Abstract
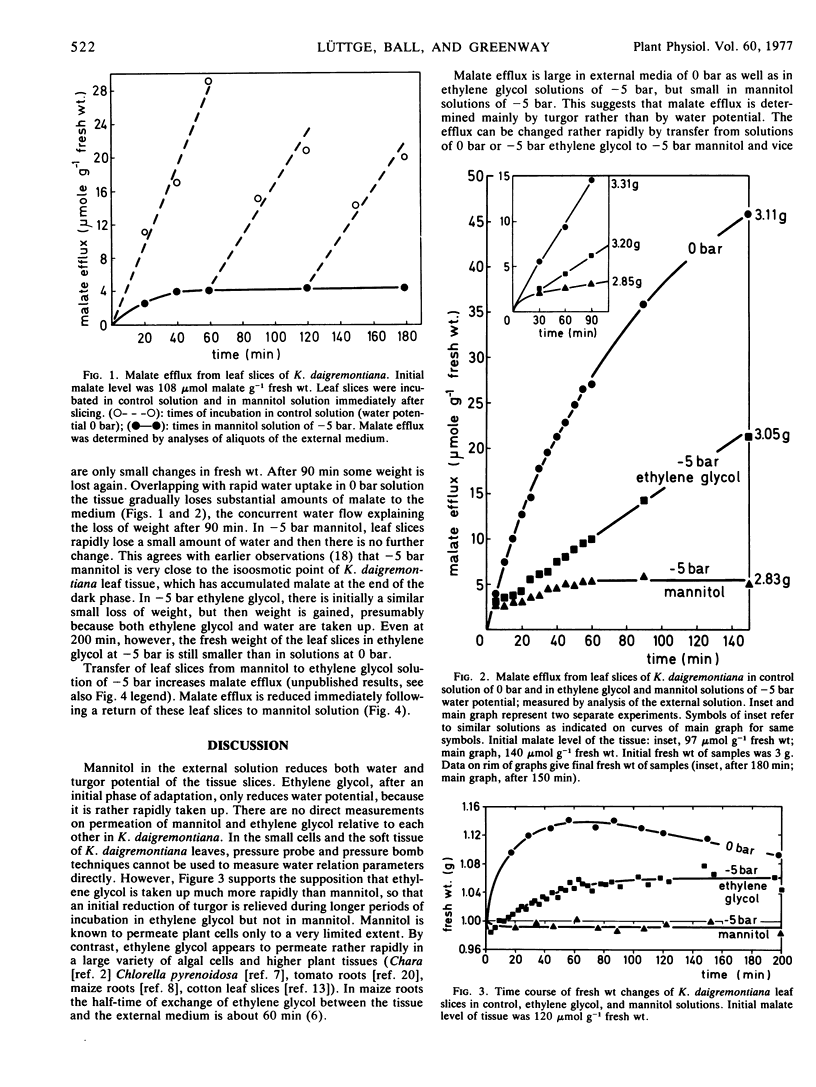
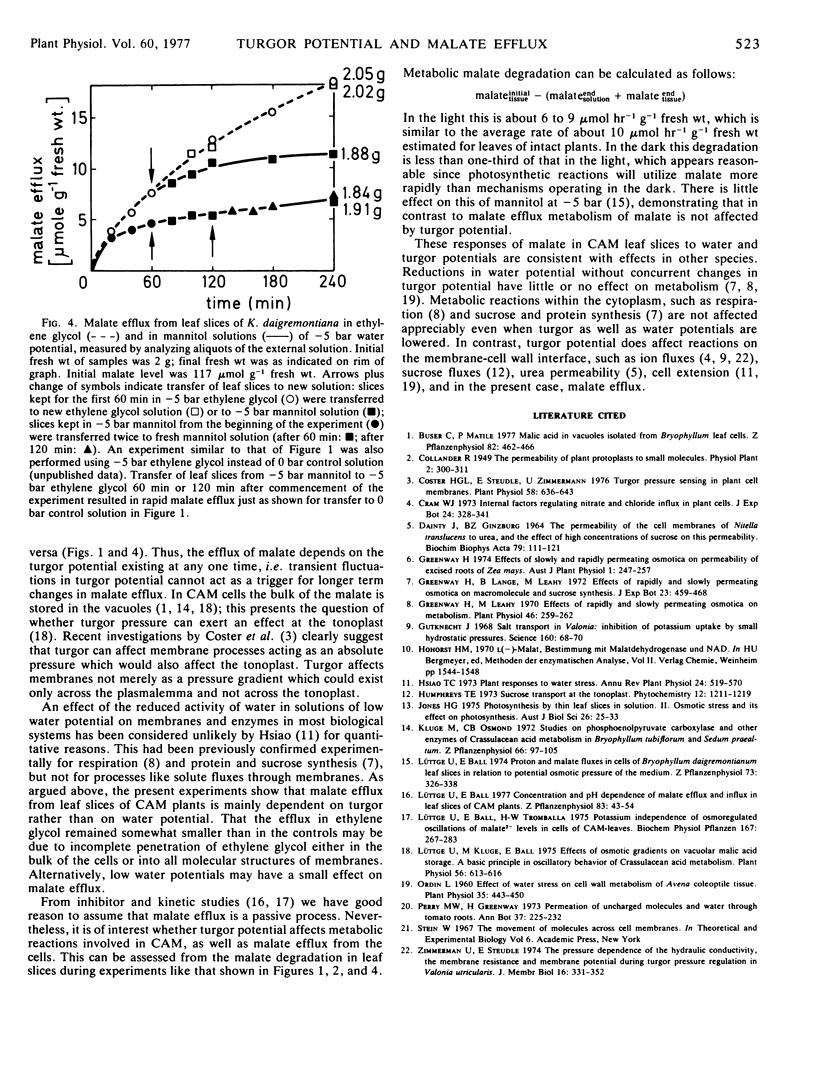
Malate efflux from leaf cells of the Crassulacean acid metabolism plant Kalanchoë daigremontiana Hamet et Perrier was studied using leaf slices submerged in experimental solutions. Leaves were harvested at the end of the dark phase and therefore contained high malate levels. Water potentials of solutions were varied between 0 and −5 bar using mannitol (a slowly permeating solute) and ethylene glycol (a rapidly permeating solute), respectively. Mannitol solutions of water potentials down to −5 bar considerably reduced malate efflux. The slowly permeating solute mannitol reduces both water potential and turgor potential of the cells. The water potential of a mannitol solution of −5 bar is just above plasmolyzing concentration. Malate efflux in ethylene glycol at −5 bar was only slightly smaller than at 0 bar, and much higher than in mannitol at −5 bar. Tissues in rapidly permeating ethylene glycol would have turgor potentials similar to tissues in 0.1 mm CaSO4. The results demonstrate that malate efflux depends on turgor potential rather than on water potential of the cells.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coster H. G. Turgor pressure sensing in plant cell membranes. Plant Physiol. 1976 Nov;58(5):636–643. doi: 10.1104/pp.58.