Abstract
Voltage-gated Cav1.2 and Cav1.3 (L-type) Ca2+ channels regulate neuronal excitability, synaptic plasticity, and learning and memory. Densin-180 (densin) is an excitatory synaptic protein that promotes Ca2+-dependent facilitation of voltage-gated Cav1.3 Ca2+ channels in transfected cells. Mice lacking densin (densin KO) exhibit defects in synaptic plasticity, spatial memory, and increased anxiety-related behaviors—phenotypes that more closely match those in mice lacking Cav1.2 than Cav1.3. Therefore, we investigated the functional impact of densin on Cav1.2. We report that densin is an essential regulator of Cav1.2 in neurons, but has distinct modulatory effects compared with its regulation of Cav1.3. Densin binds to the N-terminal domain of Cav1.2, but not that of Cav1.3, and increases Cav1.2 currents in transfected cells and in neurons. In transfected cells, densin accelerates the forward trafficking of Cav1.2 channels without affecting their endocytosis. Consistent with a role for densin in increasing the number of postsynaptic Cav1.2 channels, overexpression of densin increases the clustering of Cav1.2 in dendrites of hippocampal neurons in culture. Compared with wild-type mice, the cell surface levels of Cav1.2 in the brain, as well as Cav1.2 current density and signaling to the nucleus, are reduced in neurons from densin KO mice. We conclude that densin is an essential regulator of neuronal Cav1 channels and ensures efficient Cav1.2 Ca2+ signaling at excitatory synapses.
SIGNIFICANCE STATEMENT The number and localization of voltage-gated Cav Ca2+ channels are crucial determinants of neuronal excitability and synaptic transmission. We report that the protein densin-180 is highly enriched at excitatory synapses in the brain and enhances the cell surface trafficking and postsynaptic localization of Cav1.2 L-type Ca2+ channels in neurons. This interaction promotes coupling of Cav1.2 channels to activity-dependent gene transcription. Our results reveal a mechanism that may contribute to the roles of Cav1.2 in regulating cognition and mood.
Keywords: excitatory, postsynaptic, trafficking
Introduction
Ca2+ influx through voltage-gated (Cav) Cav1.2 L-type Ca2+ channels regulates neuronal excitability (Lacinova et al., 2008), synaptic plasticity (Moosmang et al., 2005), and learning and memory (Moosmang et al., 2005; White et al., 2008). Like other Cav channels, Cav1.2 is composed of a pore-forming α1 subunit (Cav1.2) and auxiliary β and α2δ subunits (Simms and Zamponi, 2014). The CACNA1C gene encoding Cav1.2 is a major risk gene for multiple neuropsychiatric disorders, including autism spectrum disorder, attention deficit–hyperactivity disorder, schizophrenia, bipolar disorder, and major depression (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013). Therefore, an understanding of the factors regulating Cav1.2 is crucial for understanding the balance between normal and disordered states of cognitive and affective processing.
In addition to interactions with auxiliary subunits, neuronal Cav1.2 channels interact with a variety of other regulatory proteins (Calin-Jageman and Lee, 2008; Lipscombe et al., 2013). The distal C terminus of Cav1.2 contains a consensus site for binding to proteins containing Postsynaptic Density-95, Drosophila Discs-Large and Zona Occludens (PDZ) domains (Kurschner and Yuzaki, 1999; Weick et al., 2003). The related Cav1.3 channel also contains a C-terminal PDZ-binding site, which serves as a ligand for multiple proteins that regulate the function of these channels (Olson et al., 2005; Zhang et al., 2005; Calin-Jageman et al., 2007; Jenkins et al., 2010; Gregory et al., 2011; Gregory et al., 2013). Although several PDZ domain-containing proteins are known to interact with Cav1.2 (Kurschner et al., 1998; Kurschner and Yuzaki, 1999), the functional consequences of such interactions have not been characterized.
Densin-180 (densin) is a leucine-rich repeat and PDZ domain-containing protein that is enriched in the postsynaptic density of excitatory synapses and interacts with a variety of postsynaptic proteins including calmodulin-dependent protein kinase II (CaMKII; Apperson et al., 1996; Strack et al., 2000; Walikonis et al., 2001). We showed previously that the PDZ domain of densin interacts with Cav1.3 and recruits CaMKII to the channel complex, which causes Ca2+-dependent facilitation of Cav1.3 currents in transfected cells (Jenkins et al., 2010). However, mice lacking densin (densin KO) display defects in spatial memory and elevated anxiety levels (Carlisle et al., 2011), which are more similar to the behavioral phenotypes in mice lacking Cav1.2 (Moosmang et al., 2005; Lee et al., 2012) than in mice lacking Cav1.3 (Pinggera and Striessnig, 2016). Together with evidence that Cav1.2 is more abundant in the brain than Cav1.3 (Clark et al., 2003), these results suggest that densin may be a physiological relevant component of Cav1.2 complexes.
