Abstract
Objective
To investigate pathological conditions that act as sources of pro-inflammatory cytokines and cytotoxic substances to examine telomere length (TL) in patients with either early (duration of illness [DI] ≤5 years) or chronic (DI >5 years) psychosis using T lymphocytes.
Methods
Based on these factors and the important role that T lymphocytes play in inflammation, the present study measured the TL of T lymphocytes in patients with either early or chronic psychosis. Additionally, smoking, metabolic syndrome, depression, and cognitive functioning were assessed to control for confounding effects.
Results
TL was significantly longer in patients with early and chronic psychosis than in healthy control subjects and, moreover, the significance of these findings remained after controlling for age, smoking, metabolic syndrome, DI, chlorpromazine-equivalent dose, and cognitive functioning (F=9.57, degree of freedom=2, p<0.001). Additionally, the DI, chlorpromazine-equivalent doses, and the five-factor scores of the Positive and Negative Syndrome Scale were not significantly correlated with the TL of T lymphocytes in either all patients or each psychosis group.
Conclusion
Possible mechanisms underlying the effects of antipsychotic medications on telomerase are discussed in the present study, but further studies measuring both telomerase activity and TL using a prospective design will be required.
Keywords: Antipsychotic agents, Telomere length, Telomerase, T lymphocytes, Psychosis, Quantitative Real-time PCR
INTRODUCTION
Telomeres are nucleoprotein complexes composed of tandem repeat DNA sequences (TTAGGG)n that form protective caps at the ends of eukaryotic chromosomes. In somatic cells, telomeres progressively shorten with each cell division, which is due to the incomplete replication of linear chromosomes by DNA polymerases. Telomerase is a cellular ribonucleoprotein reverse transcriptase that extends telomeres and, in turn, protects them and maintains chromosomal integrity.1) Because the expression of telomerase in most somatic cells is insufficient to completely restore the length of telomeres, these nucleoproteins progressively shorten with each cell division. Maintaining the length of telomeres extends the lifetime of cells1); thus, telomere length (TL) reflects the proliferative history of cells and serves as a mitotic clock of physical aging in many organisms.1–3)
For example, the reduction of TL in peripheral blood leukocytes is accelerated by oxidative/metabolic stress and is associated with illnesses related to aging and degenerative phenotypes, such as dementia,4) cardiovascular disease, and diabetes. In terms of psychological factors, current life stress5) and personality traits, such as pessimism6) and hostility,7) are associated with shorter leukocyte TL. Furthermore, the acceleration of telomere shortening occurs in patients with mood disorders,8) generalized anxiety disorder, post-traumatic stress disorder,9) and schizophrenia.10)
The psychotic symptoms that are commonly associated with schizophrenia are related to the overproduction of reactive oxygen species (ROS) and impaired oxidative defenses.11) Patients with schizophrenia often exhibit a variety of physical problems, including obesity, metabolic syndrome, diabetes mellitus, cardiovascular disease, and unhealthy lifestyles, which are all associated with reduced TL.12) Although many studies have investigated TL in patients with schizophrenia, the link between telomeres and schizophrenia remains elusive. Many studies have reported that psychotic patients exhibit shorter TL compared with healthy controls,10,13–15) whereas others have found no difference in this regard16–18) or longer TL.19) These discrepant findings may be due to the use of different study designs with participants of various ages and genders, different durations of illness (DI), and medication statuses and/or the use of various specimen analyses.
All these studies, except that conducted by,16) collected peripheral blood mononuclear cells (PBMCs) to assess these issues. Given that B lymphocytes exhibit the longest mean TL and CD8+CD28−T cells have the shortest mean TL,20) it was hypothesized that measuring TL in a specific subset of lymphocytes would produce more consistent results. As a result, the present study chose to focus on T lymphocytes because activated T-cells and, to a lesser extent naive/resting T-cells, regularly transmigrate into the central nervous system via the blood-brain barrier under physiological conditions and act as ROS.21) Thus, the aim of the present study was to investigate pathological conditions22) that act as sources of pro-inflammatory cytokines and cytotoxic substances to examine TL in patients with either early (DI ≤5 years) or chronic (DI >5 years) psychosis using T lymphocytes.
