Abstract
Theca cells, including theca interna cells and theca externa cells, are vital components of ovarian follicles. The aim of the present study is to identify a reliable method for the in vitro culture of theca cells from duck ovarian hierarchical (F4-F2) follicles. We improved the method for cell separation by using trypsin to further remove granular cells, and we increased the concentration of fetal bovine serum used in in vitro culture to improve cytoactivity. Cell antibody immunofluorescence (IF) showed that all inoculated cells could be stained by the CYP17A1/19A1 antibody but not by the FSHR antibody, which could stain granulosa cells. Furthermore, morphological differences were observed between the outlines of theca interna and externa cells and in their nuclei. Growth curve and CYP17A1/19A1 mRNA relative expression analyses suggested that the growth profile of theca interna cells may have been significantly different from that of theca externa cells in vitro. Theca interna cells experienced the logarithmic phase on d1–d2, the plateau phase on d2–d3, and the senescence phase after d3, while theca externa cells experienced the logarithmic phase on d1–d3, the plateau phase on d3–d5, and the senescence phase after d5. Taken together, these results suggested that we have successfully established a reliable theca cell culture model and further defined theca cell characteristics in vitro.
Keywords: characteristics, culture model, duck, theca cell
Introduction
Theca cells, including theca externa cells and theca interna cells, are vital components of ovarian follicles, and they originate from fibroblast-like stromal cells in the ovary [1]. As a cell marker of follicle development, the appearance of theca cells is a major marker of the formation of secondary follicles, and this morphological change is highly consistent with the process of follicular development [2,3]. Otherwise, in terms of physiological functions, theca cells interact with granulosa cells and oocytes through members of the autocrine BMP and TGF-β families and other growth factors. They co-regulate follicular recruitment, development, selection, and degeneration [4–6]. Studies have shown that granulosa cells are unable to synthesize steroid hormones in prehierarchical follicles, while almost all steroid hormones are synthesized in theca cells. Even in hierarchical follicles, theca cells are involved in the development and apoptosis of follicles by synthesizing androgen and estrogen [2,7,8]. These facts indicate that theca cells play a key role in the recruitment, development, selection, and apoptosis of avian follicles.
Because of the essential functions of theca cells during the development of avian ovarian follicles, establishing an in vitro culture model of theca cells is important and necessary for future investigations. Early in 1973, researchers had begun to preliminarily explore the isolation and culture of the follicular granulosa layer and the theca layer of hens [9–11]. In addition, in 1989, turkey granulosa cells and theca cells were isolated and cultured by Porter et al. [7,12], but all the studies on these cells did not measure or guarantee their viability and purity, nor did they define their characteristics. After these studies, most investigations of the granulosa layer and theca layer of follicles consistently used the previous methods, with no obvious improvements in separation or culture [3,8,13,14]. In other words, the previous studies on avian theca cells did not reliably measure their viability and purity, and their characteristics are not fully understood. However, previous studies proved that the FSHR protein was present only in granulosa cells within follicles, while CYP17A1 and CYP19A1 were present only in theca cells. In addition, assessing the CYP17A1/19A1 content was the best standard for evaluating the synthesis ability of androgen and estrogen in theca externa and interna cells respectively [2,3,8,13,15–20]. The previous studies defined the basic characteristic differences between the granulosa layer and the theca layer and provided the theoretical criteria for identifying the granulosa layer and the theca layer at the tissue level; however, no studies have systematically measured the purity, viability, and characterization of theca cells in birds. A reliable model for avian theca cell culture has not yet been established.
Therefore, in the present study, we improved the methods of theca cell isolation and culture in vitro. Specific theca cell proteins were measured and identified using immunofluorescence (IF), and cell viability was evaluated by MTT assay. In addition, the expression patterns of two marker genes (CYP17A1/19A1) were also determined by quantitative real-time PCR (qPCR) during theca cell culture in vitro. The present study aims to establish a reliable duck theca cell culture model in vitro and to further define its characteristics, which might provide a foundation for future studies involving the recruitment, development, selection, and apoptosis of avian follicles.
Materials and methods
Animals
Laying Liancheng White ducks (2 years old) were used in the present study. The ducks were kept under natural light and temperature conditions at the Waterfowl Breeding Experimental Farm at Sichuan Agricultural University (Sichuan, China) and were provided unlimited access to food and water. Individual laying cycles were recorded for each duck, and all ducks in the same laying cycle were killed by cervical dislocation 18–20 h after oviposition.
