Abstract
Background.
Respiratory syncytial virus (RSV) and human rhinovirus (HRV) are the most common viruses associated with acute respiratory tract infections in infancy. Viral interference is important in understanding respiratory viral circulation and the impact of vaccines.
Methods.
To study viral interference, we evaluated cases of RSV and HRV codetection by polymerase chain reaction in 2 prospective birth cohort studies (the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure [INSPIRE] study and the Tennessee Children’s Respiratory Initiative [TCRI]) and a double-blinded, randomized, controlled trial (MAKI), using adjusted multivariable regression analyses.
Results.
Among 3263 respiratory tract samples, 24.5% (798) and 37.3% (1216) were RSV and HRV positive, respectively. The odds of HRV infection were significantly lower in RSV-infected infants in all cohorts, with adjusted odds ratios of 0.30 (95% confidence interval [CI], .22–.40 in the INSPIRE study, 0.18 (95% CI, .11–.28) in the TCRI (adjusted for disease severity), and 0.34 (95% CI, .16–.72) in the MAKI trial. HRV infection was significantly more common among infants administered RSV immunoprophylaxis, compared with infants who did not receive immunoprophylaxis (OR, 1.65; 95% CI, 1.65–2.39).
Conclusions.
A negative association of RSV on HRV codetection was consistently observed across populations, seasons, disease severity, and geographical regions. Suppressing RSV infection by RSV immunoprophylaxis might increase the risk of having HRV infection.
Keywords: Respiratory syncytial virus, RSV, rhinovirus, RV, HRV, viral interference, infancy.
Respiratory syncytial virus (RSV) and human rhinovirus (HRV) are the most common viruses associated with acute respiratory viral infections in infancy [1, 2]. Infection rates of RSV and HRV fluctuate during the year, with a seasonal pattern in the case of RSV that is especially notable in temperate climates [3, 4]. Interference or interaction between viruses, whereby a concurrent or prior presence of one virus results in a measurable difference in the presence of another virus [5], has been proposed as one factor influencing respiratory virus circulation in general [6, 7]. Viral interference may hold implications for the development and effects of vaccines [8–10]. It may also be of particular relevance for RSV immunoprophylaxis and RSV vaccines [11].
Both RSV and HRV have been suggested to interfere with other respiratory viruses, such as influenza virus, parainfluenza virus, and human metapneumovirus [12–14]. More recently, longitudinal and surveillance studies have indicated that RSV and HRV tend to predominate at different times [15–17]. In addition, several studies have shown that RSV and HRV are less frequently present in a single sample than expected by chance [14, 18, 19]. In line with these observations, RSV-HRV viral interference has been postulated and has been demonstrated in a case-control study of largely asymptomatic children <2 years old [19–21].
To date, there is no study systematically examining RSV-HRV interference in infancy. Using a unique combination of RSV-HRV data from 3 investigations, 2 prospective infant cohorts and a double-blinded, randomized clinical trial of RSV immunoprophylaxis (palivizumab), we assessed RSV and HRV codetections to determine whether there is evidence for or against viral interference. The study is the first to explore RSV-HRV codetection interference specifically in infant populations in which there is the highest incidence of both of these infections and is one of the first to include covariates. It draws on the strengths of 3 distinct cohorts spanning a 10-year time frame, different geographic regions, heterogeneous study populations, and various levels of disease severity.
METHODS
To study RSV and HRV viral interference, we analyzed RSV and HRV codetection in respiratory tract samples collected from infants. All samples were obtained during respiratory symptoms, and therefore detection of virus by polymerase chain reaction (PCR) was considered to indicate infection. Data from 3 distinct study cohorts were used for analysis. Detailed methods of these studies have been published previously [22–24].
