Abstract
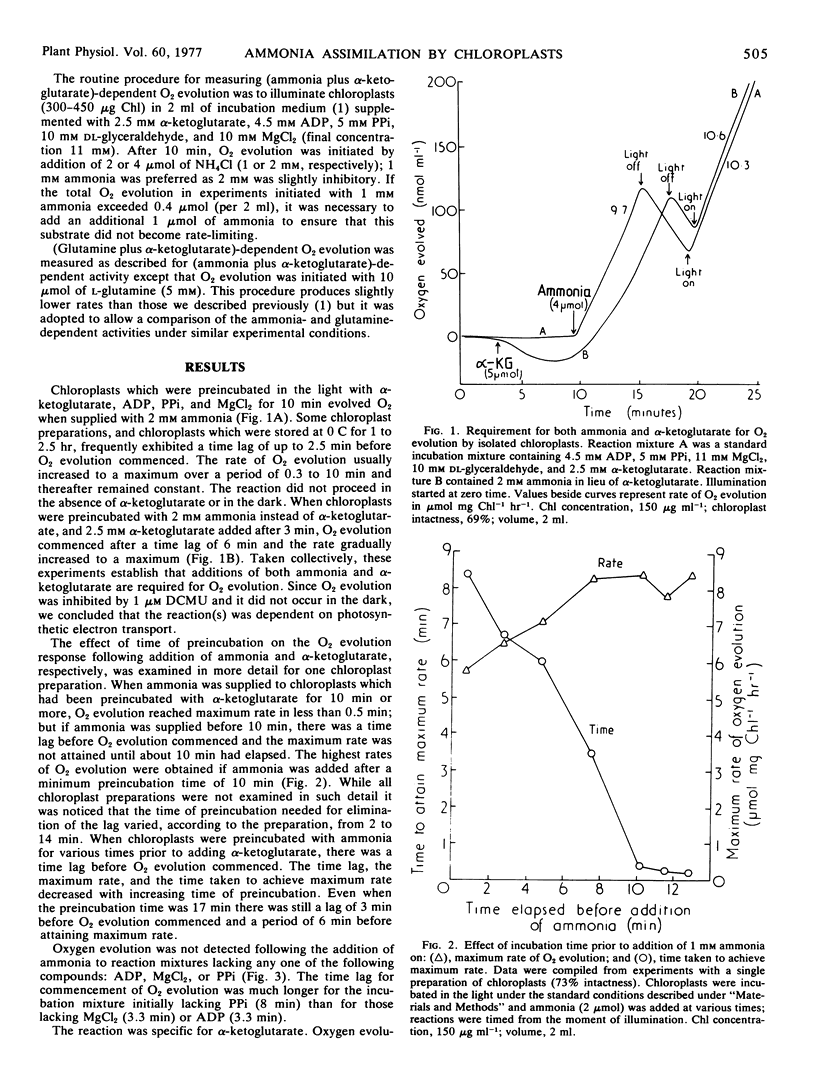
Illuminated pea (Pisum sativum) chloroplasts catalyze (ammonia plus α-ketoglutarate [α-KG])-dependent O2 evolution at rates which are commensurate with other estimates of the flux of assimilated nitrogen (mean of eight determinations, 8.3 μmole per mg chlorophyll per hour, sd 2.4). The reaction was usually initiated with 1 mm ammonia after preincubating chloroplasts in the presence of α-KG, ADP, pyrophosphate, and MgCl2.
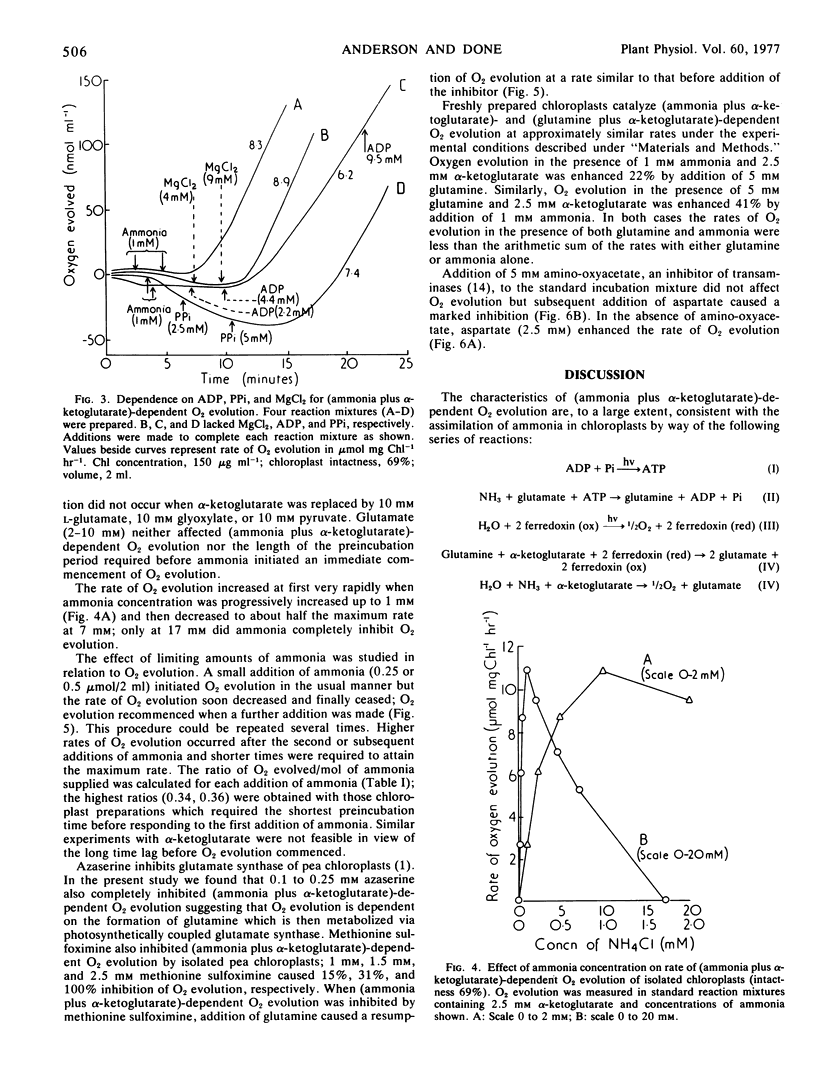
Progressive increases in ammonia concentration gave Vmax/2 at 0.2 mm (approximately) and Vmax at about 1 mm. Higher concentrations were inhibitory; at 7 mm the rate was again about Vmax/2. The highest ratio of O2 evolved per mol of ammonia supplied was 0.36.
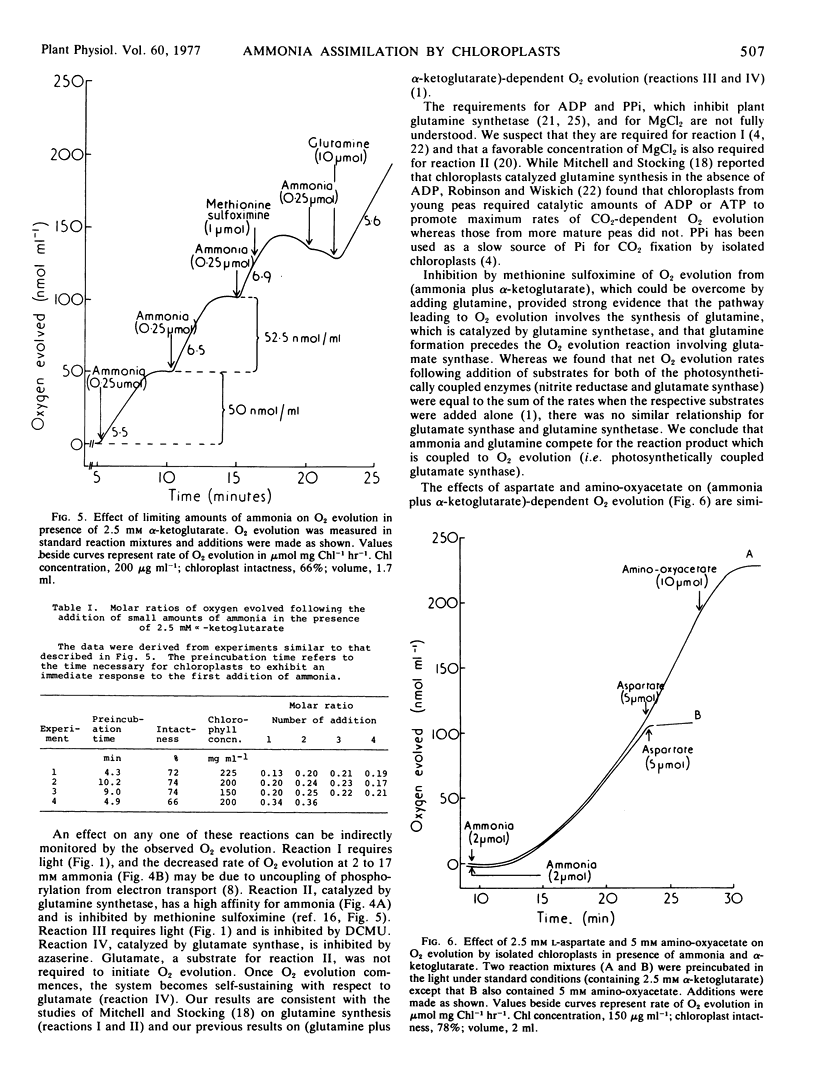
The (ammonia plus α-KG)-dependent reaction was inhibited by methionine sulfoximine, azaserine, and aspartate in the presence of amino-oxyacetate but not by amino-oxyacetate alone and not by l-glutamate. The rate of O2 evolution in the presence of 1 mm ammonia and 2.5 mm α-KG was increased only slightly by addition of 5 mm glutamine. Similarly, the rate of O2 evolution in the presence of 5 mm glutamine and 2.5 mm α-KG was increased only slightly by addition of 1 mm ammonia.
The results are attributed to the incorporation of ammonia via glutamine synthetase and reductive transamination of the glutamine formed by photosynthetically coupled glutamate synthase using α-KG as the amino acceptor. Several lines of evidence rule out the possibility that photosynthetically coupled glutamate dehydrogenase is involved.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Done J. A polarographic study of glutamate synthase activity in isolated chloroplasts. Plant Physiol. 1977 Sep;60(3):354–359. doi: 10.1104/pp.60.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J. E. Effect of methionine sulfoximine and methionine sulfone on glutamate synthesis in Klebsiella aerogenes. J Bacteriol. 1973 May;114(2):666–673. doi: 10.1128/jb.114.2.666-673.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., Baldry C. W., Walker D. A. Some effects of inorganic phosphate on O2 evolution by isolated chloroplasts. Biochim Biophys Acta. 1967;143(3):614–624. doi: 10.1016/0005-2728(67)90067-9. [DOI] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Leech R. M., Kirk P. R. An NADP-dependent L-glutamate dehydrogenase from chloroplasts of Vicia faba L. Biochem Biophys Res Commun. 1968 Aug 21;32(4):685–690. doi: 10.1016/0006-291x(68)90293-3. [DOI] [PubMed] [Google Scholar]
- Magalhaes A. C., Neyra C. A., Hageman R. H. Nitrite assimilation and amino nitrogen synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Mar;53(3):411–415. doi: 10.1104/pp.53.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. A., Stocking C. R. Kinetics and Energetics of Light-driven Chloroplast Glutamine Synthesis. Plant Physiol. 1975 Jan;55(1):59–63. doi: 10.1104/pp.55.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Localisation of glutamine synthetase in chloroplasts. Nat New Biol. 1973 Nov 14;246(150):61–62. doi: 10.1038/newbio246061a0. [DOI] [PubMed] [Google Scholar]
- O'neal D., Joy K. W. Glutamine synthetase of pea leaves: divalent cation effects, substrate specificity, and other properties. Plant Physiol. 1974 Nov;54(5):773–779. doi: 10.1104/pp.54.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neal T. D., Joy K. W. Pea leaf glutamine synthetase: regulatory properties. Plant Physiol. 1975 Jun;55(6):968–974. doi: 10.1104/pp.55.6.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Stimulation of carbon dioxide fixation in isolated pea chloroplasts by catalytic amounts of adenine nucleotides. Plant Physiol. 1976 Aug;58(2):156–162. doi: 10.1104/pp.58.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius K. A., Stocking C. R. Intracellular localization of enzymes in leaves and chloroplast membrane permeability to compounds involved in amino acid syntheses. Z Naturforsch B. 1969 Sep;24(9):1170–1179. doi: 10.1515/znb-1969-0915. [DOI] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Phosphoglycerate as a hill oxidant in a reconstituted chloroplast system. Plant Physiol. 1971 Aug;48(2):163–165. doi: 10.1104/pp.48.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner J. E., Webster G. C. Studies on the Enzymatic Synthesis of Glutamine. Plant Physiol. 1955 Sep;30(5):393–402. doi: 10.1104/pp.30.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Slabas A. R. Stepwise generation of the natural oxidant in a reconstituted chloroplast system. Plant Physiol. 1976 Feb;57(2):203–208. doi: 10.1104/pp.57.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]


