Abstract
Background:
Tuberculosis (TB) is one of the world’s deadliest diseases, and one-third of the world’s population is infected with it. The link between antitumor necrosis factor therapy and reactivation of latent TB is well recognized. However, only limited studies have evaluated the risk of TB with rituximab, a B-cell-targeting therapeutic agent used recently for rheumatological diseases, primarily rheumatoid arthritis. Moreover, no studies have assessed this risk in TB endemic regions with a high incidence and prevalence of TB (e.g., Saudi Arabia).
Objective:
To examine the risk of acquiring TB or activating latent TB in adult patients with rheumatological disease who received rituximab therapy.
Methodology:
Retrospective cohort study included 60 patients at King Faisal Specialist Hospital and Research Centre, Saudi Arabia, between October 1, 2010, and March 31, 2011.
Result:
Six patients (10%) were subsequently excluded because of the treatment for latent TB (5 patients) or prior treatment for TB (1 patient). The follow-up period was 6 months for 53 patients (98.15%) and 3 months for 1 patient (1.85%). During follow-up, none of the patients received the purified protein derivative skin test while radiological studies were performed for 30 patients (55.55%). 53 patients (98.15%) had no symptoms suggestive of TB upon follow-up, and no patient experienced a TB flare-up.
Conclusion:
Rituximab can be considered a first line of therapy for the management of rheumatological diseases in the presence of the risk of TB reactivation, especially in endemic areas with a high prevalence and incidence of TB.
Keywords: Rheumatoid arthritis, rituximab, systemic lupus erythematosus, tuberculosis
Introduction
Tuberculosis (TB) is one of the most deadly diseases worldwide, and one-third of the world’s population is infected with it. In 2014, nearly 9.6 people became sick with TB, and about 1.5 million TB-related deaths occurred worldwide.1 In 2014, Saudi Arabia had a population of 30,770,375.2 The total number of cases of TB was 3248 according to a report from the World Health Organization in 2014. Moreover, the incidence of TB was 12/100,000, and the prevalence was 16/100,000 of the Saudi population.3
Rituximab is a chimeric monoclonal antibody (human constant regions and mouse variable regions) that recognizes human CD20, a cell surface glycoprotein expressed on B-cells from early in development in the bone marrow until terminal differentiation into plasma cells. After a single course of rituximab, the peripheral blood routinely remains depleted of B-cells for 6-12 months. In addition, depletion of B-cells occurs in the tissue but may not be as dramatic. Moreover, rituximab does not eliminate long-lived plasma cells, the major source of protective antibodies.4 Rituximab was the first B-cell-targeting therapeutic agent approved for the use in humans4 and was first approved for the treatment of lymphoma based on studies in oncology and hematology and has been more recently approved for the use in rheumatology.4,5 In particular, a 2-year, multicenter, randomized, double-blind, placebo-controlled, Phase III trial of rituximab therapy showed that patients with an inadequate response to antitumor necrosis factor (anti-TNF) had significant and clinically meaningful improvements in rheumatoid arthritis (RA) activity.6
The link between anti-TNF therapy and reactivation of latent TB is well recognized. Patients receiving anti-TNF therapy are more likely to present with disseminated infection, which carries considerable mortality.7-9 Although no studies have reported increased TB or opportunistic infections with rituximab in clinical trials,10 the American College of Rheumatology in 2008 recommended screening patients for TB before rituximab therapy.11 On the other hand, an international expert committee concluded that there is no evidence indicating the necessity to screen patients systematically for TB before using rituximab in those with RA.12 Furthermore, the safety and efficacy of rituximab was demonstrated in case reports of RA patients who had developed TB while under treatment with anti-TNF or who had a history of the treatment for pulmonary TB.13-15 In addition, a case report of active TB and RA was treated with anti-TB and rituximab a week later with recovery of TB and remission of RA.15
However, because these previous studies did not directly address this issue or were limited in scope, additional studies would be beneficial in confirming the safety of rituximab in the presence of a risk for TB, particularly in TB endemic regions with a high incidence and prevalence of this disease. Hence, the study aim was to evaluate the risk of acquiring TB or reactivation latent TB in patients with rheumatological disease who received rituximab therapy in endemic area such as Saudi Arabia.
Methods
Patient population
Candidates for this study consisted of adult patients (14 years or older according to hospital policy) with rheumatological diseases who received rituximab at King Faisal Specialist Hospital and Research Centre (KFSH and RC) between October 1, 2010, and March 31, 2011. Patients were included regardless of whether or not they underwent a TB screen (e.g. tuberculin skin test and chest X-ray) before rituximab therapy, whereas patients who had received treatment for TB were excluded from the study.
Study design
The study design was a retrospective cohort design. We collected the following information from the patients’ charts and the patients’ electronic information system: Demographic data (gender and age); primary diagnosis; rituximab regimen, which was either a RA protocol (1000 mg on days 1 and 15) or a lymphoma protocol (375 mg/m2 once weekly as a course of 4 intravenous infusions on days 1, 8, 15, and 22); and the tuberculin skin test and a radiological study before rituximab therapy. Patients were followed up to 6 months for symptoms suggestive of TB and tuberculin skin test and radiological study if any.
