Abstract
Objective
Takayasu arteritis (TAK) is a large vessel vasculitis that predominately affects young women and can cause severe ischemic complications. Given the rarity of TAK, the management of this condition is challenging. We aim to describe current rheumatologist practices for the management of TAK and identify discrepancies and gaps in knowledge.
Methods
An online survey (developed by the Canadian Vasculitis Network and approved by the Canadian Rheumatology Association) containing 48 questions with regard to the diagnosis, monitoring and treatment of TAK was distributed to 495 Canadian adult and pediatric rheu-matologists by email.
Results
Sixty-six rheumatologists completed the survey (13% response rate): the majority (73%) were from academic centers and ≤25% reported managing more than ten patients in their career. For establishing the diagnosis of TAK, they relied on a combination of signs and symptoms of ischemia, elevations of inflammatory markers and vascular imaging (typically computed tomography and magnetic resonance angiography). The frequency of monitoring for disease activity and the methods employed (clinical, laboratory or imaging) were variable. All physicians used corticosteroids for the treatment of TAK, but 42% would treat for at least 6–12 months, 26% for 12–24 months and 23% would never stop corticosteroids. Fifty-three percent would always use an immunosuppressant (most commonly methotrexate or azathioprine) in addition to corticosteroids and the remainder would only start an immunosuppressant in patients with refractory or relapsing disease.
Conclusion
Physician practices for the management of TAK are variable, suggesting that there are knowledge gaps, which may impact outcomes in patients with TAK.
Keywords: Takayasu arteritis, physician practice patterns, practice guidelines, large vessel vasculitis, aortitis
Introduction
Takayasu arteritis (TAK) is a systemic large vessel vasculitis characterized by inflammation of the aorta and its main branches. Chronic inflammation in TAK results in stenoses and potentially aneurysms, which may lead to ischemia of distal tissues with complications, such as stroke, blindness, thromboembolic disease, myocardial infarction, congestive heart failure, bowel ischemia and/or chronic renal failure. Patients with TAK may also experience inflammatory manifestations in other tissues, including uveitis, arthritis and/or erythema nodosum. TAK usually occurs in females aged <40 years (mean age of onset 25–30), but it also occurs in children.1–11 The prevalence of the disease varies geographically: 40 per million in Japan and 2.7 per million in the UK.12,13 The incidence in the US was reported to be 2.6 per million per year during the 1970s.14 The reported 10-year overall survival rate of 84%–87% is concerning, especially given the young average age of patients. In addition, TAK patients with severe disease manifestations have a survival rate of only 37%.1,2,4,5
There are many challenges to overcome in order to improve patient outcomes, including early disease recognition and treatment, identifying reliable measures of disease activity by way of clinical tools, biomarkers and accessible novel imaging techniques, as well as defining optimal treatment through clinical trials. These challenges are made more difficult by disease rarity, physician inexperience and the difficulty in balancing potentially toxic treatments in a young female population of reproductive age. There are to date no clinical practice guidelines specific to TAK.
In this study, we used a Canadian nationwide questionnaire to describe physician practices for the management of TAK in order to identify potential care gaps and variations and to assist in the development of recommendations for the management of TAK.
Methods
The initial draft of the questionnaire was primarily developed by LB, CP and PL, and then modified following input from the core members of the Canadian Vasculitis Network (Can-Vasc). The core members include representatives from all Canadian provinces, most major academic centers including large pediatric centers and community practices.15 Pretest of the survey was performed with five rheumatologists and the final questions were decided upon using a modified Delphi method. The final questionnaire comprised 48 questions, predominantly categorical (forced option) with the opportunity for physicians to comment using free text. The survey took ~20 minutes to complete. The first section addressed the characteristics of the responding physicians (demographics, practice type, years in practice and the number of TAK patients seen). Physicians who managed ≤2 TAK patients over the course of their career were excluded from continuing onto further questions. Categories of additional questions were as follows: diagnostic assessment of patients (history, physical examination and investigations), monitoring of disease activity (including the definitions of remission and relapse used, measures of disease activity, assessment of comorbidities and complications), pharmacologic treatments used and the management of pediatric and pregnant patients.
The questionnaire was reviewed and approved by the Canadian Rheumatology Association for distribution to all its members through an email invitation sent in September 2015, with a link to the online questionnaire (SurveyMonkey®), available in both English and French (the majority of patients with TAK in Canada are primarily managed by rheumatologists). The survey remained open for 1 month, with one reminder email sent. The anonymously collected data were then extracted for descriptive analysis. This research study was approved by the Human Ethics Committee of the University of Western Ontario (London, Ontario, Canada) in accordance with the principles of the Helsinki declaration; health sciences research ethics board approval #106471. The physicians who participated in this survey provided informed consent electronically.