To test this hypothesis, we investigated whether densin functionally interacts with Cav1.2 in transfected cells and in neurons. We found that densin binds to, but differentially modulates, Cav1.2 compared with Cav1.3. Densin enhances the cell surface density and postsynaptic clustering of Cav1.2, as well as coupling of Cav1.2 to phosphorylation of the transcription factor cAMP response element binding protein (CREB). Our results underscore the importance of Cav1.2–protein interactions for neuronal Ca2+ signaling, which should be considered in the context of how alterations in Cav1.2 channel function may lead to neuropsychiatric disease.
Materials and Methods
Animals.
All procedures using animals were performed in accordance with the University of Iowa Institutional Animal Care and Use Committee. The densin KO mouse line was bred on a C57BL/6 background and has been described previously (Carlisle et al., 2011). Mice were housed three or four per cage and maintained on a 12 h reverse light/dark cycle with ad libitum access to food and water.
cDNAs.
For cell transfection, cDNAs corresponding to Cav1.2 subunits [rbcII (GenBank #M67515), β2a (GenBank #NC013684) and α2δ1 (GenBank #M76559.1)] were expressed from the pcDNA3.1+ vector (Invitrogen). The following constructs were described previously: Cav1.2/pcDNA3.1+ (FLAG-tagged), Cav1.2ΔNT/pcDNA3.1+ (FLAG-tagged) lacking aa 1–109, GST-tagged Cav1.2 intracellular domains: NT (aa 1–124); loop I-II (aa 406–524); loop II-III (aa 754–902); loop III-IV (aa1170–1222); CT (aa 1942–2144) (Zhou et al., 2004; Zhou et al., 2005); GST-tagged C-terminal domain of Cav1.3 (aa 1962–2155) (Calin-Jageman et al., 2007); GFP-tagged densin (GenBank #NM_057142.1 (Jiao et al., 2008)); pβA-eGFP and pβA-Cav1.2-hemagglutinin (HA) (Altier et al., 2002; Obermair et al., 2004). GST-tagged fragments of Cav1.2 NT were generated by PCR amplification and subcloning into BamHI and EcoRI sites of pGEX-4T1. The pβA-Cav1.2-HA-ΔNT was generated by PCR amplification of the sequence encoding Cav1.2-HA-ΔNT using pβA-Cav1.2-HA as a template and subcloning the sequence encoding Cav1.2ΔNT into HindIII and XbaI sites of the pβA empty vector. To generate Cav1.2ΔB1a/pcDNA3.1+, the sequence corresponding to the B1a region (aa 65–76) was deleted from Cav1.2/pcDNA3.1+ using site-directed mutagenesis. The SNAP-tagged-Cav1.2 construct was generated by amplifying the Cav1.2-HA coding region in pβA-Cav1.2-HA and ligating into PmeI and NotI sites of the pSNAPr vector (New England Biolabs) using the NEBuilder HiFi DNA Assembly cloning system (New England BioLabs).
Cell culture and transfection.
Human embryonic kidney cells transformed with SV40 T-antigen (HEK293T; American Type Culture Collection catalog #CRL-3216, RRID: CVCL_0063) were maintained in DMEM (Thermo Fisher Scientific) with 10% fetal bovine serum at 37°C in a humidified atmosphere with 5% CO2. Cells were grown to 70–80% confluence and transfected with Gene Porter reagent (Genlantis) or Fugene 6 (Promega) according to the manufacturer's protocols. For electrophysiology, cells were plated in 35 mm dishes and transfected with Cav1.2 subunit cDNAs: Cav1.2 (1.5 μg), β2a (1 μg), and α2δ1 (1 μg). pEGFPN1 (0.1 μg) or GFP-densin (1 μg) was cotransfected, which facilitated identification of transfected cells by fluorescence. For pull-down assays, cells were plated in 60 mm dishes and transfected with GFP-densin (6 μg). For coimmunoprecipitation experiments, cells were plated in 60 mm dishes and transfected with Cav1.2 (2.4 μg), β2a (0.8 μg), α2δ1 (0.8 μg), pEGFPN1 (0.5 μg), and pcDNA (3.5 μg) or GFP-densin (4 μg).
