Abstract
Limited data are available on improved outcomes after initiation of neurointensivist co-management in neurosurgical intensive care units (NSICUs) in Korea. We evaluated the impact of a newly appointed neurointensivist on the outcomes of neurosurgical patients admitted to an intensive care unit (ICU). This retrospective observational study involved neurosurgical patients admitted to the NSICU at Samsung Medical Center between March 2013 and May 2016. Neurointensivist co-management was initiated in October 1 2014. We compared the outcomes of neurosurgical patients before and after neurointensivist co-management. The primary outcome was ICU mortality. A total of 571 patients were admitted to the NSICU during the study period, 291 prior to the initiation of neurointensivist co-management and 280 thereafter. Intracranial hemorrhage (29.6%) and traumatic brain injury (TBI) (26.6%) were the most frequent reasons for ICU admission. TBI was the most common cause of death (39.0%). There were no significant differences in mortality rates and length of ICU stay before and after co-management. However, the rates of ICU and 30-day mortality among the TBI patients were significantly lower after compared to before initiation of neurointensivist co-management (8.5% vs. 22.9%; P = 0.014 and 11.0% vs. 27.1%; P = 0.010, respectively). Although overall outcomes were not different after neurointensivist co-management, initiation of a strategy of routine involvement of a neurointensivist significantly reduced the ICU and 30-day mortality rates of TBI patients.
Keywords: Neurosurgery, Intensive Care Unit, Critical Care Outcomes
Graphical Abstract
INTRODUCTION
Intensivists are physicians who specialize in the care of critically ill patients and are trained to manage all aspects of the intensive care unit (ICU) stay (1,2). Intensivists improve the clinical outcomes of critically ill patients (3,4,5). Neurointensivists are specialists focused on the management of patients with acute neurologic conditions including traumatic brain injury (TBI), stroke, status epilepticus, and neuromuscular respiratory failure (6,7,8). Despite several studies of outcome prediction in general and surgical ICUs, high-intensity ICUs managed by neurointensivists are not common in many hospitals (7,8,9). Limited data are available regarding prediction of outcomes in neuroscience ICUs (4,7,10). Several studies reported improved outcomes by neurointensivists, including decreased mortality rates, shortened lengths of ICU and hospital stay in patients with TBI, intracranial hemorrhage, and subarachnoid hemorrhage (2,3,9,11,12). However, the impact of neurointensivists and improved outcome in neuroscience ICUs in Korea has not been evaluated. This is the first report of the role of neurointensivists and clinical outcomes after initiation of neurointensivist co-management in neurosurgical intensive care units (NSICUs) in Korea. In this study, we evaluated the impact of neurointensivist co-management on outcomes in patients admitted to a NSICU.
MATERIALS AND METHODS
Study population
This was a retrospective, single-center, observational study of neurocritically ill patients and neurosurgical patients who were admitted to the NSICU at the Samsung Medical Center between March 2013 and May 2016. The patients admitted to the NSICU were considered for this study. Patients were excluded if they were admitted to departments other that neurosurgery or had not neurological problems. We also excluded the patients who were transferred to departments other that neurosurgery during ICU hospitalization because factors other than neurosurgical team and neurointensivist might have influenced the result of this study. In addition, we excluded in this analysis the patients with elective surgery such as brain tumor surgery, elective vascular surgery, microvascular decompression, epileptic surgery, elective spinal surgery, or surgery of congenital anomaly.
