Abstract
Pathological conditions such as amyotrophic lateral sclerosis, spinal cord injury and chronic pain are characterized by activation of astrocytes and microglia in spinal cord and have been modeled in rodents. In vivo imaging at cellular level in these animal models is limited due to the spinal cord’s highly myelinated funiculi. The preparation of acute slices may offer an alternative and valuable strategy to collect structural and functional information in vitro from dorsal, lateral and ventral regions of spinal cord. Here, we describe a procedure for preparing acute slices from mouse spinal cord ( Garré et al., 2016 ). This preparation should allow further understanding of how glial cells in spinal cord respond acutely to various inflammatory challenges.
Keywords: Microglia, Astrocyte, Spinal cord, Neuroinflammation, Spina cord slices
Background
Mouse transgenic technology has been used to model different human pathologies affecting the spinal cord, many of which are characterized by local glial activation, one hallmark of neuroinflammation. A major breakthrough that has enormously increased the understanding of glial biology in health and disease is the utilization of laser scanning microscopy based techniques, such as confocal microscopy ( White et al., 1987 ) and two-photon microscopy ( Denk et al., 1990 ) to visualize cell structures and subcellular domains in living animals in a noninvasive fashion; for example, mice expressing genetically encoded reporters or calcium sensors have been used to image glial structures (somata and processes) and to study calcium dynamics and signaling, respectively ( Davalos et al., 2005 ; Gee et al., 2014 ). In spinal cord, myelin is highly compact in the white matter of the dorsal, lateral, and ventral funiculi. In vivo structural imaging of glial cells and infiltrating immune cells has been successfully performed in the past using surgical procedures (laminectomy) that allow optical access to the dorsal spinal cord ( Kim et al., 2010 ). However, since myelin greatly increases light scattering, imaging is limited to the superficial layers of the dorsal funiculus, masking valuable information from deeper regions such as ventral horn. We think that acute slices prepared from wild type and transgenic mice can be used in combination with high-resolution imaging techniques to offer an alternative strategy to collect structural and functional information, in vitro, from dorsal, and also lateral and ventral regions. Coronal sections interrupt ascending and descending axons and many motor axons as well. Nevertheless, the information obtained is likely to be useful in analyzing how glial cells respond acutely to inflammatory challenges in spinal cord.
Materials and Reagents
Double edge razor blades (Everychina, Baili, catalog number: BP005)
Sterile 21 gauge needles (BD, catalog number: 305165)
-
Syringes ( ml, 3 ml, and 20 ml)
ml (COVIDIEN, catalog number: 8881600004)
3 ml (BD, catalog number: 309657)
20 ml (BD, catalog number: 302830)
Adhesive tape
Peel-a-way embedding molds (Sigma-Aldrich, catalog number: E6032)
Disposable transfer pipettes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 336)
Six-well multidish (Thermo Fisher Scientific, catalog number: 130184)
Parafilm
Coverslip (Thermo Fisher Scientific, Fisher Scientific, catalog number: 12-545-88)
70 μm cell strainer (Corning, Falcon®, catalog number: 352350)
15 ml and 50 ml polypropylene conical tubes (Corning, Falcon®, catalog numbers: 352095 and 352098, respectively)
Pipette tips (10 μl, 200 μl, 1,000 μl) (USA Scientific)
One- to two-month-old CX3CR1EGFP/+ transgenic mice (THE JACKSON LABORATORY, catalog number: 005582)
Ketamine and xylazine (provided by NYU School of Medicine, DLAR)
Isoflurane (provided by NYU School of Medicine, DLAR)
70% ethanol
Low melting point agarose (Sigma-Aldrich, catalog number: A9414)
Cyanoacrylate (Instant Krazy glue)
Ethidium bromide (MW: 394.3) (MP Biomedicals, catalog number: 802511)