Results
Characteristics of survey respondents
The invitation email was sent to 495 physicians, and 66 agreed to participate in the survey (response rate of 13%). Characteristics of the respondents are summarized in Table 1. There were 57 adult (83%) and 9 pediatric (17%) rheumatologists from all regions across Canada. The majority of respondents had >10 years in practice, 35% had <10 years’ experience and 9% were completing their postgraduate training. Although various practice types were represented, academic/teaching centers were the most common, and 31% of respondents identified their practice as a specialized referral center for vasculitis. Of the 33 physicians who did not consider their practice to be a vasculitis referral center, 33% indicated that they would refer to a specialized vasculitis center for the comanagement of TAK patients, 15% would refer to such a center for transfer of care, 18% had no specialized vasculitis center in close proximity and the remainder felt it was not necessary to refer.
Table 1.
Characteristics of physicians responding to survey
| Age | n (%) | Practice setting | n (%) |
|---|---|---|---|
| <35 | 10 (15) | Solo community | 12 (18) |
| 35–45 | 22 (33) | Group community | 4 (6) |
| 46–55 | 17 (26) | Academic/teaching hospital | 48 (73) |
| 56–65 | 15 (23) | Other | 2 (4) |
| >65 | 2 (3) | TAK patients per year | |
| Location | ≤2 | 37 (58) | |
| East Coast | 6 (9) | 2–5 | 21 (33) |
| West Coast | 7 (11) | 6–9 | 4 (6) |
| Central | 15 (23) | >10 | 2 (3) |
| Ontario | 26 (40) | Total TAK patients in | |
| Quebec | 11 (17) | career | |
| Specialty | ≤2 | 16 (25) | |
| Adult | 57 (83) | 2–5 | 21 (33) |
| Pediatric | 9 (17) | 6–9 | 12 (19) |
| Years in practice | >10 | 15 (23) | |
| Residency/fellowship | 6 (9) | ||
| 1–10 | 23 (35) | ||
| 11–29 | 30 (45) | ||
| >30 | 7 (11) |
Abbreviation: TAK, Takayasu arteritis.
Only 9% of the responding physicians had managed more than five patients per year and 23% had ever seen more than 10 patients throughout their entire career (25% of the respondents had seen less than or equal to two TAK patients in their career and were excluded from completing the subsequent part of the questionnaire).
Physician practices for the diagnosis of TAK
A total of 50 out of 66 respondents completing the questionnaire beyond the first section were asked to select from a list the clinical features (by history and physical examination) that they considered necessary to assess a patient with a suspected diagnosis of TAK. The results are shown in Table 2. Components of the history and physical examination that were most consistently performed were: eliciting symptoms of systemic disease (98%) and claudication (91%), measuring blood pressure (98%), examining for vascular pulses (98%) and bruits (89%) and assessing for musculoskeletal involvement (81%). Clinical assessment to screen for all the possible severe manifestations of TAK such as dyspnea, chest pain, ischemic abdominal pain, focal neurologic signs and, in particular, cognition and ocular symptoms was not always performed at the initial visit. Other factors that were not listed in the survey, but were suggested by respondents, included signs and symptoms of deep vein thrombosis and exposure to tuberculosis.
Table 2.
Physician practices for the diagnostic assessment of a patient suspected of TAK
| History and physical exam | Always performed (%) | Laboratory investigations | Always performed (%) |
|---|---|---|---|
| Systemic features | 98 | Complete blood count | 100 |
| Hypertension | 98 | Liver enzymes | 96 |
| Claudication | 91 | Renal function test | 98 |
| Vessel tenderness | 72 | Urinalysis | 89 |
| Pulses | 98 | C-reactive protein | 98 |
| Vascular bruits | 89 | Erythrocyte sedimentation rate | 76 |
| Bilateral blood pressure | 89 | Antinuclear antibodies | 60 |
| Arthritis/arthralgia/myalgia | 81 | Rheumatoid factor | 47 |
| Rash/ulcers | 64 | Antineutrophilic cytoplasmic antibodies | 62 |
| Raynauld’s phenomenon | 68 | Hepatitis serologies | 69 |
| Headache | 72 | Investigations for tuberculosis | 36 |
| Focal neurologic signs | 74 | VDRL for syphilis | 40 |
| Neuropathy | 53 | Human immunodeficiency virus | 29 |
| Dizziness/vertigo/syncope | 62 | Blood cultures | 9 |
| Cognitive decline | 30 | ||
| Visual disturbance | 70 | Imaging investigations | |
| Inflammatory eye disease | 45 | Chest X-ray | 73 |
| Ocular exam | 17 | Echocardiography | 67 |
| Abdominal pain/tenderness | 64 | Magnetic resonance angiography | 40 |
| Chest pain | 78 | Computed tomography angiography | 24 |
| Dyspnea | 78 | Large vessel ultrasonography | 11 |
| Hemoptysis | 55 | Positron emission tomography | 7 |
| Heart failure | 57 | Conventional arteriography | 2 |
| Cardiac murmur | 72 | Other | 16 |
Abbreviations: TAK, Takayasu arteritis; VDRL, venereal disease research laboratory.