Description of Cohorts and Laboratory Techniques
The Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure (INSPIRE) study is a longitudinal population-based birth cohort study of 1952 healthy term infants born June through December designed such that infants would be ≤6 months old during the winter viral season. Surveillance for respiratory tract infection was conducted from November through March during the first year of life for 2 consecutive seasons (2012–2014) in a region of southeastern United States. Nasal washing was performed when infants met predefined criteria for respiratory symptoms. Washes were analyzed using single-plex real-time reverse transcription PCR (RT-PCR) assays for RSV and HRV identification [24].
The Tennessee Children’s Respiratory Initiative (TCRI) is a prospective study of term healthy infants recruited when they presented to a single academic children’s hospital (Vanderbilt Children’s Hospital, Nashville, TN) with a suspected viral acute respiratory tract infection meeting specified criteria during an acute healthcare visit or hospitalization from September to May, 2004–2008. Although 674 infants were enrolled in the TCRI, this study was limited to the 662 infants for whom viral data were available. Nasal and throat swab specimens were obtained at time of illness presentation and analyzed with individual real-time RT-PCR to identify respiratory viral pathogens. This cohort, by design, represents more-severe disease with a high proportion of hospitalized infants (373 of 662 [56%]) [22].
The MAKI trial is a multicenter, randomized, double-blinded, placebo-controlled trial conducted from 2008 to 2011 in the Netherlands (Controlled Clinical Trials number ISRCTN73641710). A total of 429 otherwise healthy infants born during April–September at 33–35 weeks of gestational age were randomly assigned to receive RSV immunoprophylaxis with palivizumab (Synagis, MedImmune), a monoclonal RSV antibody, or placebo. Parents reported episodes of respiratory tract infection in the first year of life by using daily logs and collected nasal swab specimens on the second day of symptoms. The swabs were analyzed twice for RSV, once using duplex real-time RT-PCR and once by use of the RespiFinder SMART 22 assay from PathoFinder, which was also used to determine HRV positivity [23]. To avoid missing RSV-positive specimens, we considered RSV positivity of one or both assays as an RSV-positive result.
The study protocols for the INSPIRE study and the TCRI were approved by the Vanderbilt University Institutional Review Board; the MAKI study protocol was reviewed and approved by the institutional review board at the University Medical Center Utrecht and at each participating hospital. Written informed consent was obtained from parents in all 3 studies.
Statistical Analysis
The studied cohorts represent varied populations, study periods, and study locations. Therefore, all analyses were done separately for each cohort. Subjects in the INSPIRE study and the MAKI trial potentially experienced >1 episode of respiratory symptoms and thus contributed >1 viral sample and infection to the study. To ensure the independence of samples, analyses were restricted to samples collected from independent episodes of respiratory tract infection (defined as episodes at least 2 weeks apart).
The prevalence of RSV and HRV infections among samples was calculated. To evaluate the RSV and HRV codetection relationship, we conducted multivariable logistic regression by using HRV infection status as dependent variable and RSV infection status as the exposure of interest. We adjusted for infection calendar season and infection calendar month in the INSPIRE and TCRI cohorts. Interaction between RSV infection status and infection calendar month was evaluated and not found (P > .8). The type of healthcare visit (unscheduled outpatient visit, emergency department visit, and hospitalization) was collected in the TCRI cohort and served as a surrogate marker of disease severity in the analyses. The potential interaction effect between the type of healthcare visit and RSV infection status on HRV status was evaluated. MAKI trial data were analyzed similarly, using multivariable logistic regression, with RSV infection status and immunoprophylaxis treatment status adjusted in the model. RSV infection status by RSV immunoprophylaxis interaction effect was assessed. We further conducted subgroup analyses in immunoprophylaxis groups and untreated groups. Last, the potential impact of RSV immunoprophylaxis on RSV infection and HRV infection was assessed using univariate logistic regression. All analyses were performed using R 3.1.2 (available at: http://www.r-project.org).
RESULTS
Overall, 3263 independent samples were eligible for analysis. RSV and HRV were respectively detected in 24.5% of samples (798) and 37.3% of samples (1216). Table 1 contains detailed data on RSV and HRV positivity for each cohort.