Study endpoints
The study endpoints were (1) to determine the risk of acquiring TB or reactivation latent TB in patients with rheumatological disease who received rituximab therapy in endemic area such as Saudi Arabia and (2) to establish a relationship between the dose of rituximab and the risk of TB, if any.
Ethical considerations
The study was performed after being approved by the Office of Research Affairs, the Research Advisory Council, KFSH and RC. Since the present study was a retrospective observational chart review study, no patient consent was required.
Statistical analysis
Data were entered in the form of a code (to protect patient confidentiality) into a Microsoft Office Excel 2007 spreadsheet and subsequently analyzed by a statistician at the statistical department of KFSH and RC.
Results
A total of 60 adult patients with rheumatological disease received rituximab therapy. 6 patients (10%) were subsequently excluded from the study because they were being treated for latent TB (5 patients) or had been recently treated for TB (1 patient). Therefore, 54 patients were included in the study.
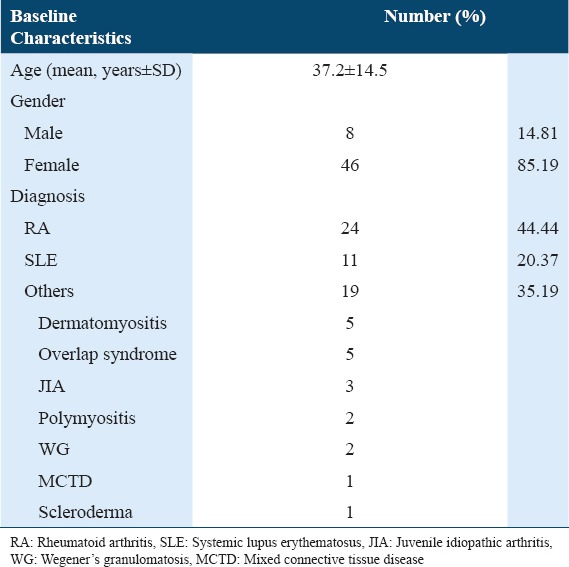
The mean age (standard deviation) of the patients (46 females, 8 males) was 37.2 (14.5) years (range, 14-65 years). The common rheumatological disease was RA (24 patients [44.44%]), followed by systemic lupus erythematosus (11 patients [20.37%]). Among the remaining patients (35.19%), 5 patients had dermatomyositis; 5, overlap syndrome; 3, juvenile idiopathic arthritis; 2, polymyositis; 2, Wegener’s granulomatosis; 1, mixed connective tissue disease; and 1, scleroderma (Table 1). Rituximab was given as per the RA protocol to 45 patients (83.33%); the lymphoma protocol was applied to 8 patients (14.81%) while 1 patient received a different regimen.
Table 1.
Baseline characteristics and diagnosis
Before rituximab therapy, the purified protein derivative (PPD) skin test was performed for 11 patients (20.37%), and the results were negative. However, the majority of patients, 43 (79.63%), did not receive the PPD skin test.
Radiological examinations before rituximab therapy were performed for 38 patients (70.37%). Of these patients, 37 (68.52%) had normal results or no findings suggestive of TB while 1 patient (1.85%) had findings suggestive of TB in the form of bronchiectatic changes and pleural thickening. However, 16 patients (29.63%) did not have any radiological study.
The patients were followed for an adequate period; 53 patients (98.15%) were followed for 6 months while 1 patient (1.85%) was followed for 3 months. During the follow-up, none of the patients received the PPD skin test while radiological studies were conducted for 30 patients (55.55%). Of these 30 patients, 29 (53.7%) showed normal results or findings not suggestive of TB while 1 patient had a suspicious finding in the form of several nodules in the right and left upper lobes on top of the bronchiectatic changes. Furthermore, 53 patients (98.15%) had no symptoms suggestive of TB upon follow-up. However, the 1 patient who had findings suggestive of TB before and after the therapy developed respiratory and constitutional symptoms. The patient was investigated, and TB was ruled out. Furthermore, the patient responded well to antibiotics and was diagnosed with bronchiectasis exacerbation secondary to bacterial infection. Thus, no patient was found to have a TB flare-up after rituximab therapy.
Discussion and Conclusion
This study assessed the risk of TB reactivation with rituximab and found no TB flare-ups following the therapy. In addition, the results supported the conclusion of the aforementioned international expert committee that there is no necessity to screen patients for TB before the use of rituximab therapy, which contrasts with the American College of Rheumatology 2008 recommendations. The evident lack of risk of acquiring TB or reactivating latent TB might be explained by the fact that the human defense mechanisms against TB mainly involve cellular immunity, whereas rituximab primarily affects the humoral immune response.15
These results are important for clinical practice since the use of rituximab could be a solution for delaying the management of rheumatological diseases in the presence of a risk of TB reactivation, especially in endemic areas. Based on our findings, we conclude that rituximab can be considered as an alternative or a first line of therapy in such cases.
