Abstract
Objective
We examined the associations of maternal plasma n-3 and n-6 PUFA concentrations during pregnancy with infant subcutaneous fat.
Methods
In a population-based prospective cohort study among 904 mothers and their infants, we measured maternal plasma n-3 and n-6 PUFA concentrations at mid-pregnancy. Body mass index, total subcutaneous fat and central-to-total subcutaneous fat ratio were calculated at 1.5, 6 and 24 months.
Results
Maternal n-3 PUFA levels were not consistently associated with infant body mass index or total subcutaneous fat. Higher maternal total n-3 PUFA levels, and specifically eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA), were associated with higher central-to-total subcutaneous fat ratio at 1.5 months, whereas higher maternal total n-3 PUFA levels were associated with lower central-to-total subcutaneous fat ratio at 6 months (all p-values<0.05). These associations were not present at 24 months. Maternal n-6 PUFA levels were not consistently associated with infant subcutaneous fat. A higher n-6/n-3 ratio was associated with lower central-to-total subcutaneous fat ratio at 1.5 months only (p-value<0.05).
Conclusions
Maternal n-3 PUFA levels during pregnancy may have transient effects on infant subcutaneous fat. Further studies are needed to assess the effects of maternal PUFA concentrations on fat mass development during early infancy.
Keywords: polyunsaturated fatty acids, pregnancy, subcutaneous fat, infancy
Introduction
Inadequate maternal intake of polyunsaturated fatty acids (PUFA) during pregnancy may influence the risk of obesity in offspring (1, 2). Several in vitro and animal studies have suggested that PUFA affect the adipose tissue development during fetal and early postnatal life (3). Among PUFA, n-3 and n-6 PUFA are of particular relevance during early life since pathways stimulated by n-6 PUFA seem to promote, while those stimulated by n-3 PUFA seem to inhibit the differentiation of adipocytes (4, 5). A US study among 1,250 mother-child pairs showed that higher maternal plasma n-3 PUFA concentrations during pregnancy tended to be associated with lower total subcutaneous fat mass in children aged 3 years (6). In the same study, higher maternal plasma n-6/n-3 PUFA ratio was associated with higher total subcutaneous fat mass in early childhood (6). A Dutch study among 234 mothers and their children showed that higher maternal plasma concentrations of dihomo-gamma linolenic acid (DGLA), a n-6 PUFA, during pregnancy were associated with higher total subcutaneous fat mass measured in children aged 7 years (7). In line with these studies, we have previously reported that lower maternal n-3 PUFA concentrations and higher n-6 PUFA concentrations during pregnancy were associated with higher total body fat and abdominal fat levels at 6 years. Thus far, not much is known about the influence of maternal plasma PUFA concentrations during pregnancy on subcutaneous fat mass development throughout infancy. Infancy is a period characterized by rapid growth and subcutaneous fat mass development and is a well-known critical period for obesity and cardio-metabolic diseases later in life (8, 9). By assessing these associations in infancy further insight into the early programming effects of maternal PUFA levels on onset and timing of adiposity in the offspring can be obtained.
Therefore, we examined, in a population-based prospective cohort study from early pregnancy onwards among 904 mothers and their infants, the associations of maternal plasma n-3 and n-6 PUFA levels during pregnancy with infant subcutaneous fat mass measures.
Methods
Study design
This study was embedded in the Generation R Study, a population-based prospective cohort study from early pregnancy onwards among 9,778 mothers and their children living in Rotterdam, the Netherlands (10, 11). The study protocol was approved by the local Medical Ethical Committee. Written informed consent was obtained from parents. Additional detailed assessments of fetal and infant growth and development were conducted in a subgroup of Dutch mothers and their children from late pregnancy onwards. Of all approached women, 80% agreed to participate. A total of 1,205 mothers and their singleton children participated in the subgroup study, of whom 1,083 mothers had plasma PUFA concentrations available. Of the group of 1,083 mothers and their children, 904 children had body mass index or skinfold thicknesses measured at the age of 1.5, 6 or 24 months (Flow chart is given in Supplementary Figure S1).
Maternal fatty acid status
Maternal non-fasting venous samples were drawn at a median gestational age of 20.5 weeks (95% range:18.6, 22.7). As previously described, EDTA plasma samples were selected and transported to the Division of Metabolic Diseases and Nutritional Medicine, Dr. von Hauner Children’s Hospital, University of Munich Medical Center to analyze PUFA concentrations (12). After being thawed, the analysis of plasma glycerophospholipid fatty acids was performed by a sensitive and precise high-throughput method, suitable in large epidemiological studies, as previously described (13). Based on findings from previous studies, we selected maternal PUFA for our analyses, which have been associated with the risk of obesity in children and adults (6, 14). Selected maternal PUFA were total n-3 PUFA, which included α-linolenic acid (ALA, C18:3n-3), eicosapentaenoic acid (EPA, C20:5n-3), docosapentaenoic acid (DPA,C22:5n-3), and docosahexaenoic acid (DHA, C22:6n-3). Total n-6 PUFA included linoleic acid (LA, C18:2n-6), γ-linolenic acid (GLA, C18:3n-6), eicosadienoic acid (EDA, C20:2n-6), dihomo-gamma-linolenic acid (DGLA, C20:3n6), arachidonic acid (AA, C20:4n-6), and docosatetraenoic acid (DTA, C22:4n-6). PUFA levels were expressed as proportion of total fatty acids present in the chromatogram (weight percentage, wt%). (15). We also calculated the ratio of total n-6/n-3 PUFA. We observed similar results when we used fatty acid concentrations in mg/L instead of percentages (results not shown).
