Abstract
Purpose of the study:
The purpose of this study was to report clinical features, neuroimaging, and visual outcome in pediatric optic neuritis (ON) in Indian population.
Materials and Methods:
This is a retrospective study of children up to the age of 16 years, diagnosed with ON, that presented at pediatric and neuroophthalmology clinic of a tertiary eye care center, in South India, within the period of 2010–2015.
Results:
We identified 62 eyes of 40 children diagnosed as ON within the study period. The mean age was 11.15 ± 3.24 years (1–15 years) with mean follow-up of 13 months. In this series, there was female preponderance (67%). Mean logarithm of the minimum angle of resolution visual acuity at presentation was 1.14 ± 0.93, which after treatment recovered to 0.10 ± 0.26 at final visit (P < 0.001). Involvement was bilateral in 22 children (55%) and recurrent in 3 eyes of 3 children. Preceding febrile illness was reported in seven cases (18%). Four (10%) cases were diagnosed as multiple sclerosis (MS), one with neuromyelitis optica, and one with acute disseminated encephalomyelitis. One case was associated with tuberculous meningitis, 1 with septicemia, and 1 with bilateral maxillary sinusitis. Neuroimaging studies of optic nerve in 14 children demonstrated isolated optic nerve enhancement. Magnetic resonance imaging brain revealed white matter T2 hyperintense lesions separate from optic nerve in ten cases, of which four cases were diagnosed as MS.
Conclusions:
Bilateral presentation was common, association with MS was low. Papillitis was more frequent than retrobulbar neuritis and prognosis was good in pediatric ON in Indian population.
Key words: Multiple sclerosis, neuroimaging, optic neuritis, pediatric
Optic neuritis (ON) can be defined as a pathologic process, whereby inflammation of one or both optic nerves leads to visual dysfunction. It can be a presenting symptom of various pediatric central nervous system disorders and may be associated with dramatic visual loss.[1] It is widely accepted that in children, attacks of ON usually occur following a febrile illness, tend to affect both eyes, are frequently associated with swollen discs, improve rapidly, with good outcomes.[2,3,4,5] The rate of conversion to multiple sclerosis (MS) is not exactly determined; studies have reported rates from 4% to 36%.[2,6,7,8,9,10] Absoud et al.[11] showed that the risk of development of MS or neuromyelitis optica (NMO) after isolated ON was high, with a cumulative probability of 0.45 at 2 years. Still there are varying differences in the studies done previously. Studies from Asia present a contrasting scenario. A study done in Korean population showed only 4% of children with MS.[9] Various studies carried out in Southeast Asia in adults had indicated that the clinical profile of ON in these regions may be different from that presented in the Western literature.[12,13,14,15,16]
In addition to that, it is well known that genetic and environmental factors play an important role in the prevalence and clinical expression of demyelinating diseases.[2] To the best of our knowledge, this study is the first series of childhood ON reported in the Indian literature. Therefore, the present study was undertaken to evaluate clinical characteristics, neuroimaging findings, and visual outcome of pediatric ON in India.
Materials and Methods
The medical records of all children <16 years of age diagnosed with ON over a 5-year period (2010–2015) were retrospectively reviewed. The clinical diagnosis of ON was made on the basis of acute or subacute visual loss of 2 weeks or less, an afferent pupillary defect, color vision defect with or without optic nerve swelling, field defects, with an abnormal visual evoked potential (VEP), and magnetic resonance imaging (MRI) brain scans. In younger children (<4 years), diagnosis of ON was made when the child was neither able to fix nor follow to light (which child could do before), acute in onset, fundus examination suggestive features of papillitis (if present) and final diagnosis was made after an abnormal MRI brain scan and VEP, for confirming the diagnosis of ON. Other optic neuropathies, such as traumatic, toxic, hereditary, were excluded from the study. Color vision testing was done using pseudoisochromatic Ishihara plates and central fields were done using Bjerrum's screen. Information about age, sex, initial best-corrected visual acuity (VA), treatment, final best-corrected VA, MRI, and associated systemic conditions was recorded. In all cases, the recovered VA was the final VA. MRI brain was done for all cases.
ON was classified as unilateral or bilateral. Patients were considered to have bilateral ON if involvement of both eyes occurred within 2 weeks of each other. All children received intravenous corticosteroids followed by oral corticosteroids in tapering doses, adjusted according to the age and weight of the child. The study was conformed to local laws and was compliant with the principles of the Declaration of Helsinki.