Definitions and outcomes
The NSICU was independently operated and separated from the neurology and general surgery ICUs in this study. Neurointensivist co-management was initiated on October 1 2014. During the neurointensivist co-management period, the neurointensivist worked 6 days per week during the daytime. The neurocritical care team consisted of an attending neurointensivist, neurosurgical resident, and consultant pharmacist. The consultant pharmacist advised the neurocritical care team on pharmaceutic issues by phone after morning rounds. Neurointensivist was involved in general critical managements, including hemodynamic monitoring, nutritional support, and use of mechanical ventilation, renal replacement therapy, etc. Neurosurgeon was responsible for surgical management. However, neurosurgeon discussed important decisions with neurointensivist in neurocritical management such as control strategy of increased intracranial pressure and neuromonitoring methods. Although the neurosurgical team discussed the treatment plans of all ICU patients with the neurointensivist, the leader of the neurosurgical team was responsible for the patients and determined their transfer from the ICU to the neurosurgical ward in this study. The neurointensivist managed all aspects of these neurosurgical patients during their ICU hospitalization. In addition, the neurointensivist evaluated and managed neurosurgical patients by lung sonography, echocardiography and bronchoscopy, together with neurocritical management. The neurointensivist also implemented central venous catheterization and peripherally inserted central catheterization, endotracheal intubation, percutaneous dilatational tracheostomy, etc. The neurointensivist had educated neurosurgical residents and monitored procedures performed by the residents in the ICU. Furthermore, the neurointensivist consulted for patients in the neurosurgical ward who had hemodynamic instability or respiratory problem.
The initial Glasgow Coma Scale (GCS) was defined as the best GCS within 24 hours of ICU admission. Severity of illness was assessed by the Acute Physiology and Chronic Health Evaluation II (APACHE II) score (13).
We compared the outcomes of neurosurgical patients admitted before and after initiation of mandatory neurointensivist co-management in the NSICU. Subgroup analysis was performed as causes of ICU admission such as TBI, intracranial hemorrhage, subarachnoid hemorrhage, and cerebral infarction. We analyzed TBI patients on ICU admission or preoperative state. TBI patients were assessed by using International Mission for Prognosis and Analysis of Clinical Trials (IMPACT) prognostic model and APACHE II score (14,15). Brain computed tomography (CT) finding was accessed according to the Marshall CT scan classification system in TBI patients (16).
The primary endpoint was ICU mortality. Secondary endpoints were 30-day mortality and length of stay (LOS) in the ICU.
Statistical analysis
All data are presented as means ± standard deviations (SD) for continuous variables and as numbers (percentages) for categorical variables. Data were compared using Student's t-test for continuous variables and the χ2 test or Fisher's exact test for categorical variables. The Kaplan-Meier method was used to generate survival curves, which were compared using log-rank test. Multiple logistic regression analysis was used to identify independent predictors of survival in neurocritically ill patients, as measured by the estimated odds ratio (OR) with 95% confidence intervals (CIs). Variables with a P value less than 0.2 on univariate analyses, as well as a priori variables that were clinically relevant, were entered into the multiple logistic regression model. All tests were 2-sided, and P values < 0.05 were considered to indicate statistical significance. Data were analyzed using SPSS statistics version 20 (IBM, Armonk, NY, USA).
Ethics statement
This was a retrospective, single-center, observational study of neurocritically ill patients and neurosurgical patients who were admitted to the NSICU at the Samsung Medical Center between March 2013 and May 2016. The study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. SMC 2016-08-126-001) to review and publish information from patients' records. The requirement for informed consent was waived because of the retrospective nature of the study.
RESULTS
Baseline characteristics
A total of 3,683 patients was admitted to the NSICU during the study period. The patients with elective surgery and were transferred to departments other that neurosurgery during ICU hospitalization were excluded. Finally, 571 patients were analyzed in this study; 291 prior to initiation of neurointensivist co-management and 280 thereafter. The mean age was 58.2 ± 19.0 years, and 306 patients (53.6%) were men. Intracranial hemorrhage (29.6%) and TBI (26.6%) were the most common reasons for ICU admission. There were no significant differences in gender, comorbidities, causes of ICU admission, and GCS and APACHE II score on ICU admission between before and after neurointensivist co-management (Table 1).
Table 1. Baseline characteristics on ICU admission before and after neurointensivist co-management.