Physician practices for the use of investigations to diagnose TAK are described in Table 2. More than 98% of physicians reported that they would order basic laboratory investigations (complete blood count, renal function, liver enzymes and inflammatory markers). A larger proportion of physicians (98%) reported ordering C-reactive protein rather than erythrocyte sedimentation rate (ESR; 76%), perhaps reflecting policies in certain regions of Canada where ESR is not publically funded. Practices for ordering autoimmune serology and infectious workup were highly variable, and physicians endorsed that they would only order these tests if they suspected an alternate diagnosis or the patient’s history suggested an exposure risk for infections. Other tests that are not widely available (antiphospholipid antibodies and additional tests for screening for hypercoaguablility, IgG4, interleukin-6 and von Willebrand factor levels) were infrequently ordered.
All physicians stated that they always use imaging to assist with a diagnosis of TAK. However, physician preference for the different types of imaging varied significantly. Most physicians would always order a chest X-ray (73%), echocardiogram (67%) and either magnetic resonance angiography (MRA) or computed tomography angiography (CTA) (64%). Physicians commented that they avoided CTA in younger patients because of radiation exposure; however, lack of timely access to MRA in some centers necessitated using CTA. Large vessel ultrasonography and fluorodeoxyglucose-positron emission tomography (PET) scan were infrequently used (7%), as was conventional angiography (2%). PET scan was not an available option for 6% of respondents. Most physicians (78%) used currently available classification criteria for TAK (the American College of Rheumatology [ACR] criteria for adult patients, and the European League Against Rheumatism, the Pediatric Rheumatology European Society and the Pediatric Rheumatology International Trials Organization criteria for pediatric patients), which include both clinical and angiographic components.16,17 The ACR criteria also include the requirement for disease onset prior to age 40 years, but the survey did not capture whether in certain cases physicians would diagnose older patients with TAK.
Variations in the assessment and monitoring of disease activity
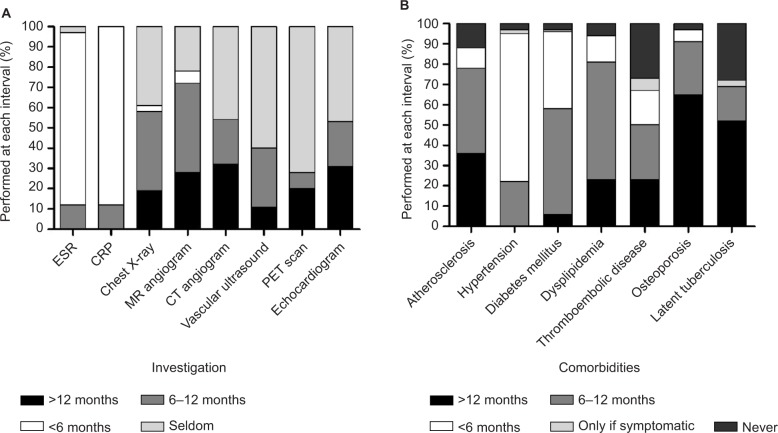
All physicians assessed disease activity by history, physical exam, measuring inflammatory markers and imaging studies; however, only 9% used a specific composite score (National Institutes of Health criteria, Indian Takayasu Activity Score, Pediatric Vasculitis Activity Score, etc).7,18,19 The monitoring parameters used throughout follow-up to assess for disease activity, complications and comorbidities are shown in Figure 1. Although most physicians (88%–90%) felt that ESR or C-reactive protein should be performed at least every 6 months, there was little consensus regarding the frequency of performing other laboratory investigations. The majority of physicians (75%) would perform MRA repeatedly (most commonly every 6–12 months). Some physicians commented that they would only perform repeat imaging in symptomatic patients or if there was an unexplained elevation in inflammatory markers; accessibility to some of the imaging tests was challenging for some centers. Not all physicians endorsed assessing for comorbidities, in particular, for latent tuberculosis and thromboembolic disease (<70%). Most physicians monitored hypertension at least every 6–12 months (97%) and atherosclerosis, diabetes and dyslipidemia at least yearly (>70%).
Figure 1.
Frequency of laboratory and imaging investigations performed by physicians managing TAK.
Note: Frequency of investigation for monitoring disease activity (A) and for assessing the comorbidities of TAK (B).
Abbreviations: CRP, C-reactive protein; CT, computed tomography; ESR, erythrocyte sedimentation rate; MR, magnetic resonance; PET, positron emission tomography; TAK, Takayasu arteritis.