Propidium iodide (MW: 668.4) (Thermo Fisher Scientific, Molecular ProbesTM, catalog number: P3566)
Phosphate buffered saline (Thermo Fisher Scientific, GibcoTM, catalog number: 14190-144)
Triton X-100, 100 ml solution (Sigma-Aldrich, catalog number: X100)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A3294)
Normal goat serum (Vector Laboratories, catalog number: S1000)
Optional: chicken anti-GFAP (EMD Millipore, catalog number: AB5541)
Mowiol® 4-88 (aqueous mounting medium) (Sigma-Aldrich, catalog number: 81381)
Tween 20
Optional: alexa fluor 647-conjugate goat anti-chicken IgY - H&L (Thermo Fisher Scientific, Invitrogen, catalog number: A21449) secondary antibody
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653)
Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S5761)
Glucose (Sigma-Aldrich, catalog number: G7528)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9333)
Sodium phosphate monobasic (NaH2PO4) (Sigma-Aldrich, catalog number: S8282)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C1016)
Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266)
HCl
NaOH
EDTA
Paraformaldehyde (PFA, 32% solution) (Electron Microscopy Sciences, catalog number: 15714)
Artificial cerebrospinal fluid (ACSF) (see Recipes)
Ca2+ and Mg2+ free-ACSF (see Recipes)
3-4% PFA, pH = 7.4 (see Recipes)
Equipment
Compressed gas tank 5% CO2, 95% O2
Leica vibratome and blade holder (Leica, model: VT1000 S)
Standard 1000 orbital shaker (TROEMNER, catalog number: 980173)
Digital pH meter (Mettler Toledo)
Hemostat clamps (World Precision Instruments, catalog number: 503736)
Forceps 12 cm long (World Precision Instruments, catalog number: 14226)
Fine dissection forceps number 5 (Roboz Surgical Instrument, catalog number: RS-4955)
SuperCut scissors (World Precision Instruments, catalog number: 14218)
Spine bone scissors (Dumont, catalog number: 15a)
Digital water bath (Thermo Fisher Scientific, Fisher ScientificTM, model: Isotemp 205)
Tubing
Digital scale (Mettler Toledo, model: MS104S)
Micropipettes (Gilson, 0.5-2 µl, 1-10 µl, 10-200 µl, 1,000 µl)
Stereo microscope with LED lights (Olympus, model: SZX10)
Zeiss-700 confocal microscope equipped with 20x objective and appropriate filters
Thermistor thermometer (SP Scienceware - Bel-Art Products - H-B Instrument, catalog number: 605010100)
Procedure
-
Before sectioning the spinal cord
Bubble (with a mixture 95% O2/5% CO2) 500 ml of normal ACSF and 50 ml of Ca2+ and Mg2+ free-ACSF for 30 min at 4 °C.
Monitor pH after 30 min. pH will be close to 7.38-7.40; adjust to 7.38-7.42 if necessary.
Insert one half of a razor blade in the blade holder of the vibratome and chill the stage by placing ice in the ice chamber.
-
Deeply anesthetize mice using an intraperitoneal injection of ketamine (100 mg/kg)/xylazine (15 mg/kg).
Note: Alternatively, isoflurane can be used to induce anesthesia.
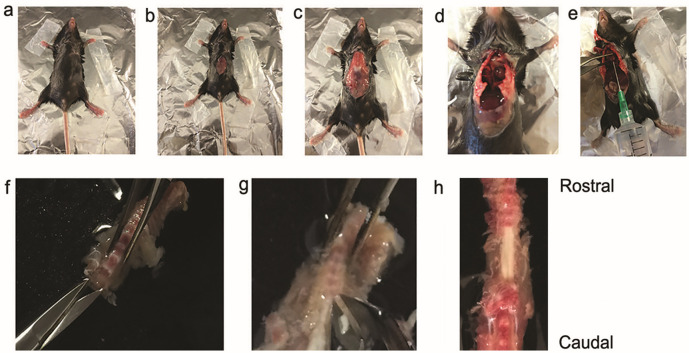
Spray the thorax and abdomen with 70% ethanol and gain access to the abdominal cavity by making a longitudinal midline cut. Open the thoracic cavity to expose the heart (use SuperCut scissors and 12 cm long forceps). (Figures 1a-1d)
-
Hold the heart with forceps and insert a 21 gauge needle into the left ventricle.
Note: Do not insert the needle too far, since it may damage interior heart compartments and impair circulation of fluids.
Make a cut in the right atrium and perfuse mice slowly with chilled Ca2+ and Mg2+ free-ACSF saturated with 95% O2/5% CO2 through the left ventricle (20 ml/ mouse, body weight: 10-15 g).
After perfusion, decapitate mice and remove the spinal column and quickly immerse it in normal ice-cold ACSF.
Make two small incisions, one on each side at the cervical end. Start to remove the ventral vertebral bodies (cervical to lumbar) using special bone scissors to cut the dorsal processes on both sides (Dumont, 15a). This procedure will open the spinal canal, cut the nerves, and expose the spinal cord (Figures 1f-1h).