Table 1.
Detection of Respiratory Syncytial Virus (RSV) and/or Human Rhinovirus (HRV) in All Cohorts
| RSV Detection Status | Samples, No. (%), by Study Group and HRV Detection Status | |||||||
|---|---|---|---|---|---|---|---|---|
| INSPIRE Study, 2012–2014, Samples, No. (%) (n = 2096) | TCRI, 2004–2008, Samples, No. (%) (n = 662) | AKI Trial, 2008–2011, Samples, No. (%) | ||||||
| Placebo Recipients (n = 256) | RSV Immunoprophylaxis Recipients (n = 249) | |||||||
| Detected | Not Detected | Detected | Not Detected | Detected | Not Detected | Detected | Not Detected | |
| Detected | 63 (3.0) | 322 (15.4) | 42 (6.3) | 333 (50.3) | 10 (3.9) | 18 (7.0) | 7 (2.8) | 3 (1.2) |
| Not detected | 653 (31.2) | 1058 (50.5) | 130 (19.6) | 157 (23.7) | 142 (55.5) | 86 (33.6) | 169 (67.9) | 70 (28.1) |
Abbreviations: INSPIRE, Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure; TCRI, Tennessee Children’s Respiratory Initiative.
Codetection in the INSPIRE Study
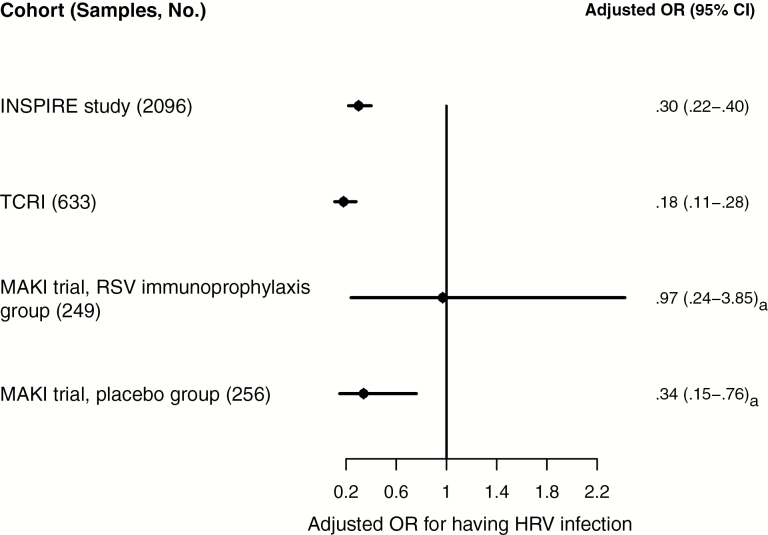
Respiratory tract infection surveillance in the INSPIRE cohort resulted in 2096 samples. RSV and HRV were detected in 18% (385) and 34% (716) of samples, respectively. The probability of HRV detection was considerably lower when RSV was present (odds ratio [OR], 0.32; 95% confidence interval [CI], .24–.42). The negative association of RSV and HRV detections was stable across calendar month and season (adjusted OR, 0.30; 95% CI, .22–.40; Figure 1 and Supplementary Figure 1).
Figure 1.
Findings of multivariate logistic regression analysis of respiratory syncytial virus (RSV) and human rhinovirus (HRV) codetection associations across cohorts. Abbreviations: CI, confidence interval; INSPIRE, Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure; OR, odds ratio; TCRI, Tennessee Children’s Respiratory Initiative. aUnadjusted OR.
Codetection in the TCRI
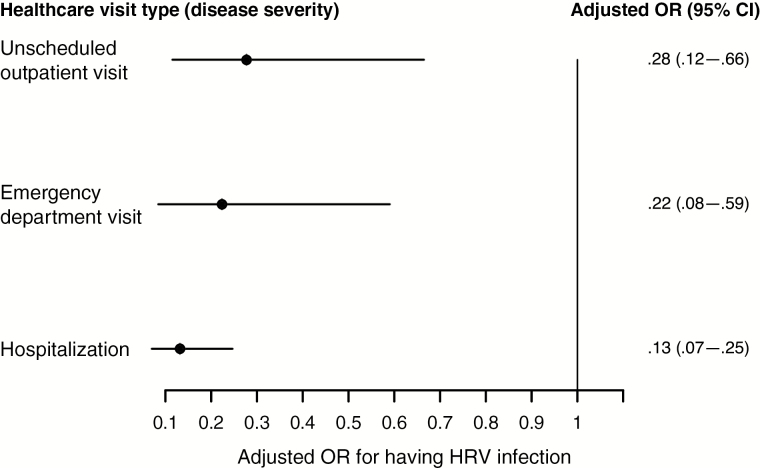
Among 662 available swab specimens with laboratory results available for analysis, 57% (375) and 26% (172) were positive for RSV and HRV, respectively. Having RSV infection was significantly associated with a decreased odds of having HRV infection (OR, 0.15; 95% CI, .10–.23). This decrease in the odds of having HRV infection when infants had already had RSV infection was significant even after adjustment for calendar season, month, and type of healthcare visit (adjusted OR, 0.18; 95% CI, .11–.28; Figure 1). Although not statistically significant, increased severity of RSV infection was associated with an even lower likelihood of having HRV infection (P = .199 for interaction term; Figure 2).
Figure 2.
Findings of multivariate logistic regression analysis of respiratory syncytial virus (RSV) and human rhinovirus (HRV) codetection associations across levels of disease severity in the Tennessee Children’s Respiratory Initiative cohort. Abbreviations: CI, confidence interval; OR, odds ratio.
Codetections in the MAKI Trial Among Infants in the Placebo Group and Those in the RSV Immunoprophylaxis Group
There were 549 swab specimens collected from participants in our MAKI trial. To ensure that only distinct episodes of respiratory tract infection were studied, we excluded swab specimens collected within 14 days from the previous swab specimen. Five hundred and five independent respiratory tract samples remained for analysis (249 in the immunoprophylaxis group and 256 in placebo group). RSV immunoprophylaxis was designed specifically to reduce the risk of RSV infection. We confirmed that RSV immunoprophylaxis significantly decreased the odds of RSV infection (OR, 0.34; 95% CI, .16–.72). At the same time, we found that HRV infection was significantly more common among infants protected from RSV by immunoprophylaxis, compared with infants who did not receive immunoprophylaxis (176 vs 152 HRV infections; OR, 1.65; 95% CI, 1.14–2.39; Table 2).
Table 2.
Respiratory Syncytial Virus (RSV) and Human Rhinovirus (HRV) Detection in the MAKI Trial, by Study Group
| Virus, Detection Status | Placebo Recipients, % (No.) (n = 256) | RSV Immunoprophylaxis Recipients, % (No.) (n = 249) | P a |
|---|---|---|---|
| RSV | |||
| Not detected | 89.1 (228) | 96.0 (239) | .005 |
| Detected | 10.9 (28) | 4.0 (10) | |
| HRV | |||
| Not detected | 40.2 (103) | 29.3 (73) | .012 |
| Detected | 59.4 (152) | 70.7 (176) |
aBy univariate analysis.
Among all 505 samples, 8% (38) and 65% (329) were positive for RSV and HRV, respectively. In the following subgroup analyses stratified by RSV immunoprophylaxis treatment status, we found that RSV infection was associated with a 66% decreased odds of having HRV infection (OR, 0.34; 95% CI, .15–.76) among subjects who did not receive immunoprophylaxis but was not significantly associated with HRV infection among subjects who received RSV immunoprophylaxis (OR, 0.97; 95% CI, .24–3.85; Figure 1).