| Parameters | Before (n = 291) | After (n = 280) | P value |
|---|---|---|---|
| Age, yr | 56.8 ± 20.2 | 59.6 ± 17.6 | 0.077 |
| Gender, man | 159 (54.6) | 147 (52.5) | 0.608 |
| Comorbidities | |||
| Diabetes mellitus | 36 (12.4) | 46 (16.4) | 0.167 |
| Hypertension | 109 (37.5) | 123 (43.9) | 0.115 |
| Malignancy | 71 (24.4) | 81 (28.9) | 0.221 |
| Dyslipidemia | 73 (25.1) | 75 (26.8) | 0.643 |
| Chronic kidney disease | 11 (3.8) | 19 (6.8) | 0.108 |
| Chronic liver disease | 9 (3.1) | 9 (3.2) | 0.934 |
| Previous TIA or stroke | 26 (8.9) | 24 (8.6) | 0.878 |
| Cause of ICU admission | 0.545 | ||
| Intracranial hemorrhage | 89 (30.6) | 80 (28.6) | |
| TBI | 70 (24.1) | 82 (29.3) | |
| Subarachnoid hemorrhage | 71 (24.4) | 71 (25.4) | |
| Cerebral infarction | 33 (11.3) | 22 (7.9) | |
| CNS infection | 16 (5.5) | 12 (4.3) | |
| Other | 12 (4.1) | 13 (4.6) | |
| GCS on ICU admission | 11.5 ± 3.9 | 11.7 ± 3.7 | 0.491 |
| APACHE II score on ICU admission | 13.0 ± 6.5 | 13.0 ± 7.0 | 0.990 |
Values are presented as number of patients (%) or mean ± SD.
ICU = intensive care unit, TIA = transient ischemic attack, TBI = traumatic brain injury, CNS = central nervous system, GCS = Glasgow Coma Scale, APACHE = Acute Physiology and Chronic Health Evaluation, SD = standard deviation.
Clinical outcomes
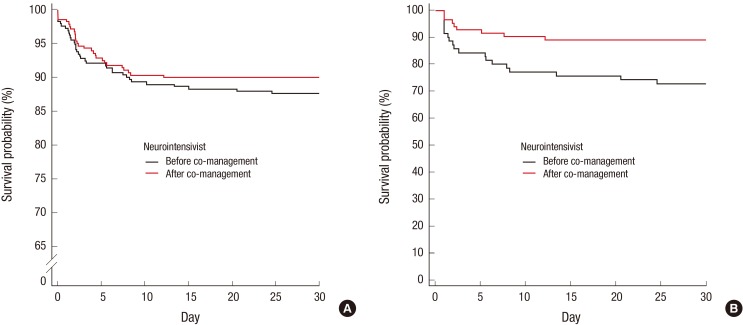
There were no significant differences in ICU mortality rate (11.0% vs. 9.6%; P = 0.595), hospital mortality rate (14.4% vs. 12.9%, P = 0.584), and LOS in the ICU (6.1 ± 7.7 days vs. 7.1 ± 7.9 days; P = 0.454) before and after neurointensivist co-management. The duration of mechanical ventilator use were increased after compared to before initiation of neurointensivist co-management (8.7 ± 11.7 days vs. 5.7 ± 4.8 days; P = 0.007). Thirty-day mortality rate showed a reducing tendency after compared to before initiation of neurointensivist co-management, but there was no significant difference (10.0% vs. 12.4%, log-rank test; P = 0.373; Fig. 1A). Clinical outcomes are shown in Table 2.
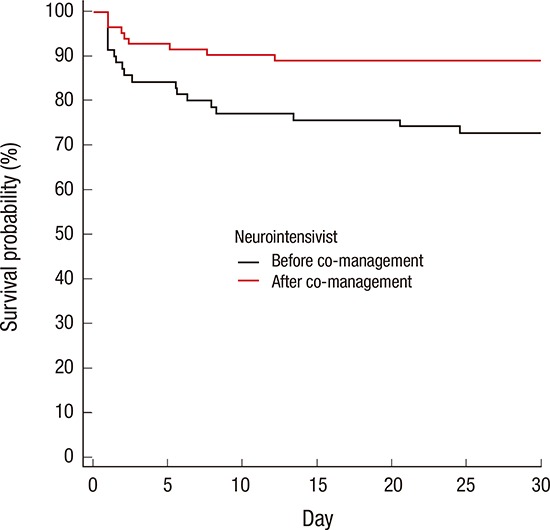
Fig. 1.
Kaplan-Meier 30-day survival analyses before and after neurointensivist co-management in all ICU patients (A) and TBI patients (B). Black solid line, before neurointensivist co-management; red solid line, after neurointensivist co-management; P = 0.373 and P = 0.011, respectively, based on log-rank tests.
