Abstract
Imaging studies have described hemodynamic activity during fear conditioning protocols with stimulus trains in which a visual conditioned stimulus (CS+) is paired with an aversive unconditioned stimulus (US, painful laser pulse) while another visual stimulus is unpaired (CS−). We now test the hypothesis that CS Event-Related Spectral Perturbations (ERSPs) are related to ratings of CS Expectancy (likelihood of pairing with the US), Valence (unpleasantness) and Salience (ability to capture attention). ERSP windows in EEG were defined by both time after the CS and frequency, and showed increased oscillatory power (Event Related Synchronization, ERS) in the delta/theta Windows (0–8 Hz) and the gamma Window (30–55 Hz). Decreased oscillatory power (Event Related Desynchronization – ERD) was found in alpha (8–14 Hz) and beta Windows (14–30 Hz). The delta/theta ERS showed a differential effect of CS+ versus CS− at Prefrontal, Frontal and Midline Channels, while alpha and beta ERD were greater at Parietal and Occipital Channels early in the stimulus train. The gamma ERS Window increased from Habituation to Acquisition over a broad area from Frontal and occipital electrodes. The CS Valence and Salience were greater for CS+ than CS−, and were correlated with each other and with the ERD at overlapping Channels, particularly in the alpha Window. Expectancy and CS Skin Conductance Response were greater for CS+ than CS− and were correlated with ERSP at fewer Channels than Valence or Salience. These results suggest that alpha ERSP activity during fear conditioning reflects Valence and Salience of the CSs more than conditioning per se. The CS Valence and Salience were correlated with each other and with ERSP in the alpha Window more commonly than in other windows. Delta/theta ERSP differentiates between CS+ and CS− over a Midline Channel at which ERSP is correlated with Expectancy ratings. Although, both Expectancy and CS Skin Conductance Response (SCR) were correlated with ERSP this correlation occurred at fewer and different Channels than those for Valence or Salience. These results suggest that ERSP activity is related to Valence and Salience of the CSs more than to conditioning per se.
Keywords: EEG, event-related synchronization, fear conditioning, human, painful laser stimulus, salience
Introduction
Fear conditioning of a neutral stimulus (conditioned stimulus, CS+) by pairing with an aversive stimulus (unconditioned stimulus, US) is a widely accepted probe to perturb the nervous system and examine neural processes for fear and anxiety (Phelps and LeDoux, 2005; Milad et al., 2006; Palazzo et al., 2008; Liu et al., 2011a; LeDoux, 2014). Studies of the amygdala in rodents have led to a neuroanatomical model of fear conditioning in which the CS+ and US produce signals which arrive in the lateral nucleus and converge there or in the basal nuclear group. The resulting signal is transmitted to the central nucleus, which is an output structure (Davis, 1992; Pare et al., 2004; Rauch et al., 2006; Sotres-Bayon et al., 2006). After conditioning, the CS+ evokes the conditioned response, which was initially evoked by the US but not by the CS+. Recordings from these structures during surgery for epilepsy demonstrate that the amygdala and Frontal lobe structures are activated and interact with each other during fear conditioning (Liu et al., 2010, 2011c,d, 2015a).
The involvement of human forebrain structures in fear conditioning is well described by fMRI studies (Sehlmeyer et al., 2009). The amygdala and hippocampus show a contrast of CS+ versus CS− related BOLD signals during the interval between the end of the CS+ and beginning of the US in a trace protocol. This contrast is related to the Skin Conductance Response (SCR), an autonomic expression of conditioned fear with tolerable painful or ‘annoying’ nonpainful USs (Carter et al., 2006; Cheng et al., 2006; Milad et al., 2007) and with a loud auditory US (Buchel et al., 1999). In a different kind of (delay) protocol, the CS+ is presented first and the US begins before the end of the CS+ so that there is an interval during which both stimuli are delivered together. In delay protocols, the Mid Cingulate Cortex showed this contrast which was related to the SCR in a protocol with a ‘highly annoying but not painful’ electrical stimulus (Milad et al., 2007; Linnman et al., 2012), while other cortical areas are not commonly involved (Sehlmeyer et al., 2009).
On the contrary, numerous fMRI studies of the anticipation with a painful US have reported that the contrast of CS+ visual stimuli versus rest is less common in the amygdala than in cortical areas including Anterior Cingulate Cortex, Prefrontal Cortex, Insula, Superior Temporal Gyrus and Inferior Parietal Lobule (Palermo et al., 2015). This anticipation was related to behavioral domains such as sensory perception rather than the SCR (cf (Seifert et al., 2013)). The role of these human cortical structures in the anticipation of pain may be related in part to their role in Salience and Valence of the conditioning stimuli (Anderson and Phelps, 2001; Frankenstein et al., 2001; Longe et al., 2001; Downar et al., 2002; Sander et al., 2003; Apkarian et al., 2005; Vogt, 2005). Finally, widespread BOLD activations occur in response to CSs at cortical sensory areas related to the modality of somatic, auditory and complex visual stimuli (Buchel et al., 1998; Knight et al., 2004; Nitschke et al., 2006), and in cortical association areas. Therefore, cortical areas may play a large but relatively unexplored role in processes for the anticipation of pain.
Cortical structures and processes can be examined in healthy subjects by EEG activity induced by CSs during fear conditioning, which has not previously been studied to our knowledge. These recordings have high temporal resolution, which can be used to measure the timing of emotional responses (Esslen et al., 2004), and the frequency of cortical processes (Pfurtscheller and Lopes da Silva, 1999; Neuper et al., 2006; Bardouille et al., 2010; Lachaux et al., 2012). We now test the hypothesis that ERSP following conditioning stimuli will be related to the behavioral response to those stimuli including the Valence, Salience, and Expectancy, and that the painful US is related to the CSs. The results of this study may enable development of combined fMRI and EEG techniques for the study of fear conditioning, and illuminate the mechanism of the fear of pain (Vlaeyen and Linton, 2000; Asmundson et al., 2004; Crombez et al., 2013).
Experimental Procedures
Participants and EEG recordings
Seven participants with no active medical or psychiatric disease (five men and two women; aged 23-58 years) were recruited for this study. The protocol for this study was approved by an Institutional Review Board of the Johns Hopkins University School of Medicine. All participants signed an informed consent form for participation in this study. EEG signals were recorded using a high density EEG cap (Quik-cap) with 128 electrodes placed on the scalp with a reference of linked earlobes (Fig. 3)(Jasper, 1958). EEG signals were amplified and digitized at the sampling rate of 1000 Hz (Neuro-Port, Blackrock Microsystems). These signals were subsequently band-pass filtered with a low-pass cutoff of 300 Hz and a high-pass cutoff of 0.1 Hz. Trials with artifacts were excluded based upon visual inspection. The timing for the onset of the laser and electrical stimuli were acquired and digitally embedded in the recordings through a data acquisition module (Model: NI USB-6212 BNC, National Instrument, Austin, TX, USA).
Fig. 3.
