidents in adolescents (Garner et al., 2015; Martiniuk et al., 2013; Pizza et al., 2010). Of note, jurisdictions with earlier high school start times have reported higher adolescent motor vehicle crash rates—potentially due to insufficient sleep (Vorona et al., 2014).
One method for examining sleep phenotypes is through the use of sleep questionnaires. In 2010, extensive lists of published and unpublished instruments used to investigate sleep issues in children and adolescents (age 0–18 years) were collected, and the psychometric properties of 57 tools were evaluated, using 11 methodological criteria outlined by the authors (Spruyt & Gozal, 2011). Only two self-report tools—Dream Content Questionnaire for Children (age 9–14 years) and the Cleveland Adolescent Sleepiness Questionnaire (age 11–17 years)—met all of the methodological criteria except standardization and norms development (Spruyt & Gozal, 2011).
Recent literature suggests that many sleep disturbances start in adolescence and persist into adulthood (Dregan & Armstrong, 2010). Measures used to describe sleep phenotypes are not well developed for adolescent populations, and adolescent sleep research is underrepresented in the literature. Therefore, the purpose of this cross-sectional observation study was to perform preliminary validation studies on self-reported sleep measures in healthy adolescents.
Methods
Population and Sampling
Healthy adolescents (age 10−18 years) who had no chronic medical conditions, and were not taking any chronic medications, were recruited as control subjects for a phenotyping study of genetic disorders (NCT00758108). The study was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD. Written informed consent was obtained from parents of the adolescents, and assent was obtained from the adolescents. Participants were compensated for completing a physical exam, questionnaires, and actigraphy recording.
Measurements
Cleveland Adolescent Sleepiness Questionnaire
The Cleveland Adolescent Sleepiness Questionnaire (CASQ) is a 16-item instrument used to measure daytime sleepiness in adolescents 11–17 years of age. Adolescents rate the frequency of each item using a 5-point Likert scale, with higher scores indicating increased daytime sleepiness (Spilsbury, Drotar, Rosen, & Redline, 2007). CASQ scores demonstrated good reliability (α = 0.89) and correlations (r = .66 to .75) with two other measures of daytime sleepiness (Pediatric Daytime Sleepiness Scale and Sleep Habits School Survey). CASQ scores also demonstrate construct validity in community samples (Spilsbury et al., 2007).
PROMIS Sleep Disturbance and Sleep-Related Impairment
Sleep disturbance and sleep-related impairment were measured using Patient Reported Outcomes Measurement Information System (PROMIS) 8-item short forms. PROMIS provides clinicians and researchers access to efficient, precise, valid, reliable, nondisease specific, and standardized questionnaires, allowing for comparability across conditions and across the lifespan. PROMIS measures were established using literature searches of well-established, existing measures, as well as expert content review, qualitative research with patients, and pilot testing (Cella et al., 2010). Within PROMIS, many self-reported measures are available in both pediatric (8–17 years) and adult populations (18 years of age or older). However, no pediatric measures are currently available in the sleep disturbance or sleep-related impairment domains (HealthMeasures, 2016).
PROMIS sleep disturbance and sleep-related impairment item banks were developed to improve self-report regarding the sleep-wake function in adults. PROMIS defines sleep as a reversible recurrent state of reduced awareness of and interaction with the environment, while wakefulness is an active engagement with the environment (Buysse et al., 2010). In this study, the adult 8-item PROMIS Sleep Disturbance questionnaire (v. 1.0; 8a) was used to measure self-reported perceptions of sleep quality, depth, and restoration within the past seven days. This includes perceived difficulties falling asleep and staying asleep, as well as sleep satisfaction. The 8-item PROMIS Sleep-Related Impairment questionnaire (v. 1.0; 8b) measured self-reported alertness, sleepiness, tiredness, and functional impairments associated with sleep problems during waking hours within the past seven days. Both measures used a 5-point Likert scale, and the raw score was converted to a standardized T-score using conversion tables published on the PROMIS website (nihpromis.org), with higher scores indicating greater sleep/wake disturbances. In reference to one of the adult legacy measures, the Pittsburgh Sleep Quality Index, the sleep disturbance bank was correlated (r = .85), and the sleep-related impairment (r = .70) were significantly correlated (Cella et al., 2010; HealthMeasures, 2016). The adult PROMIS sleep measures have demonstrated adequate content validity in adolescents age 12–18 years (van Kooten, Terwee, Kaspers, & van Litsenburg, 2016).
