Abstract
Background:
There is growing concern about the use of opioids prior to total knee arthroplasty (TKA), and research has suggested that preoperative opioid use may lead to worse pain outcomes following surgery. We evaluated the pain relief achieved by TKA in patients who had and those who had not used opioids use before the procedure.
Methods:
We augmented data from a prospective cohort study of TKA outcomes with opioid-use data abstracted from medical records. We collected patient-reported outcomes and demographic data before and 6 months after TKA. We used the Pain Catastrophizing Scale and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) to quantify the pain experiences of patients treated with TKA who had had a baseline score of ≥20 on the WOMAC pain scale (a 0 to 100-point scale, with 100 being the worst score), who provided follow-up data, and who had not had another surgical procedure within the 2 years prior to TKA. We built a propensity score for preoperative opioid use based on the Pain Catastrophizing Scale score, comorbidities, and baseline pain. We used a general linear model, adjusting for the propensity score and baseline pain, to compare the change in the WOMAC pain score 6 months after TKA between persons who had and those who had not used opioids before TKA.
Results:
The cohort included 156 patients with a mean age of 65.7 years (standard deviation [SD] = 8.2 years) and a mean body mass index (BMI) of 31.1 kg/m2 (SD = 6.1 kg/m2); 62.2% were female. Preoperatively, 36 patients (23%) had had at least 1 opioid prescription. The mean baseline WOMAC pain score was 43.0 points (SD = 12.8) for the group that had not used opioids before TKA and 46.9 points (SD = 15.7) for those who had used opioids (p = 0.12). The mean preoperative Pain Catastrophizing Scale score was greater among opioid users (15.5 compared with 10.7 points among non-users, p = 0.006). Adjusted analyses showed that the opioid group had a mean 6-month reduction in the WOMAC pain score of 27.0 points (95% confidence interval [CI] = 22.7 to 31.3) compared with 33.6 points (95% CI = 31.4 to 35.9) in the non-opioid group (p = 0.008).
Conclusions:
Patients who used opioids prior to TKA obtained less pain relief from the operation. Clinicians should consider limiting pre-TKA opioid prescriptions to optimize the benefits of TKA.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
Knee osteoarthritis is associated with substantial activity limitation and chronic pain. While total knee arthroplasty (TKA) is an effective treatment to relieve pain and restore function, patients spend an average of 13 years undergoing nonoperative therapies such as nonsteroidal anti-inflammatory drugs, physical therapy, and intra-articular injections before ultimately undergoing TKA; this highlights the need for effective analgesics1. Although treatment guidelines regarding the role of opioids for management of pain caused by knee osteoarthritis prior to TKA have been inconsistent2-4, in the U.S. over $1.5 billion is spent annually on prescription opioids for people with knee osteoarthritis5. Furthermore, opioid utilization has increased drastically, with nearly 40% of Medicare beneficiaries with knee osteoarthritis receiving at least 1 opioid prescription in 20096.
Increased utilization of opioids has been accompanied by concerns about their adverse effects on surgical outcomes when they are taken prior to total joint arthroplasty (TJA). High doses of opioids have been associated with the development of opioid dependence and hyperalgesia, which could contribute to intractable pain following TJA7. Prior studies have shown that patients who use opioids preoperatively are more likely to continue to use them after TJA7-10. The growing concern over opioid use has led to multiple studies comparing clinical outcomes between patients treated with opioids and those treated without opioids prior to TJA. Studies have shown an association between preoperative opioid use and increased pain as well as worse functional outcomes within the first week following TJA8,9,11. Preoperative opioid use has also been associated with impaired long-term clinical outcomes of various types of orthopaedic surgery, particularly spinal procedures12-15.
Although recent studies have suggested that patients who use preoperative opioids have worse outcomes of TKA9,16,17, they were based on limited sample sizes and the authors did not consider pain catastrophizing or use appropriate analytic methods to address confounding by indications that could contribute to poorer pain and functional outcomes. To address these gaps, we used restricted propensity score methodology to adjust for confounding by indication in our study comparing the reduction of pain after TKA between patients who had used opioids preoperative and those without prior opioid treatment.