Physician practices for the treatment of TAK
All physicians used high-dose corticosteroids for the treatment of patients with a new TAK diagnosis (>0.5 mg/kg of prednisone), with the majority (65%) using 1 mg/kg for induction of remission (Table 3). Twenty-five percent of physicians used high-dose pulse intravenous corticosteroids in a majority of their patients. Physicians did not comment that they would withhold treatment if patients did not have evidence of active disease at diagnosis. Most physicians would maintain patients on prednisone at least 6 months (92%), but the overall duration of therapy was variable: 42% of physicians would treat for 6–12 months, 26% for 12–24 months and 23% would not stop prednisone (Table 3). Most physicians (67%) would also use corticosteroids (15% with intravenous pulse) in patients requiring a revascularization procedure to ensure that inflammation was adequately suppressed at the time of the procedure. In these cases, physicians would advise the surgeon to postpone the procedure until the disease was felt to be adequately controlled. For any patient requiring revascularization, 33% would refer to surgery and not alter therapy in the presence of active disease.
Table 3.
Physician practices for the use of corticosteroids and primary prevention of vascular complications in TAK
| Prednisone dose at diagnosis (mg/kg/day) for active disease | n (%) | Pharmacologic agents for primary prevention of vascular complications | n (%) |
|---|---|---|---|
| ≤0.5 | 0 | Antiplatelet agent | 36 (90) |
| >0.5–<1 | 10 (25) | Duration of antiplatelet agent | |
| 1 | 26 (65) | First 6 months | 3 (8) |
| >1 | 4 (10) | As long as possible | 29 (78) |
| Duration of prednisone | As long as stenosis present | 5 (14) | |
| ≤6 months | 3 (8) | Lipid-lowering agents | 9 (23) |
| 6–12 months | 16 (42) | ACE-I or ARB | 4 (10) |
| 12–24 months | 10 (26) | ||
| Indefinitely | 9 (23) |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; TAK, Takayasu arteritis.
Physician practices for the use of immunosuppressants in addition to corticosteroids were variable: 53% would always start concomitant immunosuppressants, and the remainder would only use them for refractory or relapsing disease, for patients with severe disease at presentation or for those with adverse events to corticosteroids. Physician preferences for the various types of immunosuppressants are shown in Figure 2. Methotrexate or azathioprine was used most often (62% and 38%, respectively), followed by antitumor necrosis factor-alpha (anti-TNF-α) therapies and cyclophosphamide (16%). Other therapies (mycophenolate mofetil, tocilizumab and rituximab) were uncommonly prescribed. The duration of immunosuppressant use was variable: 53% would continue treatment indefinitely (some physicians commenting that they would potentially taper down the drug to lower doses), 15% would treat for 1 year, 15% for 2–3 years and 15% for 4–5 years.
Figure 2.
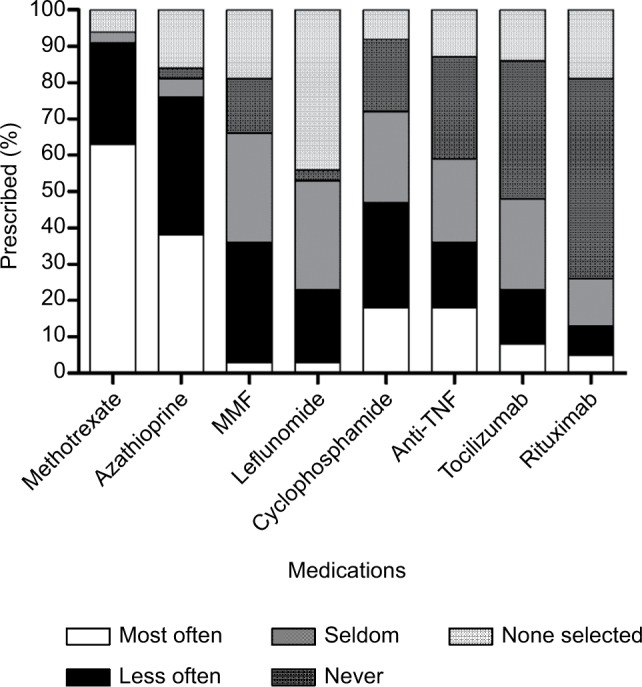
Physician preferences for the use of immunosuppressants for the treatment of TAK.
Abbreviations: MMF, mycophenolate mofetil; TAK, Takayasu arteritis; TNF, tumor necrosis factor.
Ninety-three percent of physicians defined remission as the resolution or stabilization of signs and symptoms, 85% also included the normalization of inflammatory markers and 73% included the resolution or improvement in imaging findings. Only 6% would escalate therapy based on an isolated elevation in inflammatory markers. Physicians considered the following imaging features to be indicative of active disease: new vessel stenosis, vessel wall enhancement and/or worsening of vessel wall thickening; 30% would escalate therapy in the presence of the aforementioned imaging findings. However, the majority of these physicians (94%) would only escalate treatment if there was also clinical evidence of active disease (new or worsening signs and symptoms).
