Brief Summary
Human monoclonal antibodies targeting the leukotoxin LukAB were isolated and purified from a boy with invasive Staphylococcus aureus infection. Characterization of these antibodies revealed diverse potency and modes of neutralization against toxin-mediated cytotoxicity, and they exhibited partial protection in vivo.
Keywords: Staphylococcus aureus, leukocidin, LukAB, monoclonal, hybridoma, toxins.
Abstract
The 2-component leukotoxin LukAB is critical for Staphylococcus aureus targeting and killing of human neutrophils ex vivo and is produced in the setting of human infection. We report 3 LukAB-specific human monoclonal antibodies (mAbs) with distinct mechanisms of toxin neutralization and in vivo efficacy. Three hybridomas secreting mAbs with anti-LukAB activity (designated SA-13, -15, and -17) were generated from B cells obtained from a 12-year-old boy with S. aureus osteomyelitis. Each of the 3 mAbs neutralized LukAB-mediated neutrophil toxicity, exhibited differing levels of potency, recognized different antigenic sites on the toxin, and displayed at least 2 distinct mechanisms for cytotoxic inhibition. SA-15 bound exclusively to the dimeric form of the toxin, suggesting that human B cells recognize epitopes on the dimerized form of LukAB during natural infection. Both SA-13 and SA-17 bound the LukA monomer and the LukAB dimer. Although all 3 mAbs potently neutralized cytotoxicity, only SA-15 and SA-17 significantly inhibited toxin association with the cell surface. Treatment with a 1:1 mixture of mAbs SA-15 and SA-17 resulted in significantly lower bacterial colony counts in heart, liver, and kidneys in a murine model of S. aureus sepsis. These data describe the isolation of diverse and efficacious antitoxin mAbs.
Antibiotic resistance frequencies continue to rise in Staphylococcus aureus isolates, and there is an urgent need for improved methods to both prevent and treat S. aureus infections. Staphylococcus aureus is a highly complex organism, however, and the history of failed S. aureus vaccine candidates dates back to at least 1902 [1]. One major barrier to the development of novel preventive strategies is that neither the bacterial nor host factors that govern the transition of S. aureus from a commensal organism to a pathogen are completely understood.
Staphylococcus aureus produces a wide array of virulence factors, but the 2-component leukotoxins, in particular the newly identified cytotoxin LukAB (also known as LukGH) [2, 3], are highly promising candidate antigens for inclusion in a multicomponent vaccine. Staphylococcus aureus secretes LukAB to disrupt the innate host response through lysis of neutrophils, macrophages, dendritic cells, and monocytes [2, 3]. Moreover, LukAB contributes to S. aureus fitness after leukocyte phagocytosis [4, 5] and facilitates the persistence of staphylococcal biofilms [6], both major barriers against successful use of currently available antistaphylococcal therapeutics. LukAB induces cytolysis through pore formation that occurs following toxin binding to the CD11b subunit of Mac-1 [7], an integrin found on the surface of phagocytes. Disruption of the interaction of LukAB and CD11b neutralizes cytotoxicity [8, 9].
We recently demonstrated that children with invasive S. aureus disease mount a high-titer, potently neutralizing serum antibody response to LukAB, confirming that the toxin is expressed in vivo during human infection and is targeted by the host during natural disease [10]. Furthermore, LukAB was present in all clinical isolates tested [10, 11]. Based on the discovery that children produce neutralizing antibodies to LukAB following infection, we sought to isolate human monoclonal antibodies (mAbs) with potent neutralizing capacity following natural infection to study the molecular basis for recognition and toxin inhibition. We report here the isolation and characterization of a series of human mAbs against LukAB with heterologous neutralizing activity and distinct mechanisms of protection.
MATERIALS AND METHODS
Ethics Statement
All protocols and experiments were conducted in accordance with National Institutes of Health guidelines for the care and use of human subjects and reviewed and approved by the Vanderbilt University Medical Center Institutional Review Board and Institutional Animal Care and Use Committee (see Supplementary Methods for details).
Donor Subject
A 12-year-old boy was admitted to the Monroe Carell Jr. Children’s Hospital at Vanderbilt and was enrolled into this study after confirmation of invasive S. aureus disease (osteomyelitis with associated bacteremia). Peripheral blood was collected upon enrollment and 8 weeks after recovery in heparin tubes for isolation of peripheral blood mononuclear cells (PBMCs) and in serum separator tubes.
Generation of LukAB-reactive Monoclonal Antibodies
Hybridomas producing antibodies against LukAB were generated as described before [12] and detailed in the Supplementary Methods. Briefly, B cells isolated from a patient with invasive S. aureus disease were transformed with Epstein-Barr virus and screened for specific antibody production. Cells with desired reactivity were electrofused with HMMA2.5 myeloma partner and grown in culture medium supplemented with HAT and ouabain for generating stable hybridomas. Hybridomas were cultured in serum-free medium (Hybridoma SFM, Life Technologies) for antibody expression. Antibodies were purified from culture supernatants by affinity chromatography using HiTrap MabSelect SuRe columns (Life Technologies). The sequence of the variable portions of heavy and light chains were determined as described before and detailed in the Supplementary Methods.
Enzyme-Linked Immunosorbent Assay and Epitope Binning Assays
Binding of purified antibodies to LukA, LukB, or LukAB was detected in enzyme-linked immunosorbent assay (ELISA). The antigens were immobilized (62.5 µg/well) on microtiter plates. Antibodies diluted in phosphate-buffered saline (PBS) at various concentrations were applied, and the bound antibodies were detected using antihuman immunoglobulin G (IgG) antibodies conjugated to peroxidase. The data were plotted using Prism (GraphPad), and nonlinear regression analysis was performed to calculate the half-maximal binding concentrations (EC50).