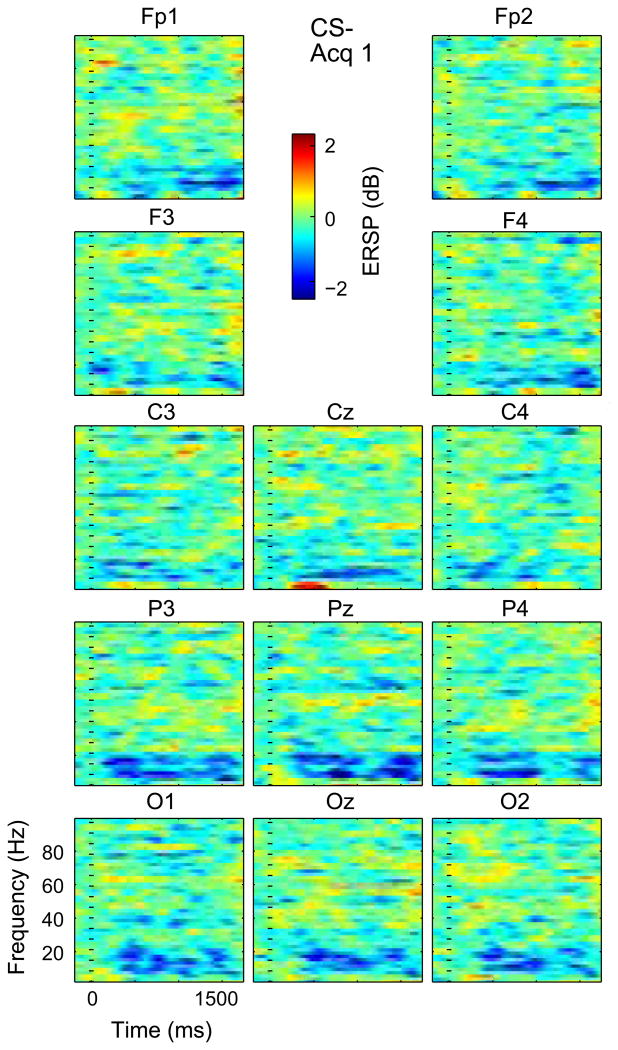
Time frequency plots across subjects for Acq 1 C– at Channels labeled above each plot and indicated by the arrangement of the time frequency plots relative to the forehead as labeled. Note that the vertical axis of each plot is from 0 to 100 Hz while the Color scale is as labeled and other conventions are as labeled in Fig. 2. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Event-Related Spectral Perturbations (ERSPs) were calculated through EEGLAB (Delorme and Makeig, 2004). An epoch extended from 2 s before to 2 s after an event, and the ERSP calculation used the whole 2 s before the epoch as a baseline (newtimef.m function in EEGLAB toolbox (Delorme and Makeig, 2004) in Matlab (Mathworks). The significance threshold was set to p < 0.05 with false discovery rate correction for multiple comparison (fdr.m function in EEGLAB toolbox (Delorme and Makeig, 2004) in MATLAB). The randomization procedures were carried out by the use of a standard random number generator (randperm.m function in Matlab Mathworks, Natick, MA).
Experimental design
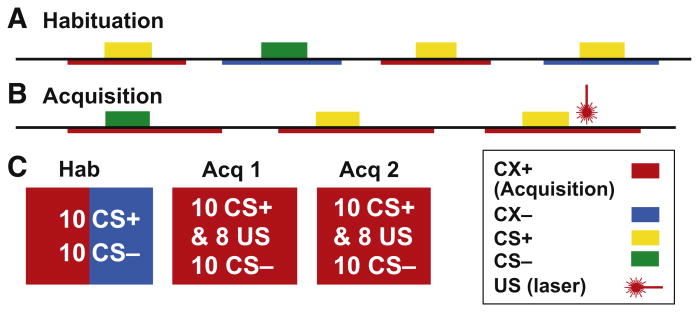
The fear conditioning paradigm included Habituation (Hab) and Acquisition (Acq) phases in the sequence shown in Fig. 1. Hab was composed of One Block while Acquisition was composed of two sequential blocks, Acq 1 and Acq 2. There were breaks between phases during which psychophysical measures of the CS and US were obtained during each break and at the end of Acquisition. A trial consisted of the presentations of contexts (CXs), which were pictures of either a living room or study room which were displayed as described below (see next section Experimental Stimuli …), where the onset and end of the contexts is indicated by the red and blue underlines in Fig. 1A and B. In each room there were two lamps; one produced yellow light and the other green light when lit. During Acquisition if the living room served as a dangerous context (CX+, red in Fig. 1B) then the study room would serve as the safe context (CX−, blue in Fig. 1) or vice versa by room. The yellow and green light from the lamp in the CX were the CSs as represented by colored blocks in Fig. 1A and B. If the yellow light was designated the dangerous cue (CS+), then the green light would designate the safe cue (CS−, Fig. 1B). The CS and CX designations were assigned at random and counterbalanced across subjects.
Fig. 1.
Fear conditioning protocol. Timelines for the Habituation (A) and Acquisition Phases (B) including approximate intervals of the Contexts (CXs), Unconditioned Stimuli (US), and Conditioning Stimuli (CS+ and CS−) which are randomized within the parameters specified in the text. Contexts and colors of the light which became the CS+ were randomized across patients (see text). The Contexts are shown as red and blue underlines to indicate that the Contexts are the background against which the CSs are presented. C shows the number of CS+, CS− and CXs within the blocks which comprise the Habituation and Acquisition Phases and which are shown in order of presentation. See text. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The Hab phase consisted of 20 trials, five for CS+ and five for CS− with each CS presented five times in the each context. The order of presentation of each combination of Context and CS was randomized and the approximate durations of stimuli, contexts and intervals during Habituation is illustrated in Fig. 1A. At the start of each 15 s trial, the CXs are presented alone for 6 s and then with the CS for 6 s, followed by CXs alone for the remaining 3 s. The inter-trial interval is set to between 2 and 4 s. The lamp colors of the CS+ and CS− and the rooms of the CXs were assigned at random and counterbalanced across subjects.
The Acq phase consisted of two blocks, Acq 1 and Acq 2 (Fig. 1C), for a total of forty trials. In each block, 20 CS+ and 20 CS− trials were ordered at random and presented within CX+. Each trial was 21 s long and the inter-trial interval was 5–7 s. When a trial started, the CX+ was presented alone for 6 s and then CSs were on within the CX+ for 6 s. The US (laser duration 1 ms, see section on Experimental Stimuli …) were delivered 3 s after the CS+ offset in 80% of CS+ trials. The time between blocks was set to 5 min. The timing of image presentation and triggers were controlled through a computer program Psychtoolbox in a MATLAB (MathWorks Inc., Natick, MA) environment. The intensity of laser pulse US was adjusted to produce a pain level of 5 out of 10 for each subject before the Hab phase.
Experimental Stimuli, rating scales and SCR
The experiment was conducted in a silent, dimly lit room with a temperature of between 22 and 24 °C; the protocols for delivery of the CSs and US are described in detail in the section on Experimental Design and in Fig. 1. Participants sat in a chair and rested their forearms on a table in front of them. The CX and CSs were displayed on a Screen (60 inches, Manufacturer LG, Model: 60LA8600-UC, Seoul, South Korea) placed 10 feet in front of the subject. Insert earplugs (Model: Max lite, Howard Leight by Honeywell, Morristown, NJ) were placed to minimize environmental noise.
The laser stimuli (US, Fig 1) were generated and delivered using a Thulium YAG laser (Themis, StarMedTech, Starnberg, Germany) with a wavelength of 2 μm, a beam diameter of 6 mm, and 1-ms duration. The laser stimuli were applied on the dorsum of the right hand. The laser was moved to a slightly different location for each pulse to avoid fatigue or sensitization of nociceptors.
During the laser intensity adjustment prior to the protocol for an individual participant, a series of laser pulses were delivered at several energy levels in an increasing order ranging from 200 to 1000 mJ with gaps of 80 mJ. The participant was asked to rate the pain intensity for the given energy levels. The laser energy for the experimental task was selected to be the level rated with an intensity of 4–6 by the participant. In addition, at each given energy level, the participant was asked to rate the Valence and Salience. The average energy level corresponding to the 4–6 pain intensity rating was 654 ± 50 mJ.
At the end of each block, numerical rating scales were used to rate the participant's psychophysical metrics regarding both CSs, and the painful US. For the painful laser stimulus, pain intensity and unpleasantness were rated separately on numerical rating scales, 0 indicated the absence of pain (or unpleasantness) and 10 indicated the greatest pain (or unpleasantness) imaginable. Conditioning was measured by (i) the CS Expectancy, which was the likelihood that either CS is paired with the US from 0 for very unlikely to 10 for very likely, and (ii) the CS+ Valence which was the unpleasantness of the CS+. Salience of a stimulus (CSs and US) was described as ‘the ability of the stimulus to capture, attention’ (Mouraux and Iannetti, 2009) (see also (Zaslansky et al., 1995; Downar et al., 2002, 2003; Lorenz and Garcia-Larrea, 2003; Legrain et al., 2005; Chien et al., 2014) and was rated on a numerical rating scale for which 0 was the absence of Salience and 10 was the most salient stimulus imaginable.