Body fat measurements during infancy
We measured weight to the nearest gram in naked infant at the age of 1.5 and 6 months by using an electronic infant scale and at 24 months by using a mechanical personal scale (SECA, Almere, The Netherlands). Body length at the age of 1.5 and 6 months was measured in supine position to the nearest millimeter by using a neonatometer and body height at 24 months was measured in standing position by using a Harpenden stadiometer (Holtain Limited, Dyfed, UK). Body mass index (kg/m2) was calculated and we constructed standard deviation scores based on our study sample. We observed similar results when we used age- and sex-adjusted body mass index standard deviation scores at 24 months based on the World Health Organization Child Growth Standards (results not shown). We measured skinfold thicknesses at the ages of 1.5, 6 and 24 months on the left side of the body at the biceps, triceps, suprailiacal and subscapular area by using a skinfold caliper (Slim Guide, Creative Health Products) according to standard procedures described in detail previously. Two measurements were performed at each site and the mean was used in the analyses. Intraclass correlation coefficient among observers was 0.88 and between observers was 0.76 (16). As previously described, we calculated total subcutaneous fat mass from the sum of all four skinfold thicknesses, central subcutaneous fat mass from the sum of suprailiacal and subscapular skinfold thicknesses and peripheral subcutaneous fat mass from the sum of biceps and triceps skinfold thicknesses (17). Measurements of body fat quantity and distribution require appropriate adjustment for body size or total fat mass, respectively, in order to undertake informative comparisons between children and within children over time. To create total subcutaneous fat mass independent of length or height and central subcutaneous fat mass independent of total subcutaneous fat mass, we estimated the optimal adjustment by log-log regression analyses (18). Based on these analyses, total subcutaneous fat mass was only weakly correlated with length at 1.5 and 6 months or height at 24 months and was not adjusted for it, whereas a central-to-total subcutaneous fat mass ratio was calculated as central divided by total subcutaneous fat mass. The central-to-peripheral subcutaneous fat mass ratio was calculated as central divided by peripheral subcutaneous fat mass.
Covariates
We obtained information on maternal age, educational level (low, medium, high), parity (nulliparous, multiparous), pre-pregnancy weight, smoking habits during pregnancy (no, yes) and folic acid supplement use (no, yes) using self-reported questionnaires during pregnancy. We measured maternal height at enrolment, and calculated pre-pregnancy body mass index (kg/m2). First trimester maternal nutritional information was obtained by food frequency questionnaire (19). Information about pregnancy complications, infants’ sex, gestational age and weight at birth was obtained from medical records. Gestational weight gain was calculated as the difference between maternal weight measured at 30 weeks of gestation (95% range: 28.5, 32.5) and pre-pregnancy weight. Information about breastfeeding duration and timing of introduction of solid foods (<3 months, 3-6 months, >6 months) was obtained by questionnaires in infancy.
Statistical analysis
We assessed the associations of maternal plasma n-3 and n-6 PUFA levels with infant adiposity measures at 1.5, 6 and 24 months and the change between these time points using linear regression models. These regression models were adjusted for gestational age at blood sampling, maternal age, educational level, parity, pre-pregnancy body mass index, maternal total energy intake, smoking habits, weight gain during pregnancy, folic acid supplement use, gestational diabetes, gestational hypertensive disorders, infants’ sex, gestational age-adjusted birth weight, breastfeeding duration and timing of introduction of solid foods. Included covariates were selected based on their associations with the exposures and outcomes of interest in previous studie s or a change in effect estimate of >10%. We constructed standard deviation scores (SDS) [(observed value - mean)/SD] for all PUFA and infant adiposity measures to enable comparison of effect estimates. We have also performed an additional analysis with central-to-peripheral subcutaneous fat mass ratio using linear regression models. In addition, we examined the associations of maternal plasma n-3 and n-6 PUFA concentrations during pregnancy with infant overweight and obesity at 24 months (body mass index above 85th percentile for age and sex) using logistic regression models. We tested for interaction terms between maternal PUFA levels and infants’ sex and birth weight in relation to infant adiposity measures at 1.5, 6 and 24 months. Since no statistically significant interactions were observed, no further stratified analyses were performed. We did not adjust the main results for multiple testing because the main exposures and outcomes were correlated. However, if we would apply Bonferroni correction, we would consider a p-value of 0.016 as significant (0.05/number of outcomes). In order to reduce potential bias associated with missing data and to maintain statistical power, we performed multiple imputations of missing covariates by generating 5 independent datasets using the Markov Chain Monte Carlo method after which the pooled effect estimates were calculated. All analyses were performed using Statistical Package for the Social Sciences version 21.0 for Windows (SPSS Inc, Chicago, IL, USA).
Results
Subject characteristics
Table 1 shows the maternal and infant characteristics. Mean (SD) second trimester maternal concentrations of total glycerophospholipid bound n-3 and n-6 PUFA were 111.4 (25.8) mg/L and 592.6 (86.0) mg/L, respectively (Table 2). Non-response analyses showed that as compared to mothers and infants with follow-up measurements, mothers without follow-up measurements were slightly younger and their infant were breastfed for a shorter period (p<0.05) (Supplementary Table S1). Also, mothers included in the analyses had a higher total n-3 PUFA concentrations compared to those not included (Supplementary Table S2). Correlation coefficients between all maternal PUFA concentrations are shown in Supplementary Table S3.
Table 1.