Competition binding studies using biolayer interferometry were performed on an Octet RED biosensor (Pall ForteBio) as described before [13]. LukAB was immobilized onto anti-His tag antibody-coated biosensor tips by immersing in protein solution at 15 µg/mL for 2 minutes. After a brief washing step, biosensor tips were immersed first into the wells containing the first mAb at a concentration of 10 μg/mL and then into the wells containing a second mAb at a concentration of 10 μg/mL. The percentage binding of the second mAb in the presence of the first mAb was determined by comparing the maximal signal of the second mAb applied after the first mAb complex to the maximal signal of the second mAb alone.
Neutralization of Toxin-Induced Primary Human Neutrophils Cytotoxicity by Monoclonal Antibodies
Primary human neutrophils (PMNs) were isolated from blood samples as previously described [14], and PMN purity was 90%–95% as determined by flow cytometric analysis using an LSR-II flow cytometer (BD Biosciences). Serial dilutions of each mAb were mixed with a fixed amount of purified LukAB (90% lethal dose [LD90] = 0.6 µg/mL) to give indicated molar ratios. Samples were preincubated for 30 minutes at room temperature (RT) before adding 2 × 105 PMNs in a final reaction volume of 100 µL. Cells were incubated for 1 hour at 37°C and 5% carbon dioxide before addition of CellTiter as described previously [10]. For experiments evaluating the cooperative effects of mAb-mediated toxin neutralization, we used mixtures of mAbs at a final concentration of 3.2 µg/mL (2:1 mAb/LukAB molar ratio).
Ex Vivo Infection of Primary Human Neutrophils
Overnight cultures of wild-type (WT) S. aureus strains of USA300 lineage BK18807 [4], LAC [3, 15], and their isogenic lukAB mutant strains grown in Roswell Park Memorial Institute 1640 (Invitrogen) medium supplemented with 1% casamino acids (RPMI + CAS) were subcultured 1:100 in RPMI + CAS and incubated for 5 hours with shaking at 180 rpm. Cell pellets were washed and normalized to equal density before infection [4]. Normalized S. aureus cultures were used to infect primary PMNs and seeded at 2 × 105 cells/well at a multiplicity of infection of 25 in a final volume of 100 µL for 2 hours at 37°C and 5% carbon dioxide. Where indicated, mAbs were added to the infection at 2.5 µg/mL immediately before starting the 2-hour incubation. The lactate dehydrogenase (LDH) release assay was performed as previously described [4] using the CytoTox-ONE homogeneous membrane integrity assay (Promega).
Monoclonal Antibody–Mediated Inhibition of LukAB Binding to Cells
Biotinylated LukAB proteins used in the binding experiments were generated using the Sulfo-NHS-LC-biotin (Thermo Scientific). Biotin-LukAB was incubated with PMNs for 10 minutes on ice. After intoxication, cells were washed with PBS, stained with a PerCP-Cy5.5 Streptavidin (Biolegend), washed with fluorescence activated cell sorting (FACS) buffer (1 × PBS + 2% fetal bovine serum + 0.05% sodium azide) before being fixed (1 × PBS + 2% paraformaldehyde + 2% fetal bovine serum + 0.05% sodium azide), and fluorescence analyzed using an LSR-II flow cytometer. For experiments including mAbs, dilutions of antibodies were preincubated with a fixed concentration of Biotin-LukAB (5 µg/mL) to give indicated molar ratios. Samples were incubated for 30 minutes at room temperature RT before adding human polymorphonuclear neutrophils (hPMNs) (2 × 105 cells/well), followed by 10 minutes of ice incubation, and then processed for FACS analysis as described above.
We evaluated mAb-mediated inhibition of LukAB binding to I domain of CD11b by ELISA. Plates were coated with I-domain and blocked as described [16]. LukAB (0.5 μg/mL) was preincubated with each mAb at the indicated molar ratio in 100 μL of Blotto buffer for 30 minutes before being added to the wells for 30 minutes. Bound LukAB was detected with 100 μL of anti-LukA rabbit polyclonal antibody [4] at a 1:2000 dilution for 1 hour and then 100 μL of horseradish peroxidase-conjugated antirabbit IgG (Promega) at a 1:3000 dilution for 1 hour. Wells were incubated with 100 μL of 1-Step Ultra TMB-ELISA substrate solution (Thermo Scientific) followed by 100 μL of 1 N sulfuric acid. Color development was measured at 450 nm on a spectrophotometer.
Murine Model of Disseminated Staphylococcus aureus Infection
For the murine disseminated infection model, an erythromycin-sensitive derivative of the USA300 strain LAC was used [17]. To prepare inocula, overnight cultures were back-diluted 1:100 in fresh tryptic soy broth (TSB) and grown for 3 hours at 37°C with 180 rpm shaking. Bacteria were then harvested by centrifugation and suspended in PBS at a final density of approximately 2.5 × 108 colony forming units (CFU)/mL.
Female BALB/cJ mice aged 7–8 weeks were subjected to disseminated S. aureus infection by retro-orbital inoculation [18,19]. Mice were pretreated with a 1:1 mixture of mAbs SA-15 and SA-17 or an isotype control (PERT-142) by intraperitoneal injection 20 hours before inoculation. Bacterial inocula were prepared as above and then administered as a 100-μL retro-orbital injection containing approximately 2.5 × 107 CFU. Mice were monitored for 96 hours, after which time they were killed, and the hearts, kidneys, and livers were removed for bacterial enumeration. Organs were homogenized using a Bullet Blender Storm (Next Advance, Averill Park, NY) and navy lysis tubes, then serially diluted onto tryptic soy agar ( plates for CFU enumeration.
