Abstract
Advances in technology have made surgery in children safer and faster. The management of pediatric cataract has made rapid progress in the past decade with the availability of safer anesthesia, newer technique's, more predictable intraocular lens (IOL) power calculation, a better understanding of neurobiology, genetics, amblyopia management, improved IOL designs for preventing visual axis opacification, and adjuvant postoperative care. Modern vitrectomy machines with minimally invasive instruments, radiofrequency, diathermy, and plasma blades help immensely in complicated cases. Preoperative evaluation with ultrasound biomicroscopy and optical coherence tomography (OCT) allows better planning of surgical procedure. The future holds good for stem cell research, customized OCT, and Zepto (precision pulse capsulotomy).
Key words: Advances, innovations, pediatric cataract
Childhood cataract accounts for 7.4%–15.3% of childhood blindness[1] and a significant amount of avertable disability-adjusted life years. The median prevalence is about 1.03/10,000 children (0.32–22.9/10,000). The incidence ranged from 1.8 to 3.6/10,000 per year. The prevalence of childhood cataract in high-income economies was found to be 0.42–2.05 compared with 0.63–13.6/10,000 in low-income economies. There was no difference in the prevalence based on laterality or gender.[2] India has a burden of around 280,000–320,000 visually impaired children,[3] leading to an estimated lifetime loss of earning capacity of US $3500 million.[4] The management of pediatric cataract has changed dramatically in the past decade.
Preoperative Evaluation
Preoperative factors play a major role in the postoperative outcomes in children. The age of onset, type of cataract, laterality, delay in presentation, best-corrected distance visual acuity, the presence of strabismus, nystagmus, and glaucoma are all predictors of postoperative visual outcomes in children.[5] The delay in presentation to hospital for surgery is associated with poor outcomes. Congenital cataract has poorer outcomes compared to developmental cataract as it is often associated with visual deprivation in the early sensitive years of visual maturation.[6] Cataract-associated nystagmus has six times lesser chance of attaining 20/40 visual acuity compared to cataract without nystagmus.[5] Congenital cataract operated <1 year of age has the increased risk of postoperative visual axis opacification (VAO).[5] Bilateral cataract has better visual outcome than unilateral cataract, 78% of the children with bilateral cataract had more than 20/40 visual acuity.[5] Whereas unilateral cataract is often associated with microphthalmos, persistent fetal vasculature (PFV), anisometropia, and late presentation, hence has poorer visual outcomes. Preoperative good distance visual acuity has a better prognosis as it indicates amblyopia has not set in these eyes, whereas cataract associated with strabismus generally indicates that visual acuity is poor in that eye.[7] Preexisting glaucoma has the added disadvantage as these children undergo additional surgeries.
Ultrasound Biomicroscopy
Ultrasound biomicroscopy (UBM) is a noninvasive technique for imaging the anterior segment of an eye, which is extensively used in glaucoma to look for angle anomalies, iris pattern, ciliary body, and ciliary processes.[8] The probes most commonly used for the ophthalmic purpose are 10 MHz, 35 MHz, and 50 MHz, with the increase in frequency penetration decreases.[9] Higher frequency ultrasound is useful for anterior segment evaluation. In addition to its role in measuring the corneal thickness, anterior chamber depth, and angle structures, it can be used preoperatively to analyze the sulcus, sulcus-to-sulcus measurement, integrity, and the presence of anterior capsule for secondary intraocular lens (IOL) and lens thickness.[9,10,11] It is of prime importance in identifying anterior persistent hyperplastic primary vitreous, posterior capsular defect, and posterior polar cataract preoperatively, which helps in better planning and management of the case.[11] Ultrasound biomicroscopy also helps us in posttraumatic cases to look for cyclodialysis, subluxation, foreign body localization in anterior segment, and rupture of the posterior capsule [Fig. 1].[12] It can also be used for planning the microvitreoretinal (MVR) entry in anteriorly dislocated lens in cases of spherophakia.[13]
Figure 1.
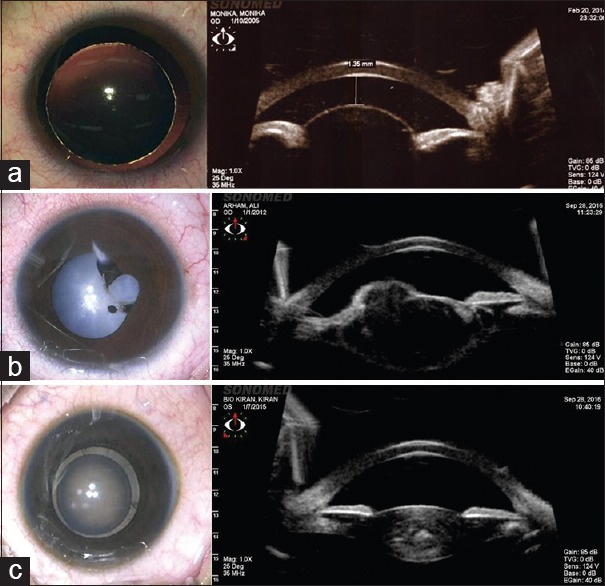
Clinical picture and ultrasound biomicroscopy (a) spherophakia (b) posttraumatic anterior capsule rupture (c) developmental zonular cataract
Optical Coherence Tomography
It is noninvasive, a noncontact technique for imaging the anterior and posterior segment of an eye with a high resolution up to 1 μ[14] Currently, with the advent of swept-source optical coherence tomography (OCT) using a longer wavelength of 1060 nm,[15] imaging of choroid has improved significantly.[16] The choroid is known to play an important role in the growth of eye;[16] hence serial imaging of choroid over a period of time might help us in better understanding the postoperative myopic shift and the pathogenesis of myopia. Using spectrally encoded extended source, high-resolution extended source (HRES) OCT system capable of providing a transverse resolution of 4.4 μm and an axial resolution of 2.1 μm in the air have become possible.[17] The power of lens calculation from extended depth OCT, using an improvement on the Bennett method is more accurate.[18] Already, OCT is being extensively used for both anterior and posterior segment evaluation. The retinal nerve fibre layer thickness is different in ethnicities;[19] it is also negatively correlated to axial length.[20] The central macular edema can sometimes be missed on clinical examination in children, which can be captured on OCT, which is seen in around 25% of children operated for complicated cataract in juvenile idiopathic arthritis.[21] Besides these, OCT can be used to check the vaulting of IOL,[22] placement of anterior chamber IOL (ACIOL), angle structures, and anomalies.