ICU = intensive care unit, TBI = traumatic brain injury.
Table 2. Clinical outcomes before and after neurointensivist co-management.
| Clinical outcomes | Before (n = 291) | After (n = 280) | P value |
|---|---|---|---|
| Need for mechanical ventilator | 129 (44.3) | 130 (46.4) | 0.615 |
| Need for renal replacement therapy | 9 (3.1) | 17 (6.1) | 0.088 |
| Need for invasive ICP monitoring | 61 (21.0) | 66 (23.6) | 0.454 |
| Duration of mechanical ventilator, day | 5.7 ± 4.8 | 8.7 ± 11.7 | 0.007 |
| Duration of renal replacement therapy, day | 5.8 ± 3.1 | 6.5 ± 4.1 | 0.638 |
| Duration of invasive ICP monitoring, day | 7.6 ± 9.2 | 7.5 ± 5.3 | 0.907 |
| ICU mortality according to causes of ICU admission | 0.451 | ||
| TBI | 16 (50.0) | 7 (25.9) | |
| Intracranial hemorrhage | 8 (25.0) | 10 (37.0) | |
| Subarachnoid hemorrhage | 5 (15.6) | 7 (25.9) | |
| Cerebral infarction | 2 (6.2) | 2 (7.4) | |
| Other | 1 (3.1) | 1 (3.7) | |
| Outcomes | |||
| ICU mortality | 32 (11.0) | 27 (9.6) | 0.595 |
| 30-day mortality | 36 (12.4) | 28 (10.0) | 0.369 |
| Hospital mortality | 42 (14.4) | 36 (12.9) | 0.584 |
| LOS in ICU, day | 6.1 ± 7.7 | 7.1 ± 7.9 | 0.454 |
| LOS in hospital, day | 65.4 ± 269.8 | 51.1 ± 123.5 | 0.417 |
Values are presented as number of patients (%) or mean ± SD.
ICP = intracranial pressure, TBI = traumatic brain injury, LOS = length of stay, ICU = intensive care unit, SD = standard deviation.
TBI was the most common cause of death in the ICU (39.0%). Twenty-three patients died of TBI during treatment in ICU. Of these patients, 16 patients were dead before neurointensivist co-management. Ten patients died of severe brain damage, while 6 patients died of medical and other problems (3 septic shock, 2 multiorgan failure, and 1 tension pneumothorax). Among 7 TBI patients who were dead after neurointensivist co-management, 6 patients died of severe brain damage and only 1 patient died of multiorgan failure. There were no significant differences in IMPACT prognostic models and APACHE II score before and after neurointensivist co-management (Table 3). However, there were significant differences in the ICU mortality rate (22.9% vs. 8.5%; P = 0.014) and hospital mortality rate (28.6% vs. 14.6%; P = 0.036) in TBI patients before and after co-management (Table 3). The 30-day mortality rate was significantly lower after compared to before initiation of neurointensivist co-management (11.0% vs. 27.1%, log-rank test; P = 0.011; Fig. 1B). In addition, there was significantly lower in ICU mortality among the TBI patients with GCS below 12 after compared to before initiation of neurointensivist co-management (17.1% vs. 38.5%; P = 0.031). Neurointensivist co-management was an independent predictor of an improved ICU mortality rate in TBI patients (adjusted OR, 0.160; 95% CI, 0.028–0.905).
Table 3. Baseline characteristics and clinical outcomes of TBI patients before and after neurointensivist co-management.