METHODS
Subjects
Patients were recruited from the outpatient clinic of the Department of Psychiatry at Chonbuk National University Hospital between July 2015 and December 2015. The inclusion criteria were as follows: 1) diagnosis of a schizophrenia spectrum disorder (schizophrenia, schizoaffective disorder, schizophreniform disorder, psychotic disorder not otherwise specified [NOS], or brief psychotic disorder [BPD]), attenuated psychosis syndrome (APS), or a delusional disorder according to the criteria of the Structural Clinical Interview from the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (SCID-IV)23,24); 2) aged between 19 and 65 years; and 3) the ability to comprehend the procedure and aims of the present study. Criteria from the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) were applied to diagnose APS.
The exclusion criteria were as follows: 1) diagnosis of mental retardation (intelligence quotient <70); 2) history of head trauma; 3) serious neurological disorder (epilepsy, stroke, Parkinson’s disease, and/or dementia); and/or 4) an acute, unstable, and significant medical illness. In total, 35 healthy individuals were recruited for the control group via advertisements; these participants were age- and sex-matched to the patients with early psychosis and interviewed using the screening module of the SCID-IV, non-patient edition.24,25) Control subjects were excluded from the present study based on the following: a current or previous diagnosis of a psychiatric or neurological disorder, alcohol or drug abuse or dependence (except for nicotine), and/or another significant medical condition. All participants provided written informed consent in accordance with a protocol approved by the Ethics Committee of the Chonbuk National University Hospital (approval number 2015-04-027-008).
Measures
Data on sociodemographic (age, sex, education, marital status, occupation, and smoking) and clinical (DI, medication, and metabolic syndrome) variables were obtained. The severity of psychiatric symptoms was measured using the Positive and Negative Syndrome Scale (PANSS)26); depressive features were evaluated using the Calgary Depression Scale for Schizophrenia (CDSS)27); and cognitive functioning was assessed with the Clinical Global Impression Scale for Cognitive Symptoms of Schizophrenia (CGI-C).28) Metabolic syndrome was defined according to the criteria of the third report of the National Cholesterol Education Program, Adult Treatment Panel (NCEP ATP III)29) in which the World Health Organization guidelines for abdominal obesity (2000) were applied. Daily doses of antipsychotic medications were converted into chlorpromazine-equivalent doses using an equivalency table provided by Hales.30) For blonanserin, 4 mg was considered to be equivalent to 1 mg of risperidone based on previous studies.31,32) Similarly, the ratio of paliperidone extended release (ER) to risperidone was determined to be 2, because the effective dose 50 values of striatal dopamine D2 receptor occupancy for paliperidone ER and risperidone are 2.38 mg/day33) and 1.2 mg/day,34) respectively.
Isolation of T Lymphocytes
A blood sample from the fasting ulnar vein (10 ml) was drawn from each individual and collected in vacutainer tubes containing preservative-free heparin. Next, PBMCs were isolated using standard density centrifugation (2,500 rpm for 30 min at 25°C without a break) with Ficoll-Paque PLUS medium (d=1.077; Amersham Pharmacia Biotech, Uppsala, Sweden). Red blood cell lysis buffer (BioLegend, San Diego, CA, USA) was used to remove residual red blood cells, and the platelets were then removed by centrifugation (1,000 rpm for 15 min at room temperature). Finally, the purification of the T lymphocytes was performed with a T cell enrichment column (R&D Systems, Minneapolis, MN, USA), and the lymphocytes were kept at −70°C until further analysis. According to a flow cytometry analysis (BD Biosciences, San Jose, CA, USA), the purity of the T lymphocytes was >92% after staining with monoclonal antibodies for human CD3 antigens PE (BD Biosciences). The entire procedure was completed within 4 hours of drawing the blood samples.