Biometry
Axial length
Predicting axial length growth and hence the refractive outcome is the major remaining challenge in pediatric cataract surgery. Axial length increases rapidly in the first 6 months (0.62 mm/month), then has a relatively slower (infantile phase) growth (0.19 mm/month) till 18 months, followed by a slow (juvenile phase) growth (0.01 mm/month).[23] Even this growth is different in different ethnicities.[24] Rate of axial length growth in pseudophakic children is more rapid in unilateral cases compared to bilateral cases.[25] Axial length growth postoperatively is also variable in children; hence, the absolute error in children is higher compared to the adult population.[26] Axial length measurement has been shown to be better estimated with immersion A-scan than A-scan only because of compression of the anterior surface of the cornea.[23] Axial length measurements made with a contact technique are on an average, 0.24–0.32 mm less than measurements made using an immersion technique. In spite of this disadvantage, indentation method is more commonly used (82.4% vs. 17.6%). Hence, if immersion scan is not possible, A-scan reading with maximum anterior chamber depth should be taken.[23] Still, measurement of axial length can be erroneous because the child is not fixing under anesthesia, that is why there is a need for an instrument which can measure the axial length along the visual axis with fovea being imaged.
Keratometry
Keratometry values are typically obtained under general anesthesia using a handheld autokeratometer with a manual keratometer in older children, whenever it is possible to take awake measurements. Keratometry steeply reduces in the first 6 months, i.e., −0.40 D/month, −0.14 D/month in the second semester, and −0.08 D/month in the 2nd year.[24] Corneal curvature reaches the adult range at about 3 years of age.[27] Girls have steeper corneas than boys.[28] Axial length has a linear relationship with keratometry, as the axial length increases keratometry decreases.[29] The K values of eyes with cataract in monocular cases were steeper than those of bilateral cataracts.[29] Keratometry is greater in the cataractous than in the fellow eyes. The mean preoperative keratometry is also different in congenital (47.78 D) than in the developmental cataract (44.35 D).[30] Mean keratometry values are significantly associated with the mean prediction error in IOL power. Hence, obtaining the right keratometry value should never be underestimated. Keratometry readings without speculum are preferred though technically difficult, as keratometry with the speculum is known to deform the globe and give an unreliable reading.[31]
Intraocular Lens Power Calculation
The full-term newborn has a mean keratometric power of 51.2 D (spherical equivalent), a mean axial length of 16.8 mm, and a mean lens power of 34.4 D which changes to 43.5 D, 23.6 mm, and 18.8 D, respectively.[32] In the pseudophakic eye of children, there is myopic shift which is more (0.5–10.75 D) in younger (2–3 years) children compared to older (8–9 years) children (−0.75–2.5),[33] and predicting this myopic shift is very difficult. IOL power calculation is targeted to achieve an emmetropic state in adulthood by undercorrecting the child according to age and leaving the child moderately hyperopic in the postoperative period. The initial desired refractive outcome after IOL implantation is, therefore, hypermetropia. An accurate refractive outcome after primary IOL implantation is crucial to avoid a large myopic shift in later childhood and in adulthood. In general, Enyedi's rule of seven is followed which is validated.[34] The suggested under correction formulae 20% for <2 years and 10% for 2–8 years[35] does not corroborate with the axial length growth and the target refraction required for age. Hence, there is a need to develop a new under correction formulae for children. The application of adult formulae for IOL power calculation to children has given mixed results.[36] We at our institute use SRK-II formula for IOL power calculation, which is suggested to have a least predictive error.[36]
Intraocular Lens Versus Aphakia
Infant aphakia treatment study has suggested that primary IOL implantation in children <7 months of age is associated with more chances glaucoma, added number of surgeries mainly due to VAO, and increased cost compared to contact lens use.[37] However, the visual acuity at 5 years of follow-up were comparable.[38] Although implantation of IOL in children more than 2 years of age is universally accepted as 80% of the growth of eye has already occurred, there was no clear evidence for implantation of IOL in children between 7 and 22 months of age. Recently, long-term visual outcomes in this group suggest good visual prognosis and postoperative complications comparable to children operated more than 2 years of age.[39] Primary IOL implantation in long term has better visual prognosis compared to secondary IOL implantation.[5] Aphakia in itself is a problem with issues of compliance with glasses and contact lens, especially in developing countries and lower socioeconomic status population;[40] hence, intraocular implantation is more often done[40] with child having axial length more than 17 mm and corneal diameter of more than 9.5 mm which was the lower cutoff in infant aphakia treatment study.
Surgery
The indications for cataract surgery include visually significant central cataracts larger than 3 mm in diameter,[41] dense nuclear cataracts, cataracts obstructing the examiner's view of the fundus, and cataracts associated with strabismus and abnormal eye movements.
