Abstract
Objective:
The study aimed to evaluate the effectiveness of freeze dried powdered probiotics on gingival status and plaque inhibition among 12–15-year-old schoolchildren.
Materials and Methods:
This randomized controlled trial was conducted among 12–15-year-old schoolchildren in Jaipur. Commercially available freeze dried probiotics containing Lactobacillus acidophilus, Bifidobacterium longum, Bifidobacterium bifidum and Bifidobacterium lactis (Prowel, Alkem Laboratories), lactic acid bacillus only (Sporolac, Sangyo), and a placebo powder calcium carbonate 250 g (Calcium Sandoz, Novartis) were assigned to two intervention groups and a placebo group each comprising 11 schoolchildren. All subjects were instructed to mix the powder in 30 ml of water and swish once daily for 3 min, for 3 weeks. Periodontal clinical parameters were assessed by examining the subjects for Turesky-Gilmore-Glickman plaque index (PI) (Modification of Quigley-Hein PI) and gingival index at baseline, 7th day, 14th day, and 21st day.
Results:
For both the probiotic groups, a statistically significant reduction (P < 0.05) in gingival status and plaque inhibition was recorded up to 2nd week of probiotic ingestion. However, no significant difference was observed in the placebo group.
Conclusion:
The use of probiotic mouth rinses improves the oral health in children by significantly reducing the plaque and gingival scores. Further studies are warranted to prove or refute the long-term effects, means of administering probiotics and the dosages needed to achieve different preventive or therapeutic purposes.
Keywords: Bacteriotherapy, Bifidobacteria, Lactobacillus, oral ecology, probiotic
Introduction
Probiotics are food supplements that contain live bacteria, which benefit people's digestive tract by maintaining a balanced gut flora.[1,2,3] According to the International Scientific Association and the World Health Organization, probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host.”[4] Probiotics are known to favorably influence the development and stability of microbiota by inhibiting the colonization of pathogens, stimulating the innate and adaptive immune system as well as by enhancing the mucosal barrier through tropic effects on the epithelium.[5,6] They can combat infections by displacing pathogenic microorganisms and replacing them with harmless beneficial bacteria. Lactobacillus and Bifidobacterium are among the most commonly used genera fulfilling these criteria.[5,7] With increasing antibiotic resistance, in recent years, the use of such beneficial bacteria in improving health of the host has gained much popularity in the field of medical research.[8]
In addition to the conventional measures against dental caries and gingival diseases involving physical and chemotherapeutic agents, there is a need for alternative disease prevention modalities.[9] Probiotics are effective, natural, and economical substitutes to combat dental diseases.[10] The rationale for using probiotics is to alter the microbial imbalance in caries and periodontal diseases by adding beneficial species.[11] Probiotics have shown favorable properties in maintaining oral health by contributing to a healthier microbial equilibrium.[12] To provide benefits in the oral cavity, probiotics should adhere to and colonize on dental tissues. They should not ferment sugars, which subsequently lower the pH facilitating demineralization.[13] Inclusion of probiotic-enriched food promotes a healthy lifestyle by delaying and halting the pathophysiology of periodontal diseases.[14]
Thus, the present study aimed to explore whether the oral administration of two commercially available probiotic preparations could change the clinical parameters of gingival tissue. For this purpose, a double-blind, randomized, placebo-controlled clinical trial was conducted among healthy schoolchildren aged 12–15 years.
