Abstract
Background:
Recent studies have demonstrated that pterygium has a close relationship with dry eye disease. This study is to determine abnormalities in meibomian gland and tear function in patients with pterygium and to assess the relationship between the variables.
Materials and Methods:
Forty eyes from forty patients with primary nasal pterygium and forty eyes from forty volunteers without ocular pathologies were enrolled in this study. Ocular surface disease index scores, meibomian gland expression scores, lid margin abnormality scores, meiboscore, tear film breakup time (BUT), Schirmer test (SIT) value, and the lower tear meniscus height (TMH), tear meniscus depth (TMD), and tear meniscus area (TMA) using Fourier domain optical coherence were performed. Analysis of variance was applied for intergroup comparisons. A statistical significance level of P < 0.05 was considered.
Results:
Ocular symptom scores, BUT scores, lid margin abnormality, meibomian gland expression, and meiboscore were significantly higher in pterygium patients than in controls (P < 0.01 for all scores). However, the SIT scores, the lower TMH, TMD, and TMA values did not revealed a significant difference between two groups (all P > 0.05). Multivariate regression analysis demonstrated that meiboscore significantly correlated with ocular symptom scores, BUT, lid margin abnormality scores, and meibomian gland expression scores.
Conclusions:
Meibomian gland function may be altered in pterygium patients, which is associated with uncomfortable ocular symptoms. Being aware of meibomian gland changes seems essential to understand the complex relationship among pterygium, tear film functions, and ocular surface changes.
Key words: Meibomian gland, ocular surface, pterygium, tear film
Pterygium is a common disease of the ocular surface characterized by the invasion of fibrovascular tissue from the bulbar conjunctiva onto the cornea.[1] The exact pathogenesis of the injury is complex and remains incompletely understood. Age, hereditary factors, sunlight, chronic inflammation, microtrauma, and heat are possible contributing factors.[2,3] Kadayifçilar et al.[4] and Ishioka et al.[5] noted inadequate tear film stability in pterygium patients and suggested that abnormal tear function may be yet another risk factor related to pterygium development. However, several other studies have shown that tear function was normal in pterygia.[6] Thus, there is an unresolved issue with regard to whether the abnormal tear function is directly associated with pterygium. If they are related, another question arises: does the change of the tear function cause pterygium or vice versa?
Many authors hypothesized that an abnormal tear function was a risk factor for pterygium.[7,8,9] In contrast, some authors suggested the reverse sequence: pathological conjunctival, corneal, or eyelid changes in pterygia lead to disturbed tear film function.[10] The relationship between pterygia and tear film function has proved difficult to define. The tear film is composed of an aqueous layer produced by lacrimal glands as well as an overlying oily layer, the lipid components of which are secreted by the meibomian glands.[11] Li et al. reported the presence of disturbances in tear quality and quantity in pterygium patients with a decrease in conjunctival goblet cell population.[12] The tear film instability in those patients was attributed in part to disturbances in tear mucins resulting from decreased goblet cell density. Turkyilmaz et al. found that the mean goblet cell density was significantly increased 1 month after excision, which may result in an increased secretion of mucin in tear film.[13]
However, to the best of our knowledge, this is the first time that meibomian gland changes have been measured in pterygium patients. The purpose of this study was to evaluate whether meibomian gland changes contribute to the ocular discomfort in pterygium patients and to investigate potential associated changes in ocular surface parameters.
Materials and Methods
This study was conducted in accordance with the tenets of the World Medical Association of Helsinki. Informed consent was obtained from all participants after an explanation of the purpose and possible consequences of the study.