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAINTY J., GINZBURG B. Z. THE PERMEABILITY OF THE CELL MEMBRANES OF NITELLA TRANSLUCENS TO UREA, AND THE EFFECT OF HIGH CONCENTRATIONS OF SUCROSE ON THIS PERMEABILITY. Biochim Biophys Acta. 1964 Jan 27;79:112–121. doi: 10.1016/0926-6577(64)90044-0. [DOI] [PubMed] [Google Scholar]
- Greenway H., Leahy M. Effects of rapidly and slowly permeating osmotica on metabolism. Plant Physiol. 1970 Aug;46(2):259–262. doi: 10.1104/pp.46.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J. Salt transport in Valonia: inhibition of potassium uptake by small hydrostatic pressures. Science. 1968 Apr 5;160(3823):68–70. doi: 10.1126/science.160.3823.68. [DOI] [PubMed] [Google Scholar]
- Lüttge U., Kluge M., Ball E. Effects of osmotic gradients on vacuolar malic Acid storage: a basic principle in oscillatory behavior of crassulacean Acid metabolism. Plant Physiol. 1975 Nov;56(5):613–616. doi: 10.1104/pp.56.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordin L. Effect of Water Stress on Cell Wall Metabolism of Avena Coleoptile Tissue. Plant Physiol. 1960 Jul;35(4):443–450. doi: 10.1104/pp.35.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U., Steudle E. The pressure-dependence of the hydraulic conductivity, the membrane resistance and membrane potential during turgor pressure regulation in Valonia utricularis. J Membr Biol. 1974;16(4):331–352. doi: 10.1007/BF01872422. [DOI] [PubMed] [Google Scholar]