In vitro binding assays.
GST-fusion proteins containing intracellular domains of Cav1.2 were expressed in E. coli (BL21) and purified on glutathione-Sepharose (GE Healthcare Life Sciences) according to standard protocols. HEK293T cells transfected with GFP-densin (4 μg) were harvested and homogenized in ice-cold cell lysis buffer containing 50 mm Tris-HCl, 150 mm NaCl, 1% Triton X-100, 0.25% w/v sodium deoxycholate, 1 mm EDTA, pH 7.4, and protease inhibitors. The homogenate was rotated at 4°C for 1 h to solubilize membrane proteins and the insoluble material was separated by centrifugation at 16,100 × g for 30 min. The supernatant was incubated with immobilized GST fusion proteins (50% slurry) at 4°C overnight. The beads were washed three times with ice-cold cell lysis buffer and the bound proteins were eluted with SDS-containing sample buffer, subjected to SDS-PAGE, and transferred to nitrocellulose. Western blotting was with mouse monoclonal anti-GFP (1:3000, Santa Cruz Biotechnology catalog #sc-53882, RRID: AB_783653).
Coimmunoprecipitation assays.
Transfected HEK293T cells were harvested 48 h after transfection. Cell lysates were prepared as described for binding assays and incubated with FLAG-agarose beads (50% slurry; Sigma-Aldrich) overnight, rotating at 4°C. After three washes with cell lysis buffer, proteins were eluted and analyzed by SDS-PAGE. Coimmunoprecipitated proteins were detected by Western blotting with GFP antibodies (1:3000, Santa Cruz Biotechnology catalog #sc-53882, RRID: AB_783653). For coimmunoprecipitation from mouse brain, neural tissue was subjected to subfractionation according to previously described methods (Cox and Emili, 2006). The prefrontal cortex was dissected and homogenized in lysis buffer containing the following (in mm): 250 sucrose, 50 Tris-HCl, 5 MgCl2, and 1 DTT, pH 7.4, plus protease inhibitors. The nuclear fraction was removed by centrifugation at 800 × g for 15 min and the supernatant was subjected to ultracentrifugation at 100,000 × g for 1 h. The membrane pellet was solubilized in solubilization buffer containing 50 mm Tris-HCl, 150 mm NaCl, pH 7.5, 1% Triton X-100, and protease inhibitors) at 4°C for 30 min and the insoluble material was removed by ultracentrifugation at 100,000 × g for 1 h. Either rabbit control IgG (Sigma-Aldrich catalog #I5006, RRID: AB_1163659) or anti-Cav1.2 antibodies (5 μg) were added to the solubilized membrane proteins along with Protein A Sepharose (50% slurry, Sigma-Aldrich). Reactions were continued overnight with end-over-end rotation at 4°C. The resin was collected by centrifugation and rinsed three times with solubilization buffer. Bound proteins were eluted, resolved on a SDS-polyacrylamide gel (4–12%), and Western blotted with anti-densin antibodies (1:5000, Colbran laboratory at Vanderbilt University, catalog #Ab650, RRID: AB_2619660; Jiao et al., 2008) as described above.
Preparation of neuronal cultures.
Low-density cultures of hippocampal neurons were prepared from BALB/c mice at embryonic day 18 as described previously (Obermair et al., 2004; Di Biase et al., 2011). Briefly, dissected hippocampi were dissociated by trypsin treatment and trituration. Neurons were plated on poly-l-lysine-coated glass coverslips in 60 mm culture dishes at a density of ∼3500 cells/cm2. After plating, cells were allowed to attach for 3–4 h before transferring the coverslips neuron-side down into a 60 mm culture dish with a glial feeder layer. Neurons and glial feeder layer were maintained in serum-free Neurobasal medium (Invitrogen) supplemented with Glutamax and B-27 supplements (Invitrogen). Ara-C (5 μm) was added 3 d after plating and, once a week, 1/3 of the medium was removed and replaced with fresh maintenance medium.