Materials and Methods
Sample
This study was conducted at Brigham and Women’s Hospital, a tertiary-care academic medical center in Boston, Massachusetts. We used data from the Adding Value in Knee Arthroplasty (AViKA) Postoperative Care Navigation Trial, a randomized controlled trial evaluating motivational interviewing to enhance TKA outcomes18,19. The study included patients who had undergone primary unilateral TKA for osteoarthritis when they were at least 40 years old. Each subject had completed, within 6 weeks before and again 6 months after TKA, a questionnaire consisting of validated measures to assess clinical outcomes, including the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)20, 5-question Mental Health Inventory (MHI-5), Pain Catastrophizing Scale, and questions about comorbidities. The Pain Catastrophizing Scale quantifies the degree to which an individual has exaggerated negative thoughts as a response to pain on a scale of 0 to 52, with higher scores indicating worse catastrophizing21.
Medical Record Review
To obtain detailed information on opioid use, we reviewed the electronic medical record of each enrolled AViKA Trial subject to determine opioid utilization from 2 years before to 1 year after TKA. Multiple sources of data were reviewed for each patient, including clinical visit notes, anesthesiology reports, discharge notes, prescription history, and medication list. We recorded information for 6 opioids: oxycodone, hydrocodone, hydromorphone, morphine, tramadol, and codeine. Other opioids were rarely found and were recorded as “other.” For each mention of an opioid prescription in the medical record, we abstracted whether the opioid was prescribed before TKA, after TKA, or both. We further documented any additional surgical procedures in the period from 2 years prior to TKA to 1 year after TKA and the type of medical insurance.
Literature Review
We conducted a systematic literature search to identify published studies on the effects of preoperative opioids on postoperative orthopaedic outcomes for comparison with our study. Our primary source for identifying publications was PubMed, which we searched with the following queries: orthopaedic outcomes and opioids, surgical outcomes and opioids, and arthroplasty and opioids. Additionally, we searched through the references of the identified papers to identify any publications missed by the queries. We then read the titles and abstracts of the 1,700 results to include papers relevant to orthopaedics and preoperative opioid use. We chose 26 studies and then excluded those in which validated outcome measure questionnaires had not been used. This left 5 publications with which to compare our own study. The questionnaires used in these studies were the Knee Society objective score16, Harris hip score10, WOMAC17, American Shoulder and Elbow Surgeons (ASES) score15, and Oswestry Disability Index (ODI)22. All preoperative and postoperative scores were transformed into a 0 to 100-point scale with 100 being the worst. We calculated a relative reduction in pain benefits due to opioids as the relative difference between the opioid users and non-users in the change in pain from before TKA to after TKA.
Statistical Analysis
We compared demographic and clinical characteristics between subjects with and those without pre-TKA opioid use. We used the chi-square test to compare across categorical factors (sex, type of insurance, and education level), the t test to compare across continuous variables with normal distribution (age, baseline WOMAC pain score, and baseline WOMAC function score), and the Wilcoxon rank sum test to compare across continuous variables with skewed distribution (body mass index [BMI], number of comorbidities, and baseline Pain Catastrophizing Scale score).
To adjust for potential confounding by the indication for opioid use, we built a propensity score for opioid use, using logistic regression with pre-TKA opioid use as an outcome. The independent variables were the Pain Catastrophizing Scale score, Charlson Comorbidity Index (grouped as 0, 1 to 3, and >3), and baseline WOMAC pain score. The model had a c-statistic of 0.682. We identified a common use area as a set of propensity scores that were common between subjects who did and those who did not use opioids prior to TKA (0.12 to 0.50) and excluded subjects with propensity scores greater or less than this common range from our analytical sample.
We then built a general linear model with the change in the WOMAC pain score over the 6 months following the TKA as the outcome variable and with the propensity for opioid use described above, use of opioids before TKA, and baseline WOMAC pain score as the independent variables.
We conducted a sensitivity analysis using marginal structural models, with inverse probability weighting according to the likelihood of opioid use prior to TKA. All analyses were conducted with SAS 9.4 software, and p < 0.05 indicated significant results.