Materials and Methods
The study was carried out from January 16, 2013 to February 06, 2013 at Government Secondary School, Labana, Jaipur, which was a double-blind, randomized, parallel, and placebo controlled clinical trial. Sample size was calculated at 80% study power and α-error of 0.05. For the ratio of standard deviation (SD) to the difference of mean to be detected as 0.8, minimum sample size required for each group came to ten subjects. Assuming 10% dropouts or attrition, it was further enhanced to 11 subjects in each group. Thus, 33 subjects were included in the study, whose baseline Turesky-Gilmore-Glickman plaque index (PI)[15] and gingival index (GI)[16] were assessed. They were randomly divided into three groups. Group A comprised 11 children using freeze dried Lactobacillus acidophilus, Bifidobacterium longum, Bifidobacterium bifidum, and Bifidobacterium lactis (Prowel, Alkem Laboratories). Group B included eleven children using freeze dried Lactic acid bacillus only (Sporolac, Sangyo). Group C included eleven children using the placebo powder calcium carbonate 250 g (Calcium Sandoz, Novartis). The study was conducted over a period of 3 weeks and examination and sampling of the subjects were done on baseline day/0 day, 7th day, 14th day, and 21st day.
The subjects included healthy schoolchildren without any systemic disorder, children between the age group of 12–15 years, no history of oral prophylaxis within 6 months, no recent history of use of antimicrobial/antibacterial agents within 3 months, and subjects with mean plaque scores >1 to include similar subjects which would minimize the chances of selection bias. The subjects who were excluded included children whose parents/guardians did not give the consent, subjects who were regularly using mouthwashes/probiotic products, children who were absent on the day of examination, subjects undergoing orthodontic treatment, children with mixed dentition, and habitual smokers.
The required ethical approval was obtained from the Institutional Review Board of Jaipur Dental College and permission was obtained from the Principal, Government Secondary School, Labana. The participants whose parents signed a written consent form before being interviewed were included in the study. The trial was also registered retrospectively, under Clinical Trials Registry of India under reference no: CTRI/2013/05/003677, dated May 27, 2013.
The first investigator comprehensively carried out the clinical examination for each subject. Before conducting the study, the investigator was calibrated to limit the intraexaminer variability. To assess the intraexaminer reliability, the investigator examined nine subjects and recorded the Turesky modification of PI and GI. The same subjects were examined by different examiners on the same day and were randomly called on the next day and the investigator repeated the examinations. The Kappa coefficient value for intraexaminer reliability with respect to the Turesky modification of PI and GI was 0.85. The values reflected high degree of conformity in observation.
The subjects were asked to refrain from oral hygiene measures for 24 h before each recall visits. The examination was conducted in the play field of the school during the morning hours. American Dental Association type-III examination[17] was carried out by the calibrated investigator throughout the study. Repackaging of all the three powders was performed under sterile conditions into small transparent antistatic zip lock polyethene pouches and was individually color coded as red, blue, and green based on the contents. Red pouch contained powder containing freeze dried L. acidophilus, B. longum, B. bifidum, and B. lactis (Prowel, Alkem Laboratories). Blue pouch contained powder containing freeze dried lactic acid bacillus only (Sporolac, Sangyo). Green pouch contained placebo powder calcium carbonate 250 g (Calcium Sandoz, Novartis). Six similar color-coded pouches were further kept in a bigger ziplock polybag along with a stirrer, measuring jar with graduations till 30 ml so that each study participant could use it for 1 week till next examination.
Before the start of the study, the second investigator who was blinded to the contents of the color-coded pouches carried out the allocation procedure based on the inclusion criteria. Following clinical assessments, using block randomization, they were randomly divided into three groups by the first investigator and it was ensured that the subjects with varying gingival and plaque scores were included in all the groups equally. The color-coded pouches were distributed to the appropriate groups by the second investigator and were supplied in a regular, scheduled manner throughout the course of the study at weekly intervals. Repackaging was done just 1 day before the weekly recall examination to ensure the viability of the powder. To ensure the criteria of randomization and double-blinding, the first investigator who carried out the examination was blinded to the allocation of study subjects into color groups and the second examiner was not involved in recording of clinical parameters at any of the recall visits.