Primary prevention of disease and treatment complications of TAK were frequently considered. Prophylaxis for Pneumocystis jiroveci was initiated by 74% of physicians, particularly for patients on high-dose corticosteroids combined with an immunosuppressant or if treated with cyclophosphamide or rituximab. For patients without any prior arterial events, aspirin was prescribed by 90% of physicians, and 78% would continue it indefinitely in the absence of toxicity; others would continue for the first 6 months, for as long as the patient required prednisone or with the presence of stenoses. The use of lipid-lowering agents for primary prevention was uncommon (23%), as were beta-blockers and angiotensin-converting inhibitors/angiotensin receptor blockers for the purpose of vascular remodeling in patients without hypertension or another indication for these therapies (10%) (Table 3).
Management of pediatric patients
Nine pediatric rheumatologists responded to the questionnaire, but only five had seen more than two patients throughout their career. Compared to adult rheumatologists, pediatric rheumatologists more consistently examined for all of the listed TAK features in the history and physical exam and all laboratory tests, except blood cultures, venereal disease research laboratory and human immunodeficiency virus. Given concerns with radiation exposure, CTA was not performed; all five physicians always performed MRA, echocardiogram and chest X-ray at diagnosis. Similar to adult rheumatologists, the frequency of imaging and the modality used for monitoring disease activity varied among physicians. Monitoring for cardiovascular complications was less commonly performed by pediatric versus adult rheumatologists. There were no significant differences in the treatment patterns of pediatric rheumatologists compared to adult rheumatologists, except they were more likely to start an immunosuppressant agent in addition to corticosteroids at diagnosis.
Management of pregnant patients
Few physicians had direct experience managing pregnant TAK patients (21%). Ninety-three percent of all the physicians surveyed would counsel their patients to not consider pregnancy during active disease, and would also recommend against pregnancy in the following circumstances: pulmonary hypertension, uncontrolled congestive heart failure or hypertension, severe valvular heart disease, recent thromboembolic event, severe acute renal failure, aneurysmal disease, carotid artery involvement, requiring high-dose corticosteroids or teratogenic medications to maintain remission. One physician would always recommend against pregnancy in TAK patients. For pregnant TAK patients with stable disease, most physicians would refer to high-risk obstetrics (94%) and modify treatment (97%) by lowering corticosteroid dose and stopping drugs known to be teratogenic (methotrexate and leflunomide). Twenty-six percent would maintain patients who were stable on azathioprine or anti-TNF agents throughout the pregnancy. For cases that flare during pregnancy, physicians would most often initiate high-dose corticosteroids (81%) and azathioprine (58%).
Discussion
In this study, we used a nationwide survey of rheumatologists to describe the practice patterns of physicians who manage patients with TAK. We included both pediatric and adult rheumatologists and physicians from different practice types (academic, community and specialized vasculitis centers). The survey results provide a comprehensive description of multiple aspects of TAK management. We found that self-reported physician practices varied in the use of corticosteroids and other immunosuppressants, the duration of drug therapy, the methods for assessing disease activity and the role of imaging.
The diagnosis of TAK is primarily clinical, assisted by laboratory investigations and imaging.12,20 The ACR classification criteria for TAK from 1990 rely heavily on history and physical exam findings suggestive of ischemia and vascular stenoses; only one parameter involves imaging (arteriogram showing large vessel occlusion).16 In general, classification criteria are designed to be highly specific and, therefore, may not be sensitive in a clinical setting, particularly, in the case of the TAK criteria where the parameters are indicative of advanced disease. Updated diagnostic criteria for TAK are currently under development.21 In our survey, most physicians assessed for the features comprising the 1990 TAK classification criteria in their diagnostic assessment. Many physicians also examined for other manifestations; however, common TAK features (vessel pain/tenderness, ocular inflammation, cutaneous manifestations) or major organ involvement were not screened for consistently by history or physical exam. At diagnosis, pediatric rheumatologists reported more complete clinical assessments, compared to adult rheumatologists. However, the survey may overestimate the comprehensiveness of the reported history and physical exam, given that the potential manifestations of TAK were listed in the survey question.
The variability in physician practices seen in this study regarding the use of imaging in TAK reflects the uncertainty regarding the utility of the imaging modalities from the available literature.20,22 Conventional arteriography was infrequently performed by the surveyed physicians; it is an invasive test that only captures late findings of TAK and cannot visualize inflammation of the vessel wall. CTA was also avoided when possible, given that it is less sensitive than MRA and PET at detecting early vessel wall changes22,23 and exposes patients to radiation and the risk of renal toxicity. In the survey, PET was infrequently used first line. PET lacks detailed anatomic information, making it less specific for TAK; it is often used in combination with computed tomography with some concern regarding radiation exposure.23,24 Arterial Doppler was also infrequently used; this modality is limited to superficial vessels and is highly user dependent.22,23 Limitations of MRA considered by the surveyed physicians included contraindications to MRA (stent placement and other metal implantations, severe renal disease, etc.) and other patient factors (claustrophobia, preferences, etc). Variations in the use of MRA between sites can be partially explained by differences in timely access between sites as well as the protocols for assessing vessel wall abnormalities. Also, the utility of MRA for the management of TAK is unclear; several studies show that MRA findings correlate poorly with measures of disease activity.25,26 Given the limitations of imaging for assessing active inflammation, the physicians surveyed always used inflammatory markers and clinical assessment to assist with determining disease activity.