Characteristics of mothers and their infant (N = 904)1
| Value | |
|---|---|
| Maternal characteristics | |
| Age (years), mean (SD) | 31.9 (4.0) |
| Gestational age at PUFA measures (weeks), median (95% range ) | 20.5 (18.6, 22.7) |
| Pre-pregnancy body mass index (kg/m2), mean (SD) | 23.4 (4.1) |
| Gestational weight gain, kg, mean (SD) | 10.0 (4.5) |
| Education, n (%) higher education | 574 (64.0) |
| Parity, n (%) nulliparous | 555 (61.4) |
| Total energy intake, kcal, mean (SD) | 2126 (486) |
| Smoking during pregnancy, n yes (%) | 198 (24.1) |
| Folic acid supplement use, n yes (%) | 676 (90.7) |
| Gestational diabetes, n (%) | 10 (1.1) |
| Gestational hypertensive disorders, n (%) | 67 (7.6) |
| Infant characteristics | |
| Males, n (%) | 464 (51.3) |
| Gestational age at birth (weeks), median (95% range ) | 40.3 (35.8, 42.4) |
| Birth weight (g), mean (SD) | 3509 (544) |
| Breastfeeding duration (months), mean (SD) | 4.5 (3.8) |
| Introduction of solid foods n (%) >6 months | 147 (18.3) |
| Infant adiposity characteristics | |
| 1.5 months | |
| Body mass index (kg/m2), mean (SD) | 15.1 (1.4) |
| Total subcutaneous fat mass (mm), mean (SD) | 24.0 (7.3) |
| Central-to-total subcutaneous fat mass ratio, mean (SD) | 0.5 (0.1) |
| Central-to-peripheral subcutaneous fat mass ratio, mean (SD) | 1.0 (0.2) |
| 6 months | |
| Body mass index (kg/m2), mean (SD) | 16.8 (1.3) |
| Total subcutaneous fat mass (mm), mean (SD) | 27.0 (6.4) |
| Central-to-total subcutaneous fat mass ratio, mean (SD) | 0.5 (0.1) |
| Central-to-peripheral subcutaneous fat mass ratio, mean (SD) | 0.9 (0.2) |
| 24 months | |
| Body mass index (kg/m2), mean (SD) | 15.9 (1.3) |
| Total subcutaneous fat mass, mean (SD), mm | 27.3 (7.2) |
| Central-to-total subcutaneous fat mass ratio, mean (SD) | 0.4 (0.1) |
| Central-to-peripheral subcutaneous fat mass ratio, mean (SD) | 0.8 (0.2) |
Values represent means (SDs), median (95% range) or number of subjects (valid %). Body mass index = weight/height2. Total subcutaneous fat mass = biceps + triceps + suprailiacal + subscapular skinfold thicknesses.
Central-to-total subcutaneous fat mass ratio = (suprailiacal + subscapular skinfold thicknesses)/total subcutaneous fat mass.
Abbreviations: PUFA: Polyunsaturated fatty acids; SD: standard deviation.
Table 2.
Second trimester maternal PUFA concentrations (N =904)1
| Absolute values (mg/L) | Relative values (wt%) | |
|---|---|---|
| Total PUFA | 704.1 (96.4) | 42.5 (1.6) |
| Total n-3 PUFA | 111.4 (25.8) | 6.8 (1.4) |
| ALA | 5.4 (1.6) | 0.3 (0.1) |
| EPA | 10.1 (5.3) | 0.6 (0.3) |
| DPA | 12.9 (3.9) | 0.8 (0.2) |
| DHA | 81.4 (19.4) | 5.0 (1.1) |
| Total n-6 PUFA | 592.6 (86.0) | 36.3 (2.0) |
| LA | 348.9 (59.6) | 21.4 (2.5) |
| GLA | 1.5 (0.7) | 0.1 (0.0) |
| EDA | 8.3 (1.7) | 0.5 (0.1) |
| DGLA | 63.4 (16.3) | 3.9 (0.7) |
| ARA | 155.9 (31.7) | 9.5 (1.4) |
| DTA | 6.9 (2.0) | 0.4 (0.1) |
Values represent means (SDs).
Abbreviations: ALA: α-linolenic acid; AA: arachidonic acid; DGLA dihomo-gamma-linolenic acid; DHA: docosahexaenoic acid; DPA: docosapentaenoic acid; DTA: docosatetraenoic acid; EDA: eicosadienoic acid; EPA: eicosapentaenoic acid; GLA: γ-linolenic acid; LA: linoleic acid; PUFA; polyunsaturated fatty acid.
Maternal PUFA levels and infant fat mass
Table 3 shows that maternal total n-3 PUFA levels as well as each n-3 PUFA individually were not consistently associated with infant body mass index and total subcutaneous fat mass in the adjusted models. Higher maternal total n-3 PUFA and specifically EPA, DPA, and DHA levels were associated with higher infant central-to-total subcutaneous fat mass ratio at 1.5 months (all p-values< 0.05). However, only higher maternal total n-3 PUFA levels were associated with lower infant central-to-total subcutaneous fat mass ratio at the age of 6 months (p-value< 0.05). Maternal n-3 PUFA levels were not associated with infant central-to-total subcutaneous fat mass ratio at 24 months. Similar results were found in the unadjusted analyses and are given in Supplementary Table S4. Supplemental Table S5 shows that higher maternal total n-3 PUFA levels and, specifically, DPA and DHA levels were associated with a decrease in central-to-total subcutaneous fat mass ratio from 1.5 to 24 months, but no associations were found for total subcutaneous fat mass.
Table 3.