SCR was measured throughout by an isolated skin conductance coupler (Model V71-23, Coulbourn Instruments, Allentown, PA) with three electrodes (Compumedisc USA Inc, Charlotte USA) on the ventral distal phalanges of index, middle and ring fingers. The coupler delivered low distortion sine wave excitation voltage of 0.5 volts (mean square root) at 100 Hz across the skin and measured the resulting current flow as SCR. The measured current was further processed by the coupler into an output voltage signal which was digitized with sampling rate of 1 kHz (Neuroport).
Event-Related Spectral Perturbation (ERSP) analysis
In this study, the ERSP was used to estimate the event-related non phase-locked responses induced by the painful laser stimuli (Delorme and Makeig, 2004). This technique measures significant event-related changes in the power spectrum across different frequency bands in the poststimulus interval. In order to detect power changes across different frequencies, each poststimulus spectral estimate was divided by the mean baseline power spectrum and this ratio was the ERSP. If an ERSP at a specific frequency and time range was larger than 1, that ERSP was an ERS (Event-Related Synchronization); if smaller than 1, it was an ERD (Event-Related Desynchronization) (Lopes da Silva and Pfurtscheller, 1999).
Prior to the ERSP analysis, the EEG recordings were re-referenced to an averaged reference and filtered 0.1–250 Hz using a Hanning window finite impulse response filter. All EEG epochs were visually inspected by two independent individuals for artifact rejections. The power spectrum was estimated using FFTs with a Hanning window. The baseline was taken 200 ms immediately before the stimulus onset. Prior to the ERSP analysis, the event-related potentials (ERPs) were estimated by averaging signals across trials and Channels for each participant and task; these ERPs were subtracted from the signals prior to spectral estimation.
In order to detect power changes across different frequencies, the mean baseline power spectrum was subtracted for each spectral estimate and significant changes in the power spectrum during the poststimulus interval were assessed using a bootstrap method. An empirical baseline power spectrum distribution was computed by selecting spectral estimates of each trial from randomly selected latency time periods in the baseline, and then averaging these estimates. Repeating this process two hundred times constructed an empirical ‘baseline’ power distribution whose 95th percentile was then taken as the significance threshold (Delorme and Makeig, 2004).
An ANOVA was applied to investigate if the mean ERSP significantly depended on Cue (CS+ and CS−), Phase (Hab and Acq 1 and Acq 2) and Channels, or their Interaction. Based upon prior scalp and subdural electrode studies, the FP1, FP2, F3, F4, C3, C4, T7, T8, Cz, P3, P4, Pz, O1, O2 and Oz Channels were selected for the analysis of the ERS and ERD as a function of Channel (Mouraux et al., 2003; Ohara et al., 2004; Hu et al., 2013). Therefore, Cue, Channel and Phase were within-subject factors. The mean of ERSP across time and frequency in each window was calculated and served as the measurement of the dependent variable, which was explained by Cue, Phase and Channel factors in the ANOVA model. For each window, a repeated measures ANOVA was applied and the results were included in the Results section.
Results
ERS/ERD spectral Windows by Phase, Cue and Channel
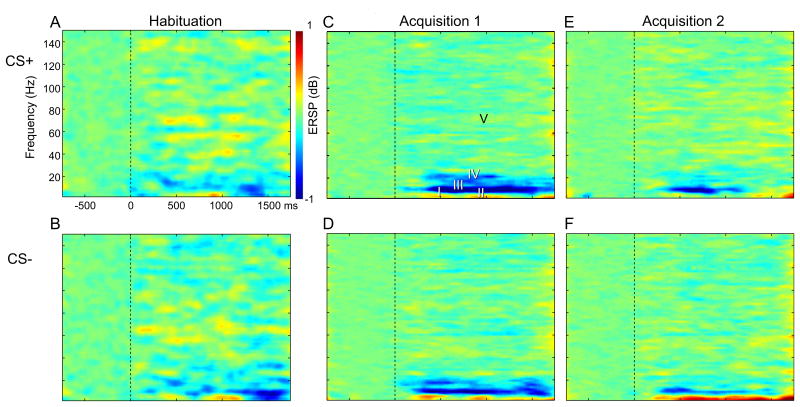
Fig. 2 shows time–frequency plots of ERSP relative to the onset of the CS+ and CS− (in rows) for each phase of conditioning (Hab, Acq 1 and Acq 2, in columns), where phases were defined in Fig. 1. For each window, the time and frequency ranges are selected to include an ERSP component found in these averages across subjects and Channels by phase and cue (Table 1). The selection of windowing parameters (Table 1) was based upon examination of the overall results (Fig. 2) without accounting for differences between factors of Channel, Cue or Phase.
Fig. 2.
Time Frequency plots of ERSP averaged across subjects and Channels. CS+ is in the upper row and CS− is in the lower row. The three columns from left to right denote Hab, Acq 1 and Acq 2. The axes of the time frequency plot are labeled in the (A) with the Y axis from 0 to 150 Hz. Windows used in this analysis are labeled in (C). Color scale is in Db, as labeled. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 1. Time and Frequency dimensions for the Windows for ERD/ERS.
| Window I | Window II | Window IIII | Window IV | Window V | |
|---|---|---|---|---|---|
| ERD/ERS | ERS | ERS | ERD | ERD | ERD/ERS |
| Frequency band | Delta/theta | Delta/theta | Alpha | Beta | Gamma |
| Frequency range | 0–8 Hz | 0–8 Hz | 8–14 Hz | 16–25 Hz | 30–120 Hz |
| Time range | 190–500 ms | 600–1400 ms | 200–1200 ms | 200–1200 ms | 0–1500 ms |
Time–frequency plots for both conditioning stimuli showed low frequency activities that were bimodal with earlier and later Windows (Fig. 2A upper row, Windows I and II – delta/theta, Table 1) and were more pronounced for CS− than CS+. Both the CS+ and CS− showed an alpha ERD (Window III –alpha) and beta ERD (Window IV). Window V is very broad since it extended over the full gamma band and across the duration of the poststimulus interval, as indicated in Table 1. The latencies of these induced non- phase-locked CS activities were relatively short, consistent with other studies of visual stimuli (Basar and Golbasi, 2014).
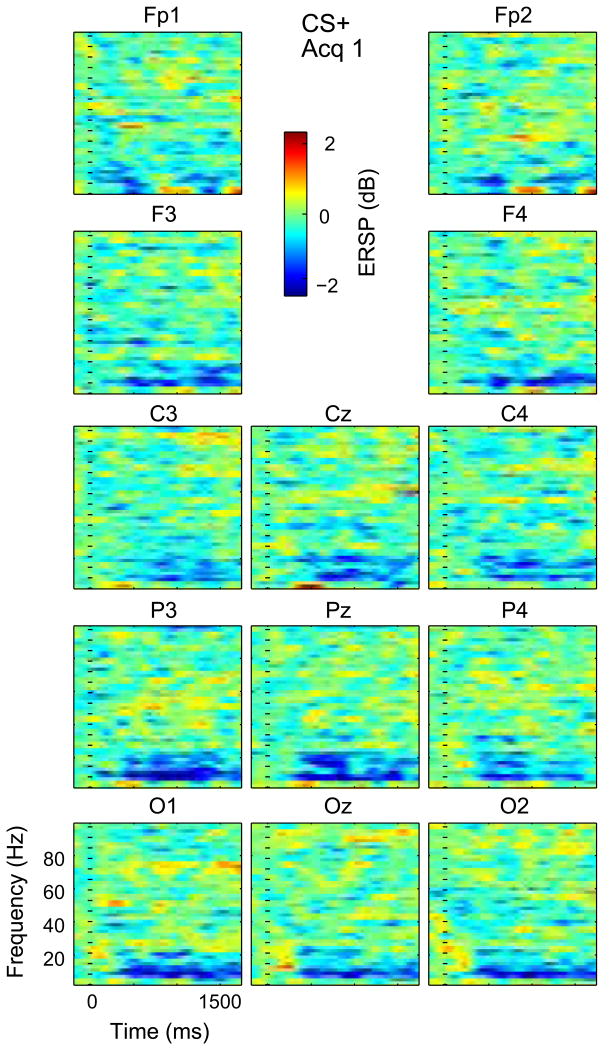
The ANOVA by factors of Cue, Phase and Channel was significant for Window I (WI) and main effects were seen for Cue (P < 0.0001), Phase (P = 0.0001) and Channel (P = 0.0001). None of the Interaction terms (Cue × Phase, Cue × Channel, and Phase × Channel) were significant. The effect of Cue can be seen by the increase in the early delta/theta red area in the time frequency plots from CS+ to CS− in all phases in Fig. 2. The effect of phase is seen in the increase in this red component from Hab from Acq 1 for both Cues. The effect of Channel is suggested by the red component at Cz for the CS− cue in Acq 1 and Acq 2 in Figs 3 and 5. No Channels were found in which Acq was different than Hab across patients on post hoc testing.