Results
Sample Characteristics
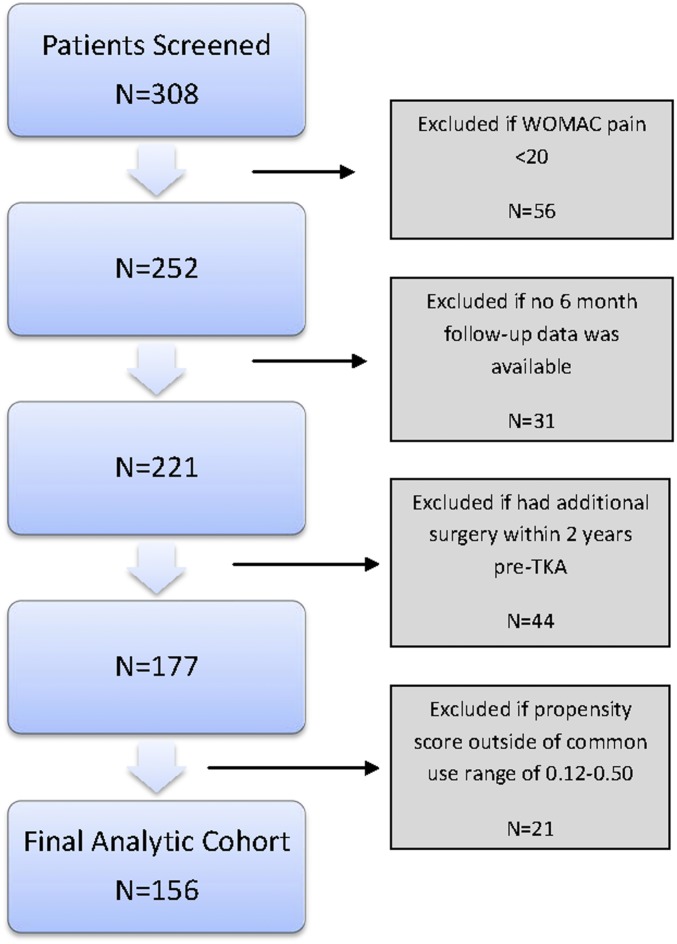
From August 2011 to November 2013, 308 patients were enrolled in the AViKA study and 252 of them had a pre-TKA WOMAC pain score of ≥20. Of those subjects, 221 (88%) provided 6-month data. We excluded 44 subjects because they had had surgery within 2 years prior to TKA, to minimize misattribution of opioid use to another surgical procedure. Of the remaining 177 subjects, 156 were included in the analysis based on the common use area of the propensity score values (0.12 to 0.50) (Fig. 1).
Fig. 1.
Selection process for analytic cohort.
The mean age at the time of TKA was 65.7 years (standard deviation [SD] = 8.2 years), 62.2% of the patients were female, and the mean BMI was 31.1 kg/m2 (SD = 6.1 kg/m2). The majority (60.4%) of the patients had graduated from college, 53% of the cohort had at least 1 comorbid condition, and 32.7% had ≥2 comorbidities. The baseline Pain Catastrophizing Scale score was 11.8 (SD = 8.6) (Table I).
TABLE I.
Cohort Characteristics
| Pre-TKA Opioid Use |
||||
| Overall (N = 156) | Yes (N = 36) | No (N = 120) | P Value | |
| Age* (yr) | 65.7 ± 8.21 | 67.5 ± 8.2 | 65.2 ± 8.2 | 0.13 |
| Female† | 97 (62.2%) | 23 (23.7%) | 74 (76.3%) | 0.81 |
| BMI* (kg/m2) | 31.1 ± 6.06 | 31.0 | 31.1 | 0.84 |
| Graduated from college†‡ | 0.40 | |||
| No | 61 (39.6%) | 16 (26.2%) | 45 (73.8%) | |
| Yes | 93 (60.4%) | 19 (20.4%) | 74 (79.6%) | |
| Comorbidities* | 0.81 ± 0.98 | 0.81 | 0.81 | 0.90 |
| Baseline WOMAC score*§ | ||||
| Pain | 43.9 ± 13.55 | 46.9 ± 15.7 | 43.0 ± 12.8 | 0.12 |
| Function | 43.7 ± 14.16 | 49.0 ± 14.1 | 42.1 ± 13.8 | 0.009 |
| Pain Catastrophizing Scale score* | 11.8 ± 8.55 | 15.5 ± 10.3 | 10.7 ± 7.7 | 0.006 |
| Type of insurance† | 0.91 | |||
| Private | 129 (82.7%) | 30 (23.3%) | 99 (76.7%) | |
| Medicaid/Medicare/other | 27 (17.3%) | 6 (22.2%) | 21 (77.8%) | |
The values are given as the mean and standard deviation.