Maternal n-3 PUFA levels and infant subcutaneous fat mass measures at 1.5, 6 and 24 months (N =904)1-2
| Maternal n-3 PUFA in SDS | Adiposity measures at 1.5 months in SDS Difference (95% confidence interval) | Adiposity measures at 6 months in SDS Difference (95% confidence interval) | Adiposity measures at 24 months in SDS Difference (95% confidence interval) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Body mass index | Total subcutaneous fat mass | Central-to-total subcutaneous fat mass ratio | Body mass index | Total subcutaneous fat mass | Central-to-total subcutaneous fat mass ratio | Body mass index | Total subcutaneous fat mass | Central-to-total subcutaneous subcutaneous fat | |
| Total | 0.02 (-0.05, 0.09) | -0.02 (-0.10, 0.05) | 0.12 (0.05, 0.20)* | -0.01 (-0.09, 0.06) | -0.02 (-0.10, 0.05) | -0.08 (-0.15, -0.01)* | 0.01 (-0.06, 0.09) | 0.05 (-0.03, 0.13) | -0.01 (-0.09, 0.07) |
| ALA | 0.05 (-0.01, 0.12) | -0.06 (-0.13, 0.02) | 0.02 (-0.06, 0.09) | 0.01 (-0.07, 0.07) | 0.02 (-0.05, 0.09) | -0.03 (-0.10, 0.04) | 0.02 (-0.06, 0.09) | 0.01 (-0.08, 0.08) | -0.02 (-0.10, 0.05) |
| EPA | 0.01 (-0.06, 0.07) | -0.01 (-0.08, 0.07) | 0.07 (0.01, 0.15)* | -0.01 (-0.07, 0.07) | -0.03 (-0.10, 0.04) | -0.07 (-0.14, 0) | 0.01 (-0.07, 0.09) | 0.04 (-0.04, 0.12) | 0.01 (-0.07, 0.09) |
| DPA | 0.03 (-0.04, 0.09) | -0.13 (-0.20, -0.05)* | 0.11 (0.04, 0.18)* | -0.06 (-0.12, 0.01) | -0.05 (-0.12, 0.02) | -0.02 (-0.10, 0.05) | -0.06 (-0.14, 0.01) | 0.02 (-0.06, 0.09) | -0.06 (-0.13, 0.02) |
| DHA | 0.01 (-0.06, 0.08) | 0.01 (-0.07, 0.08) | 0.11 (0.03, 0.18)* | -0.01 (-0.08, 0.07) | -0.01 (-0.08, 0.07) | -0.07 (-0.14, 0) | 0.02 (-0.05, 0.10) | 0.04 (-0.04, 0.12) | 0.01 (-0.08, 0.09) |
Values are regression coefficients (95% Confidence Interval) that reflect the difference in SDS of infant body mass index and fat mass measures at 1.5, 6 and 24 months per SD change in maternal n-3 PUFA levels. Body mass index = weight/height2. Total subcutaneous fat mass = biceps + triceps + suprailiacal + subscapular skinfold thicknesses. Central-to-total subcutaneous fat mass ratio = (suprailiacal + subscapular skinfold thicknesses)/total subcutaneous fat mass.
Models are adjusted for gestational age at blood sampling, maternal age, educational level, parity, pre-pregnancy body mass index, maternal total energy intake, smoking habits and weight gain during pregnancy, folic acid supplement use, gestational diabetes, gestational hypertensive disorders, infants' sex, gestational age-adjusted birth weight standard-deviation scores, breastfeeding duration and timing of introduction of solid foods (for 6 and 24 months).
P-value<0.05.
Table 4 shows that, in the adjusted models, higher maternal total n-6 PUFA levels were associated with a lower infant total subcutaneous fat mass at 1.5 months (p-value<0.05), but not with infant body mass index or central-to-total subcutaneous fat mass ratio at any time points. No consistent associations of individual n-6 PUFA levels with infant adiposity measures were present at 1.5, 6 or 24 months. Similar results were found in the unadjusted analyses and are given in Supplementary Table S6. Supplemental Table S7 shows that higher LA levels were associated with an increase in total subcutaneous fat mass over infancy, and higher ARA levels were associated with a decrease in body mass index over infancy.
Table 4.
Maternal n-6 PUFA levels and infant subcutaneous fat mass measures at 1.5, 6 and 24 months (N =904)1-2
| Maternal n-6 PUFA in SDS | Adiposity measures at 1.5 months in SDS Difference (95% confidence interval) | Adiposity measures at 6 months in SDS Difference (95% confidence interval) | Adiposity measures at 24 months in SDS Difference (95% confidence interval) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Body mass index | Total subcutaneous fat mass | Central-to-total subcutaneous fat mass ratio | Body mass index | Total subcutaneous fat mass | Central-to-total subcutaneous fat mass ratio | Body mass index | Total subcutaneous fat mass | Central-to-total subcutaneous subcutaneous fat mass | |
| Total | 0.02 (-0.05, 0.08) | -0.08 (-0.16, -0.01)* | -0.07 (-0.14, 0.01) | -0.01 (-0.07, 0.07) | 0.02 (-0.05, 0.09) | 0.04 (-0.04, 0.11) | -0.02 (-0.12, 0.08) | 0.10 (-0.01, 0.21) | 0.08 (-0.04, 0.20) |