Isolation and culture of duck theca cells
Follicles from each ovary were separated and subsequently washed in ice-cold sterile phosphate buffered saline (PBS, pH 7.4), and hierarchical follicles (F4-F2) were selected. Tweezers were used to peel away the connective tissue, and then an approximate 2.0–2.5 cm slit was cut with a surgical blade across from the stalk. The yolk and the granulosa layer flowed out. In addition, residual follicular tissues were inverted and washed several times with PBS to wash away the granulosa layer and yolk. The residual follicular tissues were incubated with 0.25% trypsin/EDTA (1×; Gibco) while shaking in a water bath for 10 min to remove the residual granulosa cells and other impurities [7,9,14]. Media (DMEM and F-12/1:1; (HyClone), 10% fetal bovine serum (Gibco), 100 μg/ml streptomycin, and 100 μg/ml penicillin (Gibco)) were added to end the digestion. In addition, the residual follicle tissue was rinsed with ice-cold PBS several times to obtain the clean theca layer. Then, the theca layer was finely minced using scissors and incubated in digestion buffer (PBS, 0.3% collagenase type I (Gibco), 0.1% DNase (Coolaber), 4% BSA (Gibco)) at 37°C while shaking in a water bath for 20 min. The digestion was terminated by the addition of ice-cold PBS. The theca cell suspension was filtered with a 200-mesh filter and then centrifuged at 800×g for 10 min at room temperature to separate floating impurities. The theca cells were cultured in a humidified atmosphere at 5% CO2 and 95% air at 37°C. To remove blood cells that could not adhere to the culture plate, the medium was changed after 6 h of incubation. Granulosa cells taken from the same follicles were cultured according to the method reported by Wen et al. [21].
Dynamic observation and growth of theca cells
Theca cells were seeded on 96-well plates, and their viability was measured every day for 7 days; then, the MTT assay was performed as previously described [22,23]. To each well in the 96-well plate that contained theca cells, 200 μl of 0.5 mg/ml MTT (Amresco) was added (MTT was added in two additional blank wells as controls), and the cells were incubated at 37°C for 4 h. The MTT solution was removed, and then 150 μl of DMSO (Solarbio) was added to each well and the plate was shaken for 10 min. Then, the absorbance at 490 nm was measured by an automatic enzyme immunoassay analyzer [24]. Before measuring the cell viability, the characteristics of duck theca cells were captured using a microscope (Olympus, Tokyo, Japan).
Cell antibody immunofluorescence
Specific follicle proteins were detected by IF assay. The theca cells were fixed with 4% paraformaldehyde (Solarbio), penetrated with 0.1% Triton-X (Amresco), sealed with 4% BSA (Solarbio), and then washed with PBS. The antibodies (anti-CYP17 rabbit polyclonal (Bioss), anti-CYP19 rabbit polyclonal (Bioss), anti-FSHR rabbit polyclonal (Boster)) were diluted 1:50 and added to the thecal cells (CYP17, CYP19, and FSHR) and the granulosa cells (FSHR) respectively, while the control group was washed in PBS without primary antibodies. All of the groups were incubated at 4°C for 8 h. Next, FITC-goat anti-rabbit IgG (EARTHOX) was diluted 1:200 and added to all the previously mentioned wells at 37°C for 1 h. Then, 1 μl/ml DAPI (Solarbio) was added to the wells for 10 min. Finally, the images were collected by fluorescence microscopy.
Quantitative real-time PCR
Total RNA was extracted from the cultured cells using TRIzol (Invitrogen) at various time points (d1, d2, d3, d4, d5, d6, and d7) that corresponded to the MTT assay and image collection. First-strand cDNA was synthesized from 10 μg of total RNA using a cDNA synthesis kit following the manufacturer’s instructions (TaKaRa, Shiga, Japan). Levels of CYP17A1/19A1 were detected using the SYBR PrimerScriptTM real-time PCR kit (TaKaRa) and a CFX96TM Real-Time system (Bio-Rad, CA, U.S.A.). The PCR was performed in a 25 μl reaction volume that consisted of 2.0 μl of cDNA, 12.5 μl of SYBR Premix EX Taq, 8.5 μl of sterile water, and 1.0 μl of each gene-specific primer. The raw results were repeated three times and normalized to β-Actin and GADPH using the 2−ΔΔCt method [25]. Primers for these genes are listed in Table 1.