After distributing the color-coded pouches, the procedure of mixing the powder in the sachet with 30 ml of water in the measuring jar, using a stirrer was demonstrated. The participants were instructed to swish and rinse their mouth once daily in the morning for 3 min. The plaque disclosing agent – two tone dye (Alpha Plac, D.P.I Ltd.) was applied using cotton tips. Examinations for the PI (Turesky Modification of Quigley Hein PI)[15] and GI[16] were carried out at baseline, 1 week, 2 weeks, and 3 weeks [Figure 1]. All the subjects were evaluated by the same examiner throughout the study period. The examination was carried out using a specific recording pro forma comprising name, age, gender, class, sociodemographic variables designed for the study, and recording format for the Turesky modification of Quigley Hein PI, 1970 and GI, 1963. A pretested and validated questionnaire was used to record the information about oral hygiene practices, dietary habits, in-between meal snacking, existing dental problems, and visit to a dentist. During the entire study period, participants were advised to exercise their usual oral hygiene practices and abstain from using any adjuvants such as mouthwashes. After the commencement of the study, dental health education and proper brushing techniques were taught to all the participants.
Figure 1.
Examinations for the plaque index (Turesky modification of Quigley Hein plaque index)
Qualitative data were summarized as mean and SD. For paired samples, repeated measure ANOVA and paired t-test were used for the comparison of mean values. For comparison of median values in paired/dependent samples, Wilcoxon signed-rank sum test was used. All analyses were performed using MedCalc version 12.2.1.0 (MedCalc Software Mariakerke, Belgium). For all tests, a P ≤ 0.05 was used for statistical significance.
The overview of the methodology followed is summarized in Figure 2.
Figure 2.
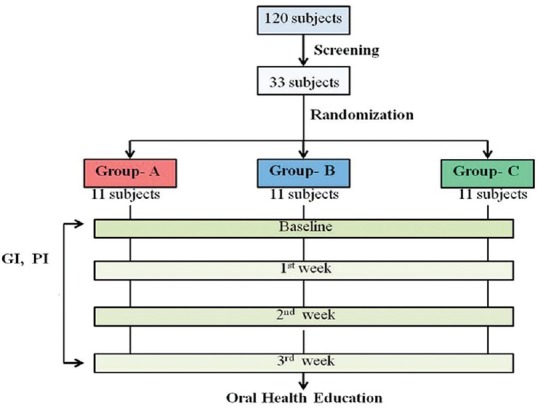
Overview of the methodology
Results
During the 4-week follow-up period, neither unintended/untoward effect was observed, nor any subject was lost to follow-up. An improvement in gingival and plaque scores in both Group A and B was observed. For Group A, both GI [Figure 3 and Table 1] and PI [Figure 3 and Table 2] decreased significantly from its baseline value to its 1st week value and even up to 2nd week value, but it increased at 3rd week and difference between baseline and 3rd week value did not show statistical significance. Similarly for Group B, both GI [Figure 4 and Table 1] and PI [Figure 4 and Table 2] decreased significantly from its baseline value to its 1st week value and even up to 2nd week value, but it increased at 3rd week and difference between baseline and 3rd week value did not show statistical significance. However, in Group C/placebo Group, both GI [Figure 5 and Table 1] and PI [Figure 5 and Table 2] increased significantly from its baseline value to its 1st week value, 2nd week up to the 3rd week.
Figure 3.
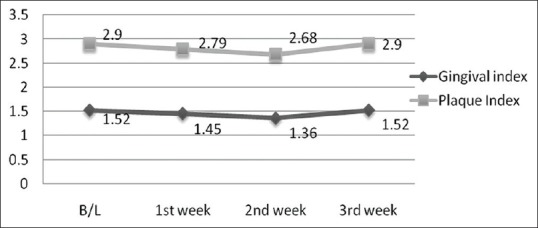
Gingival and plaque scores during subsequent weekly intervals in Group A
Table 1.