The inclusion criteria were as follows: primary nasal pterygium, willingness to attend the required study visit, and lack of any systemic disease. The exclusion criteria were as follows: any corneal disease or scar; contact lens use within 3 months; cicatricial ocular surface disease; other comorbid ocular diseases such as ocular allergies; continuous use of topical ocular medications; and histories of ocular surgery or ocular injury. This prospective study included forty patients (forty eyes) with primary nasal pterygium. The mean age was 51.2 ± 9.5 years (range 32–64 years). All patients underwent the following examinations by the same ophthalmologist [Table 1]. Dry eye symptoms were assessed using ocular surface disease index (OSDI) questionnaire, which was developed to assess the vision-related health-targeted quality of life with dry eye disease.[14]
Table 1.
Clinical parameters and their evaluation
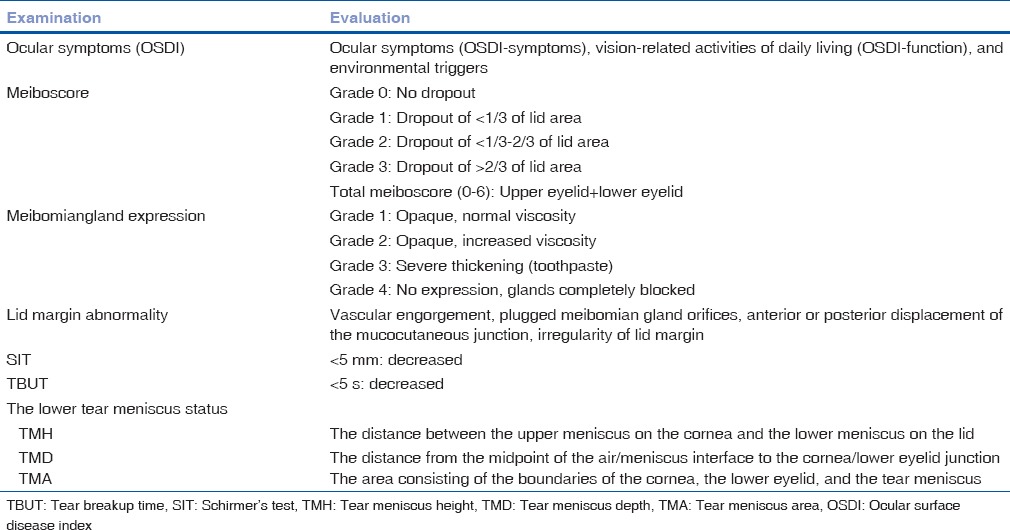
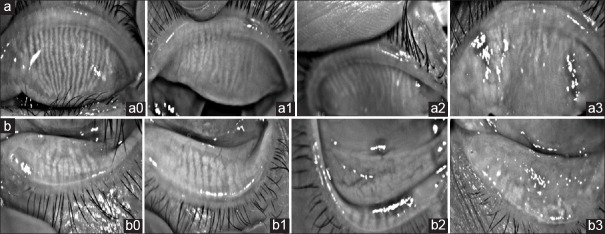
Meibomian gland expression was assessed by assigning grades for clarity and ease of meibum expression in a region of the eyelid using a slit lamp. The quality of expression was graded according to the degree of opacity and viscosity on a 0–4 scale,[15] in which 0 indicated normal viscosity; 1, opaque, normal viscosity; 2, opaque, increased viscosity; 3, severe thickening (toothpaste); and 4, no expression, glands completely blocked. Lid margin abnormalities were scored as 0 (absent) or 1 (present) for the following four parameters: vascular engorgement, plugged meibomian gland orifices, anterior or posterior displacement of the mucocutaneous junction, and irregularity of lid margin.[16] If any of these signs was present, 1 point was assigned for each item, with a total possible score range of 0–4 points. Meiboscores were performed using the Keratograph 5M (OCULUS Optikgerate GmbH, Wetzlar, Germany) for the central 15 meibomian glands on each eye. The meiboscores for the upper and lower eyelids were summed to obtain a score from 0 to 6 for each eye. It was obtained using the following grades for each eyelid: 0 (no loss of meibomian glands); 1 (meibomian gland loss involving less than one-third of the total meibomian gland area); 2 (area lost between one-third and two-thirds of the total meibomian gland area); and 3 (area lost more than two-thirds of the total meibomian gland area) [Fig. 1].[17,18]
Figure 1.
