Abstract
Biomarkers to predict the altering physiological conditions over the period leading toward the ocular disorders are of major importance in therapeutics. Isolation and validation of the biomarkers specific to ocular diseases are a challenging task. Glaucoma is a neurodegenerative disease of the eye where the correlation of biomarkers in circulating fluid may be made specific for the eye. However, conditions such as wet age-related macular degeneration (AMD) and proliferative diabetic retinopathy (DR), circulating biomarkers might be having some degree of overlap with other conditions like cancer where a common factor such as angiogenesis is involved. Diabetes, a systemic disorder affecting the target organs such as eye, kidney, heart, and nervous system can be predicted using common circulating biomarkers. However, these markers need to be validated along with various stages of disease progression to enable the possibility of targeted pharmacological interventions apart from good glycemic control alone. This review compiles the attempts made to correlate such circulating biomarkers in the ocular conditions such as glaucoma, AMD, and DR in the search for a surrogate marker for diagnostic and prognostic value. To make biomarkers for the common convenience, genetic markers are excluded from this review.
Key words: Age-related macular degeneration, circulating biomarkers in glaucoma, diabetic retinopathy
Biomarkers are defined as “cellular, biochemical or molecular alteration that is measurable in biological media such as human tissues, cells or fluids.”[1] Circulating biomarkers are by enlarge referred to the investigations that can be done in blood or its components which in term reflect the pathological process in the target organ. As far as biomarkers for ocular disease is concerned, attributing circulating biomarkers to the ocular pathologies can be divided into two broad categories, namely, ocular manifestations which are reflected in blood and systemic manifestations that are attributed to ocular pathologies.
Circulating Biomarkers In Glaucoma
Molecular pathophysiological mechanisms of glaucoma are considered to have some degree of overlap with other neurodegenerative disorders. Stress, apoptosis, DNA-repair, cell adhesion, tissue remodeling, transcription regulation, transporters, vascular tone, and energy metabolism are reported to be the pathways involved in glaucoma. Along with the factors involved in the pathways, circulating leukocytes have also showed their utility in diagnostic and prognostic purpose to monitor glaucoma.[2]
Oxidative stress
Alteration in the levels of glutathione peroxidase (GPX), superoxide dismutase (SOD), and malondialdehyde (MDA) has also been reported in the aqueous humor of patients with primary open-angle glaucoma (POAG). There was a study where oxidative stress markers such as myeloperoxidase, catalase (CAT), and MDA in the plasma of the patients with POAG have been compared with controls.[3] This study showed significantly higher levels of MDA in patients with POAG as compared to control and suggested the role of oxidative damage in glaucoma in the process of aging. Decrease in total antioxidant capacity (TAC) in the plasma of patients with POAG has also been reported by the larger study included 139 patients when compared with age- and sex-matched healthy controls.[4] Other studies have also supported the reduction in TAC in aqueous humor and blood samples from patients with glaucoma.[5,6]
In a multicentric study, 160 glaucoma patients with no known additional abnormalities showed a significant decrease in the levels of advanced oxidation protein products (AOPPs), GPX, SOD, and TAC.[7] Whereas the levels of MDA, serine, Vitamin A and E were found to be increased in the patient group as compared to healthy controls. Similarly, the significant decrease of antioxidant enzymes CAT, SOD, and GPX and a nonstatistical decrease of TAC were recorded in glaucoma patients (n = 20) as compared to healthy controls. Chang et al. reported the alteration of oxidative stress markers in primary angle-closure glaucoma (PACG) in fifty patients. This study showed that oxidation markers such as MDA, conjugated diene, AOPPs, 8-hydroxydeoxyguanosin (8-OHdG), and ischemia-modified albumin were found to be significantly higher in PACG.[8]
Vascular tone and architecture
Nitric oxide
Galassi et al. investigated the plasma and aqueous humor levels of cyclic guanosine monophosphate (cGMP) and nitrite (NO2-) in patients with POAG and their relation to ocular perfusion pressure. The results of the study showed that cGMP and NO2-levels were significantly decreased in both of these fluids of glaucoma patients as compared to healthy controls. Moreover, this study has also reported a linear relation between plasma levels of cGMP with aqueous humor with a strong positive correlation.[9]
L-arginine metabolites
Asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) are the dimethylated isomeric derivatives of the amino acid L-arginine. As they were implicated in retinal vascular tone, Javadiyan et al. quantified serum ADMA, SDMA, and L-arginine levels in patients with advanced glaucoma compared with normal healthy controls. This study also showed a significant increase in the serum levels of both of the derivatives associated with advanced glaucoma.[10]
Endothelin
Endothelin-1 (ET-1) is a potent vasoconstrictor peptide produced by vascular endothelial cells. Plasma levels of ET-1 are reported to be raised in low-tension glaucoma. Increased plasma ET-1 level to the extent of 70% is reported to cause visual field damage low tension glaucoma as compared to control. An increased level of ET-1 has been attributed to an alteration in the endothelial self-regulating sections and consequent vascular insufficiency. Irreversible functional damage has been speculated to be due to pronounced posterior ciliary arteries which supply blood to the optic nerve head.[11] Increased ET-1 levels are also reported to be involved in the regulation of aqueous dynamics in POAG patients. Increased prevalence of retinal venous thrombosis and optic nerve damage in glaucoma has been reported due to the elevated levels of prothrombin fragments 1 + 2 and D-dimer compared with both normal-tension glaucoma and healthy controls. Significant increase in plasma ET-1 and homocysteine levels in patients with POAG is related to vascular endothelial dysfunction.[12] Due to endothelial dysfunction, plasma ET-1 levels in glaucoma patients are five times higher than corresponding age-matched healthy controls.[13]