-
Carefully remove the spinal cord with forceps and fine scissors and immerse it in chilled normal ACSF saturated with 95% O2/5% CO2.
Note: Avoid stretching and pinching the cord during this procedure.
Cut any remaining spinal nerve roots close to the cord with fine scissors.
Maintain the spinal cord in ice-cold ACSF saturated with 95% O2/5% CO2 until embedding the cord in step 12.
Dissolve low melting point agarose (4% in ACSF) by heating it in a microwave (< 1 min).
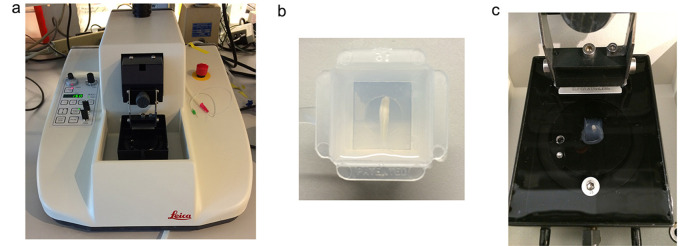
Fill a disposable embedding mold (22 x 22 x 20 mm) with agarose and chill the mold on ice. Embed the thoracic or lumbar spinal cord when the agarose temperature is lower than 37 °C (Figure 2b). At this and somewhat lower temperatures agarose is semi-solid, and cords can be embedded horizontally.
-
Keep the embedding mold on ice until the agarose becomes solid.
Notes:
Once the mold is placed on ice, agarose gelling will be completed in about 3-5 min.
Steps 10-14 should not take longer than 5-10 min.
Place the agarose mold as soon as possible in ice-cold ACSF saturated with 95% O2/5% CO2.
Apply a small amount of cyanoacrylate to the vibratome stage, remove the agarose block from the mold and glue it onto the stage. Immerse the embedded spinal cord immediately in ice-cold ACSF (bubble with a mixture of 95% O2/5% CO2 during the entire period of sectioning). (Figures 2a-2c)
-
Section spinal cord in 250-300 μm slices with the Leica vibratome (VT1000 S) at 0.1 mm/sec and 70 Hz, speed and frequency of the blade in the plane of section, respectively.
Note: Using faster speeds will prevent proper spinal cord sectioning. Before proceeding use fine brushes to remove agarose surrounding the slices.
Pick slices up using transfer pipettes or fine brushes and place them in cell strainers which are immersed in a glass Pyrex beaker containing 250 ml of ACSF saturated with 95% O2/5% CO2. Allow slices to recover for 45-60 min at 35 °C.
-
For dye uptake experiments
Prepare stock solutions of 1 mM ethidium bromide (EtdBr) and 1 mM propidium iodide (PropI2) in dH2O.
-
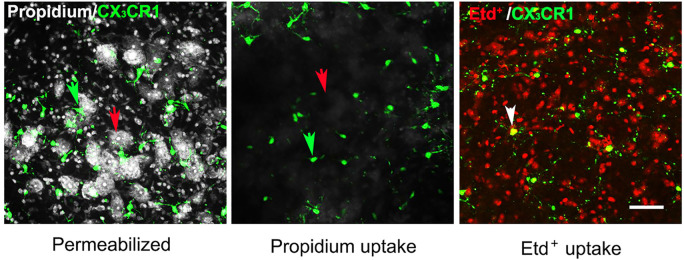
Bathe the slices in 10 µM EtdBr (for studying hemichannel activity) or 5 µM PropI2 (for testing cell viability) for 10 min at room temperature in ACSF saturated with 95% O2/5% CO2. The volumes of EtdBr and PropI2 added to ACSF do not change concentrations of electrolytes significantly.
Note: This step can be conducted in a 6-well multiwell plate filled with ASCF (2 ml/well). Remove the slices from the cell strainers (see step 17) by using transfer pipettes and place them in a 6-well multiwell. Each well must be covered with Parafilm to reduce loss of O2/CO2 from the solution.
Remove unbound Etd+ or PropI2 by rinsing slices 3 times with 1 ml of PBS at room temperature. Fix slices for 2 h at room temperature or overnight at 4 °C with 4% paraformaldehyde (PFA) in PBS.
-
For immunostaining of GFAP (optional)
Permeabilize PropI2 labeled and Etd+ labeled slices in Triton X-100 (1% in PBS) for 3 h in a shaker at room temperature.