Representative photographs depicting upper (a) and lower (b) eyelids of various grades in pterygium patients. (a0, b0) Grade 0, no loss of meibomian glands; (a1, b1) Grade 1, meibomian gland area loss less than one-third; (a2, b2) Grade 2, meibomian gland area loss was greater than one-third and less than two-thirds; (a3, b3) Grade 3, meibomian gland area loss was greater than two-thirds
Tear film instability was measured by breakup time (BUT), which is the time required for dry spots to appear on the corneal surface after blinking. Tear film instability was evaluated by placing a single fluorescein strip over the inferior tear meniscus after instilling a drop of normal saline. Patients were asked to blink three times and look straightforward. The precorneal tear film was examined with a slit lamp, and the elapsed times before initial formation of dry spots were recorded. The mean time for three attempts was recorded. Schirmer's test (SIT) was performed without topical anesthesia as the final step in the examination by placing a standard Schirmer tear test filter strip in the mid-lateral portion of the lower fornix. The amount of wetting was recorded after 5 min.
The lower tear meniscus status was evaluated using Fourier domain optical coherence tomography (FD-OCT). Vertical 2 mm scan images of the middle of the lower eyelid were obtained three times per eye. The tear meniscus height (TMH), tear meniscus depth (TMD), and tear meniscus area (TMA) were measured. The TMH was defined as the distance between the upper meniscus of the cornea and the lower meniscus of the lid. The TMD was defined as the distance from the midpoint of the air/meniscus interface to the cornea/lower eyelid junction, and the TMA was defined as the area consisting of the boundaries of the cornea, the lower eyelid, and the tear meniscus.
The data were expressed as the means ± standard deviation. A linear mixed model with Bonferroni post hoc analysis was used to evaluate repeated measurements of continuous values such as OSDI score, BUT, SIT, and TMH, TMD, and TMA. A generalized linear mixed model analysis was used for repeated measurements of noncontinuous values including lid margin abnormalities, meibum expression, and meiboscore. Statistical analyses were performed using SPSS for Windows version 16.0. (SPSS Inc, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
The mean OSDI score of patients with pterygium was 14.2 ± 5.7, which was significantly higher than that of the normal eyelids (9.9 ± 4.5; P < 0.001) [Table 2].
Table 2.
Mean±standard deviation of the ocular surface parameters that were measured between two groups
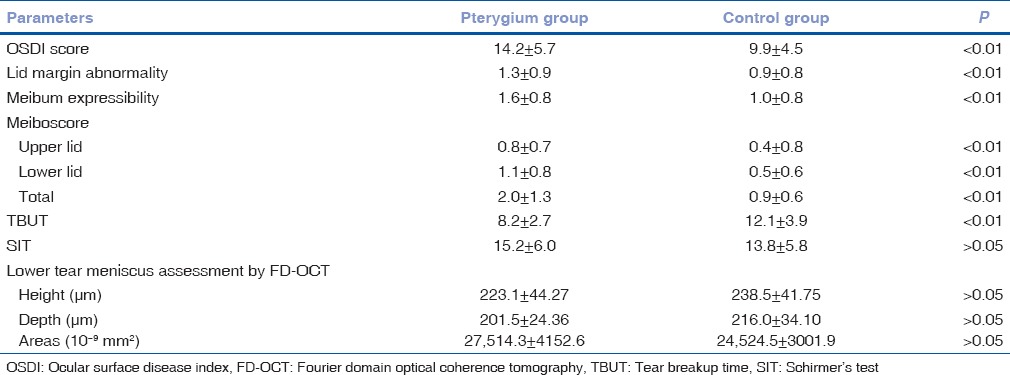
Lid margin abnormality score and meibomian gland expression score were significantly higher in pterygium group compared with the normal eyelids (P < 0.01, respectively) [Table 1]. Furthermore, meiboscores were significantly higher in pterygium group (median total meiboscore score = 2.0 ± 1.3) compared to the normal eyelids (median total meiboscore = 0.9 ± 0.6; P < 0.001) [Table 2].