Pairwise comparisons of Gingival Index between different time periods
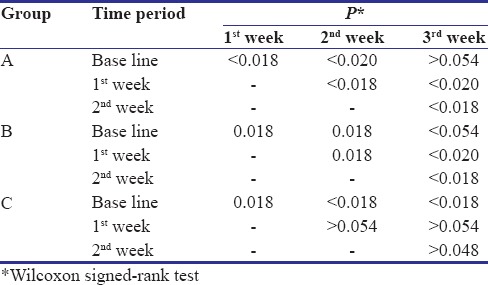
Table 2.
Pair-wise comparisons of Plaque Index between different time periods
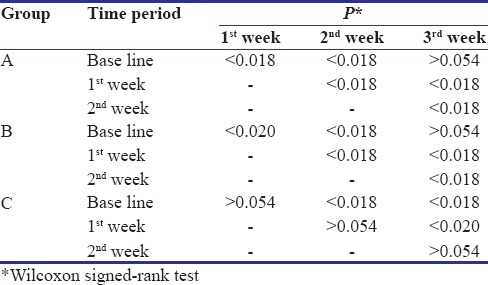
Figure 4.
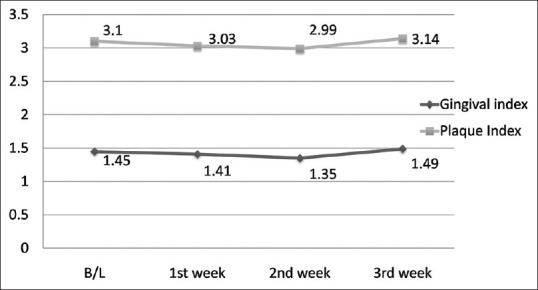
Gingival and plaque scores during subsequent weekly intervals in Group B
Figure 5.
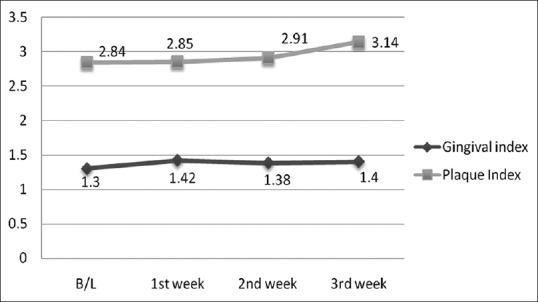
Gingival and plaque scores during subsequent weekly intervals in Group C
Discussion
The present randomized control trial was conducted to evaluate the dental health outcomes following administration of freeze dried probiotic powder in the form of a mouthrinse at repeated weekly intervals on schoolchildren in Jaipur. A total of 120 schoolchildren were screened, out of which 33 schoolchildren comprising 19 male and 14 female subjects, between 12 and 15 years of age were included in the study. In the present study, examination was performed at weekly intervals for 21 days. The examination period of 14th day was chosen for permitting comparison with other studies.[18,19] The period of 7th day was chosen because the most rapid changes in plaque formation take place during the first 4–5 days and 21st day was selected because the plaque becomes relatively stable by around the 21st day.[20]
Earlier studies[21,22] have established a beneficial effect of probiotics administered in the form of lozenges and chewing gums on oral health, which are not marketed in India. Thus, one of the aims of the present study was to find an easily available and a cost-effective alternative to these products. In the present interventional study, the gingival examination preceded the plaque examination because of the reason of using a Plaque Disclosing agent - Two-tone dye (Alpha Plac, D.P.I) as it stains the plaque and parts of the gingiva, which could have influenced the findings of gingival status.