Determination of TL Using Quantitative Real-time Polymerase Chain Reaction Analyses
The measurement of relative TL was conducted using a quantitative real-time polymerase chain reaction (PCR) assay adapted from the original method.35) DNA was extracted from the T lymphocytes with a QIAamp DNA Mini kit (Qiagen, Hilden, Germany), and the quantity and quality were determined using a Colibri Microvolume Spectrophotometer 4 (Titertek-Berthold, Pforzheim, Germany); a density ratio of 260/280 nm >1.7 was considered to be acceptable. DNA samples (5 ng) were processed in triplicate for both the telomere and the single-copy hemoglobin-b (Hgb) gene with the Applied Biosystems 7900HT Fast Real Time PCR System (Applied Biosystems, Foster City, CA, USA). The reaction system contained 5 ng of DNA, 5 μl of 1× Power SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK), and various primers (Bioneer Inc., Seoul, Korea).
The primers used in the present study were as follows: the telomere PCR primers were 100 nM of Tel1 (5′ CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′) and 900 nM of Tel 2 (5′-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3′), and the Hgb PCR primers were 300 nM of Hgb1 (5′-GCTTCTGACACAACTGTGTTCACTAGC-3′) and 700 nM of Hgb2 (5′-CACCAACTTCATCCACGTTCACC-3′). The reaction condition for the telomere was 50°C for 2 min, 95°C for 10 min, 95°C for 15 sec, 56°C for 30 sec, and 72°C for 30 sec (40 cycles). The reaction condition for Hgb was 50°C for 2 min, 95°C for 10 min, 95°C for 15 sec, 58°C for 20 sec, and 72°C for 20 sec (40 cycles). No-template control and dissociation curve analyses were performed to indicate specificity. A dilution series of a pooled of DNA (33.33, 5.556, 0.926, 0.154, and 0.026 ng) for both telomere and Hgb was included in each plate to create reaction-specific standard curves and R2 values; both the telomere and Hgb reaction values were >0.99. Four independent samples were included in each plate to control the variation between different plates, and the cycle threshold (Ct) mean values of the independent samples in each plate were used as calibrators. The relative TL was calculated using the comparative 2−ΔΔCt method: ΔCt= CtTel−CtHgb, ΔΔCt=ΔCtsample−ΔCtcalibrator. The intra-assay analysis in the present study excluded the samples from the triplicates with a standard deviation >0.5.36) The coefficients of variation (CVs) for the triplicate samples were 0.78% for the telomere reaction and 0.40% for the Hgb reaction, which indicated low measurement error.37) For the inter-assay analysis, the CVs for the independent samples between the different plates were 1.18% for the telomere reaction and 0.45% for the Hgb reaction.
Statistical Analysis
All statistical analyses were performed using the IBM SPSS Statistics ver. 20.0 ( IBM Co., Armonk, NY, USA), and p values <0.05 were considered to indicate statistical significance. The Shapiro-Wilk test was employed to assess the normality of the TL data; t tests and analysis of variance (ANOVA) and chi-square tests were carried out to assess the continuous and categorical variables, respectively. Bonferroni corrections were made for multiple comparisons, and age, number of cigarettes per day, metabolic syndrome, DI, daily doses of antipsychotics, and cognitive function were included as covariates to control for confounding effects on TL. Pearson correlation tests were conducted to analyze relationships between TL and age or PANSS scores.
RESULTS
The demographic and clinical characteristics of participants are presented in Table 1. The diagnoses for the early psychosis group were as follows; schizophrenia (n=24), schizophreniform disorder (n=10), psychotic disorder NOS (n=5), BPD (n=1), and APS (n=3). The diagnoses for the chronic psychosis group were as follows; schizophrenia (n=75), schizoaffective disorder (n=3), schizophreniform disorder (n=1), psychotic disorder NOS (n=3), and delusional disorder (n=1). There were significant differences between the two patient groups and the control group in terms of age, smoking, occupation, marriage, and chlorpromazine-equivalent dose.
Table 1.