Fig. 5.
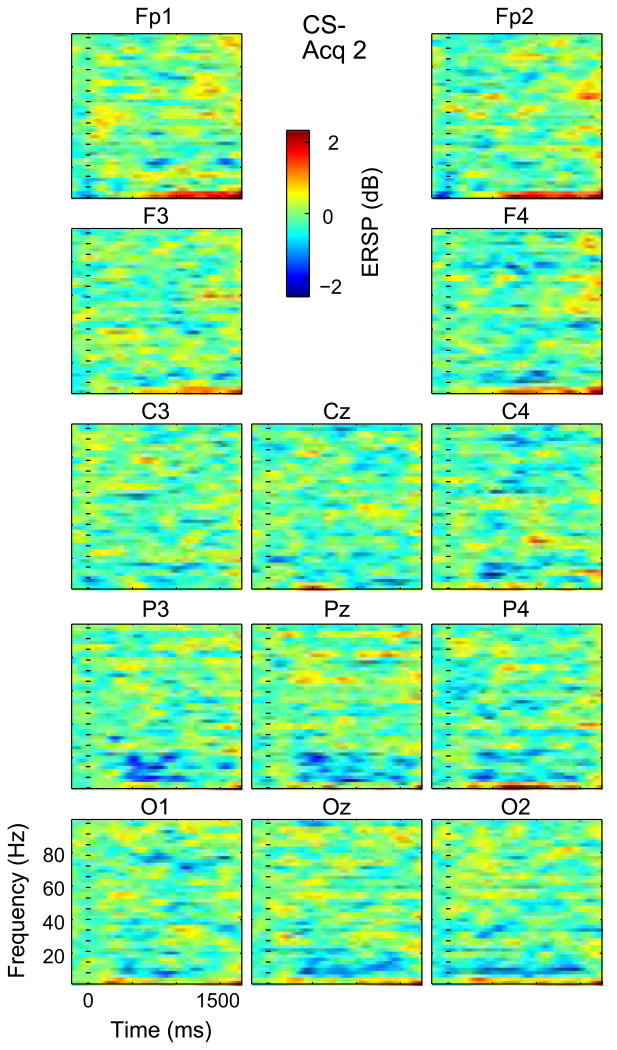
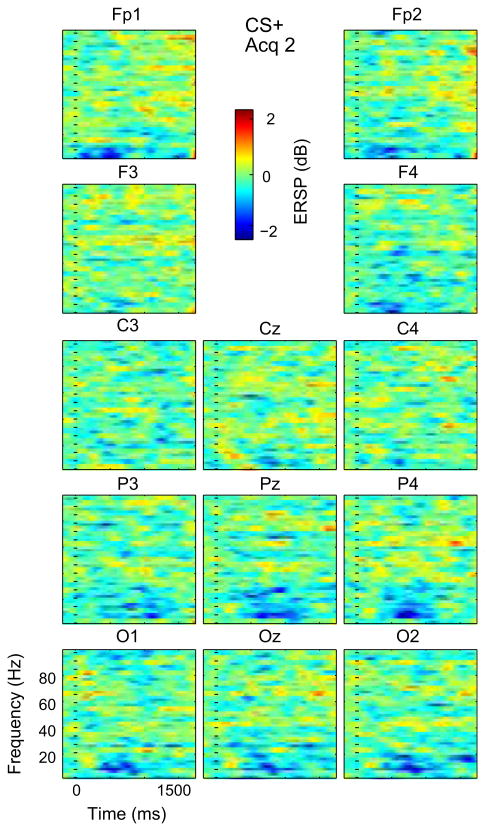
Time Frequency plots across subjects for Acq 2 CS−. Conventions as in Fig. 3.
The same ANOVA model showed significance for Window II (WII) and main effects were seen for Cue (P = 0.0001), Phase (P = 0.0001) but not Channel. The interaction terms were not significant. The effect of Cue can be seen by the low frequency ERS (longer latency red area), which is greater for CS− than CS+ in all phases (Fig. 2). The effect of Phase is seen in the increase in this red area in the time frequency plots from Hab to Acq 1 for both cues. The effect of Channel is illustrated by this red area, which is most pronounced for CS− in Channels FP1, Fp2, parietal and occipital Channels during Acquisition (Fig. 5). Therefore, WI and WII showed an effect of Cue, and this was most pronounced with greater ERS during early acquisition (phase) at Frontal Prefrontal and Midline leads except with low frequency ERD at CS+ Acq2, which is consistent with an effect of conditioning.
The same ANOVA model was significant for Window III (WIII) and main effects were seen for Phase (P = 0.003) and Channel (P = 0.017), but not for Cue. No Interaction term was found to be significant. Post hoc testing for CS+ in WIII revealed that Acq 1 was significantly different from Acq 2 at Cz and P3 (Table 2). The lack of effect of Cue can be seen by the similarity in the alpha blue area from CS+ to CS− in all phases (Fig. 2). The effect of Phase is seen in the increase in this blue area from Hab to Acq 1 followed by a decrease from Acq 1 to Acq 2 for both Cues. The effect of Channel is seen by this blue area, which is greater than most Channels at Cz, parietal and occipital Channels in CS− Acq 1 and Acq 2 (Figs 3 and 5).
Table 2.
Post hoc significant results (Wilcoxon singed rank test) for Channels with differences by Phase across subjects as specified in the Fix column. In the compare column, the <indicates that ERSP under the right side condition was larger than that under the left side condition. Differences between Acq 1 and Acq 2 are indicated in italics
| Fix | Compare | WI | WII | WIII | WIV | WV |
|---|---|---|---|---|---|---|
| CS+ | hp < ac1 | Oz, O2 | ||||
| CS+ | hp < ac2 | F3, Oz | ||||
| CS+ | ac1 > ac2 | Cz, P3 | C4 | T7 | ||
| CS− | hp < ac1 | T7 | C3, Cz | |||
| CS− | hp < ac2 | T7 | Fp1, F3, F4, Oz, O2 | C3, P3 | ||
| CS− | ac1 > ac2 | Pz |
The ANOVA Window IV (WIV) and main effects were significant for Phase (P = 0.0001) and Channel (P = 0.04), but not for Cue. Interactions were only found for Cue × Phase (P = 0.032). The lack of effect of Cue can be seen by the similarity in the higher frequency blue area from CS+ to CS− in all phases (Fig. 2). Post hoc testing in WIV for CS+ revealed that Acq 1 was greater than Acq 2 at C4, while for CS− Acq 2 was greater than Hab at Fp1, F3, F4, Oz and O2.