DISCUSSION
The epidemiology of respiratory viruses is fascinating and poorly understood, particularly for viruses with epidemic peaks and predictable circulation during certain times of the year, such as RSV. The hypothesis that respiratory viruses influence the likelihood of infection by each other is intriguing although difficult to study. This study presents epidemiological evidence supporting RSV and HRV viral interference by demonstrating a negative association between RSV and HRV infection among infants. Across 3 distinct populations, RSV infection was associated with up to an 83% decreased odds of HRV infection. The negative association was consistently present across RSV seasons, calendar month, the 10-year period studied, disease severity spectrum, and geographical regions.
A negative association between RSV and HRV infection has been previously noted among largely pediatric populations [18–21, 25, 26]. This study shows that the negative association is persistently present among the infant population, in which these viruses represent the most common respiratory pathogens. Wisdom et al speculated that interference between HRV species C and RSV is mostly due to a suppressing effect of HRV C [20]. Our results, on the other hand, provide evidence that RSV-HRV interference might also be due to a suppressing effect of RSV. RSV immunoprophylaxis is specifically designed to minimize the risk of severe RSV infection. As expected, RSV immunoprophylaxis decreased the risk of RSV infection in the MAKI trial. However, we additionally observed a significant positive association between RSV immunoprophylaxis and HRV infection (Table 2). The association of RSV immunoprophylaxis with an increased odds of HRV infection is likely due to the negative association between RSV infection and HRV infection; RSV immunoprophylaxis decreases the risk of RSV infection, which in turn may increase the risk of HRV infection. The increase in HRV infections bears similarity to the recent observation of increased risk of non–influenza virus respiratory tract infections (including HRV) in children vaccinated with inactivated influenza vaccine [27].
This study is the first to systematically investigate the RSV-HRV infection relationship specifically among infants, which represent the most common respiratory pathogens during infancy. Strengths of the study include the use of 3 distinct infant cohorts spanning a decade, the inclusion of different regions and levels of disease severity, the use of molecular testing for viral detection, the prospective nature of these studies, and the use of trial data evaluating an intervention that prevents RSV infection. Primary limitations are the relatively small sample sizes for some analyses, although consistent results across cohorts make the findings unlikely to have been due to chance. The MAKI trial used only the RespiFinder multiplex assay to detect HRV, with underdetection of HRV a possibility. However, as this assay has high sensitivity for detecting HRV [28, 29], we think significant underdetection is unlikely.
The findings of this study provide support for interference between the two most clinically significant respiratory viruses in infancy. Such interactions may shed light on the currently cloudy dynamics of respiratory viral epidemiology. Additionally, mechanisms of suppression or enhancement among respiratory viruses may interfere with preventive strategies, such as immunoprophylaxis or vaccination. The results may also add to the unsettled questions of the clinical relevance and potential mechanisms of coinfections and viral interference [30]. Given the consistency and size of the RSV-HRV interaction across location, time, and disease severity, we speculate that RSV-HRV interference may be based on a biological phenomenon, rather than on external factors. For instance, HRV is sensitive to interferon, which is known to be induced by RSV infection [31, 32]. Palivizumab (the monoclonal antibody used for RSV immunoprophylaxis) is capable of inhibiting the induction of interferon by RSV, which might explain the lack of evidence for an inverse RSV-HRV association among infants who received RSV immunoprophylaxis [33]. Additionally, mechanisms have counterevolved in viruses to interfere with the generation of viral peptides, their intracellular trafficking, or the cell surface expression of major histocompatibility complex class I molecules bearing viral peptides. One might imagine that infection with one virus could therefore alter the host immune response to a second virus to which the host is exposed. If it were feasible to monitor the immunologic state of the host in a surveillance study on a nearly daily basis, one could provide more convincing support for viral interference.
In conclusion, this study provides the first in-depth exploration of RSV-HRV codetections in infancy, describing a negative association between RSV and HRV and providing support for viral interference. Further research is needed to determine the biological and clinical relevance, as it may influence vaccine research and preventive strategies for viral respiratory tract infections in infants.
Supplementary Material
Notes
Acknowledgments. We thank the patients and families who participated in these studies; Hakmook Kang, PhD, for his support in analysis; and the Dutch Neonatal RSV Network, for its role in the MAKI trial.
Disclaimer. None of the funders had any role in the writing of the manuscript or in the decision to submit for publication.
Financial support. This work was supported by the National Institutes of Health (grants U19 AI 095227 and K24 AI 077930 to the INSPIRE study and T. V. H.), Abbott Laboratories (unrestricted grant to the MAKI trial), the Netherlands Organization for Health Research and Development (grant NWO-AGIKO 920-035-89 to the MAKI trial and M. O. B.), and a Vanderbilt Clinical and Translational Science award (UL1 TR000445 to T. V. H., L. W., and C. Y.).
Potential conflicts of interest. L. B. received grants from MedImmune and AbbVie and other support from Janssen, Gilead, Okairos, Mabxience, Alios, and AIT during the conduct of the study. MOB received other support from AbbVie during the conduct of the study. T. G., J. D. C., E. K. L., and T. V. H. received grants from the National Institutes of Health during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Laurent C, Dugué AE, Brouard J, Nimal D, Dina J, Parienti J-J, et al. Viral epidemiology and severity of respiratory infections in infants in 2009: A prospective study. Pediatr Infect Dis J. 2012; 31:827–31. [DOI] [PubMed] [Google Scholar]
- 2. Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 2010; 23:74–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monto AS. Occurrence of respiratory virus: time, place and person. Pediatr Infect Dis J 2004; 23(1 Suppl):S58–64. [DOI] [PubMed] [Google Scholar]
- 4. Tang JW, Loh TP. Correlations between climate factors and incidence–a contributor to RSV seasonality. Rev Med Virol 2014; 24:15–34. [DOI] [PubMed] [Google Scholar]
- 5. DaPalma T, Doonan BP, Trager NM, Kasman LM. A systematic approach to virus-virus interactions. Virus Res 2010; 149:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anestad G, Vainio K, Hungnes O. Interference between outbreaks of epidemic viruses: additional Norwegian observations. Scand J Infect Dis 2009; 41:381–2. [DOI] [PubMed] [Google Scholar]
- 7. Mackay IM, Arden KE. Rhinoviruses. In: Kaslow RA, Stanberry LR, Le Duc JW, eds. Viral infect humans. Boston, MA: Springer US; 2014:675–712. [Google Scholar]
- 8. Seppälä E, Viskari H, Hoppu S, et al. Viral interference induced by live attenuated virus vaccine (OPV) can prevent otitis media. Vaccine 2011; 29:8615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swaminathan S, Khanna N, Herring B, Mahalingam S. Dengue vaccine efficacy trial: does interference cause failure? Lancet Infect Dis 2013; 13:191–2. [DOI] [PubMed] [Google Scholar]
- 10. Vento S, Cainelli F. Can HIV-1 viral interference be used therapeutically? Lancet Infect Dis 2013; 13:9–10. [DOI] [PubMed] [Google Scholar]
- 11. Modjarrad K, Giersing B, Kaslow DC, Smith PG, Moorthy VS. WHO consultation on Respiratory Syncytial Virus Vaccine Development Report from a World Health Organization Meeting held on 23–24 March 2015. Vaccine. 2015; (March). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anestad G. Interference between outbreaks of respiratory syncytial virus and influenza virus infection. Lancet 1982; 1:502. [DOI] [PubMed] [Google Scholar]
- 13. Anestad G. Surveillance of respiratory viral infections by rapid immunofluorescence diagnosis, with emphasis on virus interference. Epidemiol Infect 1987; 99:523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weigl JA, Puppe W, Meyer CU, et al. Ten years’ experience with year-round active surveillance of up to 19 respiratory pathogens in children. Eur J Pediatr 2007; 166:957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brittain-Long R, Andersson LM, Olofsson S, Lindh M, Westin J. Seasonal variations of 15 respiratory agents illustrated by the application of a multiplex polymerase chain reaction assay. Scand J Infect Dis 2012; 44:9–17. [DOI] [PubMed] [Google Scholar]