The values are given as the number of patients with the percentage in parentheses. The percentages in the “Yes” and “No” groups are based on the numbers in the “Overall” group.
Data missing for one patient in each group.
The WOMAC score was transformed to a 0 to 100-point scale (100 indicating the worst score).
Opioid Utilization
Thirty-six (23%) of the 156 patients in the cohort had had at least 1 opioid prescription within the 2 years prior to TKA. The most common opioids used preoperatively in this sample were oxycodone, hydrocodone, and tramadol. Fourteen (9.0%) of the patients used >1 type of opioid. The proportions of subjects using each preoperative opioid identified within the medical records are shown in Table II.
TABLE II.
Opioid Utilization
| No. (%) of Patients |
||
| Opioid Use | Preoperative | Postoperative |
| Codeine | 0 (0%) | 0 (0%) |
| Hydrocodone | 14 (9.0%) | 52 (33.3%) |
| Hydromorphone | 1 (0.6%) | 20 (12.8%) |
| Morphine | 0 (0%) | 3 (1.9%) |
| Oxycodone | 14 (9.0%) | 150 (96.2%) |
| Tramadol | 20 (12.8%) | 30 (19.2%) |
| Other | 0 (0%) | 0 (0%) |
| Multiple opioid prescriptions | 14 (9.0%) | 146 (93.6%) |
Postoperatively, 150 (96.2%) of the patients had at least 1 opioid prescription listed in their medical record, with 146 (93.6%) having prescriptions for multiple opioids. Oxycodone was the most frequently prescribed opioid (150; 96.2%), followed by hydrocodone (52; 33.3%) (Table II).
Demographic characteristics (age, sex, and education level) were similar between patients with a documented history of pre-TKA opioid utilization and those who had not used opioids preoperatively. The unadjusted mean baseline WOMAC pain score was worse for those who had used opioids before TKA (46.9 points compared with 43.0 points for those who had not), but the difference was not significant (p = 0.12), and those who had used opioids before TKA had significantly worse functional limitations (49.0 compared with 42.1 points, p = 0.009) (Table I).
Multivariate analysis showed that, after adjusting for baseline WOMAC pain and comorbidity scores, pain catastrophizing was the only factor independently associated with pre-TKA opioid use. Those who had used opioids before TKA had mean a Pain Catastrophizing Scale score of 15.5 points compared with 10.7 points for those who had not (p = 0.006). Every unit increase in the Pain Catastrophizing Scale score was associated with a 4.7% increase in the adjusted odds of using opioids prior to TKA. After we adjusted for the propensity score, the baseline WOMAC score became more balanced between those who had used and those who had not used opioids before TKA (Table III).
TABLE III.
Adjusted Baseline and 6-Month WOMAC Pain Scores, and Change Over 6 Months, Stratified by Pre-TKA Opioid Use*
| Pre-TKA Opioid Use |
||||||
| Yes |
No |
Difference Between Groups |
||||
| Time Point | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI |
| Baseline | 44.6 | 40.3-48.9 | 43.7 | 41.4-46.0 | 1.0 | −4.0-5.9 |
| 6 months | 17.1 | 12.8-21.4 | 10.5 | 8.3-12.8 | 6.6 | 1.7-11.5 |
| Change | 27.0 | 22.7-31.3 | 33.6 | 31.4-35.9 | 6.6 | 1.7-11.5 |
Model adjusted for propensity score.
Relationship Between Pre-TKA Opioid Use and Outcomes
After adjustment for the propensity score and baseline WOMAC pain score, patients who had not used opioids before TKA had a mean 6-month WOMAC pain score of 10.5 points (95% confidence interval [CI] = 8.3 to 12.8) compared with 17.1 points (95% CI = 12.8 to 21.4) for those who had used opioids prior to TKA (Table III). Multivariate analyses adjusted for the propensity score and baseline pain score showed that the opioid group had a mean 6-month WOMAC pain-score reduction of 27.0 points (95% CI = 22.7 to 31.3) compared with 33.6 points (95% CI = 31.4 to 35.9) for the non-opioid-use group; the difference between the groups was 6.6 points (p = 0.008) (Table III).
Sensitivity analyses using an inverse probability of opioid use weighting method showed similar results, with the reduction in the WOMAC pain score being 5.7 points less for the opioid users than for those who had not used opioids prior to TKA (p = 0.0430). Adding pre-TKA functional limitations to the model did not qualitatively change the results.