Anesthesia
Safe and improved drugs help in planning surgery even in neonates. Opioids are associated with adverse effects such as vomiting and respiratory depression. The subtenon block and topical lignocaine are the safe alternatives to IV fentanyl for perioperative analgesia in pediatric cataract surgery.[42,43]
Microscope
Callisto Eye in Zeiss Lumera 700 provides rhexis assistant which is an intraoperative projection of rings of custom sizes which can be used as guides for anterior and posterior capsulorhexis.[44] In addition, it also has a toric assistant which uses the reference axis from IOL master and target axis in the microscope eyepiece to precisely align the toric IOL without corneal marking. The noncontact fundus viewing system provides clear detailed visualization of the retina. Intraoperative continuous OCT helps in angle assessment besides cornea and retinal evaluation.[45]
Instrumentation
The elasticity of the anterior capsule, low scleral rigidity, and presence of formed vitreous result in upthrust in these eyes during the surgery. The need for higher molecular weight ocular viscosurgical devices (OVDs) to compensate this thrust cannot be ignored. These viscocohesive (Healon GV) or viscoadaptive (Healon 5) OVDs help in performing anterior continuous curvilinear capsulorhexis and posterior continuous curvilinear capsulorhexis.[46] The development of fine 23.25 gauge instruments, especially the scissors and forceps, helps in chamber stability throughout the surgery. Anterior capsulotomy using vitrectomy cutter can be done in children <6 years.[47] Even smaller eyes can be operated on with more ease and confidence and these wounds can be left sutureless as wound integrity is better maintained. The enhanced cut rate on Centurion™ of 4000 cuts/min makes vitrectomy following posterior capsulotomy much safer. With the use of active fluidics, the chamber stability is better maintained. Although phacoemulsification energy per se is not needed in majority of cases, the higher aspiration and vacuum on these machine help in maintaining the AC. The radiofrequency endodiathermy (Kloti™) uses high-frequency (500 kHz) current to heat the probe tip to about 160° and cuts the capsule using thermal energy.[48] The Fugo's™ plasma blade which has been approved by the US Food and Drug Administration can be used for capsulotomy, especially in cases of the PFV and posttraumatic fibrotic capsules [Fig. 2].[49]
Figure 2.
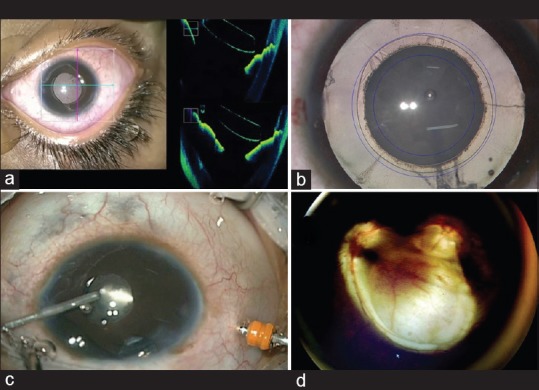
(a) Intraoperative continuous optical coherence tomography showing proper anterior chamber intraocular lens positioning (b) rhexis assistant for sizing anterior and posterior capsulorhexis (c) 23-gauge vitrectomy probe for membranectomy (d) retcam image showing fundal coloboma
Choice of Intraocular Lens
Compared to hydrophilic acrylic lenses, the hydrophobic acrylic lens shows superior reduction rates of posterior capsular opacification (PCO) and laser capsulotomy rates.[50] IOL with square edges inhibits lens epithelial cell (LEC) migration and PCO formation.[51] The “Perfect” IOL would be with a hydrophilic anterior surface and a hydrophobic posterior surface. Multifocal IOLs provide good near and distance vision[52] and also help in establishing stereopsis in unilateral cases,[53] but the brightness and contrast of the images get compromised.[52] Any decentration of lens leads to glare, halos, and deterioration in the quality of the image. Moreover, multifocal lenses cannot be used in the presence of astigmatism. Multifocal toric IOL has been used in unilateral traumatic cataract patient with a good outcome in a 7-year-old child.[54]
Femtosecond laser-assisted pediatric cataract surgery for pediatric cataracts[55] becomes a very expensive proposition due to the need for general anesthesia and two patient interfaces for each eye for anterior and posterior capsulorhexis, respectively, although re-docking with fluid interface has been described. This procedure also needs shifting of the patient between two operation theaters/tables.
Surgical Steps
Superior incision protects the wound by lids and Bell's phenomenon. Three incisions and two side ports 180° apart give 360° movements. The first incision by nondominant hand should be done, if chamber gets shallow after 1 or 2 side ports, stop and inject OVD. Preservative-free adrenaline (1:100,000) is injected for pupillary dilation, trypan blue dye (0.06%) under air stains the capsule which is washed out from all incisions for better marking of an incision. Then, a cystitome is used to give nick on anterior lens capsule; anterior capsular rhexis is completed with the help of utrata or intravitreal 23-guage forceps through 2.2 mm entry. Anterior capsulorhexis can also be done by push and pull technique.[56] Multiquadrant hydrodissection (at least three quadrants) is the preferred method in pediatric group, following which bimanual lens aspiration is completed to remove the lens matter. This is followed by partially underfilling the anterior chamber with high viscosity, cohesive viscoelastic substance, again cystitome is used to give nick over the posterior capsule and posterior capsulorhexis is completed using intravitreal forceps. The rapidly dividing LECs result in high incidence of VAO necessitating primary management of the posterior capsule. Anterior vitreous face acts as a scaffold for proliferating LECs and metaplastic pigment cells. Anterior vitrectomy breaks this scaffold, thus preventing VAO formation. Posterior capsulorhexis and vitrectomy are based on the age of the patient at surgery. A posterior capsulotomy is a must for all patients <6 years of age. Vitrectomy can be deferred after 5 years of age. In children, the surgical incisions need to be sutured using 10-0 monofilament nylon because of increased risk of anterior chamber collapse and endophthalmitis.