Cortical neuron cultures were prepared from postnatal day 0–1 mouse pups. The cerebral cortex was isolated in ice-cold dissection solution (0.1 mg/ml HBSS-containing sodium pyruvate, 0.1% glucose, and 10 mm HEPES, pH 7.3). The tissue was washed and digested for 13 min in the dissection solution containing trypsin (5 mg/ml) and DNase I (0.1 mg/ml) at room temperature. The trypsin was inactivated by two washes in prewarmed plating medium containing Eagle's basal medium (Thermo Fisher Scientific), fetal bovine serum (10%), w/v glucose (0.45%), sodium pyruvate (1 mm), glutamine (2 mm), and penicillin/streptomycin (100 μg/ml). The tissue was dissociated using polished Pasteur pipettes and the cells were pelleted at 185 × g for 13 min at 4°C. The cells were gently resuspended in prewarmed plating medium and plated on 18 mm glass coverslips coated with poly-d-lysine. Neurons were maintained in maintenance medium containing Neurobasal medium (Thermo Fisher Scientific), B27 (Thermo Fisher Scientific), glutamine (2 mm), and penicillin/streptomycin (100 μg/ml) at 37°C in a humidified atmosphere with 5% CO2 for 12–16 d before experiments.
Analysis of cell surface Cav1.2 and densin localization in transfected hippocampal neurons.
Expression plasmids were introduced into neurons at 6 d in vitro (DIV) using Lipofectamine 2000-mediated transfection (Invitrogen) as described previously (Obermair et al., 2004). For cotransfection of Cav1.2-HA + eGFP or Cav1.2-HA + eGFP + GFP-densin, 1.5 or 2.5 μg of total DNA was used at a molar ratio of 1:1 or 1:1:1, respectively. Cells were processed for immunostaining 12–13 d after transfection.
For surface staining of Cav1.2-HA, transfected neurons were incubated with anti-HA (Roche catalog #11867431001, RRID: AB_390919) for 20 min at 37°C. Coverslips were rinsed in HBSS and fixed with 4% paraformaldehyde for 10 min. After fixation, neurons were washed with PBS for 30 min, blocked with 5% goat serum for 30 min, and labeled with goat anti-rat Alexa Fluor 594 (1:4000, 1 h, Thermo Fisher Scientific catalog #A11007, RRID: AB_10561522). Coverslips were mounted in p-phenylenediamine glycerol to retard photobleaching (Flucher et al., 1993) and observed with an Axio Imager microscope (Carl Zeiss) using 63×, 1.4 numerical aperture oil-immersion objective lens. Images were recorded with cooled CCD camera (SPOT Imaging Solutions).
Fourteen-bit gray scale images of anti-HA (red channel) and eGFP (green channel) were acquired and analyzed as described previously (Di Biase et al., 2008; Di Biase et al., 2011; Stanika et al., 2016). Briefly, images were aligned and the eGFP image was used to select the regions of interest for measuring the area occupied by Cav1.2-HA clusters with MetaMorph software (Molecular Devices).
Electrophysiology.
HEK293T cells were subjected to whole-cell patch-clamp recordings 48 h after transfection. For each experiment, control cells were recorded on the same day in parallel with experimental cells. The external solution contained the following (in mm): 150 Tris, 1 MgCl2, and 10 CaCl2 or BaCl2. The internal solution contained the following (in mm): 140 N-methyl-d-glucamine, 10 HEPES, 2 MgCl2, 2 Mg-ATP, and 5 EGTA. The pH of both solutions was adjusted to 7.3 with methanesulfonic acid. Electrode resistances were 6–8 MΩ in the bath solution and series resistance was ∼6–8 MΩ compensated 70%. The holding voltage was −80 mV for transfected cells. Maximal gating charge was measured as the time integral of the gating current within 1.5–2 ms of a depolarizing step to the reversal potential (+70 mV) and tail current amplitudes were measured upon repolarization to −70 mV. Statistical analyses were as indicated.
For neuronal recordings, pyramidal neurons (12–16 DIV) were selected based on a pyramidal-shaped cell body and large proximal dendrites. The experimenter was blinded to mouse genotype before preparation of neuronal culture, recording, and data analysis. The external solution contained the following (in mm): 150 TEA-Cl, 5 BaCl2, 10 HEPES, and 10 glucose, pH 7.4 with TEA-OH. The intracellular solution contained the following (in mm): 180 N-methyl-d-glucamine, 4 MgCl2, 5 EGTA, 40 HEPES, 12 creatine-phosphate-Tris, 0.1 Leupeptin, 2 Na-ATP, and 0.4 Na-GTP, pH 7.3 with phosphoric acid. To isolate Cav1-mediated currents, external solution containing isradipine (5 μm; Tocris Bioscience) was applied by gravity perfusion. Currents were evoked by voltage ramps from −60 mV to +80 mV at 0.5 mV/ms from an initial holding potential of −60 mV.