Comparison with Other Published Studies
Table IV summarizes the findings of published studies comparing outcomes of common orthopaedic procedures between opioid users and non-users. After analyzing Knee Society scores 2 to 7 years following TKA, Zywiel et al. reported a 14.1% reduction in pain relief (p < 0.001) between the opioid users and non-users16. Pivec et al. reported that, at 58 months after total hip arthroplasty (THA), opioid users had a 7.7% reduction in pain relief, as shown by Harris hip scores (p = 0.002), compared with non-users10. Nguyen et al. reported that preoperative opioid use resulted in a significant (p < 0.01) reduction of 21.4% in pain relief at 6 to 12 months following TKA or THA17. In a study with a 2 to 9-year follow-up, Morris et al. reported a significant, 14.5% reduction in pain relief, according to the ASES score, for those who had used opioids prior to reverse shoulder arthroplasty (p = 0.005)15. Notably, Radcliff et al. did not report a significant difference in the ODI at 4 years after lumbar discectomy between those who had and those who had not used opioids prior to surgery22 (Table IV).
TABLE IV.
Comparison Between Current Study and Other Published Evidence on Reduction in Pain Benefits in Opioid Users Compared with Non-Users
| Study | Joint | Time Frame | Scale | Reduction in Benefits in Opioid Users (%) | P Value |
| Radcliff et al.22 | Spine | 4 yr | ODI | 0.8 | 0.790 |
| Morris et al.15 | Shoulder | 2-9 yr | ASES score | 14.5 | 0.005 |
| Zywiel et al.16 | Knee | 2-7 yr | Knee Society objective score | 14.1 | <0.001 |
| Pivec et al.10 | Hip | 58 mo | Harris hip score | 7.7 | 0.002 |
| Nguyen et al.17 | Hip and knee | 6-12 mo | WOMAC | 21.4 | <0.01 |
| Present study | Knee | 6 mo | WOMAC | 8.9 | <0.001 |
Discussion
The use of opioids for chronic non-cancer-related pain has increased drastically within the past decade23. This trend has been observed for knee osteoarthritis, with nearly half of Medicare beneficiaries with this condition receiving at least 1 opioid prescription in 20096. As the majority of eligible patients with knee osteoarthritis eventually undergo TKA1, understanding the postoperative effect of pre-TKA opioid use is imperative to the creation of appropriate treatment guidelines. Using data collected from medical records, we found that patients who had used opioids prior to TKA experienced less pain relief 6 months postoperatively than patients who had not used opioids prior to TKA.
The results that we present support and expand on previously published findings regarding the influence of pre-TKA opioid use (Table IV). Zywiel and colleagues reported that patients using opioids prior to TKA had significantly higher rates of revision for residual pain or stiffness (8 of 49 knees) than those who had not used opioids (0 revisions)16. Furthermore, their opioid-treated patients had significantly lower Knee Society scores at the time of final follow-up, suggesting worse clinical outcomes, than their non-opioid-using counterparts. Pivec et al. reported similar results regarding the effect of preoperative opioid use on the clinical outcomes of THA, with their patients who used narcotics prior to surgery having worse Harris hip scores than those with no history of opioid use10. Nguyen et al. included an intervention group in their study on the effects of preoperative opioid use on TJA outcomes. They showed that, while the opioid-dependent group had significantly worse follow-up WOMAC scores than patients with no preoperative opioid use, those who reduced their opioid consumption by at least 50% prior to surgery did not have different outcomes compared with those without a history of opioid use17. The analyses in these papers, however, were often limited by small sample sizes, lack of adjustment for additional comorbidities, and a lack of randomization into the treatment groups.
Our current study, which showed results consistent with those in the prior literature and expanded the evidence that pre-TKA opioid use is associated with less pain relief following the surgery, had several methodologic benefits. We adjusted for baseline WOMAC pain scores and comorbidities as well as for the propensity score for using opioids before TKA to provide a more comprehensive adjustment for potential confounding by indications and to isolate the independent effect of pre-TKA opioid use on pain relief. Overall, the 6-month change in the WOMAC pain score was associated with the baseline pain score and opioid use. Preoperatively, there was a small but not significant difference in the WOMAC pain scores between the groups, whereas the pain scores at 6 months were significantly worse for those who had used opioids preoperatively (p = 0.008). This difference indicates that preoperative opioid use may have affected the pain reduction achieved with the TKA.