Table 1. Primers used for qPCR.
| Genes | Sequence (5′–3′) | Temperature (°C) | Size (bp) |
|---|---|---|---|
| CYP17A1 | F: GCTCCCTCTGCTTCAACTCCT | 60 | 100 |
| R: CCTGACCTTGAGGCACTTCTTC | |||
| CYP19A1 | F: CTGGTCCTGGTCTCGTGCGTAT | 60 | 139 |
| R: GATGTGTCAAGCATGATCCGTCTC | |||
| GAPDH | F: AAGGCTGAGAATGGGAAAC | 54 | 254 |
| R: TTCAGGGACTTGTCATACTTC | |||
| β-Actin | F: GCTATGTCGCCCTGGATTTC | 60 | 168 |
| R: CACAGGACTCCATACCCAAGAA |
Results
Morphological characteristics and growth analysis of theca cells
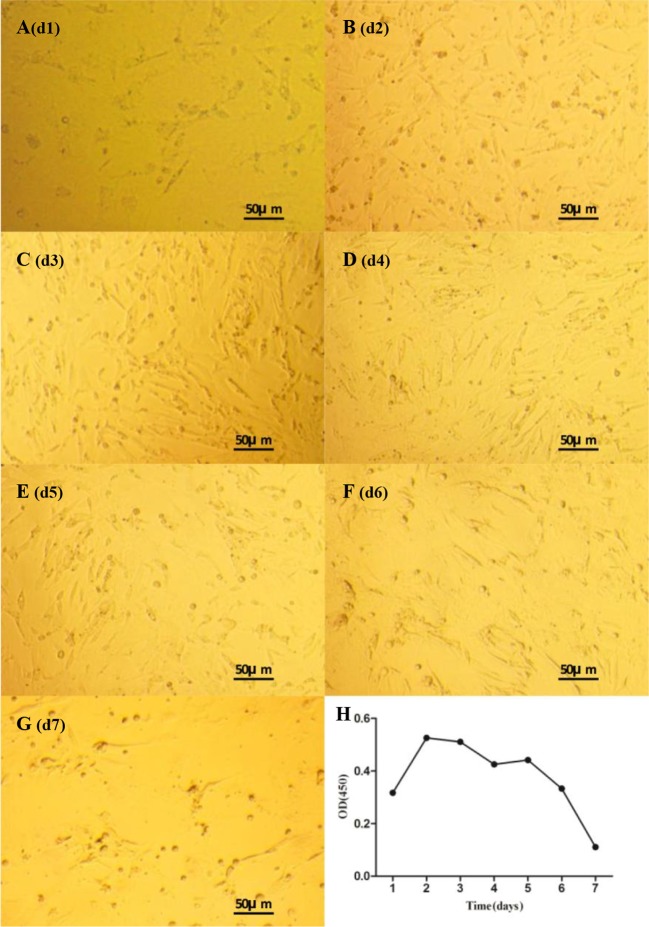
Morphological characteristics of duck theca cells cultured in vitro recorded on different days are shown in Figure 2. Theca interna cells showed an epithelial-like shape, while theca externa cells were observed to have a strip-like shape (Figure 1). After 24 h of incubation, all theca cells were tightly adhered to the bottom of the plate, and the adhered cell layer was extremely thin. The growth phase lasted until d3, and during this period, theca cells grew very quickly and spread over the bottom of the plate (Figure 2C). At d5, the theca cell density began to decline, and growth declined sharply from d6 to d7 (Figure 2E–G). In addition, it is noteworthy that theca externa cells and theca interna cells did not proliferate independently but grew mutually together.
Figure 2. Morphological characteristics and growth curve of theca cells.
Morphological characteristics and growth curve of duck theca cells in vitro as seen under a microscope (×100). (A–G) Morphology of duck theca cells on d1-d7 in vitro. (H) Growth curve of duck theca cells that were cultured in vitro (P<0.05).
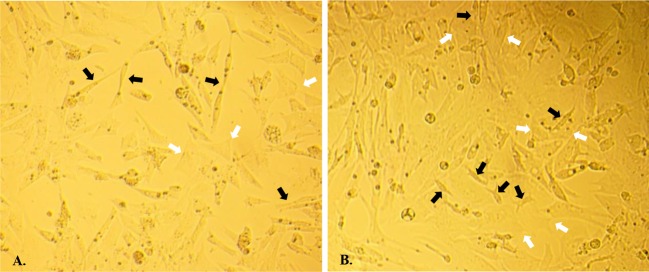
Figure 1. Morphological characteristics of theca cells.
Morphological characteristics of duck theca cells in vitro as seen under a microscope (×200). (A) Theca cells in vitro cultured at d2. (B) Theca cells in vitro cultured at d4. The theca interna cells are marked by white arrows, and the theca externa cells are marked by black arrows.