Atrial natriuretic peptide
Atrial natriuretic peptide (ANP) is a hormone secreted from the right atrium which is known to have natriuretic and diuretic properties. Brain natriuretic peptide (BNP) and ANP are cyclic endopeptidases whose principal biological effects are natriuresis and vasodilatation. The presence of both BNP and ANP in the aqueous humor has been well documented.[14] Recently, Baumane et al. identified an association between the levels of N-terminal-proANP in the plasma and the aqueous humor with POAG and suggested that ANP as a possible biomarker of POAG.[15]
Immune system
Yang et al. hypothesized the role of immune system in the initiation and progress of glaucomatous optic neuropathy in some glaucoma patients. This study analyzed the subsets of T cells and the levels of cytokine interleukin-2 (IL-2) and the soluble IL-2 receptor in peripheral blood from patients with normal pressure glaucoma or POAG and compared them with age-matched controls. The results showed that the frequency of CD8(+) human leukocyte antigen-diabetic retinopathy (HLA-DR)(+) lymphocytes were increased in patients with normotensive glaucoma (NTG) and CD3(+) CD8(+) lymphocytes increased in both NTG and POAG patients. CD5(+) lymphocytes were higher only in POAG patients. The mean concentrations of soluble IL-2 receptor in NPG and POAG patients were higher than that found in controls.[16]
Grus et al. subjected the serum of patients having POAG and NTG for the presence of auto-antibodies against bovine optic nerve antigens. This study showed the presence of 120 kDa alpha-fodrin antibodies in patients with NTG and POAG as compared to age-matched control.[17] Apart from this, upregulation of anti-HSP60, anti-MBP, and downregulation of anti-14-3-3 have been described in the patients with glaucoma.[18]
Subtracted genes encoding lymphocyte IgE receptor (Fc epsilon RII/CD23), T-cell-specific tyrosine kinase, thromboxane A2 receptor, alkaline phosphatase, and Na+/K+-ATPase have been reported to be differentially expressed in circulating leukocytes of glaucoma patients as compared to age-/sex-matched healthy controls.[19]
Wunderlich et al. evaluated the circulating leukocyte proteasome levels (20S proteasome alpha-subunit). This study showed 3.4-fold increase of plasma proteasome level in glaucoma patients (6 HTG and 6 NTG) as compared to healthy control.[20] Weinstein et al. reported that patients with POAG having 3 alpha-HSD activity in the peripheral blood lymphocytes; hence, they have concluded that it can be used as a marker for POAG for those at the risk for developing the disease.[21]
Metabolism and cell cycle
Nowak et al. quantified the serum levels of the markers of connective tissue metabolism P I CP and P III NP in 20 patients with POAG and in 10 healthy controls. This study reported 40% increase in the levels of P III NP in the serum as compared to control.[22]
Cholesterod-24S-hydroxylase (CYP46A1) is a cholesterol-metabolizing enzyme which is especially expressed in retinal ganglion cells. The metabolic product 24S-hydroxycholesterol has been linked with neurodegeneration. Fourgeux et al. showed that genetic polymorphism of CYP46A1 was responsible for the plasma levels of 24S-hydroxycholesterol in patients.[23]
When the plasma and urine citrate, creatinine levels were analyzed in patients (n = 21) with glaucoma, Fraenkl et al. found that the mean plasma citrate concentrations were significantly lower among the patients with glaucoma as compared to control while the kidney functions were considered as normal.[24]
Slepova et al. studied the role of apoptosis in the pathogenesis of POAG by quantifying the markers in blood serum and tear fluid of patients with or suspected to have different stages glaucoma. Dynamics of soluble fas (sFas)/Apo-1 and sFas ligand (sFasL) as the markers of Fas-mediated apoptosis was studied during the treatment. This study showed characteristic features are detected in Fas/FasL system associated with glaucoma stage and correlating with some clinical and functional parameters.[25]
Miscellaneous
Serum levels of brain-derived neurotrophic factor (BDNF) have been studied in POAG patients and compared with age-matched healthy controls by Ghaffariyeh et al. They have concluded that serum quantification of BDNF could be a marker for early detection of POAG.[26]
Tear levels of prekallikrein/kallikrein, angiotensin-converting enzyme (ACE) activity, BDNF, aqueous levels of IL-8, IL-6, tumor necrosis factor-alpha (TNF-α), diadenosine tetraphosphate, transforming growth factor-beta (TGF-β) vascular endothelial growth factor (VEGF), soluble CD44, gelatinase A, microRNAs (miRNAs) of various other protein, and vitreous levels of neurofilament heavy chain protein were reported to have a novelty to indicate the disease progression in glaucoma. However, their levels in plasma were not been validated for its usefulness in assessment. Similarly, increased levels of erythropoietin and soluble CD44 levels were found to be increased in aqueous humor of POAG patients as compared to healthy control, their circulating levels in serum were found to be insignificant.[27]
Circulating Biomarkers in Diabetic Retinopathy
DR is one of the micro complications of the diabetes. Besides having wide array of treatment option available for the management of DR, restoration of vision in the progressed stage of the disease is very intricate. Although, tight control of blood sugar is reported to show benefit from end-organ damage, the risk of developing diabetic complications despite of sugar control needs validated biomarkers.