Actigraphy
The Actiwatch Spectrum device (Philips Respironics, Bend, OR) was used to provide an objective indirect measure of sleep–wake patterns. Adolescents and a parent were given standardized instructions on how to wear the actigraph and how to complete the sleep diary. The device was worn on the nondominant wrist, in the home setting, during a typical school week, for seven consecutive days and nights to provide an estimate of sleep based on movement. The actigraph was set to record in 30-second epochs at a medium sensitivity level for scoring sleep and wake time. Data from actigraphs were downloaded to a computer, and total bedtime, total wake time, total sleep time (TST), sleep efficiency, and sleep onset latency were calculated using an automated algorithm that provides an overall average value for the seven-day period (Respironics Actiware 6, Philips Respironics, Bend, OR). In addition, sleep variables were split into weekday school nights (Sunday–Thursday) versus weekend nights (Friday and Saturday), and average values were calculated. Participants recorded approximate time of sleep and wake during the seven days using a sleep/wake log (Philips Respironics, Bend, OR). Actigraphy procedures were guided by a previous report aimed at standardizing publishing of results (Berger et al., 2008).
Statistical Analyses
Statistical analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, NY). Data were assessed for skewness, kurtosis, and Kolmogorov–Smirnov test for normality. The questionnaire scores and actigraphy variables were assessed using nonparametric statistics (Spearman correlations and Wilcoxon signed rank tests). Using an estimated correlation coefficient of 0.70, a sample size of 20 was calculated to have ≥ 90% power to detect a correlation between questionnaire scores at a nominal level of significance < .01. We conducted six correlation and two comparison analyses; therefore, a p-value < .006 was considered significant after Bonferroni correction of multiple analyses. Nominal p-values are presented in the results.
Results
Participants
Participants included 25 healthy adolescents (age 10–18 years). As summarized in Table 1, the cohort was 44% female, and 80% were non-Hispanic Caucasian. Six adolescents had unscorable actigraphy data on one weekday school night due to off-wrist detection sensors; the remaining six days had valid data that were included in analyses.
TABLE 1.
Participant Characteristics
| Characteristic | n | (%) |
|---|---|---|
| Sex (female) | 11 | (44.0) |
| Race/ethnicity | ||
| Non-Hispanic White | 20 | (80.0) |
| African American | 4 | (16.0) |
| Asian American | 1 | (4.0) |
|
|
||
| M | (SD) | |
|
|
||
| Age (years) | 13.6 | (2.3) |
| CASQ (total; daytime sleepiness) | 31.3 | (8.7) |
| PROMIS Sleep Disturbance | 44.3 | (7.2) |
| PROMIS Sleep-Related Impairment | 50.2 | (7.1) |
|
|
||
| Actigraphya | Mdn | [Q1, Q3] |
|
|
||
| Overall bedtime (hr:min) | 22:46 | [22:25, 23:48] |
| Weekday bedtime (hr:min) | 22:18 | [21:56, 23:32] |
| Weekend bedtime (hr:min) | 23:26 | [23:04, 00:14] |
| Overall wake time (hr:min) | 7:19 | [7:00, 8:00] |
| Weekday wake time (hr:min) | 7:05 | [6:43, 7:51] |
| Weekend wake time (hr:min) | 8:16 | [7:51, 8:43] |
| Overall sleep duration (hr:min) | 7:55 | [7:19, 8:15] |
| Sleep efficiency (%) | 91.1 | [89.8, 92.5] |
Note. N = 25. CASQ = Cleveland Adolescent Sleepiness Questionnaire; Mdn = median; PROMIS = Patient Reported Outcome Measurement Information System; Q1 = 25th percentile; Q3 = 75th percentile.
7-day measurements.
CASQ and PROMIS Sleep Scores
Although no cut-off score for daytime sleepiness has been established for the CASQ, a previous study reported normative sample CASQ scores (M = 31.2, SD = 9.4), as well as CASQ scores for primary snorers (M = 35.0, SD = 12.3) and adolescents with sleep apnea (M = 37.7, SD = 11.5 in adolescents [age 11−17 years]) (Spilsbury et al., 2007). Participants in this study had similar CASQ scores (M = 31.3, SD = 8.7, Table 1) to the normative sample of adolescents.
Participants also had similar (p = .92) PROMIS Sleep-Related Impairment scores (M = 50.2, SD = 7.1, N = 25) to that of the reported normative values for adults (M = 50, SD = 10, N = 2252); however, the mean PROMIS Sleep Disturbance score was significantly lower (p < .005)—indicating less disturbance—in adolescents (M = 44.3, SD = 7.2) than that of the reported normative values for adults (M = 50, SD = 10) (HealthMeasures, 2016). Most PROMIS measures have a mean score of 50, with a standard deviation of 10, based on a large sample of the United States general population. However, sleep disturbance/sleep-related impairment measures were not calibrated on a national sample. A score of 50 on the PROMIS sleep measures represents the average of the calibration sample, which was generally more enriched for chronic illness (HealthMeasures, 2016).
Actigraphy Weekday versus Weekend Bedtime/Wake Time and Overall Sleep Duration
Weekday versus weekend bedtime was nominally earlier (p = .02), and weekday versus weekend wake time was significantly earlier (p < .001). Median overall sleep duration (averaged over the course of seven days for each participant), including both weekday and weekend nights, was less than 8 hours per night (Table 1). Notably, only 24% of the participants met the American Academy of Sleep Medicine’s recommended 9 to 12 hours per 24 hours of sleep for 6−12 years of age or 8 to 10 hours per 24 hours of sleep for 13−18 years of age (Paruthi et al., 2016).
CASQ and PROMIS Sleep Questionnaire Score Correlations
The CASQ score was significantly correlated with PROMIS Sleep Disturbance (rS = .77, p < .001, Figure 1) and Sleep-Related Impairment (rS = .86, p < .001, Figure 1) total scores in our cohort of healthy adolescents. PROMIS Sleep Disturbance and Sleep-Related Impairment scores also were significantly correlated (p < .001, Figure 1).
FIGURE 1.
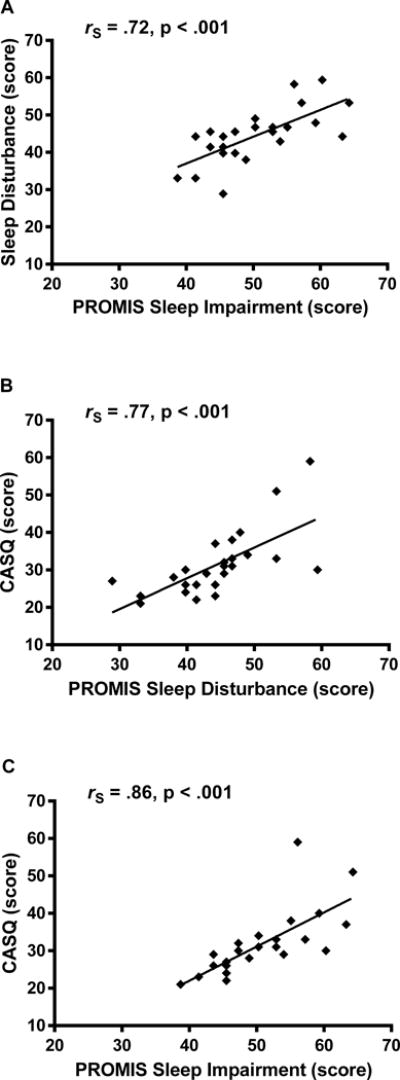
Sleep questionnaire score correlations in adolescents. Spearman correlations assessed the relationships among CASQ, PROMIS Sleep Disturbance total score, and PROMIS Sleep Related Impairment total score. Spearman correlations and nominal p-values are shown.
Total Sleep Time Using Actigraphy and Sleep Questionnaire Score Correlations
Total sleep time measured by actigraphy was negatively correlated in the expected direction with CASQ (rS = −.50, p = .01), PROMIS Sleep Disturbance (rS = −.46, p = .02), and Sleep-Related Impairment (rS = −.46, p = .02) total scores in our cohort of healthy adolescents (Figure 2). Thus, higher total sleep time measured by actigraphy was correlated with less daytime sleepiness, less sleep disturbance, and less sleep-related impairment.
FIGURE 2.
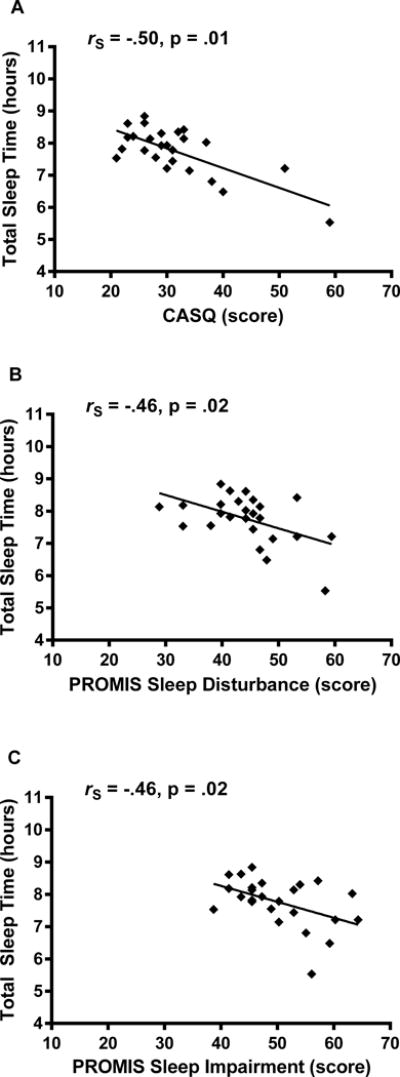
Sleep questionnaire and total sleep time correlations in adolescents. Spearman correlations assessed the relationships among CASQ, PROMIS Sleep Disturbance, and PROMIS Sleep-Related Impairment total scores with total sleep time (daily average over a one-week period) measured by actigraphy. Spearman correlations and nominal p-values are shown.
Discussion
This study performed preliminary validation of age-appropriate, self-reported sleep measures in healthy adolescents. To the best of our knowledge, construct validity of the PROMIS sleep measures in healthy adolescents has not been previously reported in the extant literature. The adolescents in our sample had an average self-reported CASQ score comparable to that of the control group used to develop the questionnaire (Spilsbury et al., 2007). The mean PROMIS Sleep-Related Impairment score in our adolescent cohort was also similar to normative adult data, but the PROMIS Sleep Disturbance score was lower than typical adult values. Our preliminary validation study found significant correlations in expected directions among all three sleep questionnaires (CASQ, PROMIS Sleep Disturbance, and PROMIS Sleep-Related Impairment), as well as total sleep time measured by actigraphy in healthy adolescents.
The field of pediatric sleep and the treatment of sleep disorders in children would benefit from well-designed, psychometrically sound measures. No pediatric measures are currently available in the PROMIS sleep domains, and our study provides potential research and clinical utility for applying the adult versions of PROMIS sleep measures in an adolescent population. PROMIS measures were developed to be used across conditions and across the lifespan, and our findings support the use the PROMIS sleep measures in longitudinal research examining the transition from adolescence to adulthood.
