Abstract
Highly purified soybean lectin (SBL) was labeled with fluorescein isothiocyanate (FITC-SBL) or tritium (3H-SBL) and repurified by affinity chromatography. FITC-SBL was found to bind to living cells of 15 of the 22 Rhizobium japonicum strains tested. The lectin did not bind to cells of the other seven R. japonicum strains, or to cells of any of the nine Rhizobium strains tested which do not nodulate soybean. The binding of the lectin to the SBL-positive strains of R. japonicum was shown to be specific and reversible by hapten inhibition with d-galactose or N-acetyl-d-galactosamine.
The lectin-binding properties of the SBL-positive R. japonicum strains were found to change substantially with culture age. The percentage of cells in a population exhibiting fluorescence after exposure to FITC-SBL varied between 0 and 70%. The average number of SBL molecules bound per cell varied between 0 and 2 × 106. While most strains had their highest percentage of SBL-positive cells and maximum number of SBL-binding sites per cell in the early and midlog phases of growth, one strain had a distinctly different pattern. The SBL-negative strains did not bind lectin at any stage of growth.
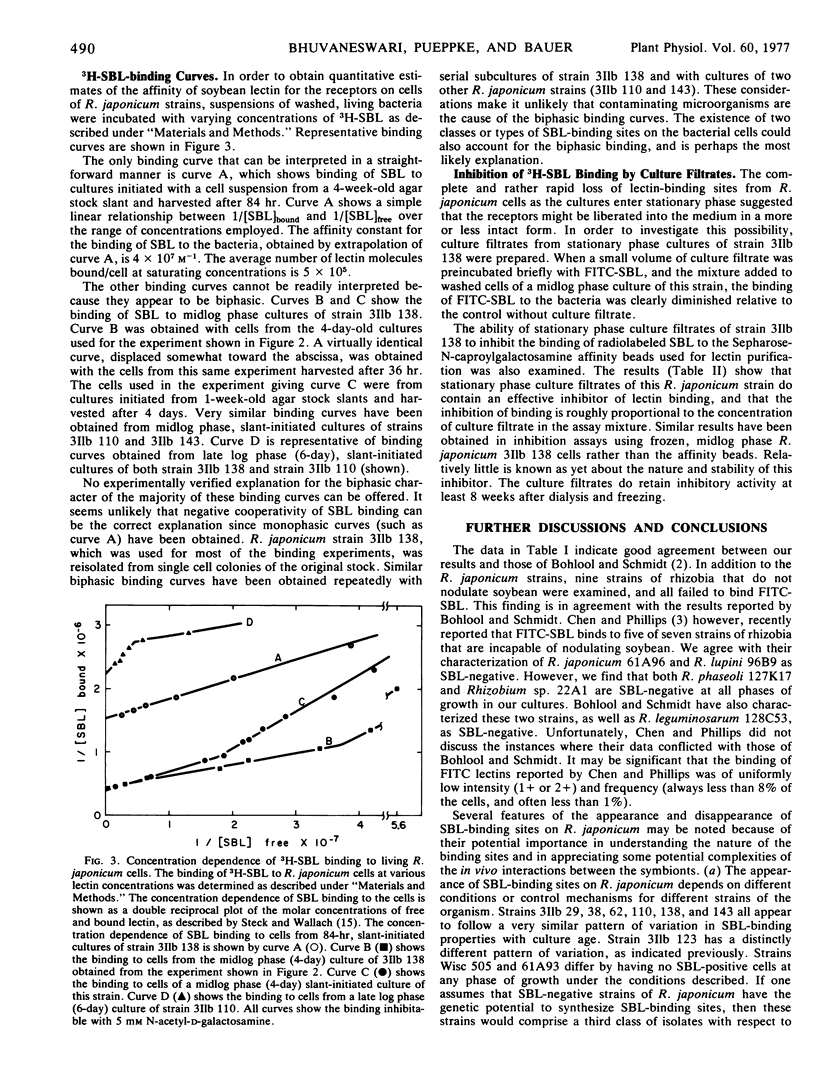
Quantitative binding studies with 3H-SBL indicated that the affinity constant for binding of SBL to its receptor sites on R. japonicum is approximately 4 × 107m−1. Many of the binding curves were biphasic. An inhibitor of SBL binding was found to be present in R. japonicum culture filtrates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Neuberger A. A simple method for the preparation of an affinity absorbent for soybean agglutinin using galactosamine and CH-Sepharose. FEBS Lett. 1975 Feb 15;50(3):362–364. doi: 10.1016/0014-5793(75)80528-x. [DOI] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Hubbell D. H. Cross-reactive antigens and lectin as determinants of symbiotic specificity in the Rhizobium-clover association. Appl Microbiol. 1975 Dec;30(6):1017–1033. doi: 10.1128/am.30.6.1017-1033.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lis H., Sela B. A., Sachs L., Sharon N. Specific inhibition by N-acetyl-D-galactosamine of the interaction between soybean agglutinin and animal cell surfaces. Biochim Biophys Acta. 1970 Sep 15;211(3):582–585. doi: 10.1016/0005-2736(70)90265-8. [DOI] [PubMed] [Google Scholar]
- Lotan R., Debray H., Cacan M., Cacan R., Sharons N. Labeling of soybean agglutinin by oxidation with sodium periodate followed by reduction with sodium [3-H]borohydride. J Biol Chem. 1975 Mar 10;250(5):1955–1957. [PubMed] [Google Scholar]
- Napoli C. A., Hubbell D. H. Ultrastructure of Rhizobium-induced infection threads in clover root hairs. Appl Microbiol. 1975 Dec;30(6):1003–1009. doi: 10.1128/am.30.6.1003-1009.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STECK T. L., HOELZLWALLACH D. F. THE BINDING OF KIDNEY-BEAN PHYTOHEMAGGLUTININ BY EHRLICH ASCITES CARCINOMA. Biochim Biophys Acta. 1965 Mar 8;97:510–522. [PubMed] [Google Scholar]
- Sherwood M. T. Improved synthetic medium for the growth of Rhizobium. J Appl Bacteriol. 1970 Dec;33(4):708–713. doi: 10.1111/j.1365-2672.1970.tb02253.x. [DOI] [PubMed] [Google Scholar]
- VALERA C. L., ALEXANDER M. NODULATION FACTOR FOR RHIZOBIUM-LEGUME SYMBIOSIS. J Bacteriol. 1965 Apr;89:1134–1139. doi: 10.1128/jb.89.4.1134-1139.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINCENT J. M. Influence of calcium and magnesium on the growth of rhizobium. J Gen Microbiol. 1962 Sep;28:653–663. doi: 10.1099/00221287-28-4-653. [DOI] [PubMed] [Google Scholar]
- Wilson J. B., Wilson P. W. Biotin as a Growth Factor for Rhizobia. J Bacteriol. 1942 Mar;43(3):329–341. doi: 10.1128/jb.43.3.329-341.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert J. S., Albersheim P. Host-symbiont interactions. I. The lectins of legumes interact with the o-antigen-containing lipopolysaccharides of their symbiont Rhizobia. Biochem Biophys Res Commun. 1976 Jun 7;70(3):729–737. doi: 10.1016/0006-291x(76)90653-7. [DOI] [PubMed] [Google Scholar]