Vascular and inflammatory biomarkers
MicroRNAs
MiRNAs are the 19–25 nucleotide long highly conserved noncoding RNAs sequences which work at the posttranscriptional levels for the gene expression regulation. In a study reported by Barutta et al. (2016) miRNA 126 levels were found to be inversely proportionate with the increased vascular complication associated with the type 1 diabetes (T1Ds) condition. The role of miRNA146a in the proliferative phase of DR has been documented by Wang et al.[28] Qing et al. have demonstrated the elevated serum levels of miR-21, miR-181c, and miR-1179 in proliferative DR (PDR) cases as compared to non-PDR (NPDR) cases.[29]
Cellular adhesion molecules
Fasching et al. demonstrated that irrespective of actual metabolic control, serum concentrations of intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 but not endothelial leukocyte adhesion molecule-1 are elevated in patients with insulin-dependent diabetes mellitus (IDDM), reflecting ongoing endothelial cell stimulation, and leukocyte activation.[30] A study by Kado and Nagata shown that serum E-selectin concentration between diabetic patients with or without microangiopathy and normal controls.[31]
Inflammatory markers
Sharma et al. reported that circulating markers of inflammation, endothelial injury, and TNF signaling are significantly associated with DR in patients with T1Ds. TNF receptor 1 (TNFR-I) and TNFR-II receptors are highly correlated, but DR associated more strongly with TNFR-I in these patients.[32] In a study reported by Arner et al., levels of insulin-like growth factor I in type 1 were found to be decreased in patients with advanced stage of DR,[33] whereas Petty et al. demonstrated that diabetes is associated with a high incidence of endothelial-binding antibodies which do not correlate with retinopathy, von Willebrand factor, ACE, or C-reactive protein (CRP) in a study performed in 777 diabetic patients.[34] The vascular complications of diabetes have been correlated with CRP, TNF-α, and IL-6 in the EURODIAB Prospective Complication Study. They have also found a positive correlation between these inflammatory factors with DR, DN, and cardiovascular disease.[35]
Renin angiotensin system and endothelin
A study performed on 41 IDDM patients compared with those of 26 controls showed elevated serum ACE activity in IDDM.[36] Laurenti et al. have shown that the increased levels of ET-1 could contribute to retinopathy development or, more probably, represent a marker of this diabetes-related complication.[37]
Extracellular matrix metalloproteinase
Jacqueminet et al. recruited 47 type 1 diabetic patients and their findings revealed that peripheral blood matrix metalloproteinase (MMP-9) levels might serve as surrogate biomarkers of retinopathy in type 1 diabetic patients free of other vascular complications.[38] On the other hand, TGF-β which is a potent inducer of extracellular matrix production and of fibrogenesis was also assessed.[39]
Progenitor cells and erythropoietin
A study done on 15 normal controls and 45 type 2 diabetic patients by Lee et al. showed the elevated levels of circulating Endothelial Progenitor Cells (EPCs) and serum erythropoietin (Epo), VEGF, and substancePwhich may be involved in the progression of DR.[40] Brunner et al. demonstrated that vasculogenic circulating progenitor cells, endothelial progenitor cells (EPCs), and mature EPCs have been associated with the DR progression.[41]
Advanced glycation end products
Al-Mesallamy et al. examined the circulating levels of soluble Receptor of Advanced Glycation Endproduct (sRAGE) in 37 type 2 diabetic patient and 20 age-matched healthy nondiabetic individuals and found that serum RAGE levels were significantly lower in patients with NPDR and PDR than in healthy controls and in those without retinopathy.[42]
Oxidative stress markers in diabetic retinopathy
The levels and the types of serum oxidative stress by-products (MDA, conjugated diene, AOPPs, protein carbonyl, and 8-OHdG) have been well documented to have predictive role in DR.[43,44]
Miscellaneous
Caliumi et al. measured the serum adrenomedullin and showed that it was increased in type 2 diabetic patients and correlated it with the presence of retinopathy.[45] Rhodopsin (RPE65) miRNA and retinoschisin were used in the assessment of DR progression, where circulating RPE65 miRNA concentration was found to be higher and lower level of retinoschisin was observed in diabetic patients.[46] Abu El-Asrar et al. showed that the bone-marrow-derived CD133(+) endothelial progenitor cells and CD14(+) monocytes may contribute to vasculogenesis in PDR.[47] Serum monomeric α2-macroglobulin was highly expressed in many diabetic patients as compared to respective control in a study done by Takada et al.,[48] Shiba et al. demonstrated that vitreous fluid sLR11 level may be a novel risk factor for the early development of PDR before the increase in circulating levels in diabetic patients.[49]
In this hospital-based cohort ofT2Ds, the levels of the N-terminal fragment of pro-BNP was found to be enhanced.[50] In a cross-sectional study performed by Blaslov et al. on 44 patients with T1DM, it has been observed that serum dipeptidyl peptidase-4 activity was independently associated with DR.[51] Abhary et al., demonstrated that severe forms of DR was associated with elevated serum ADMA, SDMA, and L-arginine.[52]
Circulating Biomarkers in Age-related Macular Degeneration
There is a strong biological correlation between inflammation, oxidative stress, and endothelial dysfunction in the disease process and progression of age-related macular degeneration (AMD). As antineovascular therapy is the popular strategy followed in this condition, developing a predictable biomarker would be of immense importance for strategizing therapeutic modalities based on the underlying pathology.