All physicians reported using high-dose corticosteroids. Maksimowicz-McKinnon et al showed that 72% of TAK patients relapsed within 1 year of tapering corticosteroid doses <10 mg and <20% achieved steroid-free remission at a median follow-up time of 3 years.5 Similar findings were reported for childhood-onset TAK.27 Given the complications of maintaining patients on long-term systemic corticosteroids, including infection, weight gain, hyperglycemia, hypertension, ocular complications, osteoporosis and aseptic necrosis, steroid-sparing agents have been shown to be frequently required for maintaining remission or for steroid-resistant disease in TAK.2,5–7,27–31 In our survey, 53% of physicians (and 100% of pediatric rheumatologists) would start immunosuppressants in all patients with TAK and an additional 36% would start immunosuppressants in patients with severe organ involvement at presentation. The choice of immunosuppressant was most commonly azathioprine or methotrexate (even though there are only small observational studies and case series for the use of these drugs in TAK), likely because they are readily available, affordable and have known efficacy in other autoimmune conditions.30 Larger observational studies are available for biologic drugs (anti-TNF agents and tocilizumab). However, the use of these drugs was limited by the physicians surveyed partly because of the high costs and lack of available reimbursement for most patients.30 Other than ongoing controlled trials of abatacept and tocilizumab for large vessel vasculitis, there are no published controlled trials in TAK.30 The pharmacologic management of TAK remains challenging, given the lack of trials and clinical practice guidelines. Only a very small number of responders to this survey indicated that they manage pediatric-onset TAK or pregnant patients with TAK; therefore, it is challenging to study these populations.
Management of known complications of TAK is similarly difficult. The majority of physicians surveyed were aware of an increased risk of arterial thromboembolic complications in TAK and would start patients on aspirin (90%) for primary prevention, which appears to be beneficial based on observational studies.3,4,6,32,33 The role of lipid-lowering agents and drugs that can promote vascular remodeling is less clear and they were infrequently prescribed. Not all physicians routinely assessed for cardiovascular risk factors and evidence of thromboembolic complications. Although studies suggest a high prevalence of these complications, there are no available guidelines as to when and how they should be screened. National recommendations for the management of cardiovascular risk factors in the general population and for secondary prevention of atherosclerotic events should be followed. It is unclear whether earlier, more frequent screening or different blood pressure/lipid targets are indicated in TAK.
Overall, this study showed variability in physician practices for the management of TAK. This variability can partially be accounted for by the wide range of physicians included in the study. Community physicians may manage less severe patients than those in academic or specialized vasculitis centers. In addition, the experience of physicians managing TAK was also highly variable. We chose to include a heterogeneous population of physicians in order to capture the breadth of management practices across the country. The relatively small sample size in our study precluded further analysis of the physician characteristics and how they may have impacted the clinical practice of TAK. We had a low response rate for the survey, which could introduce bias. However, given the rarity of this condition in North America, it is possible that most of the nonresponding physicians did not respond to the survey because they had not encountered a TAK patient in their practice. This study describes physician practices using self-report, which is a limitation as it can introduce bias. Nevertheless, important differences in physician responses suggest that there is variability in the management of TAK in Canada and potentially in other countries with similar health care systems and demographics.
The variability in physician practices can reflect the inconsistencies and gaps in the current TAK literature. The European League Against Rheumatism/European Vasculitis Study Group guidelines for Large Vessel Vasculitis (2009) contain two recommendations specific for TAK: clinical and imaging assessment (MRA or PET) of the arterial tree at diagnosis and follow-up and the use of immunosuppressants as adjunct therapy to steroids.34 The frequency of imaging for monitoring was not addressed nor was the duration of therapy.34 The Japanese cardiovascular society recommendations for vasculitis (2013) include a more comprehensive section on TAK, but do not provide details regarding the methodology used for the literature reviews and the development of the recommendations.12
Conclusion
TAK is a chronic autoimmune condition affecting mostly young women with long-term complications and high morbidity and mortality. Using a comprehensive nationwide survey, we showed significant variability in the clinical practices of physicians for the management of TAK. Based on the care gaps identified by this study, specifically, the use of imaging for the diagnosis of TAK and the assessment of disease activity, type and duration of immunosuppressant therapy and the screening and treatment of complications of TAK, we aim to develop evidence-based clinical practice guidelines for the management of TAK.