Data were acquired with an EPC-9 amplifier and Patchmaster software (HEKA Elektronik) and analyzed using Igor Pro software (Wavemetrics). Statistical analysis was performed using SigmaPlot (Systat Software). Average data are presented as mean ± SEM. For current–density plots, peak current amplitudes were normalized to whole-cell capacitance. Current–voltage (I–V) relationships were fit with the following equation: I = G(V − E)/{1 + exp[(V − V1/2)/k]} where G is conductance, V is the test potential, E is the reversal potential, V1/2 is the voltage for the half-maximal activation, and k is the slope factor.
Analyses of cell surface Cav1.2 protein levels in mouse brain.
Biotinylation of cell surface proteins in brain slices were modified from methods described previously (Robertson et al., 2010). Wild-type (WT) and densin KO mice (3–6 weeks old, male) were deeply anesthetized with isoflurane (4–5%) and rapidly decapitated. The brain was removed and the prefrontal cortex was dissected and chilled in oxygenated solution containing the following (in mm): 210 sucrose, 20 NaCl, 2.5 KCl, 1 MgCl2, and 1.2 NaH2PO4 · H2O before sectioning. Coronal slices (300 μm) were collected using a vibratome (Electron Microscopy Science 5000 Oscillating Tissue Slicer) in artificial CSF (ACSF) containing the following (in mm): 125 NaCl, 2.5 KCl, 1.2 NaH2PO4 · H2O, 1 MgCl2, and 2 CaCl2 · H2O, oxygenated. To minimize cell damage, the highest oscillation speed and the lowest advance rate of the blade were chosen during slicing. The brain slices were immediately washed twice with ACSF before incubating with ACSF containing EZ-Link Sulfo-NHS-SS-Biotin (1 mg/ml; Thermo Fisher Scientific) for 45 min. The tissue was rinsed twice quickly and for two 10 min washes in ACSF. The reaction was quenched by washing twice for 20 min each with ACSF containing glycine before lysis in radioimmunoprecipitation assay (RIPA) buffer containing 25 mm Tris, 150 mm NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, and a mixture of protease inhibitors. Lysates were then centrifuged at 12,000 × g for 30 min at 4°C. Biotinylated proteins were isolated on streptavidin beads (Thermo Fisher Scientific) overnight at 4°C with rotation. Beads were washed 3 times with RIPA buffer (0.1% Triton X-100) and biotinylated proteins were eluted in SDS-PAGE sample loading buffer at 90°C for 10 min. Western blotting was with antibodies against Cav1.2, Na+/K+ ATPase (1:3000, Developmental Studies Hybridoma Bank catalog #a6F RRID:AB_2314847) and GAPDH (1:10000, Abcam catalog #ab8245 RRID:AB_2107448).
Phosphorylated CREB assays.
Cortical neurons (12–16 DIV) from WT or densin KO mice were incubated in maintenance medium containing 0.5 μm tetrodotoxin (TTX; Tocris Bioscience), 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; Tocris Bioscience), and 10 μm D-(−)-2-amino-5-phosphonopentanoic acid (APV; Tocris Bioscience) at 37°C, 5% CO2, for 4 h. Cells were exposed to Tyrode's solution containing the following (in mm): 150 NaCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose, pH 7.3, and the following (in μm): 0.5 TTX, 10 CNQX, and 10 APV, plus either 5 or 40 mm KCl for 3 min, and immediately fixed in solution containing paraformaldehyde (4%), sucrose (4%), and EGTA (20 mm) at room temperature for 10 min. Cells were permeabilized in PBS with Triton X-100 (0.1%) for 10 min and blocked with normal goat serum (10%) in PBS for 1 h. Primary antibodies were rabbit anti-phosphorylated CREB (pCREB) (1:300; Cell Signaling Technology catalog #9198S, RRID: AB_390802); mouse anti-CaMKIIα (1:2000, Thermo Fisher Scientific catalog #MA1-048, RRID: AB_325403); mouse anti-NeuN (1:100, Millipore catalog #MAB377, RRID: AB_2298772) and were diluted in PBS, added to the coverslips, and incubated overnight at 4°C. The cells were then washed 3 times with PBS and incubated in anti-rabbit Alexa Fluor 488 (Thermo Fisher Scientific catalog #A31565, RRID: AB_10373124) and goat anti-mouse Alexa Fluor 568 (1:500; Thermo Fisher Scientific catalog #A-21134, RRID: AB_2535773) for 1 h at room temperature. After additional washing, cells were incubated in Hoechst 33342 (1 μg/ml) in distilled water for 15 min before mounting on microscope slides. The cells were visualized with a 60× oil-immersion objective on a confocal microscope (Fluoview 1000; Olympus). The level of pCREB fluorescence was quantified in confocal images analyzed in ImageJ software. The nuclear marker Hoechst and anti-NeuN staining were used to identify the region of interest over each neuron. The region adjacent to the neuron was regarded as background. Mean pixel intensity for pCREB immunofluorescence was measured in the region of interest and the background level was subtracted. Genotypes of animals were revealed after the quantification.