Statistical analysis
Mean (standard deviation) and frequency (percentage) were used to describe the summary data. Wilcoxon signed-rank test was used to compare the mean difference of VA between baseline and follow-up visits. The statistical analysis was done using Statistical software STATA 11.1 (Texas, USA). P < 0.05 was considered as statistically significant.
Results
We found 62 eyes of 40 children diagnosed as ON, fulfilling the inclusion and exclusion criteria, who presented within the study period. The mean age of presentation was 11.15 ± 3.24 years, ranging between 1 year and 15 years of age. Mean follow-up period was 13 months (0.2–64 months). Patients with demyelinating lesions on MRI were followed up for longer period (3–5 years). There was female preponderance in our study with 27 (67%) females.
Table 1 shows the comparison of initial and final VA. Thirty-five eyes (57%) presented with VA <20/200; 10 eyes (16%) presented with VA better than 20/40. After treatment, 53 eyes (86%) achieved VA better than 20/40; 7 eyes (11%) still had VA <20/200. Mean logarithm of the minimum angle of resolution (logMAR) VA at presentation was 1.14 ± 0.93, which after treatment recovered to 0.10 ± 0.26 at final visit, this was statistically significant with P < 0.001.
Table 1.
Comparison of visual acuity at initial visit and visual acuity at final visit

Twenty-two children (55%) presented with bilateral simultaneous involvement of optic nerve. Fundoscopy revealed papillitis in 38 eyes (61%); 24 eyes (39%) were diagnosed with retrobulbar neuritis. Three eyes of three children had recurrent episodes of ON (two of them had papillitis; one had retrobulbar neuritis). Neither of them was diagnosed as MS. Among three cases of recurrent ON, two cases were idiopathic while one case was later on diagnosed to have NMO with positive aquaporin-4 antibodies (NMO-IgG). It was associated with poor visual prognosis. Seven children (18%) had preceding febrile illness within 2 weeks of presentation.
MRI brain was abnormal in 26 children; 14 children showed only isolated optic nerve enhancement, 3 children showed demyelinating foci in frontal lobe, and 3 children had similar foci including parietooccipital lobe [Fig. 1]. None of them were diagnosed to have MS during the follow-up period. Cerebrospinal fluid (CSF) examination could not be done in all cases of ON since parents did not consent for the invasive procedure and due to financial constraints.
Figure 1.
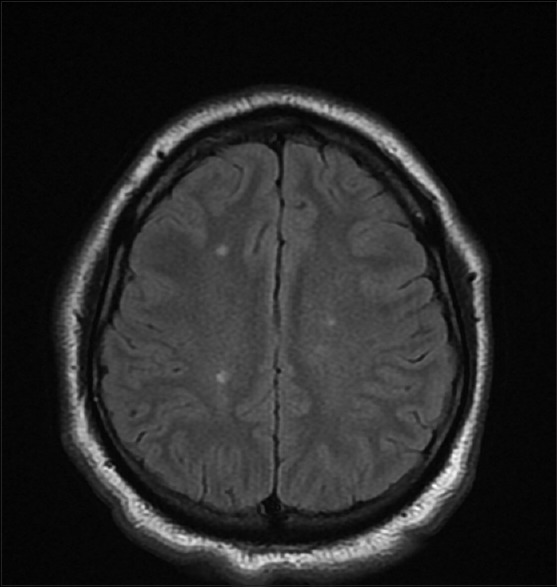
MRI of a child with optic neuritis showing multiple T2 hyperintense lesions involving parietooccipital lobe
One child with MRI brain suggestive of meningitis later was diagnosed with tuberculous meningitis; one was diagnosed with acute disseminated encephalomyelitis following CSF analysis; one case had associated bilateral maxillary sinusitis, and one had septicemia with normal brain scans.
In our series, four children were diagnosed as MS, showing demyelination of various structures of brain including periventricular white matter, subcortical structures, internal capsule, thalamus, and structures of optic pathway including optic chiasm, optic tracts, and optic radiation [Fig. 2]. Diagnosis was based on the MRI findings supported with clinical evidence. Of the four children, one of them developed transverse myelitis with urinary incontinence, 4 weeks after the episode of ON. In accordance with the recent article by Chou et al.,[17] three of the patients in our series can be classified as clinically isolated syndrome and one of them with relapsing remitting MS.
Figure 2.