Our data showed that pre-TKA opioid use was associated with pain catastrophizing but not with baseline pain. Furthermore, pre-TKA opioid use, but not pain catastrophizing, was independently associated with worse post-TKA pain. Our study suggests that pain catastrophizing may play an important role in decisions by physicians and patients to use opioids, which then places them at risk for poorer outcomes.
Of the drugs that we considered to be opioids, tramadol was the most frequently used prior to TKA. Tramadol is a centrally acting analgesic unique for its multiple mechanisms of pain relief, given its behavior as an opiate agonist and as a reuptake inhibitor of both norepinephrine and serotonin. We chose to classify tramadol as an opioid because it is structurally analogous to other opioids.
There are important limitations to our analyses. As evidence of opioid utilization was obtained from the medical record and patients were not interviewed for verification, we are unable to definitively state that opioid prescription resulted in utilization of the entire prescribed dosage. It is possible that patients received a prescription and failed to obtain the analgesic or took less than the dispensed amount. This could lead to an underestimation or overestimation of the effects of opioids on pain relief provided by TKA. Conversely, as preoperative opioid prescriptions were identified from a single medical record, it is possible that patients received additional opioid prescriptions from outside sources. Additionally, without verification interviews we were unable to fully characterize postoperative opioid use, which could have affected pain reporting during follow-up visits. Our cohort consisted of patients enrolled in the AViKA study, a randomized controlled trial of care navigation following TKA, which could have contributed to selection bias. We followed patients for only 6 months following TKA. It is possible that those who used opioids before TKA could have eventually, over a longer period of time, obtained the same degree of pain relief obtained by the patients who had not used opioids preoperatively. Since we did not have an objective way to assess individual pain tolerance in general, we could not rule out the possibility that differences in the reported severity of the pain were affected by differences in pain tolerance. We also did not have data on pain in other joints or the spine prior to TKA, which may have had an impact on post-TKA pain. Finally, patients were enrolled from a single, tertiary, academic medical center, potentially limiting the generalizability of our results.
In conclusion, the data that we presented support prior reports of worse TKA clinical outcomes in persons who used opioids preoperatively16. Specifically, our data showed that preoperative opioid use may contribute to decreased pain relief in the early postoperative period.
As individuals with osteoarthritis spend an average of 13 years between trials of nonoperative therapies and elective TKA1, our results should be viewed as a warning that opioid use may be problematic during this period due to their negative effects on subsequent TKA outcomes. Furthermore, considering that the U.S. spends over $1.5 billion5 annually on prescription opioids for patients with knee osteoarthritis, and nearly $30 billion24 on illicit use, reducing use of opioids may decrease their deleterious effects. Clinicians and policy makers may consider limiting the use of opioids prior to TKA to optimize post-TKA pain relief.
Footnotes
Investigation performed at the Orthopaedic and Arthritis Center for Outcomes Research (OrACORe), Brigham and Women’s Hospital, Boston, Massachusetts
Disclosure: This study was funded by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS) Grant K24AR057827 and Partners HealthCare. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/D274).