Vitreorhexis
Instead of doing the posterior capsulorhexis from the anterior route, posterior capsulorhexis can be done using minimally invasive and sutureless 25-gauge vitrectomy cutter through pars plana route after IOL implantation which has been shown to be equally stable with less postoperative reaction.[57] Anterior capsulorhexis can also be done using vitrectomy cutter, but the tensile strength is usually lower than anterior curvilinear capsulorhexis. Vitreorhexis has the advantage of better anterior chamber stability and less postoperative astigmatism.[58]
Intraocular Lens Insertion
Inserting the IOL in the bag when posterior capsular rhexis is already made is difficult. After making a 2.75 mm entry wound, IOL is inserted by pushing the leading haptic against the back surface of the anterior capsule, and then pushing down the trailing haptic followed by tucking of the trailing haptic into the bag. This is a safe method and results in no complications related to faulty IOL implantation.[59]
Postoperative Treatment
Early literature quotes these eyes to have a more intraocular reaction, which includes anterior chamber cells, flare, fibrinous reaction, pupillary membrane formation, and posterior synechiae formation. This is mainly due to the immaturity of the blood-aqueous barrier, insufficient fibrinolytic activity by trabecular meshwork, and foreign body reaction to IOL.[60] Heparin surface-coated IOL in uveitis cases, a subconjunctival injection of dexamethasone with or without triamcinolone, enoxaparin, and heparin in infusion fluid, has been documented to have a less postoperative reaction.[61] Postoperative single injection of hydrocortisone 5 mg/kg and dexamethasone 0.1 mg/kg have been shown to be equally effective, without the complication of increase in intraocular pressure seen with depot steroid and hyphema seen with heparin.[62] Recently, phase 3b multicentric trials, difluprednate 0.05% four times a day have shown safety and efficacy profiles similar to prednisolone acetate 1% in children 0–3 years undergoing cataract surgery.[63]
Follow-up management
Surgery is only a part of the management process, and success of the surgery depends on the postoperative follow-up, compliance for multiple examinations under anesthesia, amblyopia management, early detection, and treatment of complications.
Amblyopia Therapy
Amblyopia is frequently seen in childhood cataract; it is a form of cortical visual impairment, for which no organic cause can be attributed.[64] The inhibition of neurological signals in visual pathway leads to anatomic changes which are visible in the lateral geniculate nucleus and occipital cortex.[65] Amblyopia generally develops during the critical period of eye development, which extends up to 9 years of age. Although recent evidence suggests that the cortical plasticity can extend well beyond the postulated age.[66] Besides the basic management of amblyopia by correcting the underlying cause, appropriate optical correction, occlusion, and penalization of the dominant eye during the critical period, there is emerging evidence for the use of perceptual learning, video gaming, dichoptic training, transcranial magnetic stimulation, and drugs such as carbidopa, levodopa, and citicoline.[67] Functional magnetic resonance imaging has emerged as a modality for researching the new techniques for amblyopia management.[68] Amblyz™ liquid crystal occlusion glasses are a good alternative to traditional patching.[69]
Stem Cell Research
Recently, endogenous stem cells (lens epithelial/progenitor cells) were isolated to regenerate the lens. Lin et al. have described a novel technique, in which children <2 years of age received lensectomy with an eccentric, smaller capsulorhexis leaving LECs intact. The residual cells regenerated a lens structure with refractive power and accommodative ability. This must be studied in more eyes before it can be an adopted technique.[70] Congenital cataract is hereditary in 8.3%–25% of cases, with the most being autosomal dominant in inheritance.[71] Congenital cataract with more than forty genes and loci has been isolated.[72]
Challenging Situations
Spherophakia
Spherophakia was first described by Hartridge in 1886, it is due to the defective development of the zonules.[73] On ultrabiomicroscopy (UBM), shallow anterior chamber, steep anterior lens curvature, iridolenticular contact, elongated zonules, increased distance between the lens equator, and the ciliary processes can be seen.[13] Intralenticular bimanual irrigation and aspiration with ACIOL[74] or scleral fixated IOL with or without trabeculectomy can be done.
Technique
Surgical management of ectopia lentis in subluxation of more than 270° is challenging, which cannot be managed with capsular support rings and in-the-bag placement of IOL. The technique of intralenticular lens aspiration through a small incision came into practice in cases where capsular bag removal was deemed necessary. Sinha et al.[75] used bimanual irrigation-aspiration followed by vitrectomy cutter to remove the capsular bag. We modified this technique, two nicks are made in the anterior capsule of the lens, with the help of a 23-guage MVR, irrigation cannula was inserted from one opening to stabilize the bag, and through another opening, the cutter was inserted. The lens matter was aspirated with the help of vitrectomy cutter itself in irrigation-aspiration cut mode followed by the removal of the bag in anterior vitrectomy mode [Fig. 3].
Figure 3.
(a) Subluxated lens (b) mid-peripheral microvitreoretinal entry (c) 2 microvitreoretinal entries made (d) bag stabilized with irrigation probe (e) lens aspiration on irrigation/aspiration mode with vitrectomy cutter (f) anterior vitrectomy at 4000 cuts/sec (g) pupil constricted with pilocarpine and air injected (h) anterior chamber intraocular lens and suture placed
Persistent Fetal Vasculature
The reported visual acuity results after surgery for PFV are variable (0%–71%). If the PFV does not cover the visual axis during the 1st year of life, the prognosis for patient's vision is excellent, provided that surgery and treatment for amblyopia of the affected eye takes place as soon as possible.[76] Ultrasound and color Doppler imaging are informative screening and diagnostic tools that show characteristic flow patterns in the PFV.[77] The new echographic finding of a double linear echo on high-frequency ultrasound was observed in the region of the pars plana or plicate consistent with a thickened adherent anterior hyaloid face and not to be an anteriorly inserted peripheral retina.[78] The intraoperative presence of “salmon patch sign” eccentric pink hue is suggestive of active vasculature within the PFV.[79] The Fugo's™ plasma blade can be used to avoid intraoperative bleeding using pulses of plasma that are generated around the tip to cut and cauterize tissue without extensive collateral tissue damage [Fig. 4].[80] IOL implantation should be tried in unilateral cataract to decrease the chance of developing amblyopia.[81]
Figure 4.