Biomarkers for cellular and vascular function
MicroRNA's
Szemraj et al. revealed increased expression of miR661 and miR3121 in serum of patients with dry AMD and miR4258, miR889, and Let7 in patients with wet form. Differences in miRNA serum profile exist between patients with wet and dry form of AMD.[53]
Matrix metalloproteinase
Chau et al. determined the plasma MMP-2 and MMP-9 levels in the AMD patients and their study concluded that plasma MMP-9 levels were significantly higher in ARMD and CNV groups compared to that of the control group.[54]
Circulating endothelial cells and endothelin
Machalinska et al. showed enhanced circulating endothelial cells and EPCs (ET progenitor cells) in the AMD patients compared with the counts in healthy individuals reflects a severe vascular disturbance.[55] ET-1 is a potent vasoconstricting peptide found to be significantly increased in peripheral blood cells of AMD patients.[56]
Inflammation
A study showed significant elevation of serum concentrations of IL-1α, IL-1 β, IL-4, IL-5, IL-10, IL-13, and IL-17 in AMD patients than in controls.[57] Guymer et al. also demonstrated that there is an association between elevated urinary cytokines TGF-β1 and monocyte chemoattractant protein-1 and AMD.[58] Increased plasma level of soluble TNF-II was also found to be associated with AMD.[59] Lechner et al. have observed that circulating CD11b(+) cells and CD16 (hi) HLA-DR(−) neutrophils were found to be increased significantly in AMD patients when compared to controls.[60]
Pigment epithelium-derived and vascular endothelial growth factors
Machalinska et al. (2014) reported marked decrease in the PEDF plasma level in patients with the dry form of AMD, whereas a significant higher level of PEDF and VEGF was observed in the wet form of AMD.[61]
Oxidative stress
Baskol et al. assessed antioxidant paraoxonase 1 (PON1) activity together with MDA levels to evaluate oxidative stress in patients with AMD, where they have concluded that a negative correlation between PON1 activity and MDA levels exists in patients with AMD.[62]
Miscellaneous
A study reported by Seshasai et al. showed that higher serum leptin level was inversely associated with AMD.[63] Uehara et al. evaluated serum soluble Flt-1 in AMD patients.[64] Association of plasma-sFasL with aging and AMD was studied by Jiang et al., and they have observed that plasma sFasL increased with age and AMD.[65] Apart from these, N (epsilon)-carboxymethyllysine and pentosidine, 2-ω-carboxyethylpyrrole ethanolamine phospholipids, eotaxin-CCR3 (CCL24), vinculin, and cystatin C were also speculated to be potential biomarkers.[66,67,68,69,70]
Future/clinical Perspective
Identification and quantification of typical metabolic signatures proportional to the pathological changes in the ocular diseases in blood would be a landmark achievement in future therapeutics. As of now, the progress done with the research attempts to isolate and correlate the levels of biomarkers with the progress of glaucoma, AMD, and DR are inconclusive. Therefore, attempts are required to titrate the levels of biomarkers found in the patients with the validated stages during the progression of the disease. Common biomarkers observed in these ocular disease such as oxidative stress, inflammation, cell adhesion molecules, etc., show higher degree of overlap and can show false positive or false negative value due their nonspecificity. Therefore, identification of a molecular fingerprint is further complicated due to the size of affected tissue such as retina and amount of the biomarker released in the large circulating blood volume needs highly sensitive assay systems. Such biomarkers would be having specific diagnostic and prognostic value in the future ocular therapeutics.
Conclusion
There are accumulating evidences for the involvement of circulating or systemic biomarkers which are correlated with the various upstream and downstream pathological pathways involve in the development of glaucoma, DR, and AMD [Tables 1–3]. However, these targets need to be validated to predict the disease onset, progression of disease, to identify the individual at high risk to develop the disease, or even to assess the effects of the treatment.
Table 1.
Biomarkers evaluated for glaucoma
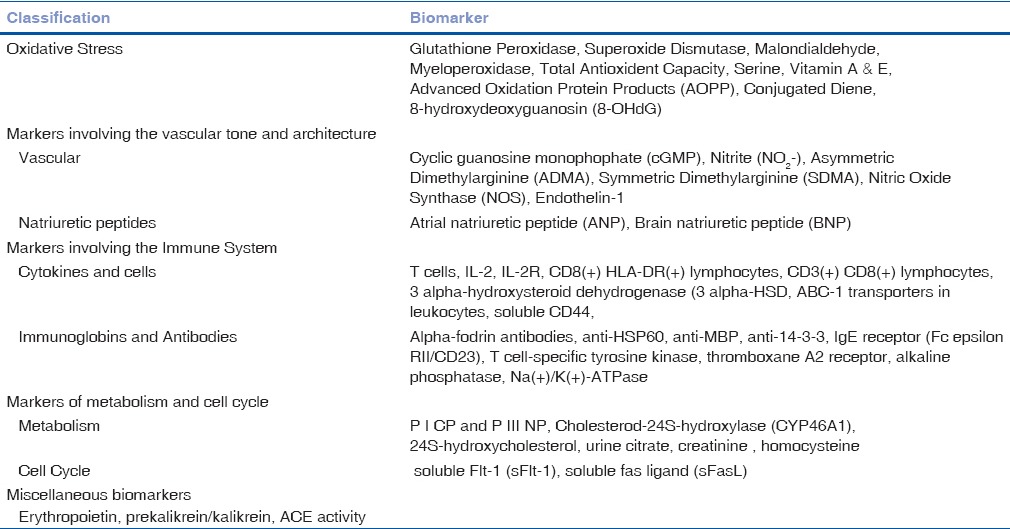
Table 3.