It is evident by the outcomes of the present study that there was a short-term improvement in the mean GI and PI scores during subsequent 2 weeks in both the intervention groups. The results were in harmony with a study[18] where a significant decrease in mean GI and PI scores of probiotic rinse compared to placebo rinse at the 14th-day examination in comparison with the baseline data was observed. Similar significant reductions were observed in gingival scores during the 2-week period.[19,23] The reduction in the mean GI could be due to bacteriocins secreted by probiotic bacteria such as Lactobacillus spp.[13] They also activate immunocompetent cells to secrete both inflammatory and anti-inflammatory cytokines, which in turn modulates the mucosal immune system. Probiotics may also exert their beneficial effect in the oral cavity by directly interacting with microorganisms in dental plaque and indirectly by modulation of the innate/acquired immune systems. Aggregation alteration is another important mechanism of action of probiotics for inhibition of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia.[24]
However, in our study, for both the study groups, the gingival and plaque scores increased at 3rd week and no statistical significant difference was observed between baseline and 3rd week. These results were contrary to a study[5] where periodontal clinical parameters were improved in both groups even after 4-week and 8-week intervention. However, similar findings were observed in previous studies[8,9] where PI and GI showed a statistically significant decrease, following the use of probiotics 2 weeks after intervention. Probiotics may achieve the antiplaque activity by inhibiting the growth of microorganisms, reducing the adhesion of bacteria to the tooth surface, inhibiting the formation of the intercellular plaque matrix, reducing the formation of cytotoxic products by modifying plaque biochemistry and ecology to a less pathogenic flora.[25] It was also demonstrated in a study[26] that probiotic mouth rinses containing an active ingredient nisin showed bactericidal activity against a wide range of Gram-positive bacteria. The main mechanism of probiotics involving action on noncariogenic bacteria resulting in control of plaque biofilm formation.[9]
In our study, the reduction in plaque accumulation and gingivitis could also be due to a confounding factor known as the Hawthorne effect or the attention bias. The subjects participation involved repeated dental examinations may, even if no active attempts were made to improve their self-performed plaque control measures, stimulated the participants to improve their mechanical tooth cleaning measures. The participants usually would improve their oral hygiene although they were unaware of the regimen administered to them. This was in agreement to another study[27] where the effect of listerine, meridol, and chlorhexidine was compared on plaque and gingivitis. It was observed that due to the Hawthorne effect, the mean PI scores in the placebo group decreased at day 7.
The results were conflicting to a study[28] where it was observed that probiotic rinse was least effective as compared to 0.2% chlorhexidine digluconate and herbal oral rinse after 1 week of intervention. The results were also contradictory to a study[29] where a reduction in the counts of periodontopathic bacteria (Tannerella forsythia) in the subgingival plaque after 4 weeks of probiotic intervention was observed when compared with that of the placebo group. This variation could be attributed to lack of compliance, motivation, and interest toward oral health education, among the rural schoolchildren. Despite providing weekly instructions, it was not possible to provide individual attention and reinforcement everyday due to unavoidable constraints such as lack of communication means such as telephone/cellular phones for strict compliance. This study involved limited training which was limited to once weekly, which could have been a barrier in providing adequate supervision for maintaining the oral hygiene adequately. Besides, the mouthrinses were not readily available as a result of which the study subjects were instructed to prepare the rinse by mixing the powder in water, which was flavorless, bland, and nonpleasing for children. After using it for few weeks, they would have disliked the taste and would not have followed the instructions efficiently as they were instructed to.
Most of the strains of probiotics can be regarded safe to a greater extent and hence come under generally recognized as safe category. However, certain strains of Enterococcus, Streptococcus, Lactobacilli, and Bifidobacteria have been associated with infections such as bacteremia, endocarditis, septicemia, fungemia, nosocomial infections, and development of caries as certain strains of lactobacilli along with Streptococcus mutans play a role in the progression of dental caries. However, these side effects are most commonly seen in immunocompromised patients.[30] However, in the present study throughout the 4-week follow-up period, no untoward side effect was observed in all the subjects. It is, therefore, important to analyze the potential strain of probiotic before using it commercially.
Conclusion
Probiotics are helpful in improving the oral health. Both the probiotic groups showed a significant short-term inhibitory effect on plaque accumulation and gingivitis. It can be proposed that probiotic mouthrinse has a potential therapeutic value in reducing gingivitis and plaque formation in children.