The mean tear breakup time (TBUT) was 8.2 ± 2.7 s (range 5–21 s) in pterygium group and 12.1 ± 3.9 s (range 9–27 s) in the control group. The difference between two groups was statistically significant (P < 0.01) [Table 1]. However, the values of TMH, TMD, TMA, and SIT were not significantly different between two groups (all P > 0.05) [Fig. 1].
Spearman correlation analysis indicated that the meiboscore was significantly correlated with ocular symptom scores (R = 0.312, P < 0.01), BUT (R = −0.287, P < 0.01), lid margin abnormality score (R = 0.273, P < 0.01), and meibomian gland expression score (R = 0.251, P < 0.01) in pterygium group.
Discussion
Our present data suggested that pterygium patients had significantly higher OSDI scores, lid margin abnormalities, meibum expressibility, and meiboscores compared with the control group. However, no changes in the SIT and lower tear meniscus volume values were observed.
We did not detect a significant difference in SIT value between normal participants and pterygium patients. The relationship between pterygium and SIT had been difficult to define. In the previous study, Roka et al. demonstrated that the values of SIT were significantly reduced in pterygium patients.[19] Conversely, Kampitak and Leelawongtawun[6] demonstrated that the SIT results did not change in pterygium patients and the size of pterygium did not correlate with SIT results. Some studies believed that these contradictory results may attribute to the methods that were used to evaluate tear function were not objective and quantitative. Since FD-OCT was reported to provide objective tear data with low intraindividual variability and high intervisit reproducibility,[20] we used it to assess tear quantity in our study. Changes in the TMH were not significantly different between two groups, which indicated that the tear meniscus production was not changed in pterygium patients. Therefore, we can speculate that the tear film quantitation remained unchanged in the pterygium patients.
Arita et al. suggested that tear fluid secretion may increase as a compensatory response to meibomian gland loss to maintain ocular surface homeostasis.[21] This disagreement could be triggered by compensatory mechanisms such as reflex production of aqueous and lipid components of the tear film, resulting in transient improvements in tear film stability.[22] This compensatory mechanism could be plausible because, in all the studies, the SIT showed no abnormal tear production. As expected, most of the objective tear tests did not correlate with each other because they provide partial information of different aspects of the disease process.
In our study, we found the BUT value was significantly reduced in the pterygium group. A shorter TBUT is associated with tear film instability.[23] Therefore, we can speculate that the quantity of the tear film in patients with pterygium is adequate but that its quality or composition is abnormal. Tear film consists of three layers. The most superficial layer is lipid layer, which is produced by the meibomian glands and stabilizes tear films by retarding evaporation and lowering surface tension.[24]
We investigated the morphologic changes in meibomian glands associated with pterygium using a noncontact meibographic technique. The resulting data demonstrated that pterygium patients were significantly associated with a greater degree of meibomian gland loss compared with the normal patients.
Pterygia are characterized by an inflammatory infiltrate with a prominent vascular reaction. This process is exacerbated by an excessive production of cytokines and growth factors that are involved in complex regulatory pathways.[25] Inflammation is also associated with meibomian gland changes and implicate in meibomian gland dysfunction (MGD) pathogenesis. Meibomian gland inflammation is often recognized with ocular surface inflammation in conditions such as blepharokeratoconjunctivitis,[26] ocular rosacea, and phlyctenular keratitis. In vivo laser CM images, Ibrahim found that the mean inflammatory cell density was significantly higher in MGD patients than the controls.[27] Direct inflammatory damage to eyelid due to elevated inflammatory status and the release of inflammatory cytokines, including tumor necrosis factor-õ, interleukin-4, and interleukin-5, may spread to the anterior and posterior lid margin, thus resulting in meibomian gland changes.[28] Chronic repeated inflammation might also cause meibum stagnation followed by the keratinization of orifices in the meibomian glands.