The effect of Phase for WIV is seen in the increase in this blue area from Hab to Acq 1 followed by a decrease at during Acq 2 for both Cues. The effect of Channel is seen by this blue area, which is greatest for parietal and occipital Channels in all combinations of Cues and Phases of Acq 1 and 2 (Figs 3–6). The interaction of Cue and Phase is reflected by the decrease in the higher frequency blue area from CS+ to CS− from Acq 1 to Acq 2 (Figs 3–6). Therefore, Windows III and IV showed an effect of phase that was most pronounced in early acquisition at occipital and parietal leads. Overall, these results are consistent with an effect of visual sensory properties related to the conditioning stimuli.
Fig. 6.
Time Frequency plots across subjects for Acq 2 CS+. Conventions as in Fig. 3.
For the broadband gamma and long poststimulus duration of WV, the same ANOVA model was significant with a main effect for phase (P = 0.0001), but not Cue (NS) or Channel (NS). No Interaction term was found to be significant. Post hoc testing for WV showed that for CS+, Hab was less than Acq 1 at O2 and Oz, and less than Acq 2 at F3 and Oz; Acq 1 was greater than Acq 2 at T7. For CS−, Hab was less than Acq 1 at C3 and Cz, and from Acq 2 at C3 and P3, while Acq 2 was less than Acq 1 at Pz. The effect of Phase is seen by the increase in the intensity and consistency of the yellow stipple from Acq 1 to Acq 2 (Fig. 2). This is very widespread across Channels for CS+(Figs. 4 and 6) and CS− (Figs. 3 and 5). Therefore, WV showed an effect of phase that showed greater ERS during the later acquisition phase and which was seen broadly across cues and Channels, latencies and frequencies (>30 Hz).
Fig. 4.
Time Frequency plots across subjects for Acq 1 CS+. Conventions as in Fig. 3.
Channels: consistency between subjects and differences between Windows and phases
The number of Channels with CS ERSP less in Habituation than either Acq 1 or 2 was significantly greater in WV (7/15) than in WI and WII (both 0/15, P = 0.006 Fisher) but not in WIV (5/15, P = 0.071). All differences between CS Acq 1 and Acq 2 by phases of acquisition had Ac1 > Ac2 (5/5, P = 0.031 binomial) and in which was significantly more common than the opposite direction Ac2 > Ac1 (0/5, P = 0.008, Fisher). Therefore, the most widespread change in WV ERSP was Hab < Acq, while Acq 1 > Acq 2.
The consistency of changes in ERSP between individual subjects versus the overall averages by subjects is an important index of the extent to which the present results can be generalized. During Acq the ERS or ERD within each Window and Channel was considered to be completely consistent if all subjects showed the same significant ERS or ERD as in the corresponding average. The results were completely consistent in all Windows except Window I. In this Window during Acq 1, the response to CS+ occurred in 89% of Prefrontal and Frontal Channels (Fp1, F3, Fp2, F4) and 95% of Midline Channels (Cz, Pz, Oz). Overall, the results are highly consistent across subjects suggesting that the results are generalizable.
In summary, a main effect was found for Cue at WI and WII indicating differential activation by the danger stimulus, and possibly a process related to conditioning. There was a main effect of Phase at all Windows which indicated increased activity from Hab to Acq, and a decrease in activation from Acq 1 to Acq 2. The latter has been reported in behavioral indexes and BOLD signals during fear conditioning (Buchel et al., 1999; Milad et al., 2005; Linnman et al., 2011) The main effect of Channel was found at all Windows except V, which indicates the widespread extent of this broadband long duration ERSP.
Correlation of psychophysical ratings and SCR and with ERSP by Channel
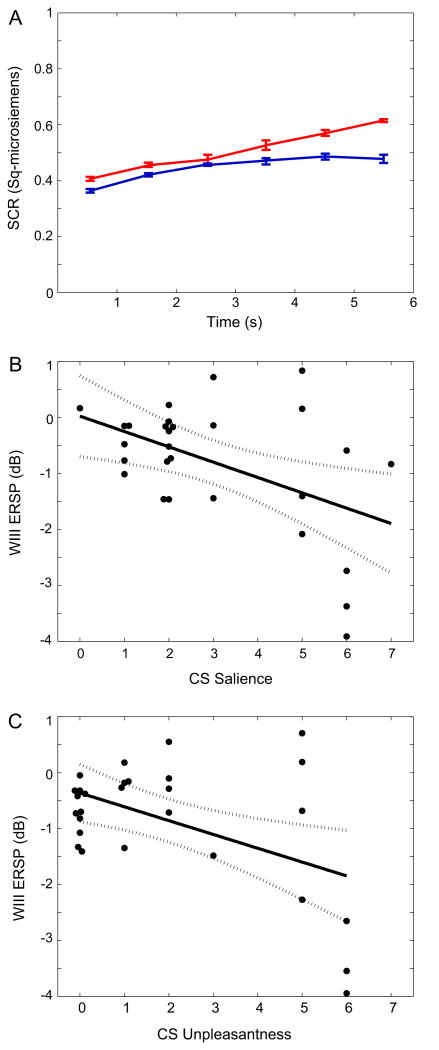
The behavioral results of fear conditioning were measured by the SCR response, and by the ratings of the Expectancy, Valence and Salience. The ability of our protocol to produce conditioned fear is demonstrated by the SCRs which were greater in response to CS+ (0.52 ± 0.01 μSiemens) than to the CS− (0.37 ± 0.02, P = 0.002 Wilcoxon signed rank test, Fig. 7A). The protocol also produced cognitive anticipation or Expectancy, which was significantly greater for the CS + (8.1 ± 2.1) than the CS− (0.71 ± 1.50, P = 0.031). Correlation of these ratings versus ERSP in different Windows may explain the functional significance of ERSP (Table 2).
Fig. 7.
SCR and CS ratings during the behavioral protocol. (A) SCR at intervals after the CS+ (red line) and CS− (blue). (B) Plot of WIII ERD versus CS Salience across Subjects, Phases and Cues. (C) Plot of WIII ERD versus CS+ unpleasantness across CS, Phases and Cues. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The Salience and Valence ratings measure properties of sensory stimuli which are the CSs. During acquisition these ratings were greater for CS+ than CS− for Valence (2.4 ± 2.2 vs 1.4 ± 2.2, P = 0.01, Wilcoxon signed rank test) and Salience (3.5 ± 1.8 vs 2.5 ± 2.0, P = 0.002). The Valence and Salience of CS were significantly correlated (R = 0.89, p < 0.001).
CS Valence was correlated with ERSP most commonly for WIII, as shown in Table 3. Specifically, CS+ Valence was significantly correlated with the magnitude of ERSP at more Channels at WIII (11/15) than ERSP at WIV (2/15, P = 0.002, Wilcoxon signed rank test) and WI, WII and WV (all 0/15, P = 0.00002). The linear regression of WIII ERD versus Valence was significant (Fig. 7C, R = −0.49, P = 0.007) with a negative regression coefficient, which results from the definition of ERD as a decrease in power from baseline (Neuper and Pfurtscheller, 2001; Delorme and Makeig, 2004).
Table 3.
Channels with correlation of SCR and ERSP by window. Significance level was p < 0.05 (n = 28) using ‘corrcoef.m’ function in Matalb. The “ +” sign indicates that the correlation coefficient is positive. Correlation coefficients are negative if there is no “+” sign shown after Channels. SCR is skin conductance response after log-transformation
| WI | WII | WIII | WIV | WV | |
|---|---|---|---|---|---|
| SCR | C3+, Cz+ | Fp1, T8+, O2+ | |||
| Valence | Fp1, Fp2, F3, F4, Cz, C4, Pz, P4, O1, O2, Oz | F4, C4 | |||
| Salience | F3, C4, P3, O1, O2, Oz | Fp1, Fp2, F4, C4, Cz, P4, Pz, O1 | F4, C4, T8 | O2 | |
| Expectancy | C3+, Cz+ | T7, P3 |
Salience of the CSs were correlated with activation of Channels in WIII 8/15 more commonly than in WII (0/15, P = 0.0010, Wilcoxon signed rank test), in WV (1/15, P = 0.007), and WIV (3/15, p = 0.058 as a trend) or WI (6/15, P = 0.5). The linear regression of WIII ERSP versus Salience was significant with a negative regression coefficient (Fig. 7B, R = −0.48, P = 0.009), which again is consistent with a direct relationship between greater magnitude of ERD (Neuper and Pfurtscheller, 2001; Delorme and Makeig, 2004).