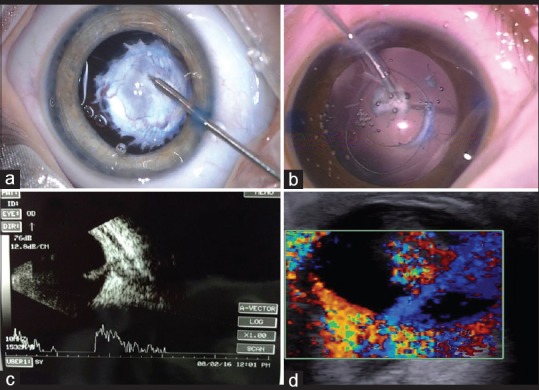
Persistent fetal vasculature (a) hemostasis using diathermy (b) Fugo's™ plasma blade (c) persistent fetal vasculature stalk on ultrasonography (d) color Doppler showing flow in persistent fetal vasculature
Conclusion
Advancement in technology has remarkably improved the surgical outcome. Zepto (precision pulse capsulotomy)[82,83] and HRES OCT[17] will be the future-driving technology. The biggest hurdle remaining is precise IOL power calculation in children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rahi JS, Sripathi S, Gilbert CE, Foster A. Childhood blindness in India: Causes in 1318 blind school students in nine states. Eye (Lond) 1995;9(Pt 5):545–50. doi: 10.1038/eye.1995.137. [DOI] [PubMed] [Google Scholar]
- 2.Sheeladevi S, Lawrenson JG, Fielder AR, Suttle CM. Global prevalence of childhood cataract: A systematic review. Eye (Lond) 2016;30:1160–9. doi: 10.1038/eye.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Titiyal JS, Pal N, Murthy GV, Gupta SK, Tandon R, Vajpayee RB, et al. Causes and temporal trends of blindness and severe visual impairment in children in schools for the blind in North India. Br J Ophthalmol. 2003;87:941–5. doi: 10.1136/bjo.87.8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shamanna BR, Dandona L, Rao GN. Economic burden of blindness in India. Indian J Ophthalmol. 1998;46:169–72. [PubMed] [Google Scholar]
- 5.Bonaparte LA, Trivedi RH, Ramakrishnan V, Wilson ME. Visual acuity and its predictors after surgery for bilateral cataracts in children. Eye (Lond) 2016;30:1229–33. doi: 10.1038/eye.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mwende J, Bronsard A, Mosha M, Bowman R, Geneau R, Courtright P. Delay in presentation to hospital for surgery for congenital and developmental cataract in Tanzania. Br J Ophthalmol. 2005;89:1478–82. doi: 10.1136/bjo.2005.074146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spanou N, Alexopoulos L, Manta G, Tsamadou D, Drakos H, Paikos P. Strabismus in pediatric lens disorders. J AAPOS. 2011;48:163–6. doi: 10.3928/01913913-20100618-05. [DOI] [PubMed] [Google Scholar]
- 8.Pavlin CJ, Harasiewicz K, Sherar MD, Foster FS. Clinical use of ultrasound biomicroscopy. Ophthalmology. 1991;98:287–95. doi: 10.1016/s0161-6420(91)32298-x. [DOI] [PubMed] [Google Scholar]
- 9.Silverman RH. High-resolution ultrasound imaging of the eye – A review. Clin Exp Ophthalmol. 2009;37:54–67. doi: 10.1111/j.1442-9071.2008.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dada T, Gadia R, Sharma A, Ichhpujani P, Bali SJ, Bhartiya S, et al. Ultrasound biomicroscopy in glaucoma. Surv Ophthalmol. 2011;56:433–50. doi: 10.1016/j.survophthal.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Gupta V, Jha R, Srinivasan G, Dada T, Sihota R. Ultrasound biomicroscopic characteristics of the anterior segment in primary congenital glaucoma. J AAPOS. 2007;11:546–50. doi: 10.1016/j.jaapos.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Kucukevcilioglu M, Hurmeric V, Ceylan OM. Preoperative detection of posterior capsule tear with ultrasound biomicroscopy in traumatic cataract. J Cataract Refract Surg. 2013;39:289–91. doi: 10.1016/j.jcrs.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Macken PL, Pavlin CJ, Tuli R, Trope GE. Ultrasound biomicroscopic features of spherophakia. Aust N Z J Ophthalmol. 1995;23:217–20. doi: 10.1111/j.1442-9071.1995.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol. 2003;21:1361–7. doi: 10.1038/nbt892. [DOI] [PubMed] [Google Scholar]
- 15.Hirata M, Tsujikawa A, Matsumoto A, Hangai M, Ooto S, Yamashiro K, et al. Macular choroidal thickness and volume in normal subjects measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:4971–8. doi: 10.1167/iovs.11-7729. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445–50. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Yu X, Liu X, Chen S, Luo Y, Wang X, Liu L. High-resolution extended source optical coherence tomography. Opt Express. 2015;23:26399–413. doi: 10.1364/OE.23.026399. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez VM, Cabot F, Ruggeri M, de Freitas C, Ho A, Yoo S, et al. Calculation of crystalline lens power using a modification of the Bennett method. Biomed Opt Express. 2015;6:4501–15. doi: 10.1364/BOE.6.004501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung MM, Huang RY, Lam AK. Retinal nerve fiber layer thickness in normal Hong Kong Chinese children measured with optical coherence tomography. J Glaucoma. 2010;19:95–9. doi: 10.1097/IJG.0b013e3181a98cfa. [DOI] [PubMed] [Google Scholar]
- 20.Pang Y, Goodfellow GW, Allison C, Block S, Frantz KA. A prospective study of macular thickness in amblyopic children with unilateral high myopia. Invest Ophthalmol Vis Sci. 2011;52:2444–9. doi: 10.1167/iovs.10-5550. [DOI] [PubMed] [Google Scholar]