References
- 1.Losina E, Paltiel AD, Weinstein AM, Yelin E, Hunter DJ, Chen SP, Klara K, Suter LG, Solomon DH, Burbine SA, Walensky RP, Katz JN. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken). 2015. February;67(2):203-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P; American College of Rheumatology . American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012. April;64(4):465-74. [DOI] [PubMed] [Google Scholar]
- 3.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, Kwoh K, Lohmander S, Rannou F, Roos EM, Underwood M. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014. March;22(3):363-88. Epub 2014 Jan 24. [DOI] [PubMed] [Google Scholar]
- 4.Jevsevar DS, Brown GA, Jones DL, Matzkin EG, Manner PA, Mooar P, Schousboe JT, Stovitz S, Sanders JO, Bozic KJ, Goldberg MJ, Martin WR 3rd, Cummins DS, Donnelly P, Woznica A, Gross L; American Academy of Orthopaedic Surgeons . The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition. J Bone Joint Surg Am. 2013. October 16;95(20):1885-6. [DOI] [PubMed] [Google Scholar]
- 5.Smith SR, Katz JN, Collins JE, Solomon DH, Jordan JM, Suter LG, Yelin EH, David Paltiel A, Losina E. Cost-effectiveness of tramadol and oxycodone in the treatment of knee osteoarthritis. Arthritis Care Res (Hoboken). 2016. April 25;(Apr):25 Epub 2016 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003-2009 in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2014. October;66(10):1489-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goesling J, Moser SE, Zaidi B, Hassett AL, Hilliard P, Hallstrom B, Clauw DJ, Brummett CM. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016. June;157(6):1259-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sing DC, Barry JJ, Cheah JW, Vail TP, Hansen EN. Long-acting opioid use independently predicts perioperative complication in total joint arthroplasty. J Arthroplasty. 2016;31(9 Suppl):170-4.e1. [DOI] [PubMed] [Google Scholar]
- 9.Patanwala AE, Jarzyna DL, Miller MD, Erstad BL. Comparison of opioid requirements and analgesic response in opioid-tolerant versus opioid-naïve patients after total knee arthroplasty. Pharmacotherapy. 2008. December;28(12):1453-60. [DOI] [PubMed] [Google Scholar]
- 10.Pivec R, Issa K, Naziri Q, Kapadia BH, Bonutti PM, Mont MA. Opioid use prior to total hip arthroplasty leads to worse clinical outcomes. Int Orthop. 2014. June;38(6):1159-65. Epub 2014 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aasvang EK, Lunn TH, Hansen TB, Kristensen PW, Solgaard S, Kehlet H. Chronic pre-operative opioid use and acute pain after fast-track total knee arthroplasty. Acta Anaesthesiol Scand. 2016. April;60(4):529-36. Epub 2015 Dec 28. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence JT, London N, Bohlman HH, Chin KR. Preoperative narcotic use as a predictor of clinical outcome: results following anterior cervical arthrodesis. Spine (Phila Pa 1976). 2008. September 01;33(19):2074-8. [DOI] [PubMed] [Google Scholar]
- 13.Faour M, Anderson JT, Haas AR, Percy R, Woods ST, Ahn UM, Ahn NU. Prolonged preoperative opioid therapy associated with poor return to work rates after single-level cervical fusion for radiculopathy for patients receiving Workers’ Compensation benefits. Spine (Phila Pa 1976). 2016. July 08;(Jul):8 Epub 2016 Jul 8. [DOI] [PubMed] [Google Scholar]
- 14.Lee D, Armaghani S, Archer KR, Bible J, Shau D, Kay H, Zhang C, McGirt MJ, Devin C. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am. 2014. June 04;96(11):e89 Epub 2014 Jun 4. [DOI] [PubMed] [Google Scholar]
- 15.Morris BJ, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Preoperative opioid use and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015. January;24(1):11-6. Epub 2014 Jul 16. [DOI] [PubMed] [Google Scholar]
- 16.Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011. November 02;93(21):1988-93. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen LC, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016. September;31(9)(Suppl):282-7. Epub 2016 Mar 17. [DOI] [PubMed] [Google Scholar]
- 18.Losina E, Collins JE, Daigle ME, Donnell-Fink LA, Prokopetz JJ, Strnad D, Lerner V, Rome BN, Ghazinouri R, Skoniecki DJ, Katz JN, Wright J. The AViKA (Adding Value in Knee Arthroplasty) postoperative care navigation trial: rationale and design features. BMC Musculoskelet Disord. 2013. October 12;14:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Losina E, Collins JE, Wright J, Daigle ME, Donnell-Fink LA, Strnad D, Usiskin IM, Yang HY, Lerner V, Katz JN. Postoperative care navigation for total knee arthroplasty patients: a randomized controlled trial. Arthritis Care Res (Hoboken). 2016. September;68(9):1252-9. Epub 2016 Jul 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988. December;15(12):1833-40. [PubMed] [Google Scholar]
- 21.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-32. [Google Scholar]
- 22.Radcliff K, Freedman M, Hilibrand A, Isaac R, Lurie JD, Zhao W, Vaccaro A, Albert T, Weinstein JN. Does opioid pain medication use affect the outcome of patients with lumbar disc herniation? Spine (Phila Pa 1976). 2013. June 15;38(14):E849-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009. February;10(2):147-59. [DOI] [PubMed] [Google Scholar]
- 24.Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011. April;12(4):657-67. Epub 2011 Mar 10. [DOI] [PubMed] [Google Scholar]