| LA | 0.01 (-0.05, 0.08) | -0.06 (-0.13, 0.02) | -0.03 (-0.10, 0.05) | 0.01 (-0.06, 0.08) | 0.05 (-0.02, 0.12) | 0.03 (-0.04, 0.10) | 0.04 (-0.06, 0.14) | 0.02 (-0.05, 0.10) | 0.03 (-0.08, 0.15) |
| GLA | 0.02 (-0.05, 0.08) | 0.04 (-0.03, 0.12) | -0.06 (-0.14, 0.01) | -0.01 (-0.08, 0.07) | -0.01 (-0.08, 0.06) | 0.03 (-0.04, 0.10) | -0.06 (-0.16, 0.05) | -0.10 (-0.21, 0.02) | 0.08 (-0.05, 0.20) |
| EDA | 0.02 (-0.04, 0.09) | 0.06 (-0.01, 0.13) | -0.03 (-0.10, 0.05) | 0.01 (-0.06, 0.07) | -0.01 (-0.08, 0.06) | 0.05 (-0.02, 0.12) | 0.04 (-0.06, 0.14) | 0.01 (-0.10, 0.12) | 0.02 (-0.09, 0.14) |
| DGLA | 0.02 (-0.05, 0.09) | 0.07 (-0.01, 0.15) | -0.04 (-0.11, 0.04) | 0.03 (-0.04, 0.10) | 0.02 (-0.06, 0.09) | 0.07 (0, 0.15)* | 0.02 (-0.09, 0.12) | -0.02 (-0.14, 0.09) | 0.04 (-0.08, 0.17) |
| ARA | -0.01 (-0.08, 0.06) | -0.05 (-0.13, 0.02) | -0.02 (-0.09, 0.06) | -0.03 (-0.10, 0.04) | -0.07 (-0.15, 0.01) | -0.04 (-0.12, 0.03) | -0.12 (-0.22, -0.02)* | -0.05 (-0.16, 0.07) | 0.01 (-0.11, 0.14) |
| DTA | 0.01 (-0.06, 0.08) | -0.05 (-0.13, 0.03) | -0.04 (-0.12, 0.04) | -0.03 (-0.10, 0.04) | -0.01 (-0.09, 0.06) | -0.01 (-0.08, 0.07) | -0.03 (-0.14, 0.07) | -0.09 (-0.21, 0.02) | 0.04 (-0.08, 0.16) |
Values are regression coefficients (95% Confidence Interval) that reflect the difference in SDS of infant body mass index and subcutaneous fat mass measures at 1.5, 6 and 24 months per SD change in maternal n-6 PUFA levels. Body mass index = weight/height2. Total subcutaneous fat mass = biceps + triceps + suprailiacal + subscapular skinfold thicknesses. Central-to-total subcutaneous fat mass ratio = (suprailiacal + subscapular skinfold thicknesses)/total subcutaneous fat mass.
Models are adjusted for gestational age at blood sampling, maternal age, educational level, parity, pre-pregnancy body mass index, maternal total energy intake, smoking habits and weight gain during pregnancy, folic acid supplement use, gestational diabetes, gestational hypertensive disorders, infants' sex, gestational age-adjusted birth weight standard-deviation scores, breastfeeding duration and timing of introduction of solid foods (for 6 and 24 months).
P-value<0.05.
We observed similar results with both maternal n-3 and n-6 PUFA when we used central-to-peripheral subcutaneous fat mass as compared to the results with central-to-total subcutaneous fat mass (Supplementary Tables S8-S9). Only higher maternal ARA levels were associated with a lower risk of infant overweight at 24 months, but no associations were observed for the other n-3 and n-6 PUFAs with the risk of infant overweight (Supplementary Table S10).
Maternal n-6/n-3 PUFA ratio and infant fat mass
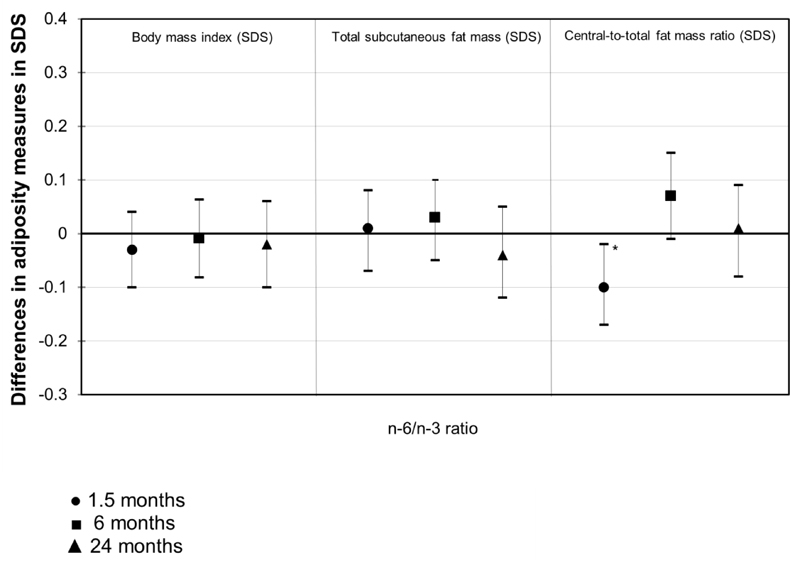
Figure 1 shows that higher maternal n-6/n-3 PUFA ratio was associated with lower central-to-total subcutaneous fat mass ratio at 1.5 months (difference: -0.10 (95% CI: -0.17, -0.02) SD per SD higher maternal n-6/n-3 PUFA ratio), but not with body mass index and total subcutaneous fat mass. No associations were present for maternal n-6/n-3 PUFA ratio with infant adiposity measures at 6 and 24 months.
Figure 1. Maternal n-6/n-3 PUFA ratio and infant fat mass measures at 1.5, 6 and 24 months (N =904).
Values are regression coefficients (95% Confidence Interval) that reflect the difference in SDS of infant body mass index and subcutaneous fat mass measures at 1.5, 6 and 24 months per SD change in maternal n-6/n-3 PUFA ratio. Body mass index = weight/height2. Total subcutaneous fat mass = biceps + triceps + suprailiacal + subscapular skinfold thicknesses. Central-to-total subcutaneous fat mass ratio = (suprailiacal + subscapular skinfold thicknesses)/total subcutaneous fat mass. Models are adjusted for gestational age at blood sampling, maternal age, educational level, parity, pre-pregnancy body mass index, maternal total energy intake, smoking habits and weight gain during pregnancy, folic acid supplement use, gestational diabetes, gestational hypertensive disorders, infants’ sex, gestational age-adjusted birth weight standard-deviation scores, breastfeeding duration and timing of introduction of solid foods (for 6 and 24 months). *P-value<0.05.