- 21.Paroli MP, Spinucci G, Fabiani C, Pivetti-Pezzi P. Retinal complications of juvenile idiopathic arthritis-related uveitis: A microperimetry and optical coherence tomography study. Ocul Immunol Inflamm. 2010;18:54–9. doi: 10.3109/09273940903311999. [DOI] [PubMed] [Google Scholar]
- 22.Baikoff G, Lutun E, Wei J, Ferraz C. Contact between 3 phakic intraocular lens models and the crystalline lens: An anterior chamber optical coherence tomography study. J Cataract Refract Surg. 2004;30:2007–12. doi: 10.1016/j.jcrs.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Wilson ME, Trivedi RH. Axial length measurement techniques in pediatric eyes with cataract. Saudi J Ophthalmol. 2012;26:13–7. doi: 10.1016/j.sjopt.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capozzi P, Morini C, Piga S, Cuttini M, VadalÁ P. Corneal curvature and axial length values in children with congenital/infantile cataract in the first 42 months of life. Invest Ophthalmol Vis Sci. 2008;49:4774–8. doi: 10.1167/iovs.07-1564. [DOI] [PubMed] [Google Scholar]
- 25.Vasavada AR, Raj SM, Nihalani B. Rate of axial growth after congenital cataract surgery. Am J Ophthalmol. 2004;138:915–24. doi: 10.1016/j.ajo.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 26.Vasavada V, Shah SK, Vasavada VA, Vasavada AR, Trivedi RH, Srivastava S, et al. Comparison of IOL power calculation formulae for pediatric eyes. Eye (Lond) 2016;30:1242–50. doi: 10.1038/eye.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehlers N, Sorensen T, Bramsen T, Poulsen EH. Central corneal thickness in newborns and children. Acta Ophthalmol (Copenh) 1976;54:285–90. doi: 10.1111/j.1755-3768.1976.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 28.Zadnik K, Manny RE, Yu JA, Mitchell GL, Cotter SA, Quiralte JC, et al. Ocular component data in schoolchildren as a function of age and gender. Optom Vis Sci. 2003;80:226–36. doi: 10.1097/00006324-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Trivedi RH, Wilson ME. Keratometry in pediatric eyes with cataract. Arch Ophthalmol. 2008;126:38–42. doi: 10.1001/archophthalmol.2007.22. [DOI] [PubMed] [Google Scholar]
- 30.Flitcroft DI, Knight-Nanan D, Bowell R, Lanigan B, O'Keefe M. Intraocular lenses in children: Changes in axial length, corneal curvature, and refraction. Br J Ophthalmol. 1999;83:265–9. doi: 10.1136/bjo.83.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen H, Ehlers N, Hjortdal JO. Value of intraoperative keratometry in predicting outcome of radial keratotomy. Acta Ophthalmol Scand. 1997;75:398–400. doi: 10.1111/j.1600-0420.1997.tb00397.x. [DOI] [PubMed] [Google Scholar]
- 32.Gordon RA, Donzis PB. Refractive development of the human eye. Arch Ophthalmol. 1985;103:785–9. doi: 10.1001/archopht.1985.01050060045020. [DOI] [PubMed] [Google Scholar]
- 33.Plager DA, Kipfer H, Sprunger DT, Sondhi N, Neely DE. Refractive change in pediatric pseudophakia: 6-year follow-up. J Cataract Refract Surg. 2002;28:810–5. doi: 10.1016/s0886-3350(01)01156-7. [DOI] [PubMed] [Google Scholar]
- 34.Sachdeva V, Katukuri S, Kekunnaya R, Fernandes M, Ali MH. Validation of guidelines for undercorrection of intraocular lens power in children. Am J Ophthalmol. 2017;174:17–22. doi: 10.1016/j.ajo.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Dahan E, Drusedau MU. Choice of lens and dioptric power in pediatric pseudophakia. J Cataract Refract Surg. 1997;23(Suppl 1):618–23. doi: 10.1016/s0886-3350(97)80043-0. [DOI] [PubMed] [Google Scholar]
- 36.Kekunnaya R, Gupta A, Sachdeva V, Rao HL, Vaddavalli PK, Om Prakash V. Accuracy of intraocular lens power calculation formulae in children less than two years. Am J Ophthalmol. 2012;154:13–19.e2. doi: 10.1016/j.ajo.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 37.Infant Aphakia Treatment Study Group. Lambert SR, Lynn MJ, Hartmann EE, DuBois L, Drews-Botsch C, et al. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: A randomized clinical trial of HOTV optotype acuity at age 4.5 years and clinical findings at age 5 years. JAMA Ophthalmol. 2014;132:676–82. doi: 10.1001/jamaophthalmol.2014.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weakley DR, Lambert SR, Wilson ME, Plager DA, Buckley EG, Lynn M. Refractive error and anisometropia after unilateral intraocular lens implantation in infants under 7 months of age-results at 5 years of age from the Infant Aphakia Treatment Study (IATS) [Last accessed on 2017 Apr 17];J AAPOS. 2016 20:e10. Available from: http://www.jaapos.org/article/S1091-8531(16)30163-X/abstract . [Google Scholar]
- 39.Struck MC. Long-term results of pediatric cataract surgery and primary intraocular lens implantation from 7 to 22 months of life. JAMA Ophthalmol. 2015;133:1180–3. doi: 10.1001/jamaophthalmol.2015.2062. [DOI] [PubMed] [Google Scholar]
- 40.Wilson ME, Pandey SK, Thakur J. Paediatric cataract blindness in the developing world: Surgical techniques and intraocular lenses in the new millennium. Br J Ophthalmol. 2003;87:14–9. doi: 10.1136/bjo.87.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasavada AR, Nihalani BR. Pediatric cataract surgery. Curr Opin Ophthalmol. 2006;17:54–61. doi: 10.1097/01.icu.0000193069.32369.e1. [DOI] [PubMed] [Google Scholar]