| Parameters | Before (n = 70) | After (n = 82) | P value |
|---|---|---|---|
| Age, yr | 57.0 ± 25.8 | 64.2 ± 15.2 | 0.045 |
| Gender, man | 48 (68.6) | 53 (68.3) | 0.971 |
| Comorbidities | |||
| Diabetes mellitus | 12 (17.1) | 23 (28.0) | 0.111 |
| Hypertension | 21 (30.0) | 37 (45.1) | 0.056 |
| Malignancy | 13 (18.6) | 18 (22.0) | 0.606 |
| Dyslipidemia | 12 (17.1) | 29 (35.4) | 0.012 |
| Chronic kidney disease | 2 (2.9) | 8 (9.8) | 0.109 |
| Chronic liver disease | 2 (2.9) | 6 (7.3) | 0.289 |
| Previous TIA or stroke | 6 (8.6) | 2 (2.4) | 0.144 |
| IMPACT | |||
| Core model | 5.9 ± 4.3 | 5.6 ± 3.5 | 0.669 |
| Extended model* | 8.1 ± 6.3 | 8.3 ± 4.7 | 0.807 |
| Lab model† | 11.6 ± 7.2 | 11.8 ± 5.2 | 0.836 |
| Marshall CT grade | 3.7 ± 1.7 | 4.1 ± 1.5 | 0.137 |
| APACHE II score on ICU admission | 14.9 ± 7.7 | 14.5 ± 6.2 | 0.635 |
| GCS on ICU admission | 10.2 ± 4.3 | 11.1 ± 3.8 | 0.162 |
| Need for mechanical ventilator | 40 (57.1) | 42 (51.2) | 0.465 |
| Need for renal replacement therapy | 1 (1.4) | 8 (9.8) | 0.030 |
| Need for invasive ICP monitoring | 6 (8.6) | 12 (14.6) | 0.249 |
| Need for vasopressor | 12 (17.1) | 18 (22.0) | 0.458 |
| Mean blood pressure on ICU admission, mmHg | 77.5 ± 10.3 | 76.7 ± 9.7 | 0.634 |
| Duration of mechanical ventilator, day | 4.9 ± 4.2 | 13.2 ± 14.7 | 0.001 |
| Duration of renal replacement therapy, day | 2.0 | 9.4 ± 3.9 | - |
| Duration of invasive ICP monitoring, day | 7.3 ± 4.0 | 5.3 ± 4.8 | 0.394 |
| Outcomes | |||
| ICU mortality | 16 (22.9) | 7 (8.5) | 0.014 |
| 30-day mortality | 19 (27.1) | 9 (11.0) | 0.010 |
| Hospital mortality | 20 (28.6) | 12 (14.6) | 0.036 |
| LOS in ICU, day | 5.2 ± 7.4 | 7.1 ± 10.4 | 0.197 |
| LOS in hospital, day | 27.4 ± 26.3 | 60.3 ± 91.4 | 0.002 |
Values are presented as number of patients (%) or mean ± SD.
TBI = traumatic brain injury, TIA = transient ischemic attack, IMPACT = International Mission for Prognosis and Analysis of Clinical Trials, CT = computed tomography, APACHE = Acute Physiology and Chronic Health Evaluation, GCS = Glasgow Coma Scale, LOS = length of stay, ICU = intensive care unit, ICP = intracranial pressure, SD = standard deviation.
*Core plus hypoxia, hypotension, and CT characteristics. †Extended plus glucose and hemoglobin.
The clinical outcomes were similar after compared to before initiation of neurointensivist co-management in patients with intracranial hemorrhage, subarachnoid hemorrhage, and cerebral infarction. There were no significant differences in ICU mortalities, 30-day mortality, and LOS in the ICU in these patients before and after neurointensivist co-management.
DISCUSSION
This study is the first report of the role of neurointensivists and clinical outcomes after initiation of neurointensivist co-management in NSICUs in Korea. Intracranial hemorrhage and TBI were the most frequent reasons for ICU admission. Approximately half of the ICU patients suffered from these diseases. TBI was the most common cause of death in the ICU. There were no significant differences in mortality rates and LOS in the ICU before and after initiation of neurointensivist co-management in this study. However, the ICU and 30-day mortality rates were significantly lower among the TBI patients after compared to before initiation of neurointensivist co-management.
Neurointensivists are critical care physicians specifically focused on the management of neurocritically ill patients (6,7,8). The birth of neurocritical care stemmed from the appreciation that an already affected brain (primary injury) is greatly influenced by systemic alterations that may adversely affect its function (secondary injury). Neurointensivist are specially trained to recognize and treat such injuries (6,7). Because of this increasing complexity of acute care of the neurocritically ill patient, a multidisciplinary approach to the neurocritical care is necessary. Neurointensivists have to work in collaboration with neurosurgeons and other physicians in coordinating acute care protocols, decisions about advanced neuromonitoring, and other aspects of care (6).