ERD at WIII was correlated with Salience of the CSs in eight Channels and was correlated with Valence for the same eight Channels. This overlap between Channels by window correlated with Valence and Salience is more common than expected at random (both 10/19 correlated electrodes, P = 0.001, combinatorial). The Salience and Valence were correlated with ERSP at Channels over the sensory structures appropriate for unconditioned and conditioned stimuli including C4, Pz, P4, O1, O2, and Oz. Overall, these results suggest that alpha ERD is strongly related to both Valence and Salience which reflect properties of the CSs.
Conditioning was measured by the SCR and the rating of CS Expectancy. Although the SCR and Expectancy were greater for CS+ than CS−, the Expectancy and SCR of the CSs were not significantly correlated (P = 0.48, R = 0.14). This suggests that participants separately formed interactions between CSs and US during acquisition for cognitive anticipation (Expectancy) and autonomic processes (SCR). The Window and Channel combinations at which ERSP was correlated with SCR showed no overlap with the combinations at which ERSP was correlated with Expectancy (5 and 4/9, P = 0.0003). These findings are consistent with separate processes and cortical circuits related to autonomic fear responses and cognitive anticipation produced by the CS+.
The correlation of Expectancy with ERSP was most common in WII and WIII (both 2/15), which was not more common than any other window (all 0/15, P = 0.22). The number of Channels at which ERSP at any Window was correlated with Expectancy of the CSs (4/15) was significantly less than that for Channels correlated with Valence (11/15, P = 0.027, Fisher) or Salience (12/15, P = 0.005). Similarly, correlation of ERSP with SCR occurred at fewer Channels (5/15) than the number of Channels in which ERSP correlated in which Salience (12/15, P = 0.026) and tended to occur at fewer Channels with ERSP correlated with Valence (11/15, P = 0.065 Fisher).
The number of Channels at which ERSP in any window was correlated with both Expectancy and Valence (or Salience) was less common than expected at random (1/15, P = 0.001, Binomial, for both Valence and Salience). A similar result was found for ERSP at Channels with correlation both for Valence (or Salience) and for SCR (3/15, 0.035, Binomial, for both Valence and Salience). Therefore, the EEG Channels or extent of cortical activity that were correlated with CS Expectancy or SCR was much less than that for Valence or Salience. The Channels correlated for Valence (or Salience) overlapped with those for SCR and Expectancy much less commonly than expected at random.
Discussion
We observed that the correlation of Salience with ERSP occurs in the same Channels as the correlation of ERSP with Valence, and that the two ratings are highly correlated across subjects. Some of these Channels are located over sensory structures appropriate to the modality of the CSs. The Channels that are correlated with Valence or Salience are much more common than, and do not overlap with, those for CS SCR or Expectancy. This suggests that cortical activity reflects properties of the conditioning stimuli rather than conditioning per se. The CS Salience and Valence measure the ability of the Conditioned Stimuli to capture attention, and to reflect the unpleasantness of the US which may motivate behavior. Salience is a property of all stimulus modalities, and in the case of fear may reflect the attention produced by a threat (CS+) of a stimulus of negative valence (US – pain), which may account for the relationship between CS Valence and Salience (Davis and Whalen, 2001; Davis et al., 2002, 2003; Asmundson and Hadjistavropoulos, 2007; Mouraux and Iannetti, 2009). These properties of threatening stimuli may be features of the response to the CS+ which are as important as Expectancy to the behavioral state produced a threat.
A prior study of human EEG activity during fear conditioning was carried out in patients with Post Traumatic Stress Disorder and controls by using neutral visual CSs, and a trauma reminder as the US (Wessa and Flor, 2007). Patients with Post Traumatic Stress Disorder showed greater conditioned responses as measured by SCR and heart rate, as well as the P300 which was an EEG ERP calculated from the response to the CS+ minus that to the CS−. The relevance of the P300 to WIII ERD is suggested by its latency and the effect of task on nonphase-locked frontocentral alpha (Yordanova and Kolev, 1998) and on gamma oscillatory power (Gurtubay et al., 2004). The P300 was recorded over central electrodes during acquisition, again suggesting a cortical alpha component which was related to salience (Picton and Hillyard, 1988; Picton, 1993; Wessa and Flor, 2007). We did not show a difference in alpha ERSP between the CS+ and CS−, which may be consistent with the results of the study above in which this difference was not marked in healthy controls (Wessa and Flor, 2007).
EEG activity has been studied in other protocols that produce fear, like faces displaying the emotions of fear or anger. For example, the theta to beta ratio recorded over Frontal structures was correlated indirectly with the effect of fear (fearful faces) upon subjective measures of attentional control (Putman et al., 2010). This theta activity may correspond to the decrease in WI and WII theta ERS related to fear (CS+ versus CS−), which was correlated with Salience for WI. In particular, this proposal may explain our findings that Frontal and Prefrontal delta/theta at WI and WII CS− are significantly greater following CS− than CS+, an effect which could be related to Salience.
In our results, fear conditioning produces Expectancy and consistent increases in alpha ERD during Acq versus Hab (Fig. 2). This Expectancy may be reflected in the contingent negative variation, an ERP which is later than the P300, and reflects the learned association between the US and the CS+ that produces fear (Wong et al., 1994; Harris, 2005; Wessa and Flor, 2007). The contingent negative variation is associated with a decrease in alpha power so that the present increase in magnitude of alpha ERD seems to be inconsistent with Expectancy but not Salience or Valence (Grunewald-Zuberbier et al., 1978; Filipovic et al., 2001).
Frequency dependence of ERSP related to conditioning stimuli
This study demonstrates an increase of theta and gamma ERS following the CSs. This result may be related to the theta activity in the amygdala and hippocampus, which has been described in a wide range of memory related functions in both mice and humans (Fell et al., 2003; Hasselmo, 2005; Cornwell et al., 2008). Synchrony between theta rhythms in the amygdala and hippocampus during fear memory consolidation and retention may indicate connectivity between these structures (Narayanan et al., 2007). Theta frequency activity is also found in hippocampal and Prefrontal cortex and may mediate the synchrony between these two structures during fear conditioning (Seidenbecher et al., 2003), extinction (Lesting et al., 2011, 2013), and retrieval in mice (Hasselmo, 2005). Therefore, theta rhythms in cortex are also be related to learning and memory (Bastiaansen and Hagoort, 2003; Paz et al., 2008).
Gamma EEG oscillations that occur in the human cortex, hippocampal formation and amygdala may be related to theta rhythms through cross frequency coupling as the theta rhythm modulates the gamma rhythm during the formation of declarative memories and the response to stimuli of negative Valence, such as pain (Fell et al., 2003; Liu et al., 2015a,b). These stimuli evoke both theta and gamma frequency activity over central, parietoinsular and medial Frontal sites, which may be connected to the hippocampus through the entorhinal cortex (Van and Pandya, 1975a,b; Aggleton et al., 1980). Cross frequency coupling in cortex produce a signal which jointly represents Salience and Valence as substrates for motivation of behavior.
Methodological considerations
In order to study the changes in nonphase-locked oscillatory activities, phase-locked components are removed during the analysis. This can be accomplished by subtracting the ERPs from every epoch to eliminate phase-locked components while retaining non-phase-locked components (Fig. 2)(Tallon-Baudry and Bertrand, 1999; Bastiaansen and Hagoort, 2003). Subtraction of this type is widely used for both EEG and MEG activities in humans (Tallon-Baudry and Bertrand, 1999; David et al., 2016; Hauck et al., 2007). The effect of ERPs upon ERSP as assessed by subtraction is very small relative to the ongoing EEG (Makeig, 1993) or ERSP resulting from the laser stimulus (Chien et al., 2014). Nevertheless, early latency ERS found in Windows I and II might originate to some degree by phase locked as well as nonphase-locked activity (Kalcher and Pfurtscheller, 1995; Bastiaansen and Hagoort, 2003).