- 42.Ghai B, Ram J, Makkar JK, Wig J, Kaushik S. Subtenon block compared to intravenous fentanyl for perioperative analgesia in pediatric cataract surgery. Anesth Analg. 2009;108:1132–8. doi: 10.1213/ane.0b013e318198a3fd. [DOI] [PubMed] [Google Scholar]
- 43.Sinha R, Subramaniam R, Chhabra A, Pandey R, Nandi B, Jyoti B. Comparison of topical lignocaine gel and fentanyl for perioperative analgesia in children undergoing cataract surgery. Paediatr Anaesth. 2009;19:371–5. doi: 10.1111/j.1460-9592.2008.02902.x. [DOI] [PubMed] [Google Scholar]
- 44.Binder AP. Intra-operative OCT devices for ophthalmic use: An overview. [Last accessed on 2017 Apr 13];Spectrum of Ophthalmology. 2014 28:2–5. Available from: http://www.springer.com/medicine/ophthalmology/journal/717 . [Google Scholar]
- 45.Ehlers JP, Dupps WJ, Kaiser PK, Goshe J, Singh RP, Petkovsek D, et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) study: 2-year results. Am J Ophthalmol. 2014;158:999–1007. doi: 10.1016/j.ajo.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson ME., Jr Anterior lens capsule management in pediatric cataract surgery. Trans Am Ophthalmol Soc. 2004;102:391–422. [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson ME, Trivedi RH, Bartholomew LR, Pershing S. Comparison of anterior vitrectorhexis and continuous curvilinear capsulorhexis in pediatric cataract and intraocular lens implantation surgery: A 10-year analysis. J AAPOS. 2007;11:443–6. doi: 10.1016/j.jaapos.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Krag S, Thim K, Corydon L. Diathermic capsulotomy versus capsulorhexis: A biomechanical study. J Cataract Refract Surg. 1997;23:86–90. doi: 10.1016/s0886-3350(97)80156-3. [DOI] [PubMed] [Google Scholar]
- 49.Singh D. Use of the Fugo blade in complicated cases. J Cataract Refract Surg. 2002;28:573–4. doi: 10.1016/s0886-3350(02)01314-7. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Wang J, Chen Z, Tang X. Effect of hydrophobic acrylic versus hydrophilic acrylic intraocular lens on posterior capsule opacification: Meta-analysis. PLoS One. 2013;8:e77864. doi: 10.1371/journal.pone.0077864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ness PJ, Werner L, Maddula S, Davis D, Zaugg B, Stringham J, et al. Pathology of 219 human cadaver eyes with 1-piece or 3-piece hydrophobic acrylic intraocular lenses: Capsular bag opacification and sites of square-edged barrier breach. J Cataract Refract Surg. 2011;37:923–30. doi: 10.1016/j.jcrs.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 52.Jacobi PC, Dietlein TS, Konen W. Multifocal intraocular lens implantation in pediatric cataract surgery. Ophthalmology. 2001;108:1375–80. doi: 10.1016/s0161-6420(01)00595-4. [DOI] [PubMed] [Google Scholar]
- 53.Lapid-Gortzak R, van der Meulen IJ, Jellema HM, Mourits MP, Nieuwendaal CP. Seven-year follow-up of unilateral multifocal pseudophakia in a child. Int Ophthalmol. 2017;37:267–270. doi: 10.1007/s10792-016-0232-5. [DOI] [PubMed] [Google Scholar]
- 54.Zeng Y, Fan L, Lu P. Multifocal toric intraocular lens for traumatic cataract in a child. Case Rep Ophthalmol. 2016;7:203–7. doi: 10.1159/000449153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dick HB, Schultz T. Femtosecond laser-assisted cataract surgery in infants. J Cataract Refract Surg. 2013;39:665–8. doi: 10.1016/j.jcrs.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 56.Nischal KK. Two-incision push-pull capsulorhexis for pediatric cataract surgery. J Cataract Refract Surg. 2002;28:593–5. doi: 10.1016/s0886-3350(01)01125-7. [DOI] [PubMed] [Google Scholar]
- 57.Raina UK, Bhambhwani V, Gupta A, Bhushan G, Seth A, Ghosh B. Comparison of transcorneal and pars plana routes in pediatric cataract surgery in infants using a 25-gauge vitrectomy system. J Pediatr Ophthalmol Strabismus. 2016;53:105–12. doi: 10.3928/01913913-20160208-01. [DOI] [PubMed] [Google Scholar]
- 58.Chee KY, Lam GC. Management of congenital cataract in children younger than 1 year using a 25-gauge vitrectomy system. J Cataract Refract Surg. 2009;35:720–4. doi: 10.1016/j.jcrs.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 59.Khokhar S, Sharma R, Patil B, Sinha G, Nayak B, Kinkhabwala RA. A safe technique for in-the-bag intraocular lens implantation in pediatric cataract surgery. Eur J Ophthalmol. 2015;25:57–9. doi: 10.5301/ejo.5000502. [DOI] [PubMed] [Google Scholar]
- 60.Rose GE. Fibrinous uveitis and intraocular lens implantation. Surface modification of polymethylmethacrylate during extracapsular cataract surgery. Ophthalmology. 1992;99:1242–7. doi: 10.1016/s0161-6420(92)31817-2. [DOI] [PubMed] [Google Scholar]