Trafficking assays.
For endocytosis assays, transfected HEK293T cells were washed twice with Dulbecco's PBS with MgCl2 and CaCl2 (DPBS2+; Thermo Fisher Scientific) 48 h after transfection. Live cells were incubated with mouse anti-HA antibodies (1:100, Sigma-Aldrich catalog #11583816001, RRID: AB_514505) at 37°C for 15 min. After washing 3 times with DPBS2+ at room temperature, the cells were maintained at 37°C for varying periods to allow for channel internalization before fixing in ice-cold paraformaldehyde (4%) in DPBS2+ for 10 min. Cells were then blocked with normal goat serum (10%), followed by incubating with anti-Cav1.2 antibodies overnight and the corresponding Alexa Fluor secondary antibodies and washes as described above. Images were taken using 60× oil-immersion objectives with a constant acquisition setting on a confocal microscope (Fluoview 1000; Olympus). The cell surface HA fluorescence in Cav1.2-positive cells was quantified in ImageJ software using the freehand brush tool to trace the membrane region stained by anti-HA antibodies manually for measuring the cell surface fluorescence density.
For forward trafficking, cells transfected with Cav1.2-SNAP were incubated with BG-Block (2 μm; New England BioLabs catalog #S9106S) at 37°C for 30 min to block existing channels. The cells were then washed 3 times and incubated in complete medium for varying durations before incubating with TMR-STAR (2 μm; New England BioLabs catalog #S9105S) at 37°C for 25 min to label newly synthesized channels. After washing with complete medium, cells were fixed for 10 min at 4°C in paraformaldehyde (4%), washed in DPBS2+, and blocked in DPBS2+ containing goat serum (10%). Cells were then incubated with anti-HA antibodies for cell surface labeling and the appropriate Alexa Fluor secondary antibodies. Cell surface TMR-STAR fluorescence was quantified in ImageJ as described above. Whole-cell fluorescence densities were quantified using freehand selection, omitting the signal intensity from adjacent areas. The background fluorescence density in each channel was also taken from the same image and subtracted from the signal density. Data were acquired and analyzed without prior knowledge of protein expression profiles.