Discussion
In this population-based prospective cohort study, we observed that higher maternal n-3 PUFA levels were associated with a higher infant central-to-total subcutaneous fat mass ratio at 1.5 months, but with a lower infant central-to-total subcutaneous fat mass ratio at 6 months. Maternal n-3 PUFA levels were not associated with infant body mass index and total subcutaneous fat mass at any time points. Higher maternal n-6 PUFA levels were not consistently associated with subcutaneous fat mass measures during infancy.
Methodological considerations
Major strengths of this study are the population-based prospective design with detailed information on maternal PUFA concentrations and infant body fat outcomes. To the best of our knowledge, this is the largest study to date addressing the association between maternal PUFA levels during pregnancy with adiposity measures repeatedly measured in infancy. Of all mothers with available PUFA measurements, 83% (904) of mothers and children participated in the infant body fat mass measurements. The non-response could lead to biased effect estimates if the associations of interest would differ between mothers and infants included and not included in the analyses. Mothers included in the analyses had higher total n-3 PUFA concentrations compared to those not included. It is difficult to speculate if these differences might have influenced our effect estimates. We measured a large number of maternal PUFA concentrations in plasma samples once during pregnancy. No information was available about PUFA concentrations earlier or later in pregnancy. Nevertheless, PUFAs measured in plasma may reflect a time frame of dietary intake of approximately 2 weeks and seem to be reasonable indicators for the recent intake (20). We used skinfold thickness as a measure of subcutaneous fat mass and therefore could not estimate deep fat layers, such as pre-peritoneal fat. However, during the first months of life approximately 90% of body fat is located subcutaneously and pre-peritoneal fat mass seems to increase only from the second year of life onwards (21, 22). We studied multiple infant fat mass outcomes. Since these outcomes are strongly correlated, we did not adjust our analyses for multiple testing (23). However, a chance finding cannot be excluded. If we would apply Bonferroni correction, the associations of total n-3 PUFA levels with central-to-total fat mass at 6 months, EPA levels with central-to-total fat mass at 1.5 months, total n-6 PUFA levels with total subcutaneous fat mass at 1.5 months and DGLA levels with central-to-total at 6 months are no longer significant. Finally, although we performed an extensive adjustment for a large number of potential confounders, residual confounding, due to specific infant diet or physical activity, might still be an issue
Interpretation of main findings
An adequate supply of n-3 and n-6 PUFA during pregnancy is important for optimal fetal and infant growth and development (24). Rapid weight gain and increased fat mass levels during infancy may be critical for the development of adiposity in later life (8, 9, 25, 26). However, thus far no previous study has addressed the associations of maternal PUFA status during pregnancy with detailed fat mass measurements throughout infancy.
It has been suggested that lower maternal n-3 PUFA and higher n-6 PUFA concentrations during pregnancy are associated with higher childhood body mass index (6, 7). Fewer studies examined these associations with body mass index in infancy. A randomized double-blind controlled trial among 144 mothers and their children suggested that supplementation by DHA, a n-3 PUFA, during pregnancy and lactation reduced body mass index in late infancy (27). However, an observational study among 244 Dutch mothers and their breastfed infant showed that n-3 and n-6 PUFA concentrations in breast milk did not affect body mass index in the first year of life (28). In this current study, we did not observe consistent associations of maternal n-3 and n-6 PUFA concentrations during pregnancy with infant body mass index at 1.5, 6 and 24 months.
Body mass index might not be an appropriate measure of fat mass and provides limited information about body fat distribution (29). Body fat distribution may be more strongly associated with cardio-metabolic risk factors than body mass index (30, 31). We have previously shown that maternal lower n-3 PUFA and higher n-6 PUFA concentrations during pregnancy were associated with higher total body fat and abdominal fat levels at the age of 6 years. However, not much is known about maternal PUFA concentrations with offspring fat mass outcomes at younger ages. Skinfold thicknesses can be used to estimate total and regional subcutaneous adiposity (32). A study among 1,250 mother-child pairs in Massachusetts showed that higher DHA and EPA, n-3 PUFA concentrations from maternal diet during pregnancy and measured in cord blood, were associated with lower total subcutaneous fat mass measured at the age of 3 years (6). In the same study, higher maternal plasma concentrations of n-6 PUFA and higher ratio of cord plasma n-6/n-3 PUFA were associated with higher total subcutaneous fat mass at the age of 3 years (6). The INFAT randomized trial showed that the combination of an increased n-3 PUFA and a reduced ARA, n-6 PUFA, dietary intake through supplementation during the perinatal period does not affect total subcutaneous fat mass during the first year of life (33).
In our study, we observed no consistent associations of maternal n-3 PUFA concentrations with infant total subcutaneous fat mass from 1.5 months to 24 months of age. Higher maternal total n-3 PUFA and EPA, DPA and DHA concentrations were associated with higher central-to-total subcutaneous fat mass ratio at 1.5 months. However, higher total n-3 PUFA concentrations were associated with lower infant central-to-total subcutaneous fat mass ratio at 6 months. The observed associations were not explained by birth weight. Only higher maternal total n-6 PUFA concentrations were associated with lower total subcutaneous fat mass at 1.5 months, but not with other infant adiposity measures or at any other time points. Based on our findings, it seems that especially maternal n-3 PUFA may stimulate central subcutaneous fat mass development in early infancy, but this is a transient effect, which is no longer present in late infancy. The underlying mechanisms that explain the associations of maternal PUFA status during pregnancy with offspring fat mass development in infancy are not clear. It has been suggested that n-3 PUFA availability during early life leads to increased activation of peroxisome proliferator–activated receptor gamma (PPARγ), which has been associated with an increased deposition of subcutaneous fat mass, but not visceral fat mass, in adults (34, 35). On the other hand, it has also been suggested that n-3 PUFA levels in early life inhibit the differentiation of adipocytes, leading to lower levels of fat mass (36). Our findings might be explained by a greater effect of n-3 PUFA on PPARγ and by a less apparent inhibitory effect of n-3 PUFA on the differentiation of adipocytes in early infancy. Further observational and experimental studies are needed to explore these detailed underlying mechanisms and their potential critical periods.