- 61.Huang Q, Cheng GP, Chiu K, Wang GQ. Surface modification of intraocular lenses. Chin Med J (Engl) 2016;129:206–14. doi: 10.4103/0366-6999.173496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phatak S, Lowder C, Pavesio C. Controversies in intraocular lens implantation in pediatric uveitis. J Ophthalmic Inflamm Infect. 2016;6:12. doi: 10.1186/s12348-016-0079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson ME, O'Halloran H, VanderVeen D, Roarty J, Plager DA, Markwardt K, et al. Difluprednate versus prednisolone acetate for inflammation following cataract surgery in pediatric patients: A randomized safety and efficacy study. Eye (Lond) 2016;30:1187–94. doi: 10.1038/eye.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res. 2013;33:67–84. doi: 10.1016/j.preteyeres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- 66.Epelbaum M, Milleret C, Buisseret P, Dufier JL. The sensitive period for strabismic amblyopia in humans. Ophthalmology. 1993;100:323–7. doi: 10.1016/s0161-6420(13)32170-8. [DOI] [PubMed] [Google Scholar]
- 67.Pediatric Eye Disease Investigator Group. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol. 2005;123:437. doi: 10.1001/archopht.123.4.437. [DOI] [PubMed] [Google Scholar]
- 68.Vagge A, Nelson LB. Amblyopia update: New treatments. Curr Opin Ophthalmol. 2016;27:380–6. doi: 10.1097/ICU.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 69.Jardine GJ, Boente CS, Wang J, Neely DE, Galli JG, Smith HA, et al. A randomized trial of Amblyz liquid crystal occlusion glasses versus traditional patching for treatment of moderate unilateral amblyopia in children: 6-month outcome. [Last accessed on 2017 Apr 13];J Pediatr Ophthalmol Strabismus. 2016 20:e34. Available from: http://www.sciencedirect.com/science/journal/10918531/19/4 . [Google Scholar]
- 70.Lin H, Ouyang H, Zhu J, Huang S, Liu Z, Chen S, et al. Lens regeneration using endogenous stem cells with gain of visual function. Nature. 2016;531:323–8. doi: 10.1038/nature17181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hejtmancik JF. Congenital cataracts and their molecular genetics. Semin Cell Dev Biol. 2008;19:134–49. doi: 10.1016/j.semcdb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Churchill A, Graw J. Clinical and experimental advances in congenital and paediatric cataracts. Philos Trans R Soc Lond B Biol Sci. 2011;366:1234–49. doi: 10.1098/rstb.2010.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farnsworth PN, Burke PA, Blanco J, Maltzman B. Ultrastructural abnormalities in a microspherical ectopic lens. Exp Eye Res. 1978;27:399–408. doi: 10.1016/0014-4835(78)90018-0. [DOI] [PubMed] [Google Scholar]
- 74.Nelson LB, Maumenee IH. Ectopia lentis. Surv Ophthalmol. 1982;27:143–60. doi: 10.1016/0039-6257(82)90069-8. [DOI] [PubMed] [Google Scholar]
- 75.Sinha R, Sharma N, Vajpayee RB. Intralenticular bimanual irrigation: Aspiration for subluxated lens in Marfan's syndrome. J Cataract Refract Surg. 2005;31:1283–6. doi: 10.1016/j.jcrs.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 76.Mittra RA, Huynh LT, Ruttum MS, Mieler WF, Connor TB, Han DP, et al. Visual outcomes following lensectomy and vitrectomy for combined anterior and posterior persistent hyperplastic primary vitreous. Arch Ophthalmol. 1998;116:1190–4. doi: 10.1001/archopht.116.9.1190. [DOI] [PubMed] [Google Scholar]
- 77.Hu A, Pei X, Ding X, Li J, Li Y, Liu F, et al. Combined persistent fetal vasculature: A classification based on high-resolution B-mode ultrasound and color Doppler imaging. Ophthalmology. 2016;123:19–25. doi: 10.1016/j.ophtha.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Mackeen LD, Nischal KK, Lam WC, Levin AV. High-frequency ultrasonography findings in persistent hyperplastic primary vitreous. J AAPOS. 2000;4:217–24. doi: 10.1067/mpa.2000.105306. [DOI] [PubMed] [Google Scholar]
- 79.Khokhar S, Gupta S, Gogia V, Nayak B. Salmon pink patch sign: Diagnosing persistent fetal vasculature. Oman J Ophthalmol. 2016;9:68–9. doi: 10.4103/0974-620X.176128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sinha R, Bali SJ, Kumar C, Shekhar H, Sharma N, Titiyal JS, et al. Results of cataract surgery and plasma ablation posterior capsulotomy in anterior persistent hyperplastic primary vitreous. Middle East Afr J Ophthalmol. 2013;20:217–20. doi: 10.4103/0974-9233.114794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solebo AL, Russell-Eggitt I, Cumberland P, Rahi JS. Congenital cataract associated with persistent fetal vasculature: Findings from IoLunder. Eye (Lond) 2016;30:1204–9. doi: 10.1038/eye.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang DF, Mamalis N, Werner L. Precision pulse capsulotomy: Preclinical safety and performance of a new capsulotomy technology. Ophthalmology. 2016;123:255–64. doi: 10.1016/j.ophtha.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 83.Thompson VM, Berdahl JP, Solano JM, Chang DF. Comparison of manual, femtosecond laser, and precision pulse capsulotomy edge tear strength in paired human cadaver eyes. Ophthalmology. 2016;123:265–74. doi: 10.1016/j.ophtha.2015.10.019. [DOI] [PubMed] [Google Scholar]