The theca cell growth curve is shown in Figure 2H. Theca cells grew quickly in the first 2 days, and their viability value peaked at d2. After that, the cells showed two steady trends on d2–d3 and d4–d5 and two declining trends on d3–d4 and d6–d7.
Expression profiles of CYP17A1/19A1 mRNA during theca cell culture
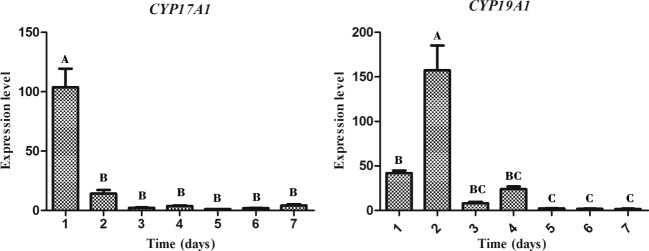
During the culture process (7 days), we measured the mRNA expression profiles of CYP17A1/19A1 in theca cells each day by qPCR (Figure 3). The level of CYP17A1 mRNA expression in theca cells on d1 was significantly higher than on the other days. After d1, the expression level of CYP17A1 decreased sharply. The expression of CYP19A1 mRNA was high on d1 and d2. On d1, the level of CYP19A1 mRNA expression was significantly higher than on d5–d7, and it reached its peak on d2. Expression on d2 was significantly higher than on the other days.
Figure 3. Expression profiles of CYP17A1/19A1 mRNA during theca cell culture.
Relative mRNA expression of the CYP17A1 and CYP19A1 genes in duck theca cells on d1–d7. The letters at the top of each bar represents the significant differences between the gene expression in various stages (P<0.05).
CYP17A1/19A1 and FSHR antibodies used for immunofluorescence identification
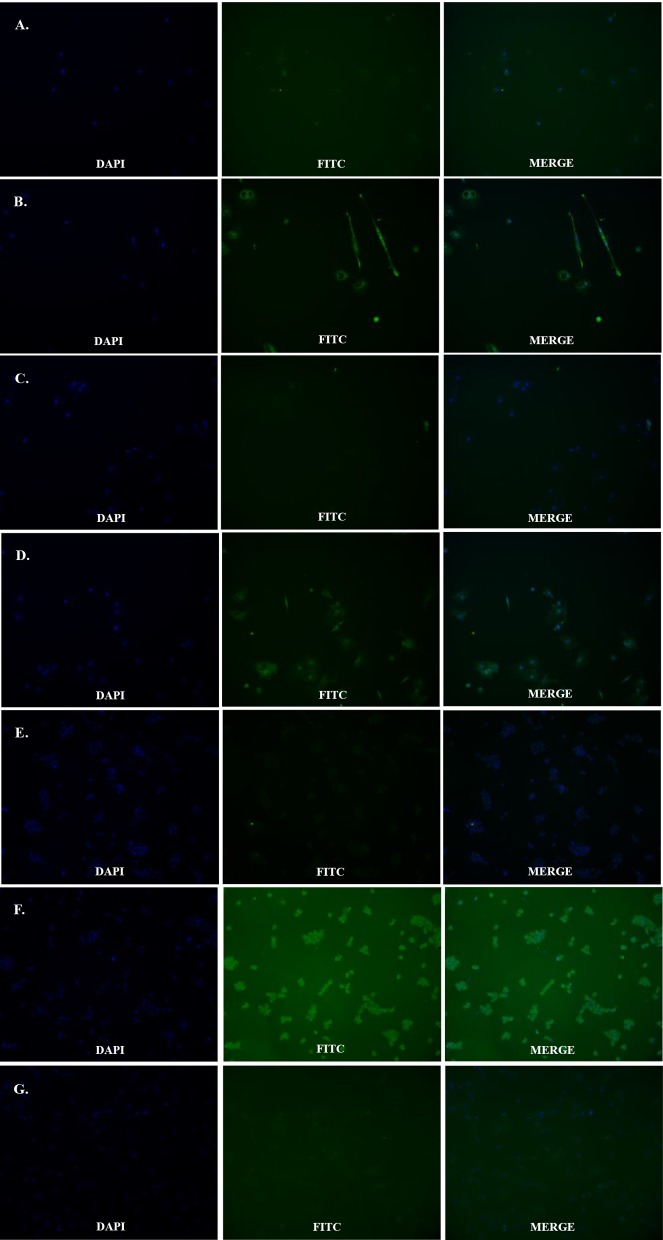
CYP17A1, CYP19A1, and FSHR antibodies were used to identify theca cells and granulosa cells respectively. Theca cells reacted with both the CYP17 and 19A1 antibodies and were marked with a green outline (Figure 4). However, when both the theca cells and the granulosa cells reacted with the FSHR antibody, only the granulosa cells were marked with a green outline (Figure 4). In contrast, there were significant differences between the cellular nuclei of theca interna cells and theca externa cells, which were identified by DAPI staining. The nuclei of theca interna cells were observed to be larger and rounder than those of theca externa cells.