The majority of sleep questionnaires used to investigate sleep issues in children and adolescents are completed by a parent or caregiver (Spruyt & Gozal, 2011). Although a parent report may provide useful information, parent report of sleep may underestimate sleep problems in children and adolescents, and as children get older, parents may be unaware of increased sleep onset delay experienced by their children, as well as frequency and duration of any night awakenings that may also affect total sleep time (Baker, Richdale, Short, & Gradisar, 2013; Goldman, Richdale, Clemons, & Malow, 2012; J. A. Owens, Spirito, McGuinn, & Nobile, 2000). Although many sleep questionnaires have been developed, and their psychometrics have been evaluated (Spruyt & Gozal, 2011), there is no fully validated legacy adolescent self-reported sleep measure. Taking this limitation into consideration, we selected the CASQ for our comparison studies with the PROMIS measures, because the CASQ meets most psychometric criterion, though it lacks standardization and norms development (Spruyt & Gozal, 2011). This is the first preliminary validation of the use of the PROMIS sleep measures in adolescents using both subjective self-reported questionnaires and objective actigraphy data.
Conclusion
Adolescents require anywhere from 8 to 12 hours of sleep each night, and over the past two decades, researchers, teachers, parents, and adolescents have consistently reported inadequate sleep throughout adolescence. Healthy adolescents in our sample slept less than 8 hours per night. Chronic sleep deprivation over time may impact daytime functioning, quality of life, and overall health of adolescents. This is the first study to show preliminary evidence of the validation of the adult versions of the PROMIS sleep measures in an adolescent population. Future studies should explore additional psychometric properties of PROMIS sleep measures in correlation with polysomnography in larger, demographically diverse populations of adolescents with and without clinical sleep abnormalities to confirm their utility as reliable measures of sleep.
Acknowledgments
The authors acknowledge this study was funded by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1ZIAHD008898-02) and the National Institute of Nursing Research of the National Institutes of Health (NIH), and by the NIH Bench-to-Bedside Program.
Data for this research were obtained as part of Characterization of WAGR Syndrome and Other Chromosome 11 Gene Deletions, registered at ClinicalTrials.gov (NCT00758108).
Present findings were presented orally at the 2016 Midwest Nursing Research Society meeting in Milwaukee, WI, and are included in the doctoral dissertation thesis of A.E.H.
The authors would like to thank the following individuals for their assistance: Jack Yanovski, Sheila Brady, Daniela Reyes-Capo, Yael Caplan, Janet Williams, and Ann Berger.
The content does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Alyson E. Hanish, Section on Growth and Obesity, Program in Developmental Endocrinology and Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD; National Institute of Nursing Research, Bethesda, MD; University of Iowa, College of Nursing, Iowa City, IA.
Deborah C. Lin-Dyken, Roy J. and Lucille A. Carver College of Medicine, Stead Family Department of Pediatric, University of Iowa, Iowa City, IA.
Joan C. Han, Section on Growth and Obesity, Program in Developmental Endocrinology and Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), Bethesda, MD; Department of Pediatrics and Physiology, University of Tennessee Health Science Center, Children’s Foundation Research Institute, Le Bonheur Children’s Hospital, Memphis, TN.
References
- Baker E, Richdale A, Short M, Gradisar M. An investigation of sleep patterns in adolescents with high-functioning autism spectrum disorder compared with typically developing adolescents. Developmental Neurorehabilitation. 2013;16:155–165. doi: 10.3109/17518423.2013.765518. [DOI] [PubMed] [Google Scholar]
- Berger AM, Wielgus KK, Young-McCaughan S, Fischer P, Farr L, Lee KA. Methodological challenges when using actigraphy in research. Journal of Pain and Symptom Management. 2008;36:191–199. doi: 10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, Pilkonis PA. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33:781–792. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Cook K. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dregan A, Armstrong D. Adolescence sleep disturbances as predictors of adulthood sleep disturbances—A cohort study. Journal of Adolescent Health. 2010;46:482–487. doi: 10.1016/j.jadohealth.2009.11.197. [DOI] [PubMed] [Google Scholar]
- Garner AA, Miller MM, Field J, Noe O, Smith Z, Beebe DW. Impact of experimentally manipulated sleep on adolescent simulated driving. Sleep Medicine. 2015;16:796–799. doi: 10.1016/j.sleep.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SE, Richdale AL, Clemons T, Malow BA. Parental sleep concerns in autism spectrum disorders: Variations from childhood to adolescence. Journal of Autism and Developmental Disorders. 2012;42:531–538. doi: 10.1007/s10803-011-1270-5. [DOI] [PubMed] [Google Scholar]
- HealthyPeople.gov. Healthy People 2020: Sleep health. 2016 Retrieved from http://www.healthypeople.gov/2020/topics-objectives/topic/sleep-health.