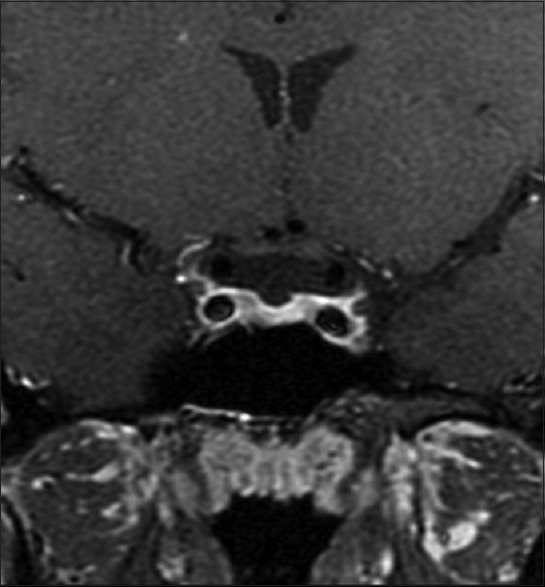
MRI of a child diagnosed with MS showing demyelination including visual pathway
Three cases of MS presented with unilateral ON; one case showed bilateral involvement. All four cases were aged above 10 years and presented with retrobulbar neuritis. None of these cases developed recurrence of any ocular involvement during their follow-up.
Flash VEP was also done in seven cases of ON, in children who had presenting VA better than 20/40 and normal MRI scan. All of them showed prolongation of P100 waveform.
Discussion
ON in adults has been analyzed extensively through the ON treatment trial.[18] An Indian study done in adults with ON showed female preponderance (70%), papillitis (53.5%), and bilateral presentation (19.3%). Baseline median logMAR VA was 1.6 ± 0.8, which improved to 0.2 ± 0.6, with approximately 64% of eyes retaining VA of 20/40 or more.[16] ON in children differs from that in adults and the data available are limited from the region.
In our study, we described the clinical characteristics, neuroimaging findings, and visual outcome of pediatric ON in South Indian population.
Table 2 shows the comparison of various features of pediatric ON with previous reports.
Table 2.
Comparison of various features of pediatric optic neuritis in our study with the previous studies
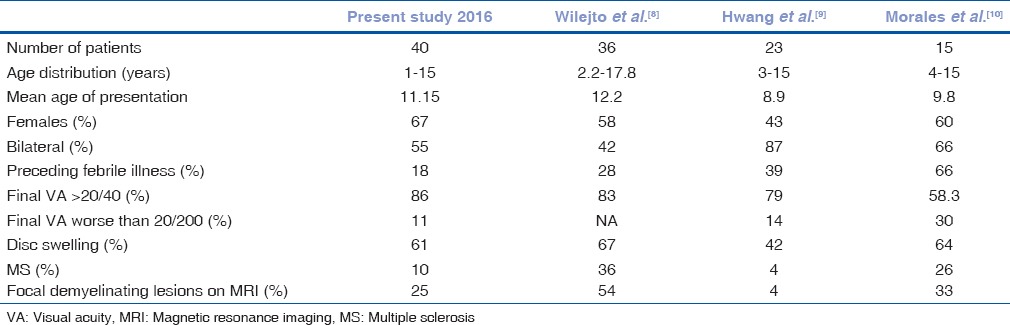
Compared to data in previous studies, there were few differences in results of our study. The rate of involvement of girls was 67% in our study versus 43%–60% in studies done previously,[8,9,10] disc swelling was present in 61% of children in our study versus 42%–67% in previous studies,[8,9,10] and preceding febrile illness was present in 18% of children in our study versus 28%–66% in other studies.[8,9,10] The prevalence of MS was 10% in our study and 4% in another study done by Hwang et al.,[9] which is lower when compared to previous reports (26%–36%).[8,9,10]
Table 3 shows cases of MS reported in previous studies. Wilejto et al. found that bilateral ON was associated with an increased risk of MS, regardless of the age at presentation.[8] Contrary to it, a systematic review and meta-analysis done by Waldman et al.[23] suggested that presentation (bilateral or unilateral) is not a factor in the development of MS. Age and presence of brain lesions outside visual system on MRI were the key variables in determining the risk of MS. They also suggested that the increased risk of MS in older children might be attributable to differences in immune system. In our study, we had four cases of MS, all of them aged above 10 years, three cases presented with unilateral ON. Ten MRI scans showed brain lesions outside visual system, while only four of them were diagnosed as MS. Lucchinetti et al.[4] reported the largest childhood series of ON to date with the longest period of follow-up. They found a greater risk of developing MS in those patients who had sequential or recurrent ON, compared with those patients who had a single episode of ON occurring in either one eye or both eyes simultaneously. In this study, among three recurrent cases, none of them were diagnosed as MS.