To block nonspecific antibody reaction, incubate slices with 0.4 ml of a blocking solution containing 5% donkey serum, 0.5% BSA, and 0.1% Triton X-100 in PBS for 0.5 h.
Incubate slices with 0.4 ml of chicken anti-GFAP (1/200 in blocking solution) for 1 h.
Remove antibody solution and incubate slices with 1 ml of washing solution (0.5% Tween 20, 0.1% Triton X-100, in PBS) in a shaker for 10 min. Repeat washing 3 times.
Incubate slices for 1 h with goat anti-chicken conjugated Alexa-647 (1/1,000 in blocking solution).
Repeat step 19d.
Mount slices on coverslips using an aqueous-based mounting medium, for example, Mowiol 4-88.
-
Take confocal images one or two days later, as convenient, with a Zeiss-700 confocal microscope equipped with a 20x objective and appropriate filters (Figure 3).
Note: Etd+ labeling is not obviously reduced in this time period between uptake and imaging.
Because opening of hemichannels (HCs) formed of connexin 43 (Cx43) and pannexin 1 (Px1) has been shown to be associated with enhanced Etd+ uptake in different cell culture systems (e.g., Contreras et al., 2003 and Garré et al., 2010 ), we used the preparation described here for evaluating HC activity in response to inflammatory challenge. Using genetic and pharmacological approaches we showed that Px1 HC opening mediated the early inflammatory response to FGF-1 and ATP. Furthermore, we identified several inflammatory mechanisms triggered by Px1 HCs (see Garré et al., 2016 ).
Figure 1. Surgical procedure for extracting the mouse spinal cord.
a. A deeply anesthetized mouse is restrained on the surgical stage with adhesive tape. b and c. Midline incision used to start the laparotomy. d. Opening of thoracic cavity. e. Insertion of 21-G needle through the left ventricle for perfusion, and cutting of the right atrium. f and g. Opening of the vertebral canal and removal of the vertebral bodies cervical to lumbar by cutting the dorsal vertebral processes. h. An exposed thoracic segment of spinal cord, viewed from the ventral side.
Figure 2. The experimental setup for sectioning the spinal cord.
a. Vibratome; b. A spinal cord segment placed horizontally in the agarose mold; c. The agarose cube containing the spinal cord is removed from the mold, rotated 90° and glued to the vibratome stage in the proper orientation for coronal sections.
Figure 3. Uptake of propidium and Etd+ by glial cells and neurons in the ventral horn of spinal cord.
(Left panel) Slices were fixed overnight with 4% PFA and permeabilized, and then incubated in 5 μM PropI2 for 10 min and mounted. Motor neurons (red arrow) and microglia (green arrow) were identified by the morphology and by expression of EGFP, respectively, in acute slices prepared from CX3CR1EGFP/+ mice. In these slices, CX3CR1 and EGFP expressing cells are brain resident macrophages, a population mostly comprised of microglia. (Middle and right panels) In separate experiments, slices were maintained in ACSF saturated with 95% O2/5% CO2 for 1 h after sectioning and incubated with 5 μM PropI2 or 10 μM EtdBr for 10 min without permeabilization, rinsed and then fixed in 4% PFA before mounting. Under these conditions, there was little Prop2+ uptake in neurons (red arrow) and microglia (green arrow, middle panel). In contrast, Etd+ uptake was observed in CX3CR1EGFP/+ microglia (white arrow, Etd+ uptake plus EGFP expression), although it was rare in motor neurons (identifiable as round dark areas without small red cells, right panel, compare to left panel). The small red cells between EGFP- Etd+ cells and surrounding motoneurons are astrocytes, as was shown by GFAP immunolabeling (see Garré et al., 2016 ). Scale bar = 50 μm.
Data analysis
A complete description of statistics used for analyzing dye uptake experiments is presented in Garré et al. (2016) .
Notes
Since the mice used for this preparation express EGFP protein in CX3CR1 cells (microglia and perivascular macrophages), the exposure of spinal cord sections to excitation light should be minimized. It may affect the number of EGFP+ cells as well as the quality of fluorescence signals.
Rapid manifestations of microglia and astrocyte activation have been observed in slices prepared from mouse cortex ( Takano et al., 2014 ). In our hands, we have not seen obvious morphological signs of microglia or astrocyte activation up to 2 h after spinal cord sectioning, the maximum time tested. Basal TNFα level was considerably reduced in slices depleted of microglial cells ( Garré et al., 2016 ). To improve the reproducibility of our protocol we recommend recording how long after sectioning the slices are used.