Results
Densin interacts differentially with and regulates Cav1.2 and Cav1.3
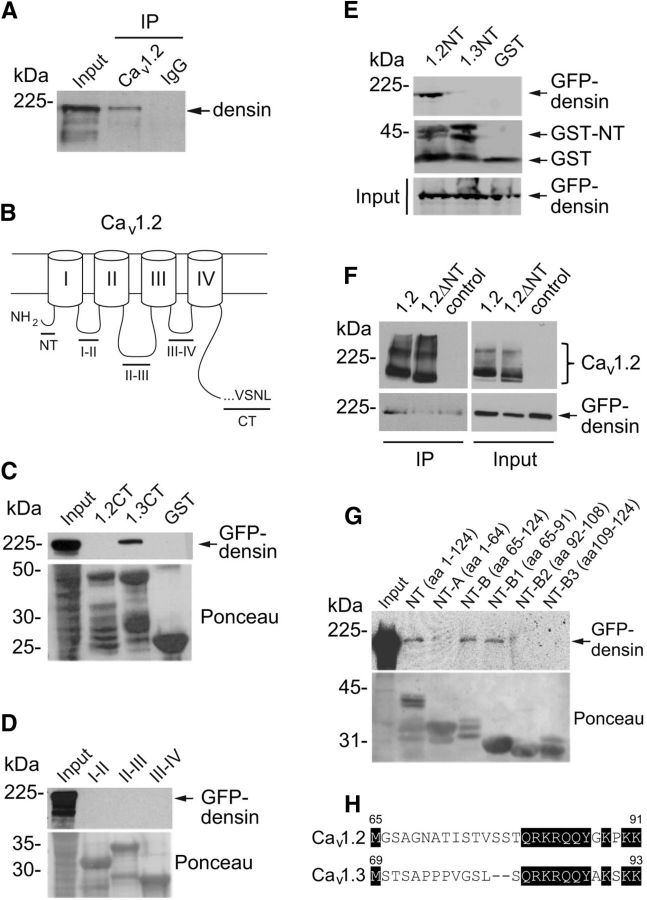
To determine whether densin is a physiological partner of Cav1.2 in vivo, we tested whether the two proteins form a complex in mouse brain. Antibodies that specifically recognize Cav1.2 (Tippens et al., 2008) were used to immunoprecipitate proteins from lysates of the mouse prefrontal cortex. Western blotting with densin antibodies revealed that densin coimmunoprecipitated with Cav1.2 antibodies, but not with control IgG (Fig. 1A). Because densin binds to the PDZ-binding site at the C terminus of Cav1.3 (Jenkins et al., 2010), we hypothesized that densin would similarly bind to the corresponding region of Cav1.2. In pull-down assays, GST fusion proteins containing the distal C terminus of Cav1.2 did not bind to GFP-densin despite interacting with the same region of Cav1.3 (Fig. 1B,C). GFP-densin also did not bind to the cytoplasmic loops of Cav1.2 (Fig. 1D). However, specific binding of GFP-densin was found for the N-terminal (NT) domain of Cav1.2 but not Cav1.3 (Fig. 1E). Moreover, deletion of the NT domain prevented coimmunoprecipitation of GFP-densin with Cav1.2 (Cav1.2ΔNT; Fig. 1F). Using GST-tagged fragments of the Cav1.2 NT, we found that GFP-densin bound specifically to proteins containing aa 65–91 (Fig. 1G). Because aa 77–91 are largely conserved in Cav1.3 (Fig. 1H), the major determinants for densin binding to the Cav1.2 NT appear to reside between aa 65 and 76.
Figure 1.
Densin interacts with Cav1.2 channels in the brain and binds to a site in the NT domain of Cav1.2. A, Coimmunoprecipitation of densin with Cav1.2 antibodies but not rabbit control IgG in lysates of mouse prefrontal cortex. Western blotting was performed using a densin antibody. B, Schematic of Cav1.2 α1 subunit showing the NT domain, the C-terminal (CT) domain, and cytoplasmic loops (I–II, II–III, and III–IV). C–E, Pull-down assays using GST-tagged cytoplasmic domains of Cav1.2. Input represents GFP-tagged densin in lysates of transfected HEK293T cells. C, Densin binds to the CT of Cav1.3, but not that of Cav1.2. D, Densin does not bind to the cytoplasmic loops of Cav1.2. E, Densin binds to the NT of Cav1.2, but not that of Cav1.3. F, Coimmunoprecipitation of GFP-densin with FLAG antibodies in HEK293T cells cotransfected with FLAG-tagged Cav1.2 (1.2), but not with Cav1.2 lacking the NT (1.2ΔNT) or control cells transfected with GFP-densin alone. G, Same as in C–E except with GST-tagged fragments of the Cav1.2 NT. Amino acid numbers are indicated in parentheses. All blots shown are representative of at least three independent experiments. Input lanes represent 7–10% of starting material. H, Sequence alignment of the B1 regions of Cav1.2 and Cav1.3. Conserved residues are shaded in black. Numbers indicate the amino acid residues.