Statistical Analysis
Differences in cell viability for in vitro and ex vivo cytotoxicity assays and mAb-mediated inhibition of LukAB binding were analyzed using analysis of variance (ANOVA) with Tukey’s post hoc test correction for multiple comparisons to determine specific differences. For the murine infection model, differences in CFU burdens in each organ were analyzed by Wilcoxon rank-sum test, assuming nonparametric distribution. Statistical analyses were performed using Prism 6.0 (GraphPad, La Jolla, CA).
RESULTS
Isolation of LukAB-Reactive Human Immunoglobulin G Monoclonal Antibodies
We sought to isolate toxin-neutralizing mAbs reactive with LukAB from a subject with recent invasive S. aureus infection. Peripheral blood mononuclear cells were isolated from a 12-year-old boy with confirmed S. aureus bacteremia and multifocal osteomyelitis. Cultures from the blood and debrided bone confirmed methicillin-resistant S. aureus (MRSA; Panton–Valentine leukocidin-positive, USA300 lineage).
Serum from the subject possessed a high titer of antibodies binding LukAB protein by ELISA (1:10240). We obtained blood from this patient in the acute phase and 2 months after recovery. B cells in the PBMC sample were transformed with Epstein-Barr virus, and the lymphoblastoid cell line supernatants were screened for binding to LukAB and separately for neutralization of LukAB-mediated cytotoxicity. Four wells from acute samples and 9 wells from convalescent samples of the total 384 wells tested were positive for LukAB binding. The wells contained, on average, approximately 30 transformed lymphoblastoid cell clones, based on cell cluster count. Therefore, the estimated circulating memory B-cell frequency of LukAB-reactive clones in the donor was 0.03% for acute samples and 0.08% for convalescent samples. Three of the cell-line supernatants exhibited potent neutralization of LukAB-mediated cytotoxicity. B cells from wells exhibiting IgG binding to LukAB were used to generate hybridoma cell lines. We obtained a panel of 3 distinct IgG clones with LukAB-neutralizing activity, designated SA-13 (from the acute sample) and SA-15 and SA-17 (from the convalescent sample). Each of the antibodies was encoded by different antibody variable genes, indicating that the clones arose independently (Supplementary Table 1).
Neutralizing Potency of Monoclonal Antibodies in Diverse Types of Primary Human Neutrophils Culture
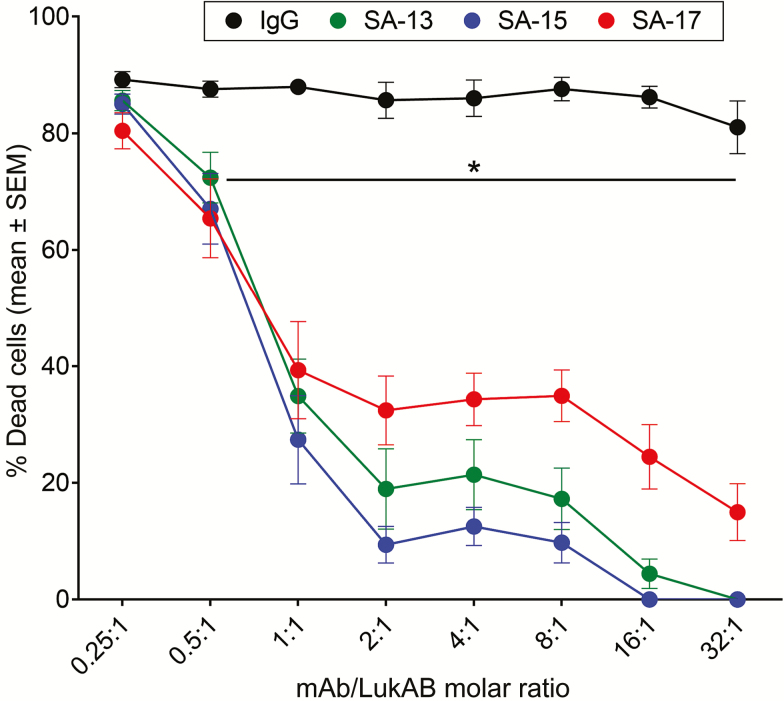
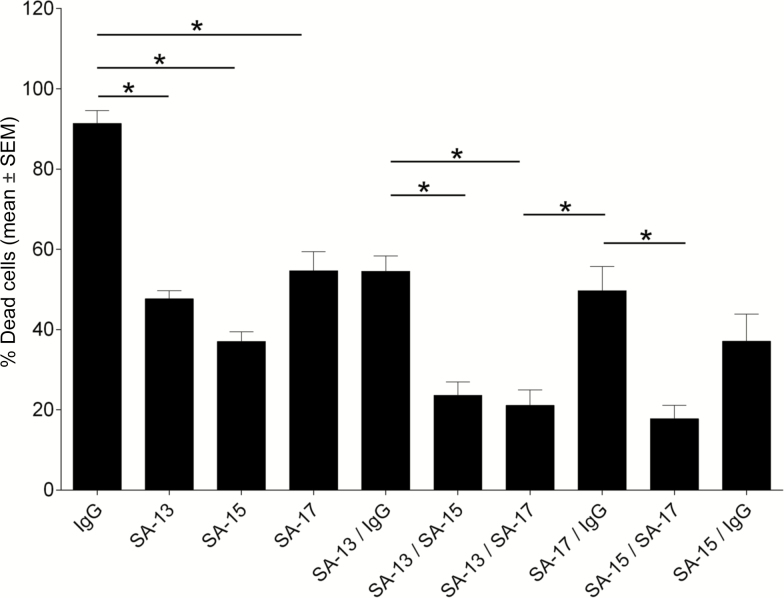
Purified IgG preparations of the mAbs were tested for their ability to inhibit LukAB-mediated cytotoxicity in PMN cultures. Each of the 3 mAbs exhibited strong neutralization of cytotoxicity against primary human PMNs, with SA-15 and SA-13 exhibiting higher potency compared with SA-17, whereas the IgG control did not affect LukAB cytotoxicity (Figure 1). These results suggest that the mAbs may mediate inhibition by recognizing distinct epitopes on LukAB or with differing affinity. Antibody potency also varied by cell type used in the neutralization assays. SA-15 was significantly more potent in the protection of PMN-like HL-60 cells (Supplementary Figure 1A). In contrast, SA-17 exhibited greater potency in the protection of macrophage-like THP-1 cells (Supplementary Figure 1B). Taken together, these studies showed that the 3 mAbs inhibit the cytotoxic effect of purified LukAB against phagocytes.