Demographic and clinical characteristics of participants
| Variable | Control (n=35) | Early psychosis (n=43) | Chronic psychosis (n=83) | p value |
|---|---|---|---|---|
| Age (yr) | 32.06±8.64* | 31.63±11.10* | 42.10±9.72 | <0.001 |
| Sex, male/female | 16/19 | 22/21 | 47/36 | 0.538 |
| Education | ||||
| Elementary school | 0 | 0 | 5 (6.0) | 0.232 |
| High school | 13 (37.1) | 20 (46.5) | 32 (38.6) | |
| University or higher | 22 (62.9) | 23 (53.5) | 46 (55.4) | |
| Number of cigarettes/day | 0 | 3.88±7.53† | 3.28±7.04† | 0.017 |
| Occupation | ||||
| Student | 10 (28.6) | 9 (20.9) | 11 (13.3) | <0.001‡ |
| Unemployed | 12 (34.3) | 24 (55.8) | 62 (74.7) | |
| Employed | 13 (37.1) | 10 (23.3) | 10 (12.0) | |
| Marital status | ||||
| Never married | 16 (45.7) | 33 (76.8) | 50 (60.2) | 0.012‡ |
| Married | 19 (54.3) | 7 (16.3) | 25 (30.1) | |
| Married but separated, or divorced | 0 | 3 (7.0) | 6 (7.2) | |
| Widowed | 0 | 0 | 2 (2.4) | |
| Chlorpromazine equivalent (mg/day) | 0*,§ | 311.50±320.85* | 641.76±445.70†,§ | <0.001 |
| Metabolic syndrome | - | 7 (16.3) | 28 (33.7) | 0.039 |
| Disease characteristics | ||||
| DI | - | 34.49±45.27 | 172.27±90.40 | <0.001 |
| CGI-C | - | 1.98±0.94 | 2.78±1.10 | <0.001 |
| CDSS | - | 3.35±3.86 | 2.18±2.96 | 0.061 |
| Positive PANSS | - | 13.12±7.31 | 12.06±5.45 | 0.362 |
| Negative PANSS | - | 12.61±6.20 | 13.76±5.18 | 0.299 |
| General PANSS | - | 24.54±8.71 | 21.92±4.79 | 0.031 |
| Total PANSS | - | 50.26±19.29 | 47.74±12.44 | 0.734 |
Values are presented as mean±standard deviation or number (%).
DI, duration of illness; CGI-C, Clinical Global Impression Scale for Cognitive Symptoms of Schizophrenia; CDSS, Calgary Depression Scale for Schizophrenia; PANSS, Positive and Negative Syndrome Scale.
Significantly different from chronic psychosis;
significantly different from control;
significantly different among three groups;
significantly different from early psychosis.
The post-hoc results revealed that the mean age of the patients with chronic psychosis was significantly older (p<0.001) than was those of the controls and the patients with early psychosis; there were no significant differences in smoking between patients with early and chronic psychosis; and the chlorpromazine-equivalent dose was significantly higher (p<0.001) in patients with chronic psychosis than in patients with early psychosis. Because metabolic syndrome was not assessed in the control group, comparisons for this variable were made only between the patient groups; the prevalence of metabolic syndrome was higher (p=0.039) in patients with chronic psychosis than in patients with early psychosis. In terms of disease characteristics, patients with chronic psychosis had significantly higher scores on the CGI-C (p<0.001) and significantly lower scores on the general PANSS (p=0.031).
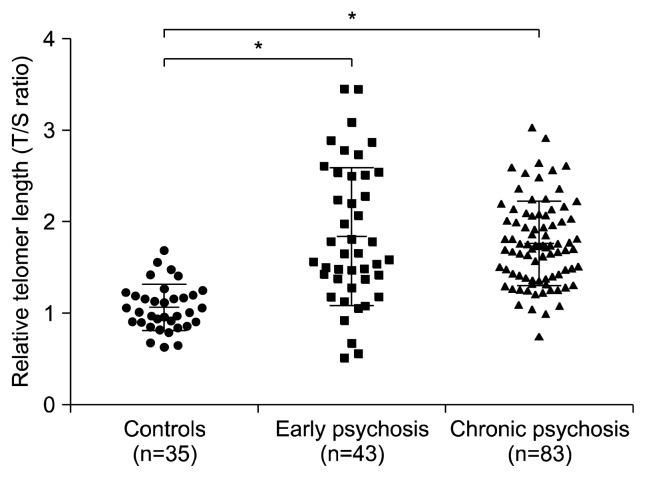
Regarding TL, there were significant differences among the three groups (F=26.74, degree of freedom [df]=2, p <0.001), and the post-hoc analyses revealed that the patients with early (1.842±0.752) and chronic (1.763±0.460) psychosis had significantly longer TL values of the T lymphocytes compared with the control group (1.063±0.752). These findings remained significant after controlling for age, smoking, metabolic syndrome, DI, chlorpromazine-equivalent dose, and cognitive functioning (F=9.57, df=2, p<0.001, Fig. 1). No significant correlations were observed between age and the TL of the T lymphocytes in the overall samples (r=0.103, p=0.192), the separate group of controls (r=−0.222, p=0.200), or all patients (r=−0.002, p=0.982). Additionally, the DI, chlorpromazine-equivalent doses, and the five-factor scores of the PANSS were not significantly correlated with the TL of T lymphocytes in either all patients or each psychosis group.