Bio-markers evaluated for Age Related Macular Degeneration
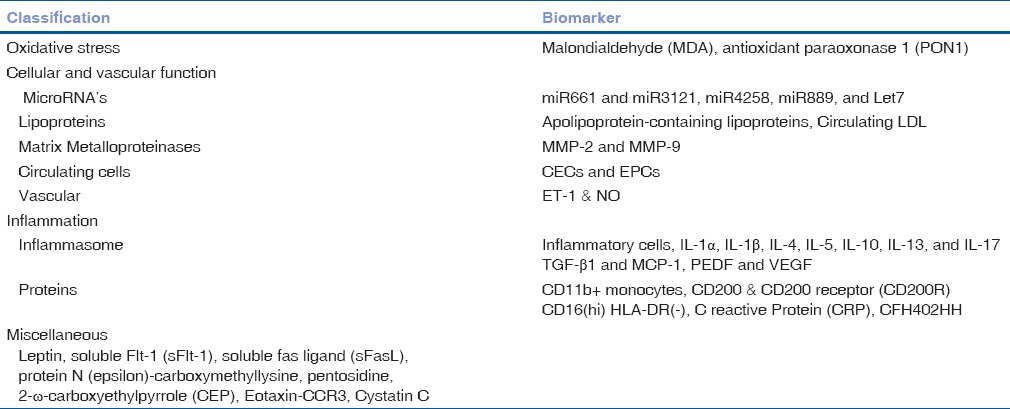
Table 2.
Biomarkers evaluated in Diabetic Retinopathy

Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hulka BS. Biological Markers in Epidemiology. New York: Oxford University Press; 1990. Overview of biological markers; pp. 3–15. [Google Scholar]
- 2.Golubnitschaja O, Flammer J. What are the biomarkers for glaucoma? Surv Ophthalmol. 2007;52(Suppl 2):S155–61. doi: 10.1016/j.survophthal.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Yildirim O, Ates NA, Ercan B, Muslu N, Unlü A, Tamer L, et al. Role of oxidative stress enzymes in open-angle glaucoma. Eye (Lond) 2005;19:580–3. doi: 10.1038/sj.eye.6701565. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Amero K, Kondkar AA, Chalam KV. An updated review on the genetics of primary open angle glaucoma. Int J Mol Sci. 2015;16:28886–911. doi: 10.3390/ijms161226135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nucci C, Di Pierro D, Varesi C, Ciuffoletti E, Russo R, Gentile R, et al. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Mol Vis. 2013;19:1841–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Goyal A, Srivastava A, Sihota R, Kaur J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr Eye Res. 2014;39:823–9. doi: 10.3109/02713683.2011.556299. [DOI] [PubMed] [Google Scholar]
- 7.Engin KN, Yemisci B, Yigit U, Agaçhan A, Coskun C. Variability of serum oxidative stress biomarkers relative to biochemical data and clinical parameters of glaucoma patients. Mol Vis. 2010;16:1260–71. [PMC free article] [PubMed] [Google Scholar]
- 8.Chang D, Sha Q, Zhang X, Liu P, Rong S, Han T, et al. The evaluation of the oxidative stress parameters in patients with primary angle-closure glaucoma. PLoS One. 2011;6:e27218. doi: 10.1371/journal.pone.0027218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galassi F, Renieri G, Sodi A, Ucci F, Vannozzi L, Masini E. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol. 2004;88:757–60. doi: 10.1136/bjo.2003.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Javadiyan S, Burdon KP, Whiting MJ, Abhary S, Straga T, Hewitt AW, et al. Elevation of serum asymmetrical and symmetrical dimethylarginine in patients with advanced glaucoma. Invest Ophthalmol Vis Sci. 2012;53:1923–7. doi: 10.1167/iovs.11-8420. [DOI] [PubMed] [Google Scholar]
- 11.Cellini M, Possati GL, Profazio V, Sbrocca M, Caramazza N, Caramazza R. Color Doppler imaging and plasma levels of endothelin-1 in low-tension glaucoma. Acta Ophthalmol Scand Suppl. 1997;224:11–3. doi: 10.1111/j.1600-0420.1997.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 12.López-Riquelme N, Villalba C, Tormo C, Belmonte A, Fernandez C, Torralba G, et al. Endothelin-1 levels and biomarkers of oxidative stress in glaucoma patients. Int Ophthalmol. 2015;35:527–32. doi: 10.1007/s10792-014-9979-8. [DOI] [PubMed] [Google Scholar]
- 13.Malishevskaia TN, Dolgova IG. Options for correction of endothelial dysfunction and oxidative stress in patients with primary open-angle glaucoma. Vestn Oftalmol. 2014;130:67. [PubMed] [Google Scholar]
- 14.Salzmann J, Flitcroft D, Bunce C, Gordon D, Wormald R, Migdal C. Brain natriuretic peptide: Identification of a second natriuretic peptide in human aqueous humour. Br J Ophthalmol. 1998;82:830–4. doi: 10.1136/bjo.82.7.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumane K, Ranka R, Laganovska G. Association of NT-proANP level in plasma and humor aqueous with primary open-angle glaucoma. Curr Eye Res. 2017;42:233–6. doi: 10.1080/02713683.2016.1180397. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Patil RV, Yu H, Gordon M, Wax MB. T cell subsets and sIL-2R/IL-2 levels in patients with glaucoma. Am J Ophthalmol. 2001;131:421–6. doi: 10.1016/s0002-9394(00)00862-x. [DOI] [PubMed] [Google Scholar]
- 17.Grus FH, Joachim SC, Bruns K, Lackner KJ, Pfeiffer N, Wax MB. Serum autoantibodies to alpha-fodrin are present in glaucoma patients from Germany and the United States. Invest Ophthalmol Vis Sci. 2006;47:968–76. doi: 10.1167/iovs.05-0685. [DOI] [PubMed] [Google Scholar]
- 18.Bell K, Gramlich OW, Von Thun Und Hohenstein-Blaul N, Beck S, Funke S, Wilding C, et al. Does autoimmunity play a part in the pathogenesis of glaucoma? Prog Retin Eye Res. 2013;36:199–216. doi: 10.1016/j.preteyeres.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Golubnitschaja O, Wunderlich K, Decker C, Mönkemann H, Schild HH, Flammer J. Molecular imaging of perfusion disturbances in glaucoma. Amino Acids. 2002;23:293–9. doi: 10.1007/s00726-001-0141-3. [DOI] [PubMed] [Google Scholar]