Figure 1.
SA-13, SA-15, and SA-17 neutralize LukAB-mediated cytotoxicity on primary human neutrophils. Monoclonal antibodies (mAbs) were preincubated with LukAB at indicated molar ratios for 30 minutes at room temperature. Primary human neutrophils (n = 6 donors) were added to the LukAB–mAb mixture and incubated for 1 hour. Neutrophil viability was evaluated with CellTiter and plotted as the mean ± SEM of percentage of dead cells. *P < .05 using 2-way analysis of variance with Tukey’s post hoc test correction for multiple comparisons. Each mean compared with immunoglobulin G control for statistical analysis. Abbreviations: IgG, immunoglobulin G; mAb, monoclonal antibody.
Neutralization of Staphylococcus aureus–Mediated Cytotoxicity During Ex Vivo Infection of Primary Human Neutrophils
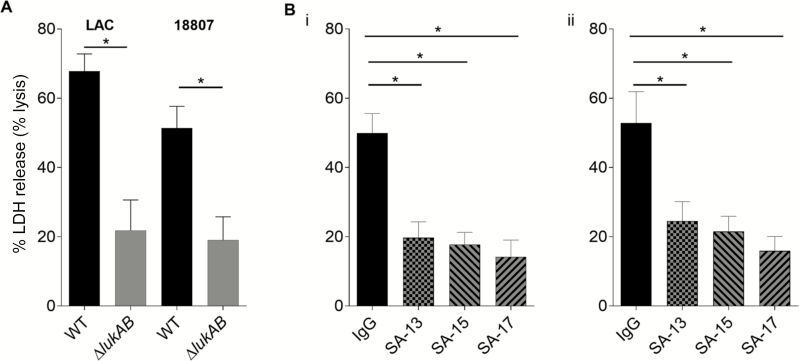
Next we sought to determine whether the mAbs could prevent cytotoxic effects mediated by S. aureus by using an ex vivo model of infection, where PMNs were infected with S. aureus strains of the USA300 lineage. For comparison, we also tested the cytotoxic effect of isogenic strains where LukAB was deleted. Each of the LukAB-reactive mAbs protected the PMNs against cytotoxic effects of WT S. aureus to a degree equivalent to strains in which lukAB was deleted (Figure 2). Similar to the findings from in vitro assays, the mAbs neutralized cytotoxicity with different potency, with SA-17 showing the highest degree of cellular protection.
Figure 2.
SA-13, SA-15, and SA-17 neutralize cytotoxicity caused by Staphylococcus aureus USA300 strains during ex vivo infection of primary human neutrophils. A, Primary human neutrophils (PMNs) from 5 donors were inoculated with wild-type (WT) USA300 S. aureus strains LAC and BK18807 and isogenic lukAB mutants (multiplicity of infection = 25) for 2 hours, and toxicity was measured in a LDH release assay (measurement of significant membrane damage/pore formation and cell lysis). Killing of PMNs in a LukAB-dependent manner is evident. B,Primary human neutrophils were inoculated with WT S. aureus strains (i: LAC; ii: BK18807) in the presence or absence of monoclonal antibodies, 2.5 μg/mL, for 2 hours, and cell death was evaluated by measuring LDH release. Bars represent mean ± SEM, with n = 5 donors. *P < .05 using 1-way analysis of variance with Tukey’s post hoc test correction for multiple comparisons. Abbreviations: IgG, immunoglobulin G; LDH, lactate dehydrogenase; WT, wild-type.