Neurointensivists have emerged in the last decades as specialists trained to manage all aspects of the ICU stay of neurocritically ill patients in the USA (2). The institution of a neurointensivist-led team model has been reported to improve outcomes, including decreased mortality rates, shortened length of ICU and hospital stay in neurocritically ill patients with TBI, intracranial hemorrhage, and subarachnoid hemorrhage (2,3,11). However, there are only a few neurointensivists in Korea. In addition, neuroscience ICUs, which are operated jointly by neurology and neurosurgery, are uncommon (17). NSICUs operate independently in most tertiary hospitals in Korea (17). Furthermore, it is very rare to treat neurocritically ill patients in cooperation between neurosurgical teams and neurointensivists in NSICUs. The concept of neurocritical care emerged in Korea only recently. Intensivists in general medical and surgical ICUs also emerged recently, and so the current status of critical care in Korea is not comparable to that of advanced countries (18). A nationwide survey of ICUs was performed in 2014, and only 17.3% of ICUs had intensive care specialists with a 5-day work week (18,19).
In general, adequate staffing showed favorable effects on ICU stay and decreased use of resources (18). However, there was no significant difference in length of ICU stay before and after initiation of neurointensivist co-management in this study. Furthermore, duration of mechanical ventilator showed an increasing trend after neurointensivist co-management. Neurosurgeons usually determined whether to transfer patients from the NSICU to general wards in this study, usually after confirming that the patients had fully recovered. Some patients stayed in ICU over a weekend, during which time the neurosurgeon wanted to monitor them closely. The indications for which the neurosurgical patients were transferred from the ICU to general wards differed among the neurosurgeons. If the patients exhibited medical problems such as sepsis, pneumonia, acute respiratory distress syndrome, and hemodynamic instability, they were transferred to internal medicine and treated in the medical ICU before neurointensivist co-management. However, after initiation of co-management many of the patients with medical problems were treated by a neurointensivist in the NSICU. Neurointensivist were sometimes consulted for patients with respiratory and hemodynamic instability in the neurosurgery general ward. Therefore, these special situations might be associated with the length of ICU stay and prolonged duration of mechanical ventilator use.
The overall mortality rate of TBI patients improved after neurointensivist co-management. Mortality according to medical problems was decreased after neurointensivist co-management in TBI patients. This change was associated with effort of neurointensivist. Furthermore, brain death according to severe brain damage was also decreased after neurointensivist co-management. The clinical outcomes of TBI patients depend on both primary and secondary brain insults (20). Aggressive management of secondary brain injury is associated with a good neurological outcome (20). Furthermore, TBI may be accompanied by hemodynamic instability for various reasons (21,22). Hemodynamic stability is important to maintain the capacity for vascular autoregulation, either globally or locally, in severe TBI patients (21,22). Therefore, collaboration between neurointensivists and neurosurgeons is necessary. Indeed, co-management with a neurointensivist improved the clinical outcomes of TBI (3,20,21). Interestingly, emergency operations on TBI patients are usually performed by young neurosurgeons in Korea. Young neurosurgeons may not have sufficient time to treat neurocritically ill patients. Therefore, young neurosurgeons need to collaborate with neurointensivists. Indeed, young neurosurgeons discussed their patients with the neurointensivist more actively than did senior members of the faculty in our hospital.
Several neuroscience ICU models such as “open” vs. “semi-closed” and “closed” ICU practice models are available (11). Korea contains all of these types; our NSICU is similar to an open ICU model. It is important to collaborate with each physician in an open ICU model. Because of the increased complexity of neurological diseases, a multidisciplinary approach to neurocritical care is required (8). Neurointensivists have to collaborate with neurosurgeons, neurologists and other physicians for decisions regarding acute care protocols in neuroscience ICU (8). However, it is not easy to cooperate with all neurosurgeons. While some neurosurgeons were cooperative, others were not. A neurointensivist alone cannot treat all patients and neurosurgeons cannot stay in the ICU all day. Thus sharing of opinions and collaboration between neurosurgeons and neurointensivists are required in treatment of neurocritically ill patients. Neurointensivists can manage not only neurological diseases but also all aspects of the critical care. They can rapidly respond to emerging problems in neuroscience ICUs. In the near future, full-time employment of neurointensivists can improve clinical outcome in neuroscience ICUs in Korea.