- 20.Wunderlich K, Golubnitschaja O, Pache M, Eberle AN, Flammer J. Increased plasma levels of 20S proteasome alpha-subunit in glaucoma patients: An observational pilot study. Mol Vis. 2002;8:431–5. [PubMed] [Google Scholar]
- 21.Weinstein BI, Iyer RB, Binstock JM, Hamby CV, Schwartz IS, Moy FH, et al. Decreased 3 alpha-hydroxysteroid dehydrogenase activity in peripheral blood lymphocytes from patients with primary open angle glaucoma. Exp Eye Res. 1996;62:39–45. doi: 10.1006/exer.1996.0005. [DOI] [PubMed] [Google Scholar]
- 22.Nowak M, Swietochowska E, Buntner B, Bandych-Biniszkiewicz D, Pojda S. Evaluation of selected parameters of connective tissue metabolism in patients with primary open angle glaucoma. Klin Oczna. 1997;99:11–4. [PubMed] [Google Scholar]
- 23.Fourgeux C, Martine L, Björkhem I, Diczfalusy U, Joffre C, Acar N, et al. Primary open-angle glaucoma: Association with cholesterol 24S-hydroxylase (CYP46A1) gene polymorphism and plasma 24-hydroxycholesterol levels. Invest Ophthalmol Vis Sci. 2009;50:5712–7. doi: 10.1167/iovs.09-3655. [DOI] [PubMed] [Google Scholar]
- 24.Fraenkl SA, Muser J, Groell R, Reinhard G, Orgul S, Flammer J, et al. Plasma citrate levels as a potential biomarker for glaucoma. J Ocul Pharmacol Ther. 2011;27:577–80. doi: 10.1089/jop.2011.0062. [DOI] [PubMed] [Google Scholar]
- 25.Slepova OS, Frolov MA, Morozova NS, Frolov AM, Lovpache DN. Markers of Fas-mediated apoptosis in primary open-angle glaucoma and opportunities of their pharmacological correction. Vestn Oftalmol. 2012;128:27–31. [PubMed] [Google Scholar]
- 26.Ghaffariyeh A, Honarpisheh N, Heidari MH, Puyan S, Abasov F. Brain-derived neurotrophic factor as a biomarker in primary open-angle glaucoma. Optom Vis Sci. 2011;88:80–5. doi: 10.1097/OPX.0b013e3181fc329f. [DOI] [PubMed] [Google Scholar]
- 27.Mokbel TH, Ghanem AA, Kishk H, Arafa LF, El-Baiomy AA. Erythropoietin and soluble CD44 levels in patients with primary open-angle glaucoma. Clin Exp Ophthalmol. 2010;38:560–5. doi: 10.1111/j.1442-9071.2010.02318.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Bozack SN, Yan Y, Boulton ME, Grant MB, Busik JV. Regulation of retinal inflammation by rhythmic expression of MiR-146a in diabetic retina. Invest Ophthalmol Vis Sci. 2014;55:3986–94. doi: 10.1167/iovs.13-13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qing S, Yuan S, Yun C, Hui H, Mao P, Wen F, et al. Serum miRNA biomarkers serve as a fingerprint for proliferative diabetic retinopathy. Cell Physiol Biochem. 2014;34:1733–40. doi: 10.1159/000366374. [DOI] [PubMed] [Google Scholar]
- 30.Fasching P, Veitl M, Rohac M, Streli C, Schneider B, Waldhäusl W, et al. Elevated concentrations of circulating adhesion molecules and their association with microvascular complications in insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4313–7. doi: 10.1210/jcem.81.12.8954033. [DOI] [PubMed] [Google Scholar]
- 31.Kado S, Nagata N. Circulating intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 1999;46:143–8. doi: 10.1016/s0168-8227(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 32.Sharma S, Purohit S, Sharma A, Hopkins D, Steed L, Bode B, et al. Elevated serum levels of soluble TNF receptors and adhesion molecules are associated with diabetic retinopathy in patients with type-1 diabetes. Mediators Inflamm. 2015;2015:279393. doi: 10.1155/2015/279393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arner P, Sjöberg S, Gjötterberg M, Skottner A. Circulating insulin-like growth factor I in type 1 (insulin-dependent) diabetic patients with retinopathy. Diabetologia. 1989;32:753–8. doi: 10.1007/BF00274537. [DOI] [PubMed] [Google Scholar]
- 34.Petty RG, Pottinger BE, Greenwood RM, Pearson JD, Mahler RF. Diabetes is associated with a high incidence of endothelial-binding antibodies which do not correlate with retinopathy, von Willebrand factor, angiotensin-converting enzyme or C-reactive protein. Diabetes Res. 1991;17:115–23. [PubMed] [Google Scholar]
- 35.Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD. EURODIAB Prospective Complications Study Group. Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes – The EURODIAB Prospective Complications Study. Diabetologia. 2005;48:370–8. doi: 10.1007/s00125-004-1628-8. [DOI] [PubMed] [Google Scholar]