Diverse Patterns of Recognition of LukAB
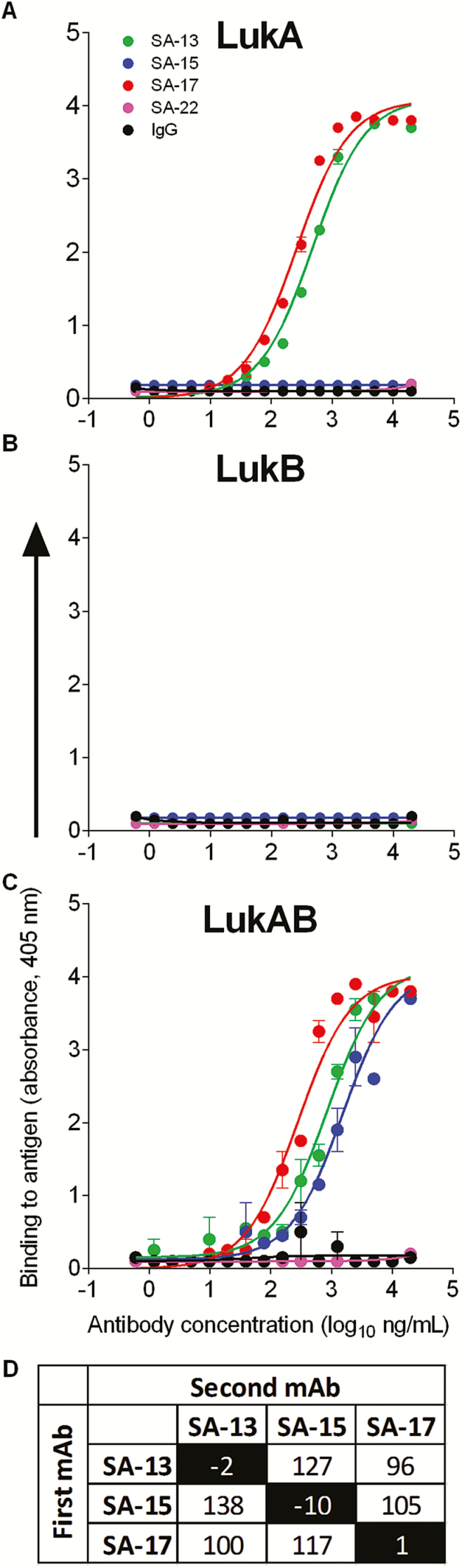
We next sought to determine the molecular basis for variation in potency seen among human mAbs targeting LukAB by defining the epitopes recognized by the mAbs. LukAB is composed of 2 separate monomers, LukA and LukB, but is isolated as a dimer in solution [8, 9]. To assess the region where each mAb binds, we tested the ability of each anti-LukAB mAb to bind to LukA or LukB monomers and to the heterodimeric toxin. As evident in initial screens, all mAbs bound to the LukAB dimer (Figure 3). Two of the mAbs, SA-13 and SA-17, also bound to monomeric LukA protein, but not to monomeric LukB (Figure 3A–C). These results suggest a binding site on the LukA monomer that remains available after toxin dimerization. In contrast, mAb SA-15 bound exclusively to the dimeric toxin, suggesting a binding site that does not form or become accessible until after toxin dimerization. These findings demonstrate that human B cells recognize sites on both the LukA monomer and the dimerized form of LukAB during natural infection. The diverse pattern of epitope recognition of these antibodies also was evident when tested using competition-binding studies with LukAB immobilized on biosensors (Figure 3D). The lack of competition for any combination of the mAbs demonstrates that the 3 mAbs described herein recognize distinct epitopes.
Figure 3.
Diverse patterns of recognition. The 3 anti-LukAB antibodies (SA-13, SA-15, and SA-17) and 2 irrelevant controls (SA-22 and immunoglobulin G) were tested for binding to immobilized LukA monomer (A), LukB monomer (B), or LukAB heterodimer (C). SA-13 and SA-17 bound both the LukA monomer and the LukAB heterodimer, while SA-15 bound only the heterodimer, suggesting that SA-15 binds to a conformational epitope present only after dimerization. None of the antibodies exhibited reduced binding in the presence of a competing antibody, suggesting unique epitopes for each of the 3 antibodies (D). Abbreviations: IgG, immunoglobulin G; mAb, monoclonal antibody.
Distinct Mechanisms of Action of LukAB Neutralization by Monoclonal Antibodies
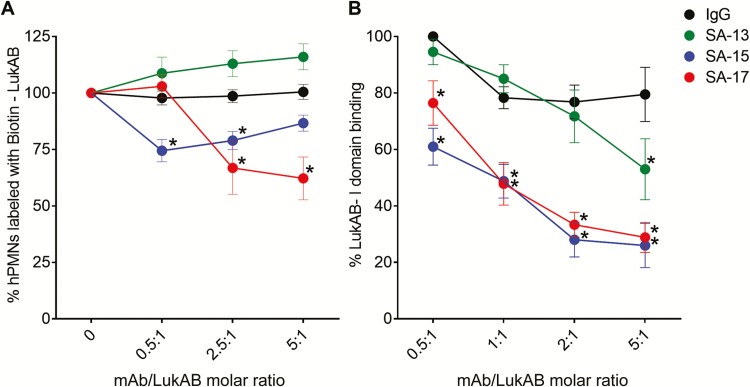
We next investigated the molecular mechanism by which the mAbs exerted their protective effects. First we tested whether the mAbs prevented association of the toxin with the surface of PMNs. Preincubation of LukAB with SA-17 caused a significant and titratable decrease in the amount of LukAB detected on the surface of PMNs. SA-15 also decreased LukAB binding at 2 concentrations tested. SA-13, however, did not interfere with cell-surface association of LukAB (Figure 4A). Using an ELISA-based assay, we demonstrated that SA-13, SA-15, and SA-17 all interfered with LukAB binding to the human I-domain of CD11b, the host receptor for LukAB [7], whereas the IgG control did not (Figure 4B). Consistent with the whole-cell binding assays, we noted varying capacities of inhibition, with SA-13 exhibiting the lowest interference of LukAB binding, despite exhibiting potent neutralization of LukAB in vitro and ex vivo.
Figure 4.