Conclusion
Maternal n-3 PUFA levels during pregnancy may have transient effects on infant central subcutaneous fat mass development. Maternal n-6 PUFA levels were not consistently associated with infant subcutaneous fat mass measures. Further studies are needed to assess the effects of maternal PUFA levels during pregnancy on detailed fat mass development throughout early infancy.
Supplementary Material
What is already known about this subject?
Lower maternal n-3 and higher n-6 PUFA levels during pregnancy are associated with higher body mass index, total and abdominal fat mass levels in childhood.
It is not known whether maternal n-3 and n-6 PUFA levels during pregnancy affect fat mass development already from early infancy onwards, which is known as a critical period for the development of obesity in later life.
What does your study add?
Maternal n-3 PUFA levels were associated with a higher central subcutaneous fat mass at 1.5 months, but with lower central subcutaneous fat mass at 6 months. No effects were observed at 24 months.
These associations were not explained by maternal socio-demographic or lifestyle related characteristics or birth characteristics.
No consistent associations were observed between maternal n-6 PUFA levels and infant subcutaneous fat mass levels.
Acknowledgements
We gratefully acknowledge the contribution of participating mothers, general practitioners, hospitals, midwives and pharmacies in Rotterdam. We also thank Stephan Stromer for his dedicated help in performing fatty acid analyses.
Funding
The general design of the Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, Erasmus University Rotterdam, Netherlands Organization for Health Research and Development (ZonMw), Netherlands Organisation for Scientific Research (NWO), Ministry of Health, Welfare and Sport and Ministry of Youth and Families. Research leading to these results has received funding from the European Union's Seventh Framework Programme (FP7/2007-2013), project NUTRIMENTHE under grant agreement n°212652 and project EarlyNutrition under grant agreement n°289346. Susana Santos received a grant from the Portuguese Foundation for Science and Technology (SFRH/BD/81123/2011). Vincent Jaddoe received grants from the Netherlands Organization for Health Research and Development (VIDI 016.136.361) and European Research Council (ERC-2014-CoG-648916). Fatty acid analysis was co-funded by the European Research Council Advanced Grant ERC-2012-AdG – no.322605 META-GROWTH to Berthold Koletzko.
Footnotes
Conflict of interest
None of the authors had a financial or personal conflict of interest.
References
- 1.Grieger JA, Clifton VL. A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients. 2015;7:153–178. doi: 10.3390/nu7010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cetin I, Alvino G, Cardellicchio M. Long chain fatty acids and dietary fats in fetal nutrition. J Physiol. 2009;587:3441–3451. doi: 10.1113/jphysiol.2009.173062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ailhaud G, Guesnet P. Fatty acid composition of fats is an early determinant of childhood obesity: a short review and an opinion. Obes Rev. 2004;5:21–26. doi: 10.1111/j.1467-789x.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- 4.Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri JM, Guesnet P. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res. 2006;45:203–236. doi: 10.1016/j.plipres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Kim HK, Della-Fera M, Lin J, Baile CA. Docosahexaenoic acid inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 preadipocytes. J Nutr. 2006;136:2965–2969. doi: 10.1093/jn/136.12.2965. [DOI] [PubMed] [Google Scholar]
- 6.Donahue SM, Rifas-Shiman SL, Gold DR, Jouni ZE, Gillman MW, Oken E. Prenatal fatty acid status and child adiposity at age 3 y: results from a US pregnancy cohort. Am J Clin Nutr. 2011;93:780–788. doi: 10.3945/ajcn.110.005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vries PS, Gielen M, Rizopoulos D, Rump P, Godschalk R, Hornstra G, et al. Association between polyunsaturated fatty acid concentrations in maternal plasma phospholipids during pregnancy and offspring adiposity at age 7: the MEFAB cohort. Prostaglandins Leukot Essent Fatty Acids. 2014;91:81–85. doi: 10.1016/j.plefa.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194–199. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 9.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaddoe VW, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27:739–756. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- 11.Kruithof CJ, Kooijman MN, van Duijn CM, Franco OH, de Jongste JC, Klaver CC, et al. The Generation R Study: Biobank update 2015. Eur J Epidemiol. 2014;29:911–927. doi: 10.1007/s10654-014-9980-6. [DOI] [PubMed] [Google Scholar]
- 12.Vidakovic AJ, Gishti O, Steenweg-de Graaff J, Williams MA, Duijts L, Felix JF, et al. Higher Maternal Plasma n-3 PUFA and Lower n-6 PUFA Concentrations in Pregnancy Are Associated with Lower Childhood Systolic Blood Pressure. J Nutr. 2015;145:2362–2368. doi: 10.3945/jn.115.210823. [DOI] [PubMed] [Google Scholar]