- Liu X. Sleep and adolescent suicidal behavior. Sleep. 2004;27:1351–1358. doi: 10.1093/sleep/27.7.1351. [DOI] [PubMed] [Google Scholar]
- Martiniuk ALC, Senserrick T, Lo S, Williamson A, Du W, Grunstein RR, Ivers RQ. Sleep-deprived young drivers and the risk for crash: The DRIVE prospective cohort study. JAMA Pediatrics. 2013;167:647–655. doi: 10.1001/jamapediatrics.2013.1429. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung and Blood Institute. 2011 National Institutes of Health Sleep Disorders Research Plan. 2011 Retrieved from http://www.nhlbi.nih.gov/health-pro/resources/sleep/nih-sleep-disorders-research-plan-2011.
- Owens J, Adolescent Sleep Working Group, & Committee on Adolescence Insufficient sleep in adolescents and young adults: An update on causes and consequences. Pediatrics. 2014;134:e921–e932. doi: 10.1542/peds.2014-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M, Nobile C. Sleep habits and sleep disturbance in elementary school-aged children. Journal of Developmental & Behavioral Pediatrics. 2000;21:27–36. doi: 10.1097/00004703-200002000-00005. [DOI] [PubMed] [Google Scholar]
- Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, Wise MS. Recommended amount of sleep for pediatric populations: A consensus statement of the American Academy of Sleep Medicine. Journal of Clinical Sleep Medicine. 2016;12:785–786. doi: 10.5664/jcsm.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizza F, Contardi S, Antognini AB, Zagoraiou M, Borrotti M, Mostacci B, Cirignotta F. Sleep quality and motor vehicle crashes in adolescents. Journal of Clinical Sleep Medicine. 2010;6:41–45. [PMC free article] [PubMed] [Google Scholar]
- HealthMeasures. PROMIS®. 2016 Retrieved from http://www.nihpromis.org/
- Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10–14. Sleep. 1998;21:861–868. [PubMed] [Google Scholar]
- Reiter J, Rosen D. The diagnosis and management of common sleep disorders in adolescents. Current Opinion in Pediatrics. 2014;26:407–412. doi: 10.1097/MOP.0000000000000113. [DOI] [PubMed] [Google Scholar]
- Spilsbury JC, Drotar D, Rosen CL, Redline S. The Cleveland Adolescent Sleepiness Questionnaire: A new measure to assess excessive daytime sleepiness in adolescents. Journal of Clinical Sleep Medicine. 2007;3:603–612. [PMC free article] [PubMed] [Google Scholar]
- Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: A review of currently available instruments. Sleep Medicine Reviews. 2011;15:19–32. doi: 10.1016/j.smrv.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten JA, Terwee CB, Kaspers GJ, van Litsenburg RR. Content validity of the Patient-Reported Outcomes Measurement Information System Sleep Disturbance and Sleep Related Impairment item banks in adolescents. Health and Quality of Life Outcomes. 2016;14:92. doi: 10.1186/s12955-016-0496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorona RD, Szklo-Coxe M, Lamichhane R, Ware JC, McNallen A, Leszczyszyn D. Adolescent crash rates and school start times in two central Virginia counties, 2009–2011: A follow-up study to a southeastern Virginia study, 2007–2008. Journal of Clinical Sleep Medicine. 2014;10:1169–1177. doi: 10.5664/jcsm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69:875–887. doi: 10.1111/j.1467-8624.1998.tb06149.x. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Understanding adolescent’s sleep patterns and school performance: A critical appraisal. Sleep Medicine Reviews. 2003;7:491–506. doi: 10.1016/S1087-0792(03)90003-7. [DOI] [PubMed] [Google Scholar]