Monoclonal antibody (mAb)–mediated inhibition of LukAB binding. A, LukAB binding to hPMN surface. Dilutions of mAbs were preincubated with a fixed concentration of Biotin–LukAB (5 µg/mL) to give indicated molar ratios. LukAB–mAb mixture was added to hPMNs (n = 4 donors) on ice for 10 minutes before cell washing, staining, and FACS analysis. Inhibition of Biotin–LukAB binding to cell surface by SA-15 and SA-17 indicate that these antibodies are blocking the receptor binding site of the toxin. B, Monoclonal antibody–mediated inhibition of LukAB binding to CD11b I-domain. LukAB was added to wells coated with purified human CD11b-I domain in presence or absence of mAbs, and residual LukAB binding was determined. Mean ± SEM are plotted. For B, n = 4 independent experiments. *P < .05 using 2-way analysis of variance with Tukey’s post hoc test correction for multiple comparisons. Each mean was compared with immunoglobulin G control for statistical analysis. Abbreviations: FACS, fluorescence activated cell sorting; hPMNs, human polymorphonuclear neutrophils; IgG, immunoglobulin G; mAb, monoclonal antibody.
Cooperative Effects of LukAB Monoclonal Antibody Combinations for Enhanced Neutralization
Because the mAbs exhibited distinct patterns of recognition and mechanism of action, we hypothesized that neutralization capacity could be improved using a combination of antibodies. To test this hypothesis, we combined LukAB-specific or control mAbs to evaluate cooperative effects. When mixed at equal concentrations, totaling 3.2 µg/mL with a 2:1 mAb:LukAB molar ratio, we observed that antibody combinations cooperated to provide increased neutralization when compared with individual mAbs and the control (Figure 5). SA-13 and SA-17 exhibited the strongest increase in potency when combined, whereas SA-15 had a more dominant individual effect. Overall, these results suggest a cooperative effect of the mAbs in combination for neutralization of the cytotoxin, presumably due to differences in binding site and mechanisms of protection.
Figure 5.
LukAB monoclonal antibodies (mAbs) exhibit enhanced neutralization when combined. Monoclonal antibodies were preincubated with LukAB (2:1 mAb/LukAB molar ratio) for 30 minutes at room temperature. Primary human neutrophils were added to the LukAB/antibody mixture and incubated for 1 hour. Neutrophil viability was evaluated with CellTiter. Bars represent mean ± SEM, with n = 6 donors. *P < .05 using 1-way analysis of variance with Tukey’s post hoc test correction for multiple comparisons. Abbreviation: IgG, immunoglobulin G.
Efficacy of Anti-LukAB Monoclonal Antibodies In Vivo
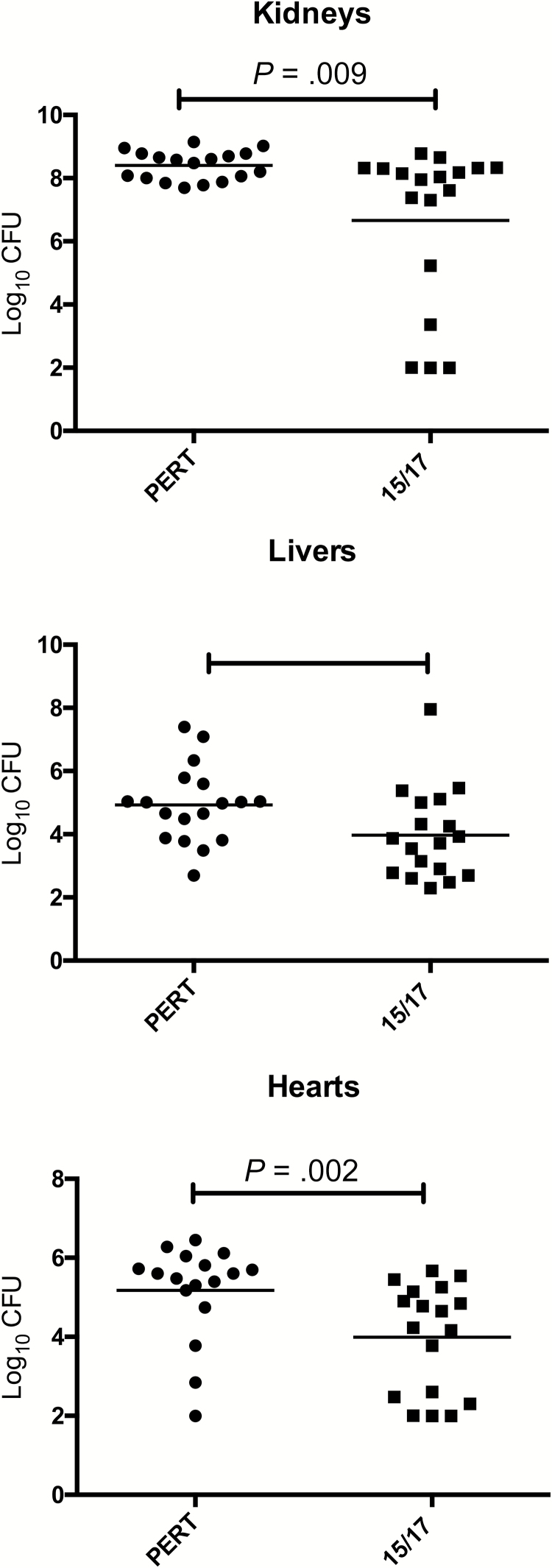
Although LukAB exhibits strong preferential tropism toward human leukocytes [7], the toxin does contribute to USA300 S. aureus bacterial burden in systemically infected mice [3, 6]. Given the breadth of protection of this series of mAbs in vitro and ex vivo, we investigated whether SA-15 and SA-17 protect in a murine model of S. aureus sepsis. A 1:1 mixture was selected due to their greater potency in intoxication experiments and was given as a single total dose of 1 mg/kg by the intraperitoneal route 20 hours before infection. This regimen resulted in significantly lower bacterial burden in murine heart, liver, and kidneys (Figure 6) compared with the same dose of a control IgG. Interestingly, treatment with individual mAbs did not exhibit a significant protective effect, suggesting that a combination effect was required for in vivo potency.