This study had several limitations. It was a retrospective review of medical records. Our study may not be broadly applicable to other centers at which no neurointensivists are available because our study was conducted at a single tertiary institution with a specialized NSICU for neurosurgical and neurocritically ill patients. The neurointensivist in our hospital is a neurologist and trained to manage all aspects of critically ill patients in medical and surgical ICUs. The treatment tendency of this neurointensivist might have influenced the clinical outcomes in neurocritically ill patients. The neurosurgical team discussed the treatment plans of all ICU patients with the neurointensivist, but it was sometimes difficult to cooperate with the neurosurgeon and the neurointensivist because collaborative work was just begun. Because of these situations, the role of neurointensivist might be limited. These special situations were different from other NSICUs in Korea. Therefore, selection bias and other confounding factors might have been influenced. Prospective large-scale studies are needed to evaluate the impact of neurointensivists in the management of neurocritically ill patients in Korea.
In conclusion, although overall outcomes were not significantly different after neurointensivist co-management, the ICU and 30-day mortality rates of TBI patients were significantly reduced. A multidisciplinary approach is important in treatment of neurocritically ill patients because of the increasing complexity of neurological problems. Therefore, collaboration between neurosurgeons and neurointensivists is necessary. In the near future, this multidisciplinary team-based approach involving neurointensivists and neurosurgeons working together will improve the outcome of neurocritically ill patients in Korea.
ACKNOWLEDGMENT
We would like to thank the nursing director of the NSICU, Hye Ok Choi, who gave excellent advice and fruitful discussions. We would also like to thank all nurses of the NSICU at Samsung Medical Center.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Ryu JA, Suh GY, Hong SC. Investigation: Ryu JA, Chung CR. Writing - original draft: Ryu JA, Hong SC.
References
- 1.Park CM, Chun HK, Lee DS, Jeon K, Suh GY, Jeong JC. Impact of a surgical intensivist on the clinical outcomes of patients admitted to a surgical intensive care unit. Ann Surg Treat Res. 2014;86:319–324. doi: 10.4174/astr.2014.86.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Josephson SA, Douglas VC, Lawton MT, English JD, Smith WS, Ko NU. Improvement in intensive care unit outcomes in patients with subarachnoid hemorrhage after initiation of neurointensivist co-management. J Neurosurg. 2010;112:626–630. doi: 10.3171/2009.8.JNS09441. [DOI] [PubMed] [Google Scholar]
- 3.Varelas PN, Eastwood D, Yun HJ, Spanaki MV, Hacein Bey L, Kessaris C, Gennarelli TA. Impact of a neurointensivist on outcomes in patients with head trauma treated in a neurosciences intensive care unit. J Neurosurg. 2006;104:713–719. doi: 10.3171/jns.2006.104.5.713. [DOI] [PubMed] [Google Scholar]
- 4.Suarez JI, Zaidat OO, Suri MF, Feen ES, Lynch G, Hickman J, Georgiadis A, Selman WR. Length of stay and mortality in neurocritically ill patients: impact of a specialized neurocritical care team. Crit Care Med. 2004;32:2311–2317. doi: 10.1097/01.ccm.0000146132.29042.4c. [DOI] [PubMed] [Google Scholar]
- 5.Morrow DA, Fang JC, Fintel DJ, Granger CB, Katz JN, Kushner FG, Kuvin JT, Lopez-Sendon J, McAreavey D, Nallamothu B, et al. Evolution of critical care cardiology: transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: a scientific statement from the American Heart Association. Circulation. 2012;126:1408–1428. doi: 10.1161/CIR.0b013e31826890b0. [DOI] [PubMed] [Google Scholar]
- 6.Jarquin-Valdivia AA, Bonovich DC, Hemphill JC., 3rd The role of the neurointensivist. Semin Neurosurg. 2003;14:131–138. [Google Scholar]
- 7.Suarez JI. Outcome in neurocritical care: advances in monitoring and treatment and effect of a specialized neurocritical care team. Crit Care Med. 2006;34:S232–S238. doi: 10.1097/01.CCM.0000231881.29040.25. [DOI] [PubMed] [Google Scholar]
- 8.Bithal PK. Neurointensive care unit and neurointensivist: do we need them? J Neuroanaesth Crit Care. 2016;3:1–2. [Google Scholar]
- 9.Knopf L, Staff I, Gomes J, McCullough L. Impact of a neurointensivist on outcomes in critically ill stroke patients. Neurocrit Care. 2012;16:63–71. doi: 10.1007/s12028-011-9620-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varelas PN, Conti MM, Spanaki MV, Potts E, Bradford D, Sunstrom C, Fedder W, Hacein Bey L, Jaradeh S, Gennarelli TA. The impact of a neurointensivist-led team on a semiclosed neurosciences intensive care unit. Crit Care Med. 2004;32:2191–2198. doi: 10.1097/01.ccm.0000146131.03578.21. [DOI] [PubMed] [Google Scholar]
- 11.Mirski MA, Chang CW, Cowan R. Impact of a neuroscience intensive care unit on neurosurgical patient outcomes and cost of care: evidence-based support for an intensivist-directed specialty ICU model of care. J Neurosurg Anesthesiol. 2001;13:83–92. doi: 10.1097/00008506-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Bershad EM, Feen ES, Hernandez OH, Suri MF, Suarez JI. Impact of a specialized neurointensive care team on outcomes of critically ill acute ischemic stroke patients. Neurocrit Care. 2008;9:287–292. doi: 10.1007/s12028-008-9051-5. [DOI] [PubMed] [Google Scholar]
- 13.Kümpers P, Hafer C, Lukasz A, Lichtinghagen R, Brand K, Fliser D, Faulhaber-Walter R, Kielstein JT. Serum neutrophil gelatinase-associated lipocalin at inception of renal replacement therapy predicts survival in critically ill patients with acute kidney injury. Crit Care. 2010;14:R9. doi: 10.1186/cc8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, Murray GD, Marmarou A, Roberts I, Habbema JD, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:e165. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raj R, Siironen J, Kivisaari R, Hernesniemi J, Skrifvars MB. Predicting outcome after traumatic brain injury: development of prognostic scores based on the IMPACT and the APACHE II. J Neurotrauma. 2014;31:1721–1732. doi: 10.1089/neu.2014.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deepika A, Prabhuraj AR, Saikia A, Shukla D. Comparison of predictability of Marshall and Rotterdam CT scan scoring system in determining early mortality after traumatic brain injury. Acta Neurochir (Wien) 2015;157:2033–2038. doi: 10.1007/s00701-015-2575-5. [DOI] [PubMed] [Google Scholar]
- 17.Song HK, Lee BI, Lee JH, Lee KS, Whang SH. Status of neurocritical care in Korea: a nationwide questionnaire survey. J Neurocrit Care. 2013;6:82–86. [Google Scholar]
- 18.Lim CM, Kwak SH, Suh GY, Koh Y. Critical care in Korea: present and future. J Korean Med Sci. 2015;30:1540–1544. doi: 10.3346/jkms.2015.30.11.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwak SH, Jeong CW, Lee SH, Lee HJ, Koh Y. Current status of intensive care units registered as critical care subspecialty training hospitals in Korea. J Korean Med Sci. 2014;29:431–437. doi: 10.3346/jkms.2014.29.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wijayatilake DS, Shepherd SJ, Sherren PB. Updates in the management of intracranial pressure in traumatic brain injury. Curr Opin Anaesthesiol. 2012;25:540–547. doi: 10.1097/ACO.0b013e328357960a. [DOI] [PubMed] [Google Scholar]
- 21.Helmy A, Vizcaychipi M, Gupta AK. Traumatic brain injury: intensive care management. Br J Anaesth. 2007;99:32–42. doi: 10.1093/bja/aem139. [DOI] [PubMed] [Google Scholar]
- 22.Haddad SH, Arabi YM. Critical care management of severe traumatic brain injury in adults. Scand J Trauma Resusc Emerg Med. 2012;20:12. doi: 10.1186/1757-7241-20-12. [DOI] [PMC free article] [PubMed] [Google Scholar]