Fig. 1.
Relative telomere length among three groups determined by quantitative real-time polymerase chain reaction. Scatter plot graphs illustrate relative individual’s relative telomere length (mean and standard deviation).
*Significant differences (p<0.001); T/S, telomere to single copy gene.
DISCUSSION
Because high mortality rates, poor physical condition, and vulnerability to stress are associated with schizophrenia and affect TL, the present study measured TL in T lymphocytes. The results showed that the TL values in the early and chronic psychosis groups were significantly longer than those in the control group. These findings remained significant after controlling for covariates such as age, smoking, metabolic syndrome, DI, chlorpromazine- equivalent dose, and cognitive functioning. This was an unexpected finding because most previous studies10,13–15) found a shorter TL in patients with schizophrenia.
On the other hand, the study conducted by Nieratschker et al.,19) which is the largest-scale study (n=539) in the field of TL research performed to date, found that patients with schizophrenia had longer TL than healthy controls. These authors suggested four alternative explanations; 1) an association with endophenotype (hippocampal volume and/or impaired episodic memory), 2) the effects of psychotropic medications, 3) advanced paternal age, and 4) an association with increased mortality. Wikgren et al.38) reported that healthy apolipoprotein E (APOE) ɛ3/ɛ3 individuals with increased TL exhibit reduced hippocampal volumes and that APOE ɛ4 carriers with longer telomeres show poorer performance during episodic memory tasks.39) The present study did not objectively measure cognitive functioning or hippocampal volume, and controlling for cognitive function using CGI-C scores did not produce different results. However, it should be noted that the mean CGI-C scores of the patient groups were <3 (mild level). Moreover, the issues of paternal age and mortality could not be addressed in the present study because the mean age of the chronic psychosis group was 42 years, and information regarding paternal age was not obtained.
The most intriguing interpretations emerging from the present findings involve the association between anti-psychotic use and longer TL. Several studies in both animal40) and human subjects41,42) indicate that atypical anti-psychotics have antioxidant properties. Oxidative stress stimulates the export of telomerase reverse transcriptase (TERT), which is a catalytic protein component of telomerase, from the nucleus into the cytosol43); this process contributes to decreased telomerase activity and, subsequently, shorter TL. It is possible that the atypical anti-psychotics used by the patients in the present study may have blocked this effect and led to the present results: that is, a longer TL. Other suggested mechanisms by which antipsychotics increase TERT expression and telomerase activity include the modulation of intracellular Wnt/β-catenin or PI3K/Akt signaling pathways that is triggered by increases in brain-derived neurotrophic factor (BDNF) expression and 5-hydroxytryptamine (5-HT) modulation.44,45)
However, the results of several studies contradict these theories. When peripheral blood lymphocytes were obtained from healthy volunteers administered typical and/or atypical antipsychotics within the respective therapeutic ranges normally administered to patients, no effect of telomerase activity was observed for either type of drug.46) Furthermore, either decreases14) or no alterations47) in TL were observed in patients with first-episode/newly diagnosed antipsychotic-naïve schizophrenia. In subanalyses of the present data, no significant associations were found between the chlorpromazine-equivalent doses and DI or TL (data not shown). Thus, it seems essential that future studies measure both telomerase activity and TL within a prospective design. Another mechanism underlying this relationship could be related to the immunosuppressive effects of antipsychotics. Clozapine and haloperidol inhibit the proliferation of human lymphocytes48); more importantly, the in vitro stimulation of the T lymphocytes of medicated schizophrenia patients with anti-CD3 produces lower proliferative responses compared with those observed in well-matched controls.49) Therefore, decreased cellular senescence may have contributed to the longer TL observed in the present study. Regarding the correlation analyses, the lack of an association between age and TL may have been due to the fact that the mean age of the participants was younger than 42 years. Previous studies also found no significant correlations in healthy controls13,18) or patients with schizophrenia. Similarly, the lack of an association between PANSS scores and TL in the present study may be related to the lower mean PANSS scores in the patient groups.