Table 3.
Comparison of reported multiple sclerosis cases in previous studies with the place of the study where it was carried out
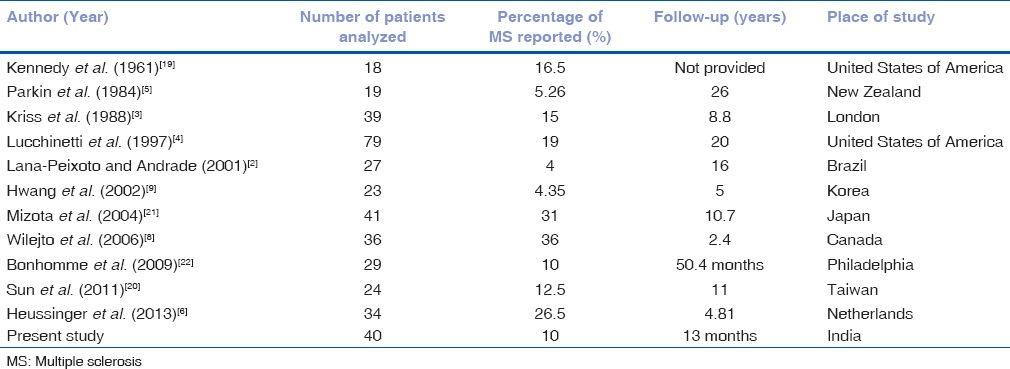
The dissimilarities in the findings can be attributed to differences due to varied period of follow-up, different races, geographical distribution of the population as many of these studies were done in different parts of the world. It is, therefore, possible that in our population, ON in children could harbor some different characteristics from those reported previously. No case series of pediatric ON were available from Indian population; hence, we could not compare our data within similar ethnic population. The present study was conducted with the aim of understanding the clinical picture of pediatric ON in India. There were few limitations of our study, first that we could not perform CSF analysis in all cases and second that the period of follow-up was minimal, so we could not assess rate of conversion to MS.
Conclusions
We found that the clinical profile of pediatric ON in the Indian scenario was similar from that reported in the Western population. However, the percentage of ON associated with MS was lower than the Western population but higher than that reported from other Asian countries.
Financial support and sponsorship
Bilateral involvement was more common, papillitis was frequent, association with MS was low, lower recurrence rate, and with good visual prognosis were the characteristics of ON in children in India.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Yeh EA, Graves JS, Benson LA, Wassmer E, Waldman A. Pediatric optic neuritis. Neurology. 2016;87(9 Suppl 2):S53–8. doi: 10.1212/WNL.0000000000002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lana-Peixoto MA, Andrade GC. The clinical profile of childhood optic neuritis. Arq Neuropsiquiatr. 2001;59:311–7. doi: 10.1590/s0004-282x2001000300001. [DOI] [PubMed] [Google Scholar]
- 3.Kriss A, Francis DA, Cuendet F, Halliday AM, Taylor DS, Wilson J, et al. Recovery after optic neuritis in childhood. J Neurol Neurosurg Psychiatry. 1988;51:1253–8. doi: 10.1136/jnnp.51.10.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucchinetti CF, Kiers L, O'Duffy A, Gomez MR, Cross S, Leavitt JA, et al. Risk factors for developing multiple sclerosis after childhood optic neuritis. Neurology. 1997;49:1413–8. doi: 10.1212/wnl.49.5.1413. [DOI] [PubMed] [Google Scholar]
- 5.Parkin PJ, Hierons R, McDonald WI. Bilateral optic neuritis. A long-term follow-up. Brain. 1984;107(Pt 3):951–64. doi: 10.1093/brain/107.3.951. [DOI] [PubMed] [Google Scholar]
- 6.Heussinger N, Kontopantelis E, Rompel O, Paulides M, Trollmann R. Predicting multiple sclerosis following isolated optic neuritis in children. Eur J Neurol. 2013;20:1292–6. doi: 10.1111/ene.12184. [DOI] [PubMed] [Google Scholar]
- 7.Brady KM, Brar AS, Lee AG, Coats DK, Paysse EA, Steinkuller PG. Optic neuritis in children: Clinical features and visual outcome. J AAPOS. 1999;3:98–103. doi: 10.1016/s1091-8531(99)70078-9. [DOI] [PubMed] [Google Scholar]
- 8.Wilejto M, Shroff M, Buncic JR, Kennedy J, Goia C, Banwell B. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology. 2006;67:258–62. doi: 10.1212/01.wnl.0000224757.69746.fb. [DOI] [PubMed] [Google Scholar]