Another key etiological factor known to be associated with pterygium is the exposure to ultraviolet (UV) radiation. In the laboratory, fibroblast cells cultured from pterygium tissue have upregulated matrix metalloproteinases when exposed to UV stimulation.[29] Alteration or deregulation of the stem-microenvironmental networking provokes disease development. Some studies postulate that pterygium was associated with limbal microenvironmental anomaly where the resident epithelial cells became hyperproliferative. The hyperkeratinization of the epithelium at the lid margin and meibomian gland might affect the structural changes with meibomian glands.[30]
Conclusions
Meibomian glands' alterations in pterygium patients may have aggravated the tear stability and ocular surface damage, possibly because of the changes in the lipid layer of the tear film, which may have resulted from a greater extent of meibomian glands dropout and lid margin changes. Our study revealed new evidence regarding the pathologic changes of meibomian gland (MG) in pterygium. Further research will be necessary to evaluate the exact mechanism by which meibomian gland changes related to pterygium.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kheirkhah A, Safi H, Molaei S, Nazari R, Behrouz MJ, Raju VK. Effects of pterygium surgery on front and back corneal astigmatism. Can J Ophthalmol. 2012;47:423–8. doi: 10.1016/j.jcjo.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Gonnermann J, Maier AK, Klein JP, Bertelmann E, Pleyer U, Klamann MK. Evaluation of ocular surface temperature in patients with pterygium. Curr Eye Res. 2014;39:359–64. doi: 10.3109/02713683.2013.844262. [DOI] [PubMed] [Google Scholar]
- 3.Ergin A, Bozdogan O. Study on tear function abnormality in pterygium. Ophthalmologica. 2001;215:204–8. doi: 10.1159/000050859. [DOI] [PubMed] [Google Scholar]
- 4.Kadayifçilar SC, Orhan M, Irkeç M. Tear functions in patients with pterygium. Acta Ophthalmol Scand. 1998;76:176–9. doi: 10.1034/j.1600-0420.1998.760210.x. [DOI] [PubMed] [Google Scholar]
- 5.Ishioka M, Shimmura S, Yagi Y, Tsubota K. Pterygium and dry eye. Ophthalmologica. 2001;215:209–11. doi: 10.1159/000050860. [DOI] [PubMed] [Google Scholar]
- 6.Kampitak K, Leelawongtawun W. Precorneal tear film in pterygium eye. J Med Assoc Thai. 2014;97:536–9. [PubMed] [Google Scholar]
- 7.Ozsutcu M, Arslan B, Erdur SK, Gulkilik G, Kocabora SM, Muftuoglu O. Tear osmolarity and tear film parameters in patients with unilateral pterygium. Cornea. 2014;33:1174–8. doi: 10.1097/ICO.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 8.Sapkota K, Franco S, Sampaio P, Lira M. Goblet cell density association with tear function and ocular surface physiology. Cont Lens Anterior Eye. 2015;38:240–4. doi: 10.1016/j.clae.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Jiang B, Gu Y. Changes of tear film function after pterygium operation. Ophthalmic Res. 2011;45:210–5. doi: 10.1159/000321531. [DOI] [PubMed] [Google Scholar]
- 10.Zakaria N, De Groot V, Tassignon MJ. Tear film biomarkers as prognostic indicators for recurrent pterygium. Bull Soc Belge Ophtalmol. 2011;317:53–4. [PubMed] [Google Scholar]
- 11.Chan CM, Liu YP, Tan DT. Ocular surface changes in pterygium. Cornea. 2002;21:38–42. doi: 10.1097/00003226-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Zhang M, Lin Y, Xiao Q, Zhu X, Song S, et al. Tear function and goblet cell density after pterygium excision. Eye (Lond) 2007;21:224–8. doi: 10.1038/sj.eye.6702186. [DOI] [PubMed] [Google Scholar]