Given that T lymphocytes are a promising neural candidate for studying psychiatric disorders50) and all previous studies have measured TL using PBMCs, the strength of the present study is the fact that it is the first to investigate TL in patients with psychosis using T lymphocytes. However, several limitations of this study should be mentioned. First, lifestyle factors, such as exercise and eating, affect TL.51,52) Because low levels of physical activity and poor eating habits are associated with schizophrenia,53) these factors should be measured and controlled for in future studies. Second, the TL of naïve T cells is longer than that of memory T cells,54) and changes in the proportion of activated or memory T cells have been reported in patients with schizophrenia.55,56) Thus, measuring TL in subpopulations of T lymphocytes would produce more accurate and consistent results. Third, the sample size of the early psychosis group in the present study was relatively small.
In conclusion, the T lymphocytes of patients with early and chronic psychosis had longer TL values compared with those of the healthy control group, and this finding remained significant after controlling for various confounding factors. Possible interpretations of the effects of anti-psychotics on TL were offered.
Acknowledgments
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C1459). The author would like to thank all the participants in the study and the Heavenly Father who guides along the right pathway. The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–62. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 2.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 3.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 4.Thomas P, O’Callaghan NJ, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev. 2008;129:183–190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Donovan A, Lin J, Tillie J, Dhabhar FS, Wolkowitz OM, Blackburn EH, et al. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2009;23:446–449. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brydon L, Lin J, Butcher L, Hamer M, Erusalimsky JD, Blackburn EH, et al. Hostility and cellular aging in men from the Whitehall II cohort. Biol Psychiatry. 2012;71:767–773. doi: 10.1016/j.biopsych.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, et al. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry. 2014;19:1163–1170. doi: 10.1038/mp.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kao HT, Cawthon RM, Delisi LE, Bertisch HC, Ji F, Gordon D, et al. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13:118–119. doi: 10.1038/sj.mp.4002105. [DOI] [PubMed] [Google Scholar]
- 11.Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci. 2011;66:202–213. doi: 10.1093/gerona/glq180. [DOI] [PubMed] [Google Scholar]
- 13.Yu WY, Chang HW, Lin CH, Cho CL. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J Psychiatry Neurosci. 2008;33:244–247. [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Egea E, Bernardo M, Heaphy CM, Griffith JK, Parellada E, Esmatjes E, et al. Telomere length and pulse pressure in newly diagnosed, antipsychotic-naive patients with nonaffective psychosis. Schizophr Bull. 2009;35:437–442. doi: 10.1093/schbul/sbn169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kota LN, Purushottam M, Moily NS, Jain S. Shortened telomere in unremitted schizophrenia. Psychiatry Clin Neurosci. 2015;69:292–297. doi: 10.1111/pcn.12260. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Cheng L, Craig DW, Redman M, Liu C. Cerebellar telomere length and psychiatric disorders. Behav Genet. 2010;40:250–254. doi: 10.1007/s10519-010-9338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansour H, Chowdari K, Fathi W, Elassy M, Ibrahim I, Wood J, et al. Does telomere length mediate associations between inbreeding and increased risk for bipolar I disorder and schizophrenia? Psychiatry Res. 2011;188:129–132. doi: 10.1016/j.psychres.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Malaspina D, Dracxler R, Walsh-Messinger J, Harlap S, Goetz RR, Keefe D, et al. Telomere length, family history, and paternal age in schizophrenia. Mol Genet Genomic Med. 2014;2:326–331. doi: 10.1002/mgg3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieratschker V, Lahtinen J, Meier S, Strohmaier J, Frank J, Heinrich A, et al. Longer telomere length in patients with schizophrenia. Schizophr Res. 2013;149:116–120. doi: 10.1016/j.schres.