There is a possibility that the consistency of these results is a function of the windows selected for this analysis (Table 1). Time–frequency plots were constructed for CSs and the selection of windowing parameters in these plots (Table 1) was based on visual inspection of the overall averages of ERSP (Fig. 2) without accounting for differences in results between Subjects, Cues or Phases. Therefore, the consistency between data overall versus individual subjects and factors is unlikely to be a product of windowing of the overall results.
Implications for conditioned behaviors
In this study, we used a trace conditioning protocol which is often associated with increased BOLD signals in the Amygdala, Hippocampus, Insula and Dorsolateral Prefrontal Cortex. In addition, increased BOLD signals were found in auditory association cortex in protocols with auditory CSs and US (Buchel et al., 1999), and in Midfrontal Gyrus, Frontal Operculum, Inferior Parietal Lobule, Supplementary Motor Area and Occipital Cortex with visual CSs and somatic sensory USs (Knight et al., 2004; Sehlmeyer et al., 2009). In these studies of fear conditioning BOLD signals were found in sensory areas appropriate to the CSs and US and so may be related to the Valence and Salience of the CSs resulting from conditioning.
The effectiveness of a fear conditioning protocol is often confirmed by a differential increase in the SCR for. the CS+ over versus CS− (Milad et al., 2005; Carter et al., 2006), as in the present results. Stimulus related autonomic arousal is mediated by the Insula and Mid Cingulate Cortex during attention (‘goal orientation’), and by Anterior Cingulate Cortex during emotion (Benarroch, 2001; Beissner et al., 2013). This neuroanatomy may be consistent with the correlation of SCR with WI ERS at Prefrontal Channels, and with WV ERS at Midline vertex and central Channels. We also assessed conditioning by the CS+ Expectancy, which is a cognitive response requiring attention, decision making, and rating of the likelihood that the CS+ is paired with the US.
The anticipation of pain was the subject of a metaanalysis of activation and correlation of fMRI signals during the anticipation of pain versus the ‘resting state’ (Palermo et al., 2015). fMRI BOLD signals for the contrast of CS+ and a resting state in this analysis were increased at Prefrontal Cortex, Mid Cingulate, Anterior Insula, Inferior Parietal Lobule, and decreased at the Anterior Cingulate. Significant changes in this BOLD contrast in the amygdala were much less common than those found in cortex. The present results are also consistent with fMRI and electroencephalographic studies, which found functional interactions involving the frontoparietal attention circuit during directed attention to visual stimuli (Corbetta and Shulman, 2002; Fox et al., 2006) and to pain (Ohara et al., 2006; Liu et al., 2011b; Kucyi et al., 2012). Therefore, the cortical structures with ERSP related to conditioned stimuli are also involved in networks for attention and Salience of the sensory modalities which are relevant to the CSs.
Acknowledgments
This work was supported by the National Institutes of Health – National Institute of Disorders and Stroke (NS38493 to FAL) and by the Hopkins Neurosurgery Pain Research Institute.
Footnotes
None of the authors has conflicts of interest related to this work. The manuscript is in accordance with the statement of ethical standards for manuscripts submitted to Neuroscience.
References
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede R-D, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Hadjistavropoulos HD. Is high fear of pain associated with attentional biases for pain-related or general threat? A categorical reanalysis. J Pain. 2007;8:11–18. doi: 10.1016/j.jpain.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Vlaeyen JW, Crombez G. Understanding and treating the fear of pain. Oxford; NY, NY: 2004. [Google Scholar]
- Bardouille T, Picton TW, Ross B. Attention modulates beta oscillations during prolonged tactile stimulation. Eur J Neurosci. 2010;31:761–769. doi: 10.1111/j.1460-9568.2010.07094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basar E, Golbasi BT. Event related desynchronization: use as a neurophysiologic marker is restricted. Cogn Neurodyn. 2014;8:437–445. doi: 10.1007/s11571-014-9301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Event-induced theta responses as a window on the dynamics of memory. Cortex. 2003;39:967–992. doi: 10.1016/s0010-9452(08)70873-6. [DOI] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Pain-autonomic interactions: a selective review. Clin Auton Res. 2001;11:343–349. doi: 10.1007/BF02292765. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J Neurosci. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, O'Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage. 2006;29:1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behav Neurosci. 2006;120:1187–1195. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- Chien JH, Liu CC, Kim JH, Markman TM, Lenz FA. Painful cutaneous laser stimuli induce event-related oscillatory EEG activities which are different from those induced by non-painful electrical stimuli. J Neurophysiol. 2014;112:824–833. doi: 10.1152/jn.00209.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman Gl. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci. 2008;28:5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombez G, Van Ryckeghem DM, Eccleston C, Van DS. Attentional bias to pain-related information: a meta-analysis. Pain. 2013;154:497–510. doi: 10.1016/j.pain.2012.11.013. [DOI] [PubMed] [Google Scholar]
- David O, Kilner JM, Friston KJ. Mechanisms of evoked and induced responses in MEG/EEG. Neuroimage. 2016;31:1580–1591. doi: 10.1016/j.neuroimage.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol. 2002;87:615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Downar J, Mikulis DJ, Davis KD. Neural correlates of the prolonged salience of painful stimulation. Neuroimage. 2003;20:1540–1551. doi: 10.1016/s1053-8119(03)00407-5. [DOI] [PubMed] [Google Scholar]
- Esslen M, Pascual-Marqui RD, Hell D, Kochi K, Lehmann D. Brain areas and time course of emotional processing. Neuroimage. 2004;21:1189–1203. doi: 10.1016/j.neuroimage.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Elfadil H, Schaller C, Elger CE, Fernandez G. Rhinal-hippocampal theta coherence during declarative memory formation: interaction with gamma synchronization? Eur J Neurosci. 2003;17:1082–1088. doi: 10.1046/j.1460-9568.2003.02522.x. [DOI] [PubMed] [Google Scholar]
- Filipovic SR, Jahanshahi M, Rothwell JC. Uncoupling of contingent negative variation and alpha band event-related desynchronization in a go/no-go task. Clin Neurophysiol. 2001;112:1307–1315. doi: 10.1016/s1388-2457(01)00558-2. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenstein UN, Richter W, McIntyre MC, Remy F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. Neuroimage. 2001;14:827–836. doi: 10.1006/nimg.2001.0883. [DOI] [PubMed] [Google Scholar]
- Grunewald-Zuberbier E, Grunewald G, Rasche A, Netz J. Contingent negative variation and alpha attenuation responses in children with different abilities to concentrate. Electroencephalogr Clin Neurophysiol. 1978;44:37–47. doi: 10.1016/0013-4694(78)90103-7. [DOI] [PubMed] [Google Scholar]
- Gurtubay IG, Alegre M, Labarga A, Malanda A, Artieda J. Gamma band responses to target and non-target auditory stimuli in humans. Neurosci Lett. 2004;367:6–9. doi: 10.1016/j.neulet.2004.05.104. [DOI] [PubMed] [Google Scholar]