Figure 6.
In vivo protective effect of prophylactic treatment with monoclonal antibodies (mAbs). BALB/cJ mice were given a 1:1 mixture of SA-15 and SA-17 (1 mg/kg, intraperitoneally) prophylactically and subjected to disseminated bacterial infection with USA300 Staphylococcus aureus. Kidney (A), liver (B), and heart (C) tissues were harvested after 96 hours, and bacterial load was enumerated. A significant reduction in bacterial load was observed in all 3 tissues compared with animals treated with a control immunoglobulin G preparation.
DISCUSSION
In this study we report for the first time that human mAbs, isolated from the B cells of a child with invasive S. aureus infection, are capable of potently neutralizing cytotoxicity mediated by LukAB. Although all 3 mAbs neutralized the toxin, they exhibited differing levels of potency, recognized different antigenic sites, used distinct mechanisms of toxin inhibition, and displayed improved function when used in combination. In addition to neutralizing LukAB-mediated cytotoxicity in vitro and ex vivo, these human mAbs ameliorated disease severity in vivo. Thus the implementation of hybridoma technology allowed the characterization of antibodies that are produced naturally during invasive human S. aureus disease, highlighting the utility of this approach.
The progressive increase in the prevalence of antibiotic resistance within circulating strains of S. aureus is well documented [9, 20], and a clear need exists for novel preventive and therapeutic approaches to combat this pathogen. The vast majority of attempted staphylococcal vaccines have targeted capsular antigens and other surface components such as iron surface determinant B [21–23]. Staphylococcus aureus produces a wealth of anti-antibody surface components, such as Protein A and staphylokinase, that impede humoral host defenses. An ideal vaccine target would be one that is expressed by all clinically important strains (both methicillin-susceptible S. aureus and MRSA), is important for pathogenesis, and acts extracellularly to avoid interference by surface components such as SpA.
LukAB is increasingly recognized as a critical component of the S. aureus virulence repertoire devoted to evasion of human phagocytes, although its exact role during natural human infection remains to be fully elucidated. Previous studies have shown that S. aureus kills human phagocytes in a LukAB-dependent manner [2, 3, 24] and that disruption of lukAB markedly impairs the ability of S. aureus to avoid whole blood– and PMN-mediated killing [2–5, 7]. The prominent role of LukAB in S. aureus–mediated killing of PMNs, the primary mediator of antistaphylococcal host defense [25], is greatly influenced by the increased expression of LukAB upon PMN encounter [5]. Furthermore, the direct targeting of CD11b, a highly abundant protein on the surface of PMNs, potentiates the effect of LukAB during S. aureus infection [7].
Although all clinical isolates that we have characterized thus far harbor the gene encoding LukAB [10, 11] and this toxin is expressed in various in vitro models [3–5, 7, 26], these observations alone do not guarantee its relevance during human disease. We recently reported that anti-LukAB antibodies are produced in high titer following invasive S. aureus infection in children [10], providing strong evidence that the toxin is expressed during human disease and recognized by humoral host defenses. The human mAbs described here provide further evidence that human B cells recognize this toxin in both its monomeric and its dimerized form during invasive disease and generate a highly functional antibody response.
Recently, Badarau et al reported the identification of anti-LukAB antibodies by screening a large IgG library for binding to the toxin and concluded that potent toxin neutralization required a binding site present only after toxin dimerization [27]. By contrast, we found that potently neutralizing antibodies had distinct binding sites and that naturally occurring antibodies block LukAB by targeting either the dimeric toxin or monomeric LukA. Moreover, we observed that SA-15 and SA-17 significantly inhibited toxin association with the surface of neutrophils in vitro but that SA-13 (also potently neutralizing in all in vitro assays) did not have any effect on this process. This finding suggests that SA-13 interferes with a distinct, downstream step in cytolysis, such as toxin oligomerization or pore formation.
Our collective results suggest at least 3 patterns of action for anti-LukAB antibodies: (1) recognition of an epitope on the LukA monomer that blocks toxin attachment (SA-17), (2) recognition of an epitope on the LukA monomer that blocks a step in cytolysis after cell attachment (SA-13), and (3) recognition of a LukAB quaternary epitope present only on the heterodimer that interferes with receptor recognition and downstream steps in cytolysis (SA-15). The results also suggest that the functional antitoxin antibody response following invasive human infection is diverse and indicate that toxin neutralization can be achieved by interfering with >1 step in the cytolysis pathway. In support of the latter point, we observed distinct functional activity (and combination effect) among the mAbs, indicating that LukAB-mediated pore formation and cytotoxicity can be disrupted by the host response at multiple points. Similarly, differences in mAb potency by cell type (PMN vs macrophage-like cells) may be explained by differential mechanisms of protection (interference with receptor binding vs interference with oligomerization/pore formation)
Altogether, this study describes the identification of novel human antibodies that potently neutralize the cytotoxic potential of S. aureus toward human neutrophils and establishes an efficient workflow for the identification and purification of naturally occurring human anti-MRSA mAbs. Structure–function work, assessment of mAb function across allelic variants of LukAB, and further in vivo analyses are underway to investigate these mAbs as potential future therapeutic options to combat this major human pathogen.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. The authors thank Dr Eric Skaar for helpful discussions and Nicole Soper for laboratory support. The HMMA2.5 myeloma line was provided by Dr Marshall Posner and Dr Lisa Cavacini.