If this preparation is used for electrophysiological recordings and/or for periods longer than 2 h post-sectioning, an alternative recipe for preparing enriched ACSF can be used (see Mitra and Brownstone, 2012).
Recipes
-
Artificial cerebrospinal fluid (ACSF)
Note: Fresh preparation is recommended.
119.0 mM NaCl
26.2 mM NaHCO3
11.0 mM glucose
2.5 mM KCl
1.0 mM NaH2PO4
2.5 mM CaCl2
1.3 mM MgCl2
Adjust pH to 7.4 using either 2 N HCl or 5 N NaOH, to lower or raise pH, respectively
Measure osmolarity (300-310 mOsm)
Filter the solution
-
Ca2+ and Mg2+ free-ACSF
119.0 mM NaCl
26.2 mM NaHCO3
11.0 mM glucose
2.5 mM KCl
1.0 mM NaH2PO4
5.0 mM EDTA
Adjust pH to 7.4 using either 2 N HCl or 5 N NaOH, to lower or raise pH, respectively
Measure osmolarity (300-310 mOsm)
Filter the solution
-
3-4% PFA, pH = 7.4
Dilute the concentrated 32% PFA stock solution (Electron Microscopy Sciences) in Ca2+ and Mg2+ free PBS (Thermo Fisher Scientific) to make a solution 3-4% PFA, pH = 7.4
Acknowledgments
The protocol here was adapted from Garré et al. (2016). This work was supported by National Institutes of Health Grants NS45287 and NS55363 to M.V.L.B. and NS072238 to F.F.B., National Institutes of Health Grants GM107469 and AG048410 to G.Y. and the Research Council of Lithuania MIP-76/2015 to F.F.B.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Contreras J. E., Saez J. C., Bukauskas F. F. and Bennett M. V.(2003). Gating and regulation of connexin 43(Cx43) hemichannels. Proc Natl Acad Sci U S A 100(20): 11388-11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davalos D., Grutzendler J., Yang G., Kim J. V., Zuo Y., Jung S., Littman D. R., Dustin M. L. and Gan W. B.(2005). ATP mediates rapid microglial response to local brain injury in vivo . Nat Neurosci 8(6): 752-758. [DOI] [PubMed] [Google Scholar]
- 3.Denk W., Strickler J. H. and Webb W. W.(1990). Two-photon laser scanning fluorescence microscopy. Science 248(4951): 73-76. [DOI] [PubMed] [Google Scholar]
- 4.Garré J. M., Retamal M. A., Cassina P., Barbeito L., Bukauskas F. F., Saez J. C., Bennett M. V. and Abudara V.(2010). FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc Natl Acad Sci U S A 107(52): 22659-22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garré J. M., Yang G., Bukauskas F. F. and Bennett M. V.(2016). FGF-1 triggers Pannexin-1 hemichannel opening in spinal astrocytes of rodents and promotes inflammatory responses in acute spinal cord slices. J Neurosci 36(17): 4785-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gee J. M., Smith N. A., Fernandez F. R., Economo M. N., Brunert D., Rothermel M., Morris S. C., Talbot A., Palumbos S., Ichida J. M., Shepherd J. D., West P. J., Wachowiak M., Capecchi M. R., Wilcox K. S., White J. A. and Tvrdik P.(2014). Imaging activity in neurons and glia with a Polr2a-based and cre-dependent GCaMP5G-IRES-tdTomato reporter mouse . Neuron 83(5): 1058-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J. V., Jiang N., Tadokoro C. E., Liu L., Ransohoff R. M., Lafaille J. J. and Dustin M. L.(2010). Two-photon laser scanning microscopy imaging of intact spinal cord and cerebral cortex reveals requirement for CXCR6 and neuroinflammation in immune cell infiltration of cortical injury sites. J Immunol Methods 352(1-2): 89-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra P. and Brownstone R. M.(2012). An in vitro spinal cord slice preparation for recording from lumbar motoneurons of the adult mouse . J Neurophysiol 107(2): 728-741. [DOI] [PubMed] [Google Scholar]
- 9.Takano T., He W., Han X., Wang F., Xu Q., Wang X., Oberheim Bush N. A., Cruz N., Dienel G. A. and Nedergaard M.(2014). Rapid manifestation of reactive astrogliosis in acute hippocampal brain slices. Glia 62(1): 78-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White J. G., Amos W. B. and Fordham M.(1987). An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol 105(1): 41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]