- 13.Türkyilmaz K, Oner V, Sevim MS, Kurt A, Sekeryapan B, Durmus M. Effect of pterygium surgery on tear osmolarity. J Ophthalmol. 2013;2013:863498. doi: 10.1155/2013/863498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 15.Borchman D, Foulks GN, Yappert MC, Bell J, Wells E, Neravetla S, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:3805–17. doi: 10.1167/iovs.10-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mudgil P, Borchman D, Yappert MC, Duran D, Cox GW, Smith RJ, et al. Lipid order, saturation and surface property relationships: A study of human meibum saturation. Exp Eye Res. 2013;116:79–85. doi: 10.1016/j.exer.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–5. doi: 10.1016/j.ophtha.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Arita R, Itoh K, Inoue K, Kuchiba A, Yamaguchi T, Amano S. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009;116:379–84. doi: 10.1016/j.ophtha.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Roka N, Shrestha SP, Joshi ND. Assessment of tear secretion and tear film instability in cases with pterygium and normal subjects. Nepal J Ophthalmol. 2013;5:16–23. doi: 10.3126/nepjoph.v5i1.7816. [DOI] [PubMed] [Google Scholar]
- 20.Kim SE, Yoon JS, Lee SY. Tear measurement in prosthetic eye users with Fourier-domain optical coherence tomography. Am J Ophthalmol. 2010;149:602–7.e1. doi: 10.1016/j.ajo.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Arita R, Morishige N, Koh S, Shirakawa R, Kawashima M, Sakimoto T, et al. Increased tear fluid production as a compensatory response to meibomian gland loss: A multicenter cross-sectional study. Ophthalmology. 2015;122:925–33. doi: 10.1016/j.ophtha.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Rahman A, Yahya K, Fasih U, Waqar-ul-Huda, Shaikh A. Comparison of Schirmer's test and tear film breakup time test to detect tear film abnormalities in patients with pterygium. J Pak Med Assoc. 2012;62:1214–6. [PubMed] [Google Scholar]
- 23.Kiliç A, Gürler B. Effect of pterygium excision by limbal conjunctival auotografting on tear function tests. Ann Ophthalmol (Skokie) 2006;38:235–8. doi: 10.1007/s12009-006-0011-4. [DOI] [PubMed] [Google Scholar]
- 24.Di Girolamo N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: Role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 2004;23:195–228. doi: 10.1016/j.preteyeres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Bandyopadhyay R, Nag D, Mondal SK, Gangopadhyay S, Bagchi K, Bhaduri G. Ocular surface disorder in pterygium: Role of conjunctival impression cytology. Indian J Pathol Microbiol. 2010;53:692–5. doi: 10.4103/0377-4929.72036. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim OM, Matsumoto Y, Dogru M, Adan ES, Wakamatsu TH, Goto T, et al. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010;117:665–72. doi: 10.1016/j.ophtha.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Teramukai S, Kinoshita S. Meibomian glands and ocular surface inflammation. Ocul Surf. 2015;13:133–49. doi: 10.1016/j.jtos.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim OM, Matsumoto Y, Dogru M, Adan ES, Wakamatsu TH, Shimazaki J, et al. In vivo confocal microscopy evaluation of meibomian gland dysfunction in atopic-keratoconjunctivitis patients. Ophthalmology. 2012;119:1961–8. doi: 10.1016/j.ophtha.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Jang SY, Lee SY, Yoon JS. Meibomian gland dysfunction in longstanding prosthetic eye wearers. Br J Ophthalmol. 2013;97:398–402. doi: 10.1136/bjophthalmol-2012-302404. [DOI] [PubMed] [Google Scholar]
- 30.Das P, Gokani A, Bagchi K, Bhaduri G, Chaudhuri S, Law S. Limbal epithelial stem-microenvironmental alteration leads to pterygium development. Mol Cell Biochem. 2015;402:123–39. doi: 10.1007/s11010-014-2320-z. [DOI] [PubMed] [Google Scholar]