- 36.Duntas L, Keck FS, Haug C, Hetzel W, Wolf CF, Rosenthal J, et al. Serum angiotensin-converting enzyme activity and active renin plasma concentrations in insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1992;16:203–8. doi: 10.1016/0168-8227(92)90118-b. [DOI] [PubMed] [Google Scholar]
- 37.Laurenti O, Vingolo EM, Desideri GB, Ferri C, Bellini C, Cassone-Faldetta M, et al. Increased levels of plasma endothelin-1 in non-insulin dependent diabetic patients with retinopathy but without other diabetes-related organ damage. Exp Clin Endocrinol Diabetes. 1997;105(Suppl 2):40–2. doi: 10.1055/s-0029-1211795. [DOI] [PubMed] [Google Scholar]
- 38.Jacqueminet S, Ben Abdesselam O, Chapman MJ, Nicolay N, Foglietti MJ, Grimaldi A, et al. Elevated circulating levels of matrix metalloproteinase-9 in type 1 diabetic patients with and without retinopathy. Clin Chim Acta. 2006;367:103–7. doi: 10.1016/j.cca.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 39.Pfeiffer A, Middelberg-Bisping K, Drewes C, Schatz H. Elevated plasma levels of transforming growth factor-beta 1 in NIDDM. Diabetes Care. 1996;19:1113–7. doi: 10.2337/diacare.19.10.1113. [DOI] [PubMed] [Google Scholar]
- 40.Lee IG, Chae SL, Kim JC. Involvement of circulating endothelial progenitor cells and vasculogenic factors in the pathogenesis of diabetic retinopathy. Eye (Lond) 2006;20:546–52. doi: 10.1038/sj.eye.6701920. [DOI] [PubMed] [Google Scholar]
- 41.Brunner S, Schernthaner GH, Satler M, Elhenicky M, Hoellerl F, Schmid-Kubista KE, et al. Correlation of different circulating endothelial progenitor cells to stages of diabetic retinopathy:First in vivo data. Invest Ophthalmol Vis Sci. 2009;50:392–8. doi: 10.1167/iovs.08-1748. [DOI] [PubMed] [Google Scholar]
- 42.Al-Mesallamy HO, Hammad LN, El-Mamoun TA, Khalil BM. Role of advanced glycation end product receptors in the pathogenesis of diabetic retinopathy. J Diabetes Complications. 2011;25:168–74. doi: 10.1016/j.jdiacomp.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Mancino R, Di Pierro D, Varesi C, Cerulli A, Feraco A, Cedrone C, et al. Lipid peroxidation and total antioxidant capacity in vitreous, aqueous humor, and blood samples from patients with diabetic retinopathy. Mol Vis. 2011;17:1298–304. [PMC free article] [PubMed] [Google Scholar]
- 44.Pan HZ, Zhang H, Chang D, Li H, Sui H. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. Br J Ophthalmol. 2008;92:548–51. doi: 10.1136/bjo.2007.130542. [DOI] [PubMed] [Google Scholar]
- 45.Caliumi C, Balducci S, Petramala L, Cotesta D, Zinnamosca L, Cianci R, et al. Plasma levels of adrenomedullin, a vasoactive peptide, in type 2 diabetic patients with and without retinopathy. Minerva Endocrinol. 2007;32:73–8. [PubMed] [Google Scholar]
- 46.Shalchi Z, Sandhu HS, Butt AN, Smith S, Powrie J, Swaminathan R. Retina-specific mRNA in the assessment of diabetic retinopathy. Ann N Y Acad Sci. 2008;1137:253–7. doi: 10.1196/annals.1448.008. [DOI] [PubMed] [Google Scholar]
- 47.Abu El-Asrar AM, Struyf S, Verbeke H, Van Damme J, Geboes K. Circulating bone-marrow-derived endothelial precursor cells contribute to neovascularization in diabetic epiretinal membranes. Acta Ophthalmol. 2011;89:222–8. doi: 10.1111/j.1755-3768.2009.01700.x. [DOI] [PubMed] [Google Scholar]
- 48.Takada T, Kodera Y, Matsubara M, Kawashima Y, Maeda T, Fujita Y, et al. Serum monomeric α2-macroglobulin as a clinical biomarker in diabetes. Atherosclerosis. 2013;228:270–6. doi: 10.1016/j.atherosclerosis.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 49.Shiba T, Bujo H, Takahashi M, Sato Y, Jiang M, Hori Y, et al. Vitreous fluid and circulating levels of soluble lr11, a novel marker for progression of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2013;251:2689–95. doi: 10.1007/s00417-013-2373-9. [DOI] [PubMed] [Google Scholar]
- 50.Hamano K, Nakadaira I, Suzuki J, Gonai M. N-terminal fragment of probrain natriuretic peptide is associated with diabetes microvascular complications in type 2 diabetes. Vasc Health Risk Manag. 2014;10:585–9. doi: 10.2147/VHRM.S67753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blaslov K, Bulum T, Duvnjak L. Circulating dipeptidyl peptidase-4 activity is associated with diabetic retinopathy in type 1 diabetic patients. Eur J Ophthalmol. 2015;25:328–32. doi: 10.5301/ejo.5000547. [DOI] [PubMed] [Google Scholar]