Acknowledgments
The authors thank the Canadian Rheumatology Association Guidelines Committee for assistance with the development of the survey and its dissemination. This study was funded by the Canadian Initiative for Outcomes in Rheumatology cAre (CIORA). CanVasc collaborators are Volodko Bakowsky (Nova Scotia Rehabilitation Centre and Dalhousie University, Halifax, Nova Scotia, Canada), Simon Carette (Mount Sinai Hospital, Toronto, University of Toronto, Ontario, Canada), Natasha Dehghan (University of British Columbia, Vancouver, British Columbia, Canada), Leilani Famorca (Langs Community Centre, Cambridge, Ontario, Canada), Michele Goulet (Hôpital du Sacré-Coeur de Montréal, Montréal, Quebec, Canada), Nader Khalidi (St Joseph’s Healthcare Hamilton, McMaster University, Hamilton, Ontario, Canada), Majed Khraishi (Memorial University of Newfoundland, St John’s, Newfoundland, Canada), Christian Pineau (McGill University, MUHC Lupus and Vasculitis clinic, Montréal, Québec, Canada), Dax G Rumsey (Department of Pediatrics, University of Alberta, Edmonton, Alberta, Canada), David Robinson (University of Manitoba, Arthritis Centre, Winnipeg, Manitoba, Canada), Regina Taylor-Gjevre (Royal University Hospital, University of Saskatchewan, Saskatoon, Canada), Tanveer Towheed (Queen’s University, Kingston, Ontario, Canada), Judith Trudeau (CHAU de Lévis, Lévis, Quebec, Canada) and Rae Yeung (the Hospital for Sick Children, Toronto, Ontario, Canada).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med. 1994;120(11):919–929. doi: 10.7326/0003-4819-120-11-199406010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Park MC, Lee SW, Park YB, Chung NS, Lee SK. Clinical characteristics and outcomes of Takayasu’s arteritis: analysis of 108 patients using standardized criteria for diagnosis, activity assessment, and angiographic classification. Scand J Rheumatol. 2005;34(4):284–292. doi: 10.1080/03009740510026526. [DOI] [PubMed] [Google Scholar]
- 3.Mwipatayi BP, Jeffery PC, Beningfield SJ, et al. Takayasu arteritis: clinical features and management: report of 272 cases. ANZ J Surg. 2005;75(3):110–117. doi: 10.1111/j.1445-2197.2005.03312.x. [DOI] [PubMed] [Google Scholar]
- 4.Subramanyan R, Joy J, Balakrishnan KG. Natural history of aortoarteritis (Takayasu’s disease) Circulation. 1989;80(3):429–437. doi: 10.1161/01.cir.80.3.429. [DOI] [PubMed] [Google Scholar]
- 5.Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum. 2007;56(3):1000–1009. doi: 10.1002/art.22404. [DOI] [PubMed] [Google Scholar]
- 6.Vanoli M, Daina E, Salvarani C, et al. ITAA Study Group Takayasu’s arteritis: a study of 104 Italian patients. Arthritis Rheum. 2005;53(1):100–107. doi: 10.1002/art.20922. [DOI] [PubMed] [Google Scholar]
- 7.Bicakcigil M, Aksu K, Kamali S, et al. Takayasu’s arteritis in Turkey – clinical and angiographic features of 248 patients. Clin Exp Rheumatol. 2009;27(1 Suppl 52):S59–S64. [PubMed] [Google Scholar]
- 8.Moriwaki R, Numano F. Takayasu arteritis: follow-up studies for 20 years. Heart Vessels Suppl. 1992;7:138–145. doi: 10.1007/BF01744560. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Zhang H, Jiang X, et al. Clinical manifestations and longterm outcome for patients with Takayasu arteritis in China. J Rheumatol. 2014;41(12):2439–2446. doi: 10.3899/jrheum.140664. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe Y, Miyata T, Tanemoto K. Current clinical features of new patients with Takayasu Arteritis observed from cross-country research in Japan: age and sex specificity. Circulation. 2015;132(18):1701–1709. doi: 10.1161/CIRCULATIONAHA.114.012547. [DOI] [PubMed] [Google Scholar]
- 11.Brunner J, Feldman BM, Tyrrell PN, et al. Takayasu arteritis in children and adolescents. Rheumatology (Oxford) 2010;49(10):1806–1814. doi: 10.1093/rheumatology/keq167. [DOI] [PubMed] [Google Scholar]
- 12.JCS Joint Working Group Guideline for management of vasculitis syndrome (JCS 2008). Japanese Circulation Society. Circ J. 2011;75(2):474–503. doi: 10.1253/circj.cj-88-0007. [DOI] [PubMed] [Google Scholar]
- 13.Watts R, Al-Taiar A, Mooney J, Scott D, Macgregor A. The epidemiology of Takayasu arteritis in the UK. Rheumatology (Oxford) 2009;48(8):1008–1011. doi: 10.1093/rheumatology/kep153. [DOI] [PubMed] [Google Scholar]
- 14.Hall S, Barr W, Lie JT, Stanson AW, Kazmier FJ, Hunder GG. Takayasu arteritis. A study of 32 North American patients. Medicine (Baltimore) 1985;64(2):89–99. [PubMed] [Google Scholar]