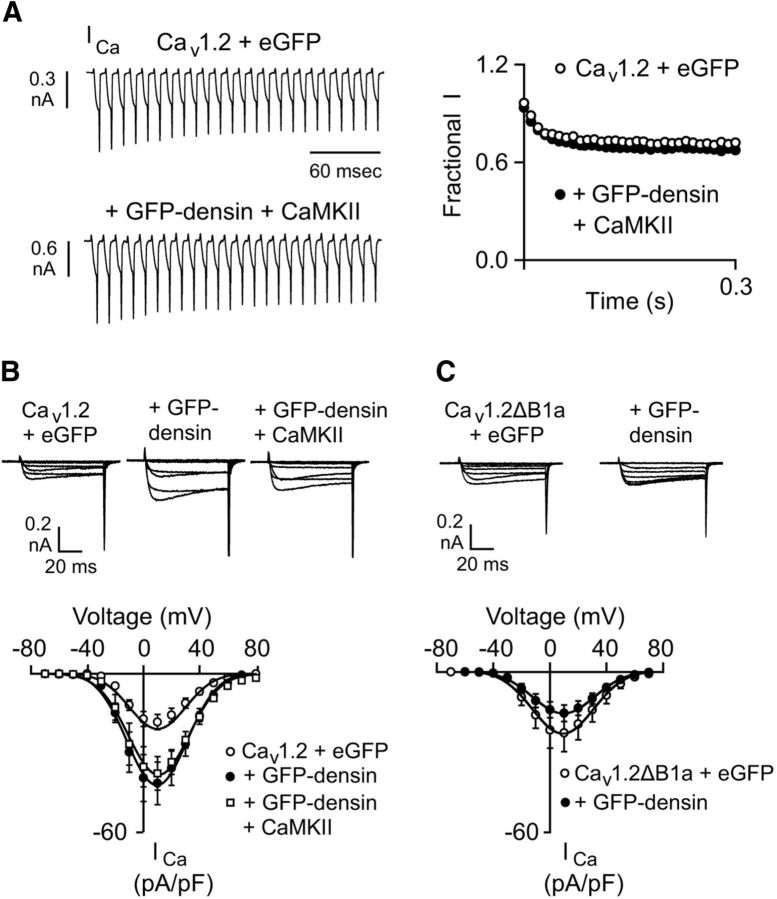
To elucidate the functional impact of densin on Cav1.2, we turned to an electrophysiological protocol we used previously to uncover the modulatory effects of densin on Cav1.3. When cotransfected with densin and its interacting protein, CaMKII (Strack et al., 2000; Walikonis et al., 2001), Cav1.3 channels undergo Ca2+-dependent facilitation during repetitive depolarizations (Jenkins et al., 2010). Although there was no effect of densin and CaMKII on Cav1.2 Ca2+ currents (ICa) with this protocol (Fig. 2A), I–V relationships revealed significantly greater current densities in cells cotransfected with GFP-densin and CaMKII than in control cells (at +10 mV, 38.0 ± 6.4 pA/pF for Cav1.2 + GFP-densin + CaMKII, n = 13, vs 20.7 ± 2.8 pA/pF for Cav1.2 + eGFP, n = 14, p = 0.02 by t test; Fig. 2B). This effect did not require CaMKII because it was reproduced in cells cotransfected with only Cav1.2 and GFP-densin (Fig. 2B). Densin did not augment ICa in cells cotransfected with channels lacking the NT aa 65–76 (Cav1.2ΔB1a) comprising the densin-binding site (at +10 mV, 15.4 ± 2.8 pA/pF for Cav1.2ΔB1a + GFP-densin, n = 14, vs 22.5 ± 5.6 pA/pF for Cav1.2ΔB1a + eGFP, n = 14, p = 0.29; Fig. 2C). These results indicate that densin binding to Cav1.2 is required for the enhanced current density in cells cotransfected with GFP-densin.
Figure 2.
Densin does not cause CDF but increases current density of Cav1.2 in HEK293T cells. A, ICa was evoked by 5 ms pulses from −80 to 10 mV at 100 Hz in cells transfected with Cav1.2 alone (n = 13) or cotransfected with densin and CaMKII (n = 11). Test current amplitudes were normalized to the first in the train (Fractional I) and plotted against time (right). B, ICa was evoked by 50 ms depolarizations from −80 mV to various voltages and normalized to cell capacitance. Representative current traces and plots of current density against voltage are shown for cells transfected with Cav1.2 and: eGFP (n = 13), GFP-densin (n = 15), and GFP-densin + CaMKII (n = 11). C, Same as in B except for ICa recorded in cells transfected with Cav1.2ΔB1a and eGFP (n = 14) or GFP-densin (n = 14).
GFP-densin could increase ICa by increasing the number of Cav1.2 channels and/or their activity in the cell membrane. To pinpoint the underlying mechanism, we measured the gating current upon depolarization to the reversal potential (Igating) and the tail current (Itail) evoked by repolarization to the holding voltage (Fig. