Abstract
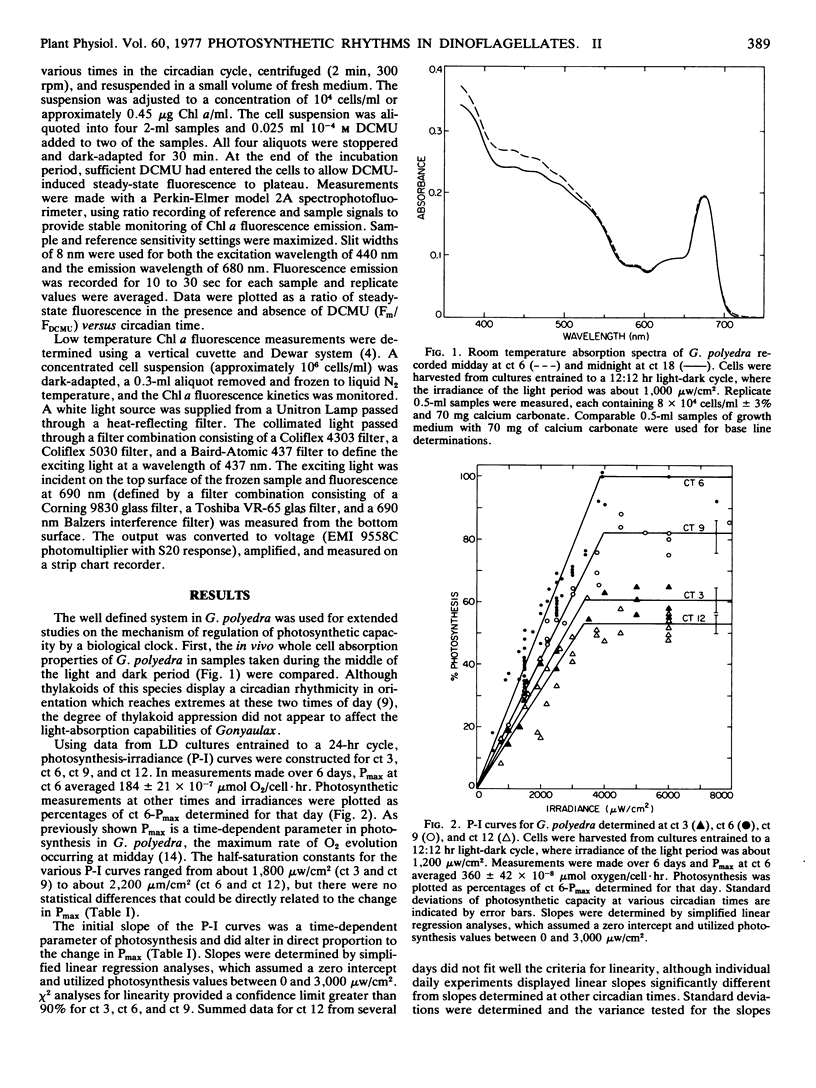
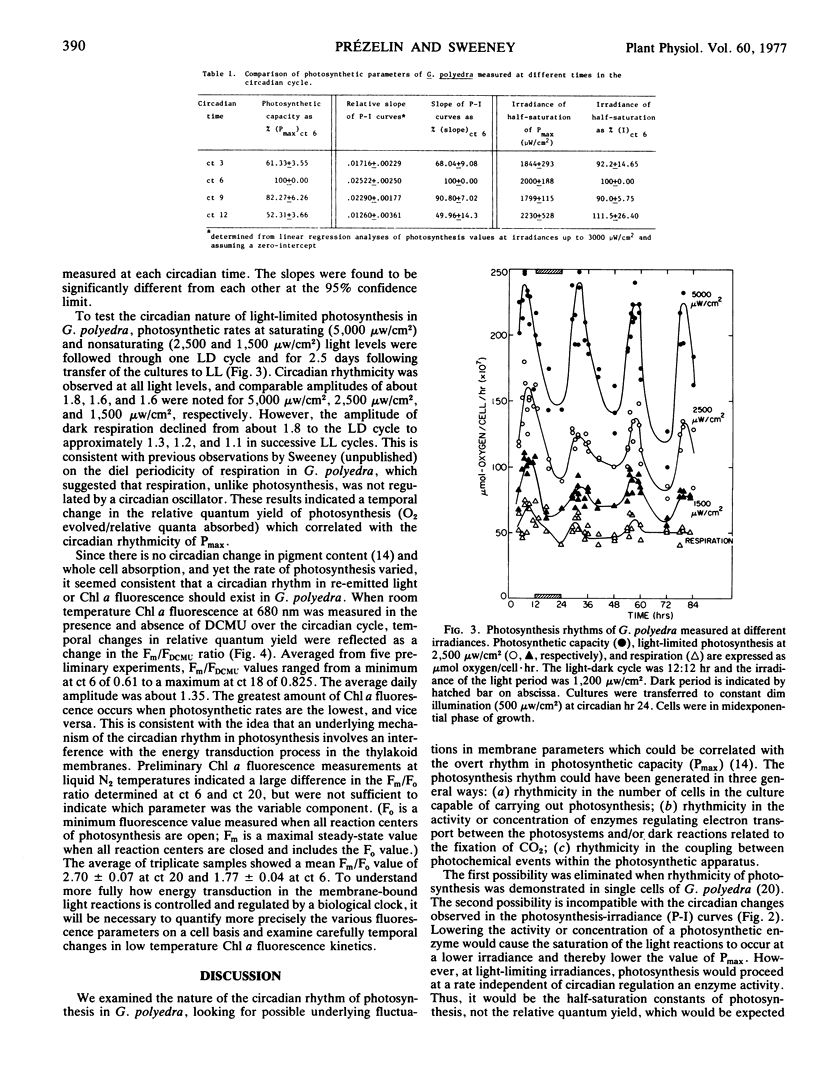
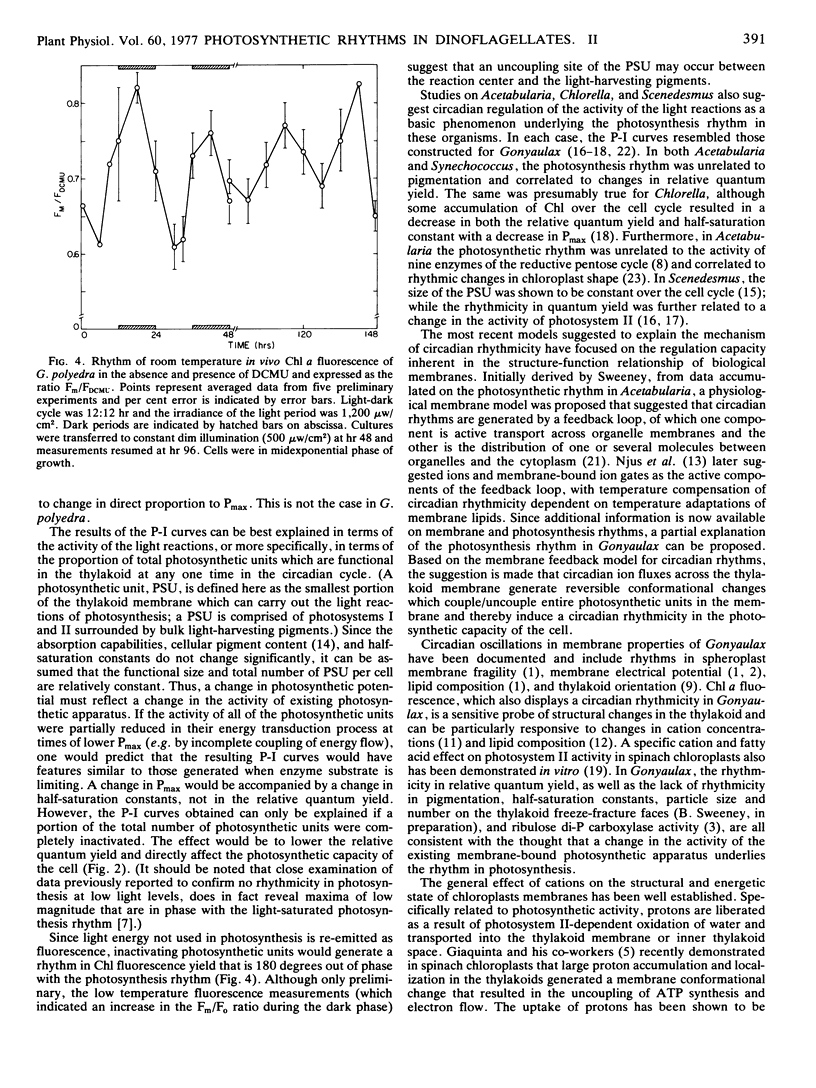
Using data from light-dark cultures of Gonyaulax polyedra entrained to a 24-hour cycle, whole cell absorption curves and photosynthesis-irradiance curves were constructed for various circadian times. While whole cell absorbance and half-saturation constants of photosynthesis showed no statistical difference that could be directly related to the photosynthetic rhythm, the initial slope of the photosynthesis-irradiance curve was a time-dependent parameter which altered in direct proportion to the change in photosynthetic capacity. The results indicated a temporal change in the relative quantum yield of photosynthesis, and the circadian rhythmicity of light-limited photosynthesis was established under constant conditions. Circadian rhythmicity was detected in room temperature chlorophyll fluorescence yield. Low temperature fluorescence kinetics also showed fluctuations. The results suggest that regulation of photosynthesis by the biological clock of Gonyaulax may be mediated through the membrane-bound light reactions and a partial explanation of the underlying mechanism is proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamich M., Laris P. C., Sweeney B. M. In vivo evidence for a circadian rhythm in membranes of Gonyaulax. Nature. 1976 Jun 17;261(5561):583–585. doi: 10.1038/261583a0. [DOI] [PubMed] [Google Scholar]
- Bush K. J., Sweeney B. M. The Activity of Ribulose Diphosphate Carboxylase in Extracts of Gonyaulax polyedra in the Day and the Night Phases of the Circadian Rhythm of Photosynthesis. Plant Physiol. 1972 Oct;50(4):446–451. doi: 10.1104/pp.50.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L. Absorption spectroscopy of biological materials. Methods Enzymol. 1972;24:3–25. doi: 10.1016/0076-6879(72)24052-6. [DOI] [PubMed] [Google Scholar]
- Giaquinta R. T., Ort D. R., Dilley R. A. The possible relationship between a membrane conformational change and photosystem II dependent hydrogen ion accumulation and adenosine 5'-triphosphate synthesis. Biochemistry. 1975 Oct 7;14(20):4392–4396. doi: 10.1021/bi00691a008. [DOI] [PubMed] [Google Scholar]
- HASTINGS J. W., ASTRACHAN L., SWEENEY B. M. A persistent daily rhythm in photosynthesis. J Gen Physiol. 1961 Sep;45:69–76. doi: 10.1085/jgp.45.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASTINGS J. W. Biochemical aspects of rhythms: phase shifting by chemicals. Cold Spring Harb Symp Quant Biol. 1960;25:131–143. doi: 10.1101/sqb.1960.025.01.012. [DOI] [PubMed] [Google Scholar]
- Herman E. M., Sweeney B. M. Circadian rhythm of chloroplast ultrastructure in Gonyaulax polyedra, concentric organization around a central cluster of ribosomes. J Ultrastruct Res. 1975 Mar;50(3):347–354. doi: 10.1016/s0022-5320(75)80065-7. [DOI] [PubMed] [Google Scholar]
- Murata N. Relationships between the Transition of the Physical Phase of Membrane Lipids and Photosynthetic Parameters in Anacystis nidulans and Lettuce and Spinach Chloroplasts. Plant Physiol. 1975 Oct;56(4):508–517. doi: 10.1104/pp.56.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njus D., Sulzman F. M., Hastings J. W. Membrane model for the circadian clock. Nature. 1974 Mar 8;248(5444):116–120. doi: 10.1038/248116a0. [DOI] [PubMed] [Google Scholar]