- 15.Famorca L, Twilt M, Barra L, et al. Development of Canadian recommendations for the management of ANCA-Associated Vasculitides: results of the national needs assessment questionnaire. Open Rheumatol J. 2015;9:16–20. doi: 10.2174/18743129014090100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–1134. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 17.Ozen S, Pistorio A, Iusan SM, et al. Paediatric Rheumatology International Trials Organisation (PRINTO) EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69(5):798–806. doi: 10.1136/ard.2009.116657. [DOI] [PubMed] [Google Scholar]
- 18.Misra R, Danda D, Rajappa SM, et al. Development and initial validation of the Indian Takayasu Clinical Activity Score (ITAS2010) Rheumatology (Oxford) 2013;52(10):1795–1801. doi: 10.1093/rheumatology/ket128. [DOI] [PubMed] [Google Scholar]
- 19.Dolezalova P, Price-Kuehne FE, Özen S, et al. Disease activity assessment in childhood vasculitis: development and preliminary validation of the Paediatric Vasculitis Activity Score (PVAS) Ann Rheum Dis. 2013;72(10):1628–1633. doi: 10.1136/annrheumdis-2012-202111. [DOI] [PubMed] [Google Scholar]
- 20.Serra R, Butrico L, Fugetto F, et al. Updates in pathophysiology, diagnosis and management of takayasu arteritis. Ann Vasc Surg. 2016;35:210–225. doi: 10.1016/j.avsg.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 21.de Souza AW, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun. 2014;48–49:79–83. doi: 10.1016/j.jaut.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Alibaz-Oner F, Direskeneli H. Update on Takayasu’s arteritis. Presse Med. 2015;44(6 Pt 2):e259–e265. doi: 10.1016/j.lpm.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Hartlage GR, Palios J, Barron BJ, et al. Multimodality imaging of aortitis. JACC Cardiovasc Imaging. 2014;7(6):605–619. doi: 10.1016/j.jcmg.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Soussan M, Nicolas P, Schramm C, et al. Management of large-vessel vasculitis with FDG-PET: a systematic literature review and meta-analysis. Medicine (Baltimore) 2015;94(14):e622. doi: 10.1097/MD.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tso E, Flamm SD, White RD, et al. Takayasu arteritis: utility and limitations of magnetic resonance imaging in diagnosis and treatment. Arthritis Rheum. 2002;46(6):1634–1642. doi: 10.1002/art.10251. [DOI] [PubMed] [Google Scholar]
- 26.Eshet Y, Pauzner R, Goitein O, et al. The limited role of MRI in long-term follow-up of patients with Takayasu’s arteritis. Autoimmun Rev. 2011;11(2):132–136. doi: 10.1016/j.autrev.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Goel R, Kumar TS, Danda D, et al. Childhood-onset Takayasu arteritis – experience from a tertiary care center in South India. J Rheumatol. 2014;41(6):1183–1189. doi: 10.3899/jrheum.131117. [DOI] [PubMed] [Google Scholar]
- 28.Szugye HS, Zeft AS, Spalding SJ. Takayasu Arteritis in the pediatric population: a contemporary United States-based single center cohort. Pediatr Rheumatol Online J. 2014;12:21. doi: 10.1186/1546-0096-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clemente G, Hilário MO, Len C, et al. Brazilian multicenter study of 71 patients with juvenile-onset Takayasu’s arteritis: clinical and angiographic features. Rev Bras Reumatol Engl Ed. 2016;56(2):145–151. doi: 10.1016/j.rbre.2016.01.004. Portuguese. [DOI] [PubMed] [Google Scholar]
- 30.Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014;53(5):793–801. doi: 10.1093/rheumatology/ket320. [DOI] [PubMed] [Google Scholar]
- 31.de Franciscis S, Serra R, Luongo A, Sabino G, Puzziello A. The management of Takayasu’s arteritis: personal experience. Ann Vasc Surg. 2007;21(6):754–760. doi: 10.1016/j.avsg.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Duarte MM, Geraldes R, Sousa R, Alarcão J, Costa J. Stroke and transient ischemic attack in Takayasu’s Arteritis: a systematic review and meta-analysis. Stroke Cerebrovasc Dis. 2016;25(4):781–791. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 33.de Souza AW, Machado NP, Pereira VM, et al. Antiplatelet therapy for the prevention of arterial ischemic events in Takayasu arteritis. Circ J. 2010;74(6):1236–1241. doi: 10.1253/circj.cj-09-0905. [DOI] [PubMed] [Google Scholar]
- 34.Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2009;68(3):318–323. doi: 10.1136/ard.2008.088351. [DOI] [PubMed] [Google Scholar]