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Cheon J, Brown R, Coccia M, Puterman E, Aschbacher K, et al. Systematic and cell type-specific telomere length changes in subsets of lymphocytes. J Immunol Res. 2016;2016:5371050. doi: 10.1155/2016/5371050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levite M. Neurotransmitters activate T-cells and elicit crucial functions via neurotransmitter receptors. Curr Opin Pharmacol. 2008;8:460–471. doi: 10.1016/j.coph.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Brait VH, Arumugam TV, Drummond GR, Sobey CG. Importance of T lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. J Cereb Blood Flow Metab. 2012;32:598–611. doi: 10.1038/jcbfm.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB, Gibbon M, Spitzer RL, Williams J. Structured clinical interview for DSM-IV axis I disorders, research version. New York: New York State Psychiatric Institute; 1997. [Google Scholar]
- 24.Han OS, Hong JP. Structured clinical interview for DSM-IV axis I disorders, research version. Seoul: Korea Hana Medical Publishing; 2000. [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, William JB. Structured clinical interview for DSM-IV axis I disorders, research version, non-patient edition (SCID-I/NP) New York: New York State Psychiatric Institute; 1997. [Google Scholar]
- 26.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 27.Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl. 1993;(22):39–44. [PubMed] [Google Scholar]
- 28.Haro JM, Kamath SA, Ochoa S, Novick D, Rele K, Fargas A, et al. The Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand Suppl. 2003;(416):16–23. doi: 10.1034/j.1600-0447.107.s416.5.x. [DOI] [PubMed] [Google Scholar]
- 29.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Hales RE, Yudofsky SC, Gabbard GO American Psychiatric Publishing. The American Psychiatric Publishing textbook of psychiatry. 5th ed. Washington, DC: American Psychiatric Pub; 2008. [Google Scholar]
- 31.Tateno A, Arakawa R, Okumura M, Fukuta H, Honjo K, Ishihara K, et al. Striatal and extrastriatal dopamine D2 receptor occupancy by a novel antipsychotic, blonanserin: a PET study with [11C]raclopride and [11C]FLB 457 in schizophrenia. J Clin Psychopharmacol. 2013;33:162–169. doi: 10.1097/JCP.0b013e3182825bce. [DOI] [PubMed] [Google Scholar]
- 32.Hori H, Yamada K, Kamada D, Shibata Y, Katsuki A, Yoshimura R, et al. Effect of blonanserin on cognitive and social function in acute phase Japanese schizophrenia compared with risperidone. Neuropsychiatr Dis Treat. 2014;10:527–533. doi: 10.2147/NDT.S59861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arakawa R, Ito H, Takano A, Takahashi H, Morimoto T, Sassa T, et al. Dose-finding study of paliperidone ER based on striatal and extrastriatal dopamine D2 receptor occupancy in patients with schizophrenia. Psychopharmacology (Berl) 2008;197:229–235. doi: 10.1007/s00213-007-1029-z. [DOI] [PubMed] [Google Scholar]
- 34.Nyberg S, Eriksson B, Oxenstierna G, Halldin C, Farde L. Suggested minimal effective dose of risperidone based on PET-measured D2 and 5-HT2A receptor occupancy in schizophrenic patients. Am J Psychiatry. 1999;156:869–875. doi: 10.1176/ajp.156.6.869. [DOI] [PubMed] [Google Scholar]
- 35.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kananen L, Surakka I, Pirkola S, Suvisaari J, Lönnqvist J, Peltonen L, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry. 2013;18:576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wikgren M, Karlsson T, Nilbrink T, Nordfjäll K, Hultdin J, Sleegers K, et al. APOE ɛ4 is associated with longer telomeres, and longer telomeres among ɛ4 carriers predicts worse episodic memory. Neurobiol Aging. 2012;33:335–344. doi: 10.1016/j.neurobiolaging.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Wikgren M, Karlsson T, Lind J, Nilbrink T, Hultdin J, Sleegers K, et al. Longer leukocyte telomere length is associated with smaller hippocampal volume among non-demented APOE ɛ3/ɛ3 subjects. PLoS One. 2012;7:e34292. doi: 10.1371/journal.pone.0034292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parikh V, Khan MM, Mahadik SP. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2003;37:43–51. doi: 10.1016/S0022-3956(02)00048-1. [DOI] [PubMed] [Google Scholar]