- 52.Abhary S, Kasmeridis N, Burdon KP, Kuot A, Whiting MJ, Yew WP, et al. Diabetic retinopathy is associated with elevated serum asymmetric and symmetric dimethylarginines. Diabetes Care. 2009;32:2084–6. doi: 10.2337/dc09-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szemraj M, Bielecka-Kowalska A, Oszajca K, Krajewska M, Gos R, Jurowski P, et al. Serum microRNAs as potential biomarkers of AMD. Med Sci Monit. 2015;21:2734–42. doi: 10.12659/MSM.893697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chau KY, Sivaprasad S, Patel N, Donaldson TA, Luthert PJ, Chong NV. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye (Lond) 2008;22:855–9. [PubMed] [Google Scholar]
- 55.Machalinska A, Safranow K, Dziedziejko V, Mozolewska-Piotrowska K, Paczkowska E, Klos P, et al. Different populations of circulating endothelial cells in patients with age-related macular degeneration: A novel insight into pathogenesis. Invest Ophthalmol Vis Sci. 2011;52:93–100. doi: 10.1167/iovs.10-5756. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Tian XY, Huang Y, Wang N. Rosiglitazone attenuated endothelin-1-induced vasoconstriction of pulmonary arteries in the rat model of pulmonary arterial hypertension via differential regulation of ET-1 receptors. PPAR Res. 2014;2014:374075. doi: 10.1155/2014/374075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nassar K, Grisanti S, Elfar E, Lüke J, Lüke M, Grisanti S. Serum cytokines as biomarkers for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2015;253:699–704. doi: 10.1007/s00417-014-2738-8. [DOI] [PubMed] [Google Scholar]
- 58.Guymer RH, Tao LW, Goh JK, Liew D, Ischenko O, Robman LD, et al. Identification of urinary biomarkers for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:4639–44. doi: 10.1167/iovs.10-7120. [DOI] [PubMed] [Google Scholar]
- 59.Faber C, Jehs T, Juel HB, Singh A, Falk MK, Sørensen TL, et al. Early and exudative age-related macular degeneration is associated with increased plasma levels of soluble TNF receptor II. Acta Ophthalmol. 2015;93:242–7. doi: 10.1111/aos.12581. [DOI] [PubMed] [Google Scholar]
- 60.Lechner J, Chen M, Hogg RE, Toth L, Silvestri G, Chakravarthy U, et al. Alterations in circulating immune cells in neovascular age-related macular degeneration. Sci Rep. 2015;5:16754. doi: 10.1038/srep16754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machalinska A, Mozolewska-Piotrowska K, Paczkowska E, Lubiniski W. Increased expression of endothelin-1 – A novel diagnostic marker for early AMD detection? Klin Oczna. 2014;116:16–20. [PubMed] [Google Scholar]
- 62.Baskol G, Karakucuk S, Oner AO, Baskol M, Kocer D, Mirza E, et al. Serum paraoxonase 1 activity and lipid peroxidation levels in patients with age-related macular degeneration. Ophthalmologica. 2006;220:12–6. doi: 10.1159/000089269. [DOI] [PubMed] [Google Scholar]
- 63.Seshasai S, Liao J, Toh QC, Cheng CY, Cheung GC, Sethi S, et al. Serum leptin and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:1880–6. doi: 10.1167/iovs.14-15933. [DOI] [PubMed] [Google Scholar]
- 64.Uehara H, Mamalis C, McFadden M, Taggart M, Stagg B, Passi S, et al. The reduction of serum soluble Flt-1 in patients with neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159:92–100.e1.2. doi: 10.1016/j.ajo.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang S, Moriarty-Craige SE, Li C, Lynn MJ, Cai J, Jones DP, et al. Associations of plasma-soluble fas ligand with aging and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:1345–9. doi: 10.1167/iovs.07-0308. [DOI] [PubMed] [Google Scholar]
- 66.Ni J, Yuan X, Gu J, Yue X, Gu X, Nagaraj RH, et al. Plasma protein pentosidine and carboxymethyllysine, biomarkers for age-related macular degeneration. Mol Cell Proteomics. 2009;8:1921–33. doi: 10.1074/mcp.M900127-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim HJ, Woo SJ, Suh EJ, Ahn J, Park JH, Hong HK, et al. Identification of vinculin as a potential plasma marker for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:7166–76. doi: 10.1167/iovs.14-15168. [DOI] [PubMed] [Google Scholar]
- 68.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–71. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharma NK, Prabhakar S, Gupta A, Singh R, Gupta PK, Gupta PK, et al. New biomarker for neovascular age-related macular degeneration: Eotaxin-2. DNA Cell Biol. 2012;31:1618–27. doi: 10.1089/dna.2012.1786. [DOI] [PubMed] [Google Scholar]
- 70.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]