Figure 4. immunofluorescence identification by CYP17A1/19A1 and FSHR antibodies.
Fluorescent image of theca cells and granulosa cells marked by their specific protein antibodies. (A and B) Control and treatment groups stained with CYP17A1 antibody to mark theca cells. (C and D) Control and treatment groups stained with CYP19A1 antibody to mark theca cells. (E) Treatment group stained with FSHR antibody to mark theca cells. (F and G) Control and treatment group stained with FSHR antibody to mark granulosa cells.
Discussion
Theca cells are important for the recruitment, development, selection, and apoptosis of avian follicles. Early in 1979, researchers had explored methods for separating avian theca cells. In hens, Huang et al. [9] used a simple physical method to directly separate theca cells. To date, this method is still widely used in avian species [14,26–28], but it might be difficult to separate theca cells from granulosa cells completely in vitro using this method. Previous reports [26,27] and our unpublished results suggested that the sensitivity of granulosa cells to trypsin was more intense than theca cells. Therefore, to ensure that the residual granulosa cells adhered to the theca layer were completely removed, trypsin was used to further digest theca cells after their physical separation. In previous studies, theca cell digestion methods were not stable in mammals or birds, and both the digestion reagent and the digestion time were not clearly defined [9,12,14,26–34]. Previous theca cell culture experiments in birds were limited to turkey and chicken species, and all these experiments only used collagenase as the digestion reagent. The digestion time was vague and ranged from 35 to 120 min [9,12,14,26–28]. In this experiment, we found that if only collagenase was used to digest theca cells, it produced a large amount of goo, which had serious impact on the ability to separate and collect the cells. Therefore, in the present study, a mixture of collagenase and DNase was employed to digest the cells and the digestion time was adjusted to 20 min. These modifications achieved satisfactory results.
In mammals, we commonly authenticate the purity of theca cells by a radioimmunoassay (RIA), because various follicular cells have obvious differences in their hormonal content [30,33–35]. However, in birds, the various components of follicular cells (including granulosa cells, theca interna cells, and theca externa cells) contain similar hormones (although the content varies greatly) [7,8]. Therefore, we cannot use RIA to authenticate avian theca cells. It has been reported that in hen follicular cells, the CYP17A1/19A1 protein exists specifically in theca cells (including theca interna cells and theca externa cells) on an organizational level [8,13,15,18]. Therefore, we used the CYP17A1/19A1 antibody to authenticate duck theca cells using the IF technique. These results showed that all theca cells that were separated and cultured in the present study were stained with the CYP17A1/19A1 antibody (Figure 4A–D). It verified that all cells that were isolated in the experiment were theca cells. In addition, we further used the FSHR (the specific avian granulosa cells protein) [19] antibody (Figure 4E and F) to ensure that there were no granulosa cells mixed in with the theca cells. Based on these results, we corroborate that the methods for isolation and digestion used in the present study are reasonable and reliable.
During the experiment, we found that the two types of cells had distinct morphological characteristics. The shape of theca externa cells appeared similar to smooth muscle cells because of their strip-like shape, while theca interna cells looked like typical steroidogenic cells because of their epithelial-like shape. The nuclei of theca interna cells appeared larger and rounder than theca externa cells (Figure 1), and all these results were consistent with previous studies [36–39]. Furthermore, the cell growth trend in the present study was evaluated by the MTT assay, and it was determined to be compliant with basic cell growth laws [40,41]. The viability of theca cells also remained high, which indicated that theca cells could maintain normal morphology and good activity in this cell culture model. The fact that the expression levels of CYP17A1/19A1 mRNA reached certain threshold values could reflect the steroid synthesis ability and viability of theca cells [3,18]. In our study, the expression of CYP17A1 mRNA was significantly higher on d1 (P<0.05) than at other time points, which suggested that in vitro, androgen was synthesized in copious amounts from theca interna cells at the beginning of the culture. On the second day, the expression of CYP19A1 mRNA was significantly higher (P<0.05) than at the other time points, and when combined with the high quantity of androgen synthesized on the first day, the results suggested that most of the androgen in theca externa cells was transformed into estrogen by the CYP19A1 protein on the second day. These steroid synthesis patterns and characteristics are consistent with previous reports [2,3,16,18,20], which indicate that the ability of theca cells to synthesize steroids in this culture model remained normal. After comprehensive analysis of theca cell density, viability, and assessment of their ability to synthesize steroids during d1–d7, we found that all parameters of cell density, viability, and expression of the CYP17A1/19A1 genes did not have a steady trend after seeding, which suggests that the latency period of theca cells should be between d0 and d1. The rapid growth period occurred between d1 and d2, which revealed that the logarithmic phase of theca cell growth should be between d1 and d2. After d2, there were two distinct trends, which could correspond to the two different cells. Combined with the qPCR results, we presumed that the growth plateau phase of theca interna cells was from d2 to d3, and on approximately the third day, cell activity began to decrease. For theca externa cells, their plateau phase could have been around d3–d5, with cell activity beginning to decrease on d5. However, in our research, the trends of theca cell density, viability, and their ability to synthesize steroids were mutually consistent. Further investigations need to be performed to clarify the details of the growth characteristics of theca interna and externa cells.