Further randomized controlled trials are required to prove or refute the long-term effects of probiotics on oral health. It is recommended for the manufacturers to improve the strain performance and activity by conducting further research to determine the exact dosage, improve consumer acceptance, stability and efficacy of probiotic-containing products by incorporating flavoring agents and making the products more palatable and more pleasing for the use by schoolchildren and for the parents to periodically reinforce healthy behaviors among their children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Rajeev Yadav, Associate Professor, Department of Preventive and Social Medicine, S.M.S. Medical College, Jaipur, for extending his valuable suggestions throughout our study.
References
- 1.Caglar E, Kargul B, Tanboga I. Bacteriotherapy and probiotics’ role on oral health. Oral Dis. 2005;11:131–7. doi: 10.1111/j.1601-0825.2005.01109.x. [DOI] [PubMed] [Google Scholar]
- 2.Caglar E, Sandalli N, Twetman S, Kavaloglu S, Ergeneli S, Selvi S. Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol Scand. 2005;63:317–20. doi: 10.1080/00016350510020070. [DOI] [PubMed] [Google Scholar]
- 3.Caglar E, Kuscu OO, Cildir SK, Kuvvetli SS, Sandalli N. A probiotic lozenge administered medical device and its effect on salivary mutans streptococci and lactobacilli. Int J Paediatr Dent. 2008;18:35–9. doi: 10.1111/j.1365-263X.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- 4.Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food London, Ontario, Canada; 30 April and 01 May. 2002 [Google Scholar]
- 5.Shimauchi H, Mayanagi G, Nakaya S, Minamibuchi M, Ito Y, Yamaki K, et al. Improvement of periodontal condition by probiotics with Lactobacillus salivarius WB21: A randomized, double-blind, placebo-controlled study. J Clin Periodontol. 2008;35:897–905. doi: 10.1111/j.1600-051X.2008.01306.x. [DOI] [PubMed] [Google Scholar]
- 6.Tlaskalová-Hogenová H, Stepánková R, Hudcovic T, Tucková L, Cukrowska B, Lodinová-Zádníková R, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Isolauri E. Probiotics in human disease. Am J Clin Nutr. 2001;73:1142S–6S. doi: 10.1093/ajcn/73.6.1142S. [DOI] [PubMed] [Google Scholar]
- 8.Purunaik S, Thippeswamy HM, Chavan SS. To evaluate the effect of probiotic mouthrinse on plaque and gingivitis among 15-16 year old school children of Mysore City, India-randomized controlled trial. Glob J Med Res. 2014;14:9–14. [Google Scholar]
- 9.Salem RG, Abd-El-Aziz AM, Erfan DM. Assessment of the effect of probiotic yoghurt and different probiotic strains on salivary Streptococcus mutans in children: An in vivo and an in vitro study. Egypt J Med Microbiol. 2016;25:35–41. [Google Scholar]
- 10.Scariya L, Nagarathna DV, Varghese M. Probiotics in periodontal therapy. Int J Pharm Bio Sci. 2015;6:242–50. [Google Scholar]
- 11.Laleman I, Teughels W. Probiotics in the dental practice: A review. Quintessence Int. 2015;46:255–64. doi: 10.3290/j.qi.a33182. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Q, Stamatova I, Kainulainen V, Korpela R, Meurman JH. Interactions between Lactobacillus rhamnosus GG and oral micro-organisms in an in vitro biofilm model. BMC Microbiol. 2016;16:149. doi: 10.1186/s12866-016-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meurman JH. Probiotics: Do they have a role in oral medicine and dentistry? Eur J Oral Sci. 2005;113:188–96. doi: 10.1111/j.1600-0722.2005.00191.x. [DOI] [PubMed] [Google Scholar]