- Prézelin B. B., Meeson B. W., Sweeney B. M. Characterization of photosynthetic rhythms in marine dinoflagellates: I. Pigmentation, photosynthetic capacity and respiration. Plant Physiol. 1977 Sep;60(3):384–387. doi: 10.1104/pp.60.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWEENEY B. M. The photosynthetic rhythm in single cells of Gonyaulax polyedra. Cold Spring Harb Symp Quant Biol. 1960;25:145–148. doi: 10.1101/sqb.1960.025.01.013. [DOI] [PubMed] [Google Scholar]
- Schmid G. H., Gaffron H. Photosynthetic units. J Gen Physiol. 1968 Aug;52(2):212–239. doi: 10.1085/jgp.52.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger H., Bishop N. I. Quantum yield of photosynthesis in synchronous Scenedesmus cultures. Nature. 1967 Apr 8;214(5084):140–142. doi: 10.1038/214140a0. [DOI] [PubMed] [Google Scholar]
- Siegenthaler P. A. Inhibition of photosystem II electron transport in chloroplasts by fatty acids and restoration of its activity by Mn2+. FEBS Lett. 1974 Mar 1;39(3):337–340. doi: 10.1016/0014-5793(74)80144-4. [DOI] [PubMed] [Google Scholar]
- Sweeney B. M. A physiological model for circadian rhythms derived from the acetabularia rhythm paradoxes. Int J Chronobiol. 1974;2(1):25–33. [PubMed] [Google Scholar]
- Vanden Driessche T. Circadian rhythms in Acetabularia: photosynthetic capacity and chloroplast shape. Exp Cell Res. 1966 Apr;42(1):18–30. doi: 10.1016/0014-4827(66)90315-6. [DOI] [PubMed] [Google Scholar]
- Walther W. G., Edmunds L. N. Studies on the Control of the Rhythm of Photosynthetic Capacity in Synchronized Cultures of Euglena gracilis (Z). Plant Physiol. 1973 Feb;51(2):250–258. doi: 10.1104/pp.51.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]