In conclusion, the present study summarized and improved upon the previous methods for theca cell separation and culture, established a reliable duck theca cell culture model in vitro, and further defined the morphology, growth trends, and basic characteristics of theca cells.
Abbreviations
- BMP
bone morphogenetic protein
- CYP17A1
cytochrome P450 17α-hydroxylase
- CYP19A1
cytochrome P450 aromatase
- DMEM
dulbecco's modified eagle media
- DMSO
dimethyl sulphoxide
- FSHR
follicle simulating hormone receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IF
immunofluorescence
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- qPCR
quantitative real-time PCR
- RIA
radioimmunoassay
- TGF
transforming growth factor
Funding
This work was supported by the National Natural Science Funds of China [grant number 31672424]; the National Waterfowl Industrial Technology System [grant number CARS-43-6]; the Project of National Science and Technology Plan for the Rural Development in China [grant number 2015BAD03B06]; and the Livestock & Poultry Breeding Research Project of Sichuan Province (2016–2020) [grant number 2016NYZ0044].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Author contribution
Xiang Gan and Da Chen did the majority of the experimentation. Yan Deng, Junsong Yuan, Jiamin Qiu, Wenqiang Sun, Chunchun Han, Jiwei Hu and Liang Li did some of the experimentation and provided reagents, animal materials and critical comments on the paper. Jiwen Wang, Xiang Gan and Bo Kang devised the hypothesis and experimental plan, and wrote the paper.
References
- 1.Honda A., Hirose M., Hara K., Matoba S., Inoue K., Miki H. et al. (2007) Isolation, characterization, and in vitro and in vivo differentiation of putative thecal stem cells. Proc. Natl. Acad. Sci. U.S.A. 104, 12389–12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nitta H., Osawa Y. and Bahr J.M. (1991) Immunolocalization of steroidogenic cells in small follicles of the chicken ovary: anatomical arrangement and location of steroidogenic cells change during follicular development. Domest. Anim. Endocrinol. 8, 587–594 [DOI] [PubMed] [Google Scholar]
- 3.Lee K.A., Volentine K.K. and Bahr J.M. (1998) Two steroidogenic pathways present in the chicken ovary: theca layer prefers delta 5 pathway and granulosa layer prefers delta 4 pathway. Domest. Anim. Endocrinol. 15, 1–8 [DOI] [PubMed] [Google Scholar]
- 4.Miyoshi T., Otsuka F., Inagaki K., Otani H., Takeda M., Suzuki J. et al. (2007) Differential regulation of steroidogenesis by bone morphogenetic proteins in granulosa cells: involvement of extracellularly regulated kinase signaling and oocyte actions in follicle-stimulating hormone-induced estrogen production. Endocrinology 148, 337–345 [DOI] [PubMed] [Google Scholar]
- 5.Otsuka F. (2010) Multiple endocrine regulation by bone morphogenetic protein system. Endocr. J. 57, 3–14 [DOI] [PubMed] [Google Scholar]
- 6.Sriperumbudur R., Zorrilla L. and Gadsby J.E. (2010) Transforming growth factor-beta (TGFbeta) and its signaling components in peri-ovulatory pig follicles. Anim. Reprod. Sci. 120, 84–94 [DOI] [PubMed] [Google Scholar]
- 7.Porter T.E., Hargis B.M., Silsby J.L. and el Halawani M.E. (1989) Differential steroid production between theca interna and theca externa cells: a three-cell model for follicular steroidogenesis in avian species. Endocrinology 125, 109–116 [DOI] [PubMed] [Google Scholar]
- 8.Gomez Y., Velazquez P.N., Juarez-Oropeza M.A. and Pedernera E. (1998) Steroid metabolism in granulosa and theca interna cells from preovulatory follicles of domestic hen (Gallus domesticus). Anim. Reprod. Sci. 52, 81–91 [DOI] [PubMed] [Google Scholar]
- 9.Huang E.S. and Nalbandov A.V. (1979) Steroidogenesis of chicken granulosa and theca cells: in vitro incubation system. Biol. Reprod. 20, 442–453 [DOI] [PubMed] [Google Scholar]