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Financial support. I. P. T. is supported by a grant 1K23AI113150from the National Institute of Allergy and Infectious Diseases (NIAID). J. E. C. is supported by NIAID grant 1K08AI113107 and by a Burroughs Wellcome Fund Career Award for Medical Scientists. D. B. A. J. was supported by NIAID Research Training Award T32AI007180 and is currently supported by NIAID grant F32AI122486. Work on LukAB in the Torres lab is supported by NIAID grant R01AI099394. V. J. T. is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases. Finally, this project was supported by CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences.
Potential conflicts of interest. J. E. C. is a member of the scientific advisory boards for PaxVax, CompuVax, GigaGen, Meissa Vaccines, and Rensavir, and has joint research or grants with Sanofi, Mapp Biopharmaceutical, Takeda Vaccines, Moderna, Avatar, Inovio, Profectus, and Arbutus Biopharma, and has served as consultant to Sanofi and Ridgeback Biotherapeutics. None of these activities pertain to the content of this manuscript. C. B. C. has joint research or grants with Pfizer and GSK and has served on scientific advisory boards for Theravance Pharmaceuticals and GSK Vaccines, none of which is within the scope of the submitted work. V. J. T. is listed as an inventor on patent applications filed by New York University School of Medicine, which are currently under commercial license to Janssen Biotech Inc. D. B. A. J. serves as a consultant to Pfizer. I. P. T. serves as an investigator on studies funded by GlaxoSmithKline and Horizon Pharma. None of these studies conflict with the contents of this manuscript. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wright AE. Notes on the treatment of furunculosis, sycosis, and acne by the inoculation of a Staphylococcus aureus vaccine. Lancet 1902; 159:874–884. [Google Scholar]
- 2. Ventura CL, Malachowa N, Hammer CH, et al. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One 2010; 5:e11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dumont AL, Nygaard TK, Watkins RL, et al. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol 2011; 79:814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melehani JH, James DBA, DuMont AL, Torres VJ, Duncan JA. Staphylococcus aureus Leukocidin A/B (LukAB) kills human monocytes via host NLRP3 and ASC when extracellular, but not intracellular. PLOS Pathog 2015; 11:e1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DuMont AL, Yoong P, Surewaard BG, et al. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect Immun 2013; 81:1830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scherr TD, Hanke ML, Huang O, et al. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. MBio 2015; 6. pii: e01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DuMont AL, Yoong P, Day CJ, et al. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A 2013; 110:10794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DuMont AL, Yoong P, Liu X, et al. Identification of a crucial residue required for Staphylococcus aureus LukAB cytotoxicity and receptor recognition. Infect Immun 2014; 82:1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Badarau A, Rouha H, Malafa S, et al. Structure-function analysis of heterodimer formation, oligomerization, and receptor binding of the Staphylococcus aureus bi-component toxin LukGH. J Biol Chem 2015; 290:142–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomsen IP, Dumont AL, James DB, et al. Children with invasive Staphylococcus aureus disease exhibit a potently neutralizing antibody response to the cytotoxin LukAB. Infect Immun 2014; 82:1234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chadha AD, Thomsen IP, Jimenez-Truque N, et al. Host response to Staphylococcus aureus cytotoxins in children with cystic fibrosis. J Cyst Fibros 2016; 15:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu X, McGraw PA, House FS, Crowe JE., Jr An optimized electrofusion-based protocol for generating virus-specific human monoclonal antibodies. J Immunol Methods 2008; 336:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flyak AI, Ilinykh PA, Murin CD, et al. Mechanism of human antibody-mediated neutralization of Marburg virus. Cell 2015; 160:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reyes-robles T, Lubkin A, Iii FA, Lacy DB, Torres VJ. Exploiting dominant-negative toxins to combat Staphylococcus aureus pathogenesis. EMBO Rep 2016; 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 2006; 367:731–9. [DOI] [PubMed] [Google Scholar]
- 16. Ueda T, Rieu P, Brayer J, Arnaout MA. Identification of the complement iC3b binding site in the beta 2 integrin CR3 (CD11b/CD18). Proc Natl Acad Sci U S A 1994; 91:10680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boles BR, Thoendel M, Roth AJ, Horswill AR. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One 2010; 5:e10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hammer ND, Reniere ML, Cassat JE, et al. Two heme-dependent terminal oxidases power Staphylococcus aureus organ-specific colonization of the vertebrate host. MBio 2013; 4 pii: e00241–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kehl-Fie TE, Zhang Y, Moore JL, et al. MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect Immun 2013; 81:3395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakoulas G, Moellering RC., Jr Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis 2008; 46:S360–7. [DOI] [PubMed] [Google Scholar]
- 21. Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis 2012; 54:560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Proctor RA. Is there a future for a Staphylococcus aureus vaccine? Vaccine 2012; 30:2921–7. [DOI] [PubMed] [Google Scholar]
- 23. Thomsen I, Dudney H, Creech CB. Searching for the holy grail of a staphylococcal vaccine. Hum Vaccin 2010; 6:1068–70. [DOI] [PubMed] [Google Scholar]
- 24. Yanai M, Rocha MA, Matolek AZ, et al. Separately or combined, LukG/LukH is functionally unique compared to other staphylococcal bicomponent leukotoxins. PLoS One 2014; 9:e89308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 2012; 34:237–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balasubramanian D, Ohneck EA, Chapman J, et al. Staphylococcus aureus coordinates leukocidin expression and pathogenesis by sensing metabolic fluxes via RpiRc. MBio 2016; 7:1130–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Badarau A, Rouha H, Malafa S, et al. Context matters: the importance of dimerization-induced conformation of the LukGH leukocidin of Staphylococcus aureus for the generation of neutralizing antibodies. MAbs 2016; 142:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.