- Harris JB. (Differential conditioning of alpha amplitude: a fresh look at an old phenomenon. Clin Neurophysiol. 2005;116:1433–1443. doi: 10.1016/j.clinph.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm?–Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus. 2005;15:936–949. doi: 10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- Hauck M, Lorenz J, Engel AK. Attention to painful stimulation enhances gamma-band activity and synchronization in human sensorimotor cortex. J Neurosci. 2007;27:9270–9277. doi: 10.1523/JNEUROSCI.2283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Peng W, Valentini E, Zhang Z, Hu Y. Functional features of nociceptive-induced suppression of alpha band electroencephalographic oscillations. J Pain. 2013;14:89–99. doi: 10.1016/j.jpain.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the international federation. EEG Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Kalcher J, Pfurtscheller G. Discrimination between phase-locked and non-phase-locked event-related EEG activity. Electroencephalogr Clin Neurophysiol. 1995;94:381–384. doi: 10.1016/0013-4694(95)00040-6. [DOI] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. J Neurosci. 2004;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Hodaie M, Davis KD. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J Neurophysiol. 2012;108:3382–3392. doi: 10.1152/jn.00674.2012. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Axmacher N, Mormann F, Halgren E, Crone NE. High-frequency neural activity and human cognition: past, present and possible future of intracranial EEG research. Prog Neurobiol. 2012;98:279–301. doi: 10.1016/j.pneurobio.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Coming to terms with fear. Proc Natl Acad Sci U S A. 2014;111:2871–2878. doi: 10.1073/pnas.1400335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Bruyer R, Guerit JM, Plaghki L. Involuntary orientation of attention to unattended deviant nociceptive stimuli is modulated by concomitant visual task difficulty. Evidence from laser evoked potentials. Clin Neurophysiol. 2005;116:2165–2174. doi: 10.1016/j.clinph.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape HC. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One. 2011;6:e21714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesting J, Daldrup T, Narayanan V, Himpe C, Seidenbecher T, Pape HC. Directional theta coherence in prefrontal cortical to amygdalo-hippocampal pathways signals fear extinction. PLoS One. 2013;8:e77707. doi: 10.1371/journal.pone.0077707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Rougemont-Bucking A, Beucke JC, Zeffiro TA, Milad MR. Unconditioned responses and functional fear networks in human classical conditioning. Behav Brain Res. 2011;221:237–245. doi: 10.1016/j.bbr.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Zeidan MA, Furtak SC, Pitman RK, Quirk GJ, Milad MR. Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. Am J Psychiatry. 2012;169:415–423. doi: 10.1176/appi.ajp.2011.10121780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Ohara S, Franaszczuk PJ, Zagzoog N, Gallagher M, Lenz FA. Painful stimuli evoke potentials recorded from the medial temporal lobe in humans. Neurosci. 2010;165:1402–1411. doi: 10.1016/j.neuroscience.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Crone NE, Franaszczuk PJ, Cheng D, Schretlen DS, Lenz FA. Fear conditioning is associated with dynamic directed functional interactions between and within the human amygdala, hippocampus, and frontal lobe. Neurosci. 2011a;189:359–369. doi: 10.1016/j.neuroscience.2011.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Ohara S, Franaszczuk PJ, Crone NE, Lenz FA. Attention to painful cutaneous laser stimuli evokes directed functional interactions between human sensory and modulatory pain-related cortical areas. Pain. 2011b;152:2781–2791. doi: 10.1016/j.pain.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Ohara S, Franaszczuk PJ, Lenz FA. Attention to painful cutaneous laser stimuli evokes directed functional connectivity between activity recorded directly from human pain-related cortical structures. Pain. 2011c;152:664–675. doi: 10.1016/j.pain.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Shi CQ, Franaszczuk PJ, Crone NE, Schretlen D, Ohara S, Lenz FA. Painful laser stimuli induce directed functional interactions within and between the human amygdala and hippocampus. Neurosci. 2011d;178:208–217. doi: 10.1016/j.neuroscience.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Chien JH, Kim JH, Chang YF, Anderson WS, Lenz FA. Functional role of induced gamma oscillatory responses upon processing noxious and innocuous sensory events in humans. Neurosci. 2015a;303:412–421. doi: 10.1016/j.neuroscience.2015.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Chien JH, Kim JH, Chuang YF, Cheng DT, Lenz FA. Cross-frequency coupling in deep brain structures upon processing the painful sensory inputs. Neurosci. 2015b;303:412–421. doi: 10.1016/j.neuroscience.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longe SE, Wise R, Bantick S, Lloyd D, Johansen-Berg H, McGlone F, Tracey I. Counter-stimulatory effects on pain perception and processing are significantly altered by attention: an fMRI study. Neuroreport. 2001;12:2021–2025. doi: 10.1097/00001756-200107030-00047. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva FH, Pfurtscheller G. Basic concepts on EEG synchronization and desynchronization. In: Pfurtscheller G, Lopes da Silva FH, editors. Handbook of Electroencephalography and Clinical Neurophysiology. Vol. 6. Elsevier Science B.V.; 1999. pp. 3–11. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Garcia-Larrea L. Contribution of attentional and cognitive factors to laser evoked brain potentials. Neurophysiol Clin. 2003;33:293–301. doi: 10.1016/j.neucli.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol. 1993;86:283–293. doi: 10.1016/0013-4694(93)90110-h. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol. 2009;101:3258–3269. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Guerit JM, Plaghki L. Non-phase locked electroencephalogram (EEG) responses to CO2 laser skin stimulations may reflect central interactions between A partial differential- and C-fibre afferent volleys. Clin Neurophysiol. 2003;114:710–722. doi: 10.1016/s1388-2457(03)00027-0. [DOI] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Kluge C, Bergado J, Stork O, Pape HC. Dissociated theta phase synchronization in amygdalo-hippocampal circuits during various stages of fear memory. Eur J Neurosci. 2007;25:1823–1831. doi: 10.1111/j.1460-9568.2007.05437.x. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol. 2001;43:41–58. doi: 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Neuper C, Wortz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res. 2006;159:211–222. doi: 10.1016/S0079-6123(06)59014-4. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Attention to a painful cutaneous laser stimulus modulates electrocorticographic event-related desynchronization in humans. Clin Neurophysiol. 2004;115:1641–1652. doi: 10.1016/j.clinph.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Analysis of synchrony demonstrates 'pain networks' defined by rapidly switching, task-specific, functional connectivity between pain-related cortical structures. Pain. 2006;123:244–253. doi: 10.1016/j.pain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Palazzo E, Fu Y, Ji G, Maione S, Neugebauer V. Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviors. Neuropharmacology. 2008;55:537–545. doi: 10.1016/j.neuropharm.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo S, Benedetti F, Costa T, Amanzio M. Pain anticipation: an activation likelihood estimation meta-analysis of brain imaging studies. Hum Brain Mapp. 2015;36:1648–1661. doi: 10.1002/hbm.22727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Paz R, Bauer EP, Pare D. Theta synchronizes the activity of medial prefrontal neurons during learning. Learn Mem. 2008;15:524–531. doi: 10.1101/lm.932408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1993;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Picton TW, Hillyard SA. Endogenous event-related potentials. In: Picton TW, editor. Human event-related potentials. Vol. 6. Amsterdam: Elsevier; 1988. pp. 361–416. [Google Scholar]
- Putman P, van PJ, Maimari I, van der WS. EEG theta/beta ratio in relation to fear-modulated response-inhibition, attentional control, and affective traits. Biol Psychol. 2010;83:73–78. doi: 10.1016/j.biopsycho.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Seifert F, Schuberth N, De CR, Peltz E, Nickel FT, Maihofner C. Brain activity during sympathetic response in anticipation and experience of pain. Hum Brain Mapp. 2013;34:1768–1782. doi: 10.1002/hbm.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Van HG, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (1999), (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res. 1975a;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Van HG, Pandya DN, Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Res. 1975b;95:25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Wong PS, Shevrin H, Williams WJ. Conscious and nonconscious processes: an ERP index of an anticipatory response in a conditioning paradigm using visually masked stimuli. Psychophysiology. 1994;31:87–101. doi: 10.1111/j.1469-8986.1994.tb01028.x. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Event-related alpha oscillations are functionally associated with P300 during information processing. Neuroreport. 1998;9:3159–3164. doi: 10.1097/00001756-199810050-00007. [DOI] [PubMed] [Google Scholar]
- Zaslansky R, Sprecher E, Tenke CE, Hemli JA, Yarnitsky D. The P300 in pain evoked potentials. Pain. 1995;66:39–49. doi: 10.1016/0304-3959(96)03020-5. [DOI] [PubMed] [Google Scholar]