- 10.Bahr J.M., Wang S.C., Huang M.Y. and Calvo F.O. (1983) Steroid concentrations in isolated theca and granulosa layers of preovulatory follicles during the ovulatory cycle of the domestic hen. Biol. Reprod. 29, 326–334 [DOI] [PubMed] [Google Scholar]
- 11.Tilly J.L. and Johnson A.L. (1989) Regulation of androstenedione production by adenosine 3΄,5΄-monophosphate and phorbol myristate acetate in ovarian thecal cells of the domestic hen. Endocrinology 125, 1691–1699 [DOI] [PubMed] [Google Scholar]
- 12.Porter T.E., Hargis B.M., Silsby J.L. and el Halawani M.E. (1991) Characterization of dissimilar steroid productions by granulosa, theca interna and theca externa cells during follicular maturation in the turkey (Meleagris gallopavo). Gen. Comp. Endocrinol. 84, 1–8 [DOI] [PubMed] [Google Scholar]
- 13.Kato M., Shimada K., Saito N., Noda K. and Ohta M. (1995) Expression of P450 17 alpha-hydroxylase and P450aromatase genes in isolated granulosa, theca interna, and theca externa layers of chicken ovarian follicles during follicular growth. Biol. Reprod. 52, 405. [DOI] [PubMed] [Google Scholar]
- 14.Jia Y., Lin J., Zeng W. and Zhang C. (2010) Effect of prostaglandin on luteinizing hormone-stimulated proliferation of theca externa cells from chicken prehierarchical follicles. Prostag. Oth. Lipid Mediators 92, 77–84 [DOI] [PubMed] [Google Scholar]
- 15.Nitta H., Osawa Y. and Bahr J.M. (1991) Multiple steroidogenic cell populations in the thecal layer of preovulatory follicles of the chicken ovary. Endocrinology 129, 2033–2040 [DOI] [PubMed] [Google Scholar]
- 16.Velázquez P., Gómez Y., González del Pliego M. and Pedernera E. (1991) Steroidogenic cell subpopulations obtained from the theca of preovulatory follicles in the ovary of the domestic fowl. Gen. Comp. Endocrinol. 83, 243. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Vertiz A., González del Pliego M., Velazquez P. and Pedernera E. (1993) Morphological changes in the thecal layer during the maturation of the preovulatory ovarian follicle of the domestic fowl (Gallus domesticus). Gen. Comp. Endocrinol. 92, 80–87 [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Maldonado E., Velazquez P.N., Juarez-Oropeza M.A. and Pedernera E. (1996) Steroid metabolism in theca externa cells from preovulatory follicles of domestic hen (Gallus domesticus). Gen. Comp. Endocrinol. 101, 173–179 [DOI] [PubMed] [Google Scholar]
- 19.Woods D.C. and Johnson A.L. (2005) Regulation of follicle-stimulating hormone-receptor messenger RNA in hen granulosa cells relative to follicle selection. Biol. Reprod. 72, 643–650 [DOI] [PubMed] [Google Scholar]
- 20.Akhtar M., Wright J.N. and Lee-Robichaud P. (2011) A review of mechanistic studies on aromatase (CYP19) and 17α-hydroxylase-17,20-lyase (CYP17). J. Steroid Biochem. Mol. Biol. 125, 2–12 [DOI] [PubMed] [Google Scholar]
- 21.Wen R., Hu S.Q., Xiao Q.H., Han C.C., Gan C., Gou H. et al. (2015) Leptin exerts proliferative and anti-apoptotic effects on goose granulosa cells through the PI3K/Akt/mTOR signaling pathway. J. Steroid Biochem. 149, 70–79 [DOI] [PubMed] [Google Scholar]
- 22.Chen Q., Cao H.Z. and Zheng P.S. (2014) LGR5 promotes the proliferation and tumor formation of cervical cancer cells through the Wnt/β-catenin signaling pathway. Oncotarget 5, 9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang W.T. and Zheng P.S. (2014) Promoter hypermethylation of KLF4 inactivates its tumor suppressor function in cervical carcinogenesis. PLoS ONE 9, e88827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H., Wang F., Tao X. and Cheng H. (2012) Ammonia-containing dimethyl sulfoxide: an improved solvent for the dissolution of formazan crystals in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Anal. Biochem. 421, 324–326 [DOI] [PubMed] [Google Scholar]