- 14.George VT, Varghese MM, Vaseem MS, Thomas A, Ittycheria PG, Sreejith CK. The promising future of probiotics: A new era in periodontal therapy. J Int Oral Health. 2016;8:404–8. [Google Scholar]
- 15.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970;41:41–3. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 16.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 17.Dunning JM. Principles of Dental Public Health. 4th ed. Vol. 339. London: Harvard University Press; 1986. p. 173. [Google Scholar]
- 18.Noordin K, Kamin S. The effect of probiotic mouth rinse on plaque and gingival inflammation. Ann Dent Univ Malaya. 2007;14:19–25. [Google Scholar]
- 19.Jindal G, Pandey RK, Agarwal J, Singh M. A comparative evaluation of probiotics on salivary mutans streptococci counts in Indian children. Eur Arch Paediatr Dent. 2011;12:211–5. doi: 10.1007/BF03262809. [DOI] [PubMed] [Google Scholar]
- 20.Norman OH, Arden GC. Primary Preventive Dentistry. 4th ed. Norwalk, Connecticut: Appleton and Lange; 1994. pp. 22–3. [Google Scholar]
- 21.Caglar E, Cildir SK, Ergeneli S, Sandalli N, Twetman S. Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol Scand. 2006;64:314–8. doi: 10.1080/00016350600801709. [DOI] [PubMed] [Google Scholar]
- 22.Montalto M, Vastola M, Marigo L, Covino M, Graziosetto R, Curigliano V, et al. Probiotic treatment increases salivary counts of lactobacilli: A double-blind, randomized, controlled study. Digestion. 2004;69:53–6. doi: 10.1159/000076559. [DOI] [PubMed] [Google Scholar]
- 23.Harini PM, Anegundi RT. Efficacy of a probiotic and chlorhexidine mouth rinses: A short-term clinical study. J Indian Soc Pedod Prev Dent. 2010;28:179–82. doi: 10.4103/0970-4388.73799. [DOI] [PubMed] [Google Scholar]
- 24.Twetman S, Derawi B, Keller M, Ekstrand K, Yucel-Lindberg T, Stecksen-Blicks C. Short-term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontol Scand. 2009;67:19–24. doi: 10.1080/00016350802516170. [DOI] [PubMed] [Google Scholar]
- 25.Cummins D, Creeth JE. Delivery of antiplaque agents from dentifrices, gels, and mouthwashes. J Dent Res. 1992;71:1439–49. doi: 10.1177/00220345920710071601. [DOI] [PubMed] [Google Scholar]
- 26.Gross E, Morell JL. The structure of nisin. J Am Chem Soc. 1971;93:4634–5. doi: 10.1021/ja00747a073. [DOI] [PubMed] [Google Scholar]
- 27.Brecx M, Brownstone E, MacDonald L, Gelskey S, Cheang M. Efficacy of Listerine, Meridol and chlorhexidine mouthrinses as supplements to regular tooth cleaning measures. J Clin Periodontol. 1992;19:202–7. doi: 10.1111/j.1600-051x.1992.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 28.Mishra R, Tandon S, Rathore M, Banerjee M. Antimicrobial and plaque inhibitory potential of herbal and probiotic oral rinses in children: A randomized clinical trial. Indian J Dent Res. 2014;25:485–92. doi: 10.4103/0970-9290.142543. [DOI] [PubMed] [Google Scholar]
- 29.Mayanagi G, Kimura M, Nakaya S, Hirata H, Sakamoto M, Benno Y, et al. Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: A double-blinded, placebo-controlled, randomized clinical trial. J Clin Periodontol. 2009;36:506–13. doi: 10.1111/j.1600-051X.2009.01392.x. [DOI] [PubMed] [Google Scholar]
- 30.Snydman DR. The safety of probiotics. Clin Infect Dis. 2008;46(Suppl 2):S104–11. doi: 10.1086/523331. [DOI] [PubMed] [Google Scholar]