- 16. Ambrosioni J, Bridevaux P, Wagner G, Mamin A, Kaiser L. Epidemiology of viral respiratory infections in a tertiary care centre in the era of molecular diagnosis, Geneva, Switzerland, 2011–2012. Clin Microbiol. 2014; 20:578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zimmerman RK, Rinaldo CR, Nowalk MP, et al. Influenza and other respiratory virus infections in outpatients with medically attended acute respiratory infection during the 2011-12 influenza season. Influenza Other Respir Viruses 2014; 8:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanner H, Boxall E, Osman H. Respiratory viral infections during the 2009–2010 winter season in Central England, UK: incidence and patterns of multiple virus co-infections. Eur J Clin Microbiol Infect Dis. 2012; 31:3001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin ET, Fairchok MP, Stednick ZJ, Kuypers J, Englund JA. Epidemiology of multiple respiratory viruses in childcare attendees. J Infect Dis 2013; 207:982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wisdom A, Kutkowska AE, McWilliam Leitch EC, et al. Genetics, recombination and clinical features of human rhinovirus species C (HRV-C) infections; interactions of HRV-C with other respiratory viruses. PLoS One 2009; 4:e8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karppinen S, Toivonen L, Schuez-Havupalo L, Waris M, Peltola V. Interference between respiratory syncytial virus and rhinovirus in respiratory tract infections in children. Clin Microbiol Infect. Elsevier Ltd; 2016; 22:208.e1–208.e6. [DOI] [PubMed] [Google Scholar]
- 22. Hartert TV, Carroll K, Gebretsadik T, Woodward K, Minton P; Vanderbilt Center for Asthma and Environmental Health Research Investigators and Collaborators The Tennessee Children’s Respiratory Initiative: Objectives, design and recruitment results of a prospective cohort study investigating infant viral respiratory illness and the development of asthma and allergic diseases. Respirology 2010; 15:691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blanken MO, Rovers MM, Molenaar JM, et al. ; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–9. [DOI] [PubMed] [Google Scholar]
- 24. Larkin EK, Gebretsadik T, Moore ML, et al. ; INSPIRE Study. Objectives, design and enrollment results from the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure Study (INSPIRE). BMC Pulm Med 2015; 15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brunstein JD, Cline CL, McKinney S, Thomas E. Evidence from multiplex molecular assays for complex multipathogen interactions in acute respiratory infections. J Clin Microbiol 2008; 46:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greer RM, McErlean P, Arden KE, et al. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J Clin Virol 2009; 45:10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54:1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pillet S, Lardeux M, Dina J, et al. Comparative evaluation of six commercialized multiplex PCR kits for the diagnosis of respiratory infections. PLoS One 2013; 8:e72174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loens K, van Loon AM, Coenjaerts F, et al. ; GRACE Study Group. Performance of different mono- and multiplex nucleic acid amplification tests on a multipathogen external quality assessment panel. J Clin Microbiol 2012; 50:977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brand HK, de Groot R, Galama JM, et al. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr Pulmonol 2012; 47:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gulraiz F, Bellinghausen C, Dentener MA, et al. Efficacy of IFN-λ1 to protect human airway epithelial cells against human rhinovirus 1B infection. PLoS One 2014; 9:e95134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cakebread JA, Xu Y, Grainge C, et al. Exogenous IFN-β has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J Allergy Clin Immunol 2011; 127:1148–54.e9. [DOI] [PubMed] [Google Scholar]
- 33. Schijf MA, Lukens MV, Kruijsen D, et al. Respiratory syncytial virus induced type I IFN production by pDC is regulated by RSV-infected airway epithelial cells, RSV-exposed monocytes and virus specific antibodies. PLoS One. 2013; 8:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.