- 25.Livak K.J. and Schmittgen T.D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 26.Marrone B.L. and Hertelendy F. (1985) Decreased androstenedione production with increased follicular maturation in theca cells from the domestic hen (Gallus domesticus). J. Reprod. Fertil. 74, 543–550 [DOI] [PubMed] [Google Scholar]
- 27.Wang L., Croze F., Morley P. and Tsang B.K. (1993) Granulosa-thecal cell interactions in the regulation of plasminogen activator activity during ovarian follicular development in the hen. Biol. Reprod. 49, 924–932 [DOI] [PubMed] [Google Scholar]
- 28.Roberts R.D., Sharp P.J., Burt D.W. and Goddard C. (1994) Insulin-like growth factor-I in the ovary of the laying hen: gene expression and biological actions on granulosa and thecal cells. Gen. Comp. Endocrinol. 93, 327–336 [DOI] [PubMed] [Google Scholar]
- 29.Roberts A.J. and Skinner M.K. (1990) Hormonal regulation of thecal cell function during antral follicle development in bovine ovaries. Endocrinology 127, 2907–2917 [DOI] [PubMed] [Google Scholar]
- 30.Hillier S.G., Yong E.L., Illingworth P.J., Baird D.T., Schwall R.H. and Mason A.J. (1991) Effect of recombinant activin on androgen synthesis in cultured human thecal cells. J. Clin. Endocrinol. Metab. 72, 1206–1211 [DOI] [PubMed] [Google Scholar]
- 31.Engelhardt H., Tekpetey F.R., Gore-Langton R.E. and Armstrong D.T. (1992) Regulation of steroid production in cultured porcine thecal cells by transforming growth factor-beta. Mol. Cell Endocrinol. 85, 117–126 [DOI] [PubMed] [Google Scholar]
- 32.Nahum R., Thong K.J. and Hillier S.G. (1995) Metabolic regulation of androgen production by human thecal cells in vitro. Hum. Reprod. 10, 75–81 [DOI] [PubMed] [Google Scholar]
- 33.Li S.K. and Hearn M.T. (2000) Isolation of thecal cells: an assessment of purity and steroidogenic potential. J. Biochem. Biophys. Methods 45, 169–181 [DOI] [PubMed] [Google Scholar]
- 34.Young J.M. and McNeilly A.S. (2012) Inhibin removes the inhibitory effects of activin on steroid enzyme expression and androgen production by normal ovarian thecal cells. J. Mol. Endocrinol. 48, 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts A.J. and Skinner M.K. (1990) Estrogen regulation of thecal cell steroidogenesis and differentiation: thecal cell-granulosa cell interactions. Endocrinology 127, 2918–2929 [DOI] [PubMed] [Google Scholar]
- 36.Erickson G.F., Magoffin D.A., Dyer C.A. and Hofeditz C. (1985) The ovarian androgen producing cells: a review of structure/function relationships. Endocr. Rev. 6, 371–399 [DOI] [PubMed] [Google Scholar]
- 37.Meidan R., Girsh E., Blum O. and Aberdam E. (1990) In vitro differentiation of bovine theca and granulosa cells into small and large luteal-like cells: morphological and functional characteristics. Biol. Reprod. 43, 913–921 [DOI] [PubMed] [Google Scholar]
- 38.Tajima K., Orisaka M., Mori T. and Kotsuji F. (2007) Ovarian theca cells in follicular function. Reprod. Biomed. Online 15, 591–609 [DOI] [PubMed] [Google Scholar]
- 39.Lagaly D.V., Aad P.Y., Grado-Ahuir J.A., Hulsey L.B. and Spicer L.J. (2008) Role of adiponectin in regulating ovarian theca and granulosa cell function. Mol. Cell Endocrinol. 284, 38–45 [DOI] [PubMed] [Google Scholar]
- 40.Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Nofziger T.H. et al. (1988) Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 48, 4827. [PubMed] [Google Scholar]
- 41.Ding F., Li Q.Q., Li L., Gan C., Yuan X., Gou H. et al. (2015) Isolation, culture and differentiation of duck (Anas platyrhynchos) preadipocytes. Cytotechnology 67, 773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]