- 41.Miljević Č, Nikolić-Kokić A, Nikolić M, Niketić V, Spasić MB, Lečić-Toševski D, et al. Effect of atypical antipsychotics on antioxidant enzyme activities in human erythrocytes (in vitro study) Hum Psychopharmacol. 2013;28:1–6. doi: 10.1002/hup.2272. [DOI] [PubMed] [Google Scholar]
- 42.Brinholi FF, Farias CC, Bonifácio KL, Higachi L, Casagrande R, Moreira EG, et al. Clozapine and olanzapine are better antioxidants than haloperidol, quetiapine, risperidone and ziprasidone in in vitro models. Biomed Pharmacother. 2016;81:411–415. doi: 10.1016/j.biopha.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 43.Saretzki G. Extra-telomeric functions of human telomerase: cancer, mitochondria and oxidative stress. Curr Pharm Des. 2014;20:6386–6403. doi: 10.2174/1381612820666140630095606. [DOI] [PubMed] [Google Scholar]
- 44.Bersani FS, Lindqvist D, Mellon SH, Penninx BW, Verhoeven JE, Révész D, et al. Telomerase activation as a possible mechanism of action for psychopharmacological interventions. Drug Discov Today. 2015;20:1305–1309. doi: 10.1016/j.drudis.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Polho GB, De-Paula VJ, Cardillo G, dos Santos B, Kerr DS. Leukocyte telomere length in patients with schizophrenia: a meta-analysis. Schizophr Res. 2015;165:195–200. doi: 10.1016/j.schres.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 46.Porton B, Delisi LE, Bertisch HC, Ji F, Gordon D, Li P, et al. Telomerase levels in schizophrenia: a preliminary study. Schizophr Res. 2008;106:242–247. doi: 10.1016/j.schres.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Hu M, Zong X, He Y, Wang D, Dai L, et al. Association of telomere length and mitochondrial DNA copy number with risperidone treatment response in first-episode antipsychotic-naïve schizophrenia. Sci Rep. 2015;5:18553. doi: 10.1038/srep18553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leykin I, Mayer R, Shinitzky M. Short and long-term immunosuppressive effects of clozapine and haloperidol. Immunopharmacology. 1997;37:75–86. doi: 10.1016/S0162-3109(97)00037-4. [DOI] [PubMed] [Google Scholar]
- 49.Craddock RM, Lockstone HE, Rider DA, Wayland MT, Harris LJ, McKenna PJ, et al. Altered T-cell function in schizophrenia: a cellular model to investigate molecular disease mechanisms. PLoS One. 2007;2:e692. doi: 10.1371/journal.pone.0000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gladkevich A, Kauffman HF, Korf J. Lymphocytes as a neural probe: potential for studying psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:559–576. doi: 10.1016/j.pnpbp.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Lin J, Epel E, Blackburn E. Telomeres and lifestyle factors: roles in cellular aging. Mutat Res. 2012;730:85–89. doi: 10.1016/j.mrfmmm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Østhus IB, Sgura A, Berardinelli F, Alsnes IV, Brønstad E, Rehn T, et al. Telomere length and long-term endurance exercise: does exercise training affect biological age? A pilot study. PLoS One. 2012;7:e52769. doi: 10.1371/journal.pone.0052769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dipasquale S, Pariante CM, Dazzan P, Aguglia E, McGuire P, Mondelli V. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res. 2013;47:197–207. doi: 10.1016/j.jpsychires.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Weng NP, Levine BL, June CH, Hodes RJ. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci U S A. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drexhage RC, Hoogenboezem TA, Cohen D, Versnel MA, Nolen WA, van Beveren NJ, et al. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychopharmacol. 2011;14:746–755. doi: 10.1017/S1461145710001653. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez-Egea E, Vértes PE, Flint SM, Turner L, Mustafa S, Hatton A, et al. Peripheral immune cell populations associated with cognitive deficits and negative symptoms of treatment-resistant schizophrenia. PLoS One. 2016;11:e0155631. doi: 10.1371/journal.pone.0155631. [DOI] [PMC free article] [PubMed] [Google Scholar]