- 9.Hwang JM, Lee YJ, Kim MK. Optic neuritis in Asian children. J Pediatr Ophthalmol Strabismus. 2002;39:26–32. doi: 10.3928/0191-3913-20020101-07. [DOI] [PubMed] [Google Scholar]
- 10.Morales DS, Siatkowski RM, Howard CW, Warman R. Optic neuritis in children. J Pediatr Ophthalmol Strabismus. 2000;37:254–9. [PubMed] [Google Scholar]
- 11.Absoud M, Cummins C, Desai N, Gika A, McSweeney N, Munot P, et al. Childhood optic neuritis clinical features and outcome. Arch Dis Child. 2011;96:860–2. doi: 10.1136/adc.2009.175422. [DOI] [PubMed] [Google Scholar]
- 12.Wakakura M, Minei-Higa R, Oono S, Matsui Y, Tabuchi A, Kani K, et al. Baseline features of idiopathic optic neuritis as determined by a multicenter treatment trial in Japan. Optic Neuritis Treatment Trial Multicenter Cooperative Research Group (ONMRG) Jpn J Ophthalmol. 1999;43:127–32. doi: 10.1016/s0021-5155(98)00072-0. [DOI] [PubMed] [Google Scholar]
- 13.Wang JC, Tow S, Aung T, Lim SA, Cullen JF. The presentation, aetiology, management and outcome of optic neuritis in an Asian population. Clin Exp Ophthalmol. 2001;29:312–5. doi: 10.1046/j.1442-9071.2001.00442.x. [DOI] [PubMed] [Google Scholar]
- 14.Woung LC, Lin CH, Tsai CY, Tsai MT, Jou JR, Chou P. Optic neuritis among national health insurance enrollees in Taiwan, 2000-2004. Neuroepidemiology. 2007;29:250–4. doi: 10.1159/000112858. [DOI] [PubMed] [Google Scholar]
- 15.Lim SA, Goh KY, Tow S, Fu E, Wong TY, Seah A, et al. Optic neuritis in Singapore. Singapore Med J. 2008;49:667–71. [PubMed] [Google Scholar]
- 16.Saxena R, Phuljhele S, Menon V, Gadaginamath S, Sinha A, Sharma P. Clinical profile and short-term outcomes of optic neuritis patients in India. Indian J Ophthalmol. 2014;62:265–7. doi: 10.4103/0301-4738.121131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou IJ, Wang HS, Whitehouse WP, Constantinescu CS. Paediatric multiple sclerosis: Update on diagnostic criteria, imaging, histopathology and treatment choices. Curr Neurol Neurosci Rep. 2016;16:68. doi: 10.1007/s11910-016-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The clinical profile of optic neuritis. Experience of the Optic Neuritis Treatment Trial. Optic Neuritis Study Group. Arch Ophthalmol. 1991;109:1673–8. doi: 10.1001/archopht.1991.01080120057025. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy C, Carter S. Relation of optic neuritis to multiple sclerosis in children. Pediatrics. 1961;28:377–87. [PubMed] [Google Scholar]
- 20.Sun MH, Wang HS, Chen KJ, Su WW, Hsueh PY, Lin KK, et al. Clinical characteristics of optic neuritis in Taiwanese children. Eye (Lond) 2011;25:1457–64. doi: 10.1038/eye.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizota A, Niimura M, Adachi-Usami E. Clinical characteristics of Japanese children with optic neuritis. Pediatr Neurol. 2004;31:42–5. doi: 10.1016/j.pediatrneurol.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Bonhomme GR, Waldman AT, Balcer LJ, Daniels AB, Tennekoon GI, Forman S, et al. Pediatric optic neuritis: Brain MRI abnormalities and risk of multiple sclerosis. Neurology. 2009;72:881–5. doi: 10.1212/01.wnl.0000344163.65326.48. [DOI] [PubMed] [Google Scholar]
- 23.Waldman AT, Stull LB, Galetta SL, Balcer LJ, Liu GT. Pediatric optic neuritis and risk of multiple sclerosis: Meta-analysis of observational studies. J AAPOS. 2011;15:441–6. doi: 10.1016/j.jaapos.2011.05.020. [DOI] [PubMed] [Google Scholar]