At the time of our study, there was no similar study published in the literature.16 However, upon further review before the publication, we found a study which was conducted to assess TB risk in patients treated with non-antitumor necrosis factor-α (TNF-α) targeted biologics and recently licensed TNF-α inhibitors which was done through review of data from clinical trials and national registries. The study was retrospective and its conclusion is similar to ours indicating safety of rituximab.17
On the other hand, a case report was published about occurrence of knee TB after rituximab therapy in patient with RA and therefore they suggested TB screening before rituximab therapy.18 However, that patient had two pulses of glucocorticoid which by itself contribute to the risk of reactivation of TB and thus limited any conclusion from this case.
Limitations of the Study and Recommendations for Future Research
The study was retrospective in nature, and thus, we had incomplete TB screening before rituximab therapy and incomplete follow-up investigations. In addition, the sample size was small, and one patient had short follow-up only 3 months after therapy which is still shorter duration than the duration of action of rituximab which is 6 months. Therefore, future studies should be conducted prospectively with a larger size group to confirm the above findings and to make sure of adequate follow-up duration.
Acknowledgments
This work was supported by King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia.
References
- 1.Centre for Disease Control and Prevention. [Last accessed on 2016 Apr 11]. Available from: http://www.cdc.gov/tb/statistics/default.htm .
- 2.Central Department of Statistics and Information. Saudi Arabia; 2014. [Last accessed on 2016 Apr 11]. Available from: http://www.stats.gov.sa/en . [Google Scholar]
- 3.World Health Organization. Tuberculosis Country Profiles. [Last accessed on 2016 Apr 11]. Available from: https://www.extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=SA&LAN=EN&outtype=html .
- 4.Looney RJ, Srinivasan R, Calabrese LH. The effects of rituximab on immunocompetency in patients with autoimmune disease. Arthritis Rheum. 2008;58:5–14. doi: 10.1002/art.23171. [DOI] [PubMed] [Google Scholar]
- 5.Winthrop KL, Yamashita S, Beekmann SE, Polgreen PM Infectious Diseases Society of America Emerging Infections Network. Mycobacterial and other serious infections in patients receiving anti-tumor necrosis factor and other newly approved biologic therapies: Case finding through the emerging infections network. Clin Infect Dis. 2008;46:1738–40. doi: 10.1086/587989. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 7.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 8.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumour necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–5. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD BIOB ADASER Group. Treatment of rheumatoid arthritis with tumour necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: A multicenter active surveillance report. Arthritis Rheum. 2003;48:2122–7. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 10.Furst DE. The risk of infections with biologic therapies for rheumatoid arthritis. Semin Arthritis Rheum. 2010;39:327–46. doi: 10.1016/j.semarthrit.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 12.Buch MH, Smolen JS, Betteridge N, Breedveld FC, Burmester G, Dörner T, et al. Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:909–20. doi: 10.1136/ard.2010.144998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung N, Owczarczyk K, Hellmann M, Lehmann C, Fätkenheuer G, Hallek M, et al. Efficacy and safety of rituximab in a patient with active rheumatoid arthritis and chronic disseminated pulmonary aspergillosis and history of tuberculosis. Rheumatology (Oxford) 2008;47:932–3. doi: 10.1093/rheumatology/ken143. [DOI] [PubMed] [Google Scholar]
- 14.Burr ML, Malaviya AP, Gaston JH, Carmichael AJ, Ostör AJ. Rituximab in rheumatoid arthritis following anti-TNF-associated tuberculosis. Rheumatology (Oxford) 2008;47:738–9. doi: 10.1093/rheumatology/ken113. [DOI] [PubMed] [Google Scholar]
- 15.Pehlivan Y, Kisacik B, Bosnak VK, Onat AM. Rituximab seems to be a safer alternative in patients with active rheumatoid arthritis with tuberculosis. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2012-006585. pii: Bcr2012006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller EA, Ernst JD. Anti-TNF immunotherapy and tuberculosis reactivation: Another mechanism revealed. J Clin Invest. 2009;119:1079–82. doi: 10.1172/JCI39143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantini F, Niccoli L, Goletti D. Tuberculosis risk in patients treated with non-anti-tumor necrosis factor-a (TNF-a) targeted biologics and recently licensed TNF-a inhibitors: Data from clinical trials and national registries. J Rheumatol Suppl. 2014;91:56–64. doi: 10.3899/jrheum.140103. [DOI] [PubMed] [Google Scholar]
- 18.Ghaleba RM, Fahmy KA. Knee tuberculosis under rituximab therapy for rheumatoid arthritis. Joint Bone Spine. 2013;80:432–40. doi: 10.1016/j.jbspin.2012.10.018. [DOI] [PubMed] [Google Scholar]