- 13.Glaser C, Demmelmair H, Koletzko B. High-throughput analysis of fatty acid composition of plasma glycerophospholipids. J Lipid Res. 2010;51:216–221. doi: 10.1194/jlr.D000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon RJ, Harvey NC, Robinson SM, Ntani G, Davies JH, Inskip HM, et al. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J Clin Endocrinol Metab. 2013;98:299–307. doi: 10.1210/jc.2012-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaddoe VW, Bakker R, van Duijn CM, van der Heijden AJ, Lindemans J, Mackenbach JP, et al. The Generation R Study Biobank: a resource for epidemiological studies in children and their parents. Eur J Epidemiol. 2007;22:917–923. doi: 10.1007/s10654-007-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ay L, Hokken-Koelega AC, Mook-Kanamori DO, Hofman A, Moll HA, Mackenbach JP, et al. Tracking and determinants of subcutaneous fat mass in early childhood: the Generation R Study. Int J Obes (Lond) 2008;32:1050–1059. doi: 10.1038/ijo.2008.76. [DOI] [PubMed] [Google Scholar]
- 17.Ketel IJ, Volman MN, Seidell JC, Stehouwer CD, Twisk JW, Lambalk CB. Superiority of skinfold measurements and waist over waist-to-hip ratio for determination of body fat distribution in a population-based cohort of Caucasian Dutch adults. Eur J Endocrinol. 2007;156:655–661. doi: 10.1530/EJE-06-0730. [DOI] [PubMed] [Google Scholar]
- 18.Wells JC, Cole TJ, steam As. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord. 2002;26:947–952. doi: 10.1038/sj.ijo.0802027. [DOI] [PubMed] [Google Scholar]
- 19.Heppe DH, Medina-Gomez C, Hofman A, Franco OH, Rivadeneira F, Jaddoe VW. Maternal first-trimester diet and childhood bone mass: the Generation R Study. Am J Clin Nutr. 2013;98:224–232. doi: 10.3945/ajcn.112.051052. [DOI] [PubMed] [Google Scholar]
- 20.Skeaff CM, Hodson L, McKenzie JE. Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. J Nutr. 2006;136:565–569. doi: 10.1093/jn/136.3.565. [DOI] [PubMed] [Google Scholar]
- 21.Olhager E, Flinke E, Hannerstad U, Forsum E. Studies on human body composition during the first 4 months of life using magnetic resonance imaging and isotope dilution. Pediatr Res. 2003;54:906–912. doi: 10.1203/01.PDR.0000088064.63106.5E. [DOI] [PubMed] [Google Scholar]
- 22.Holzhauer S, Zwijsen RM, Jaddoe VW, Boehm G, Moll HA, Mulder PG, et al. Sonographic assessment of abdominal fat distribution in infancy. Eur J Epidemiol. 2009;24:521–529. doi: 10.1007/s10654-009-9368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koletzko B, Boey CC, Campoy C, Carlson SE, Chang N, Guillermo-Tuazon MA, et al. Current information and Asian perspectives on long-chain polyunsaturated fatty acids in pregnancy, lactation, and infancy: systematic review and practice recommendations from an early nutrition academy workshop. Ann Nutr Metab. 2014;65:49–80. doi: 10.1159/000365767. [DOI] [PubMed] [Google Scholar]
- 25.Gillman MW. Early infancy - a critical period for development of obesity. J Dev Orig Health Dis. 2010;1:292–299. doi: 10.1017/S2040174410000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gishti O, Gaillard R, Manniesing R, Abrahamse-Berkeveld M, van der Beek EM, Heppe DH, et al. Fetal and infant growth patterns associated with total and abdominal fat distribution in school-age children. J Clin Endocrinol Metab. 2014;99:2557–2566. doi: 10.1210/jc.2013-4345. [DOI] [PubMed] [Google Scholar]
- 27.Lucia Bergmann R, Bergmann KE, Haschke-Becher E, Richter R, Dudenhausen JW, Barclay D, et al. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy? J Perinat Med. 2007;35:295–300. doi: 10.1515/JPM.2007.085. [DOI] [PubMed] [Google Scholar]
- 28.Scholtens S, Wijga AH, Smit HA, Brunekreef B, de Jongste JC, Gerritsen J, et al. Long-chain polyunsaturated fatty acids in breast milk and early weight gain in breast-fed infants. Br J Nutr. 2009;101:116–121. doi: 10.1017/S0007114508993521. [DOI] [PubMed] [Google Scholar]
- 29.Prentice AM, Jebb SA. Beyond body mass index. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 30.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 31.Gishti O, Gaillard R, Durmus B, Abrahamse M, van der Beek EM, Hofman A, et al. BMI, total and abdominal fat distribution, and cardiovascular risk factors in school-age children. Pediatr Res. 2015;77:710–718. doi: 10.1038/pr.2015.29. [DOI] [PubMed] [Google Scholar]
- 32.Wells JC, Fewtrell MS. Measuring body composition. Arch Dis Child. 2006;91:612–617. doi: 10.1136/adc.2005.085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hauner H, Much D, Vollhardt C, Brunner S, Schmid D, Sedlmeier EM, et al. Effect of reducing the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: an open-label randomized controlled trial. Am J Clin Nutr. 2012;95:383–394. doi: 10.3945/ajcn.111.022590. [DOI] [PubMed] [Google Scholar]
- 34.Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. Am J Clin Nutr. 2006;83:1520S–1525S. doi: 10.1093/ajcn/83.6.1520S. [DOI] [PubMed] [Google Scholar]
- 35.Laplante M, Festuccia WT, Soucy G, Gelinas Y, Lalonde J, Berger JP, et al. Mechanisms of the depot specificity of peroxisome proliferator-activated receptor gamma action on adipose tissue metabolism. Diabetes. 2006;55:2771–2778. doi: 10.2337/db06-0551. [DOI] [PubMed] [Google Scholar]
- 36.Ailhaud G, Guesnet P, Cunnane SC. An emerging risk factor for obesity: does disequilibrium of polyunsaturated fatty acid metabolism contribute to excessive adipose tissue development? Br J Nutr. 2008;100:461–470. doi: 10.1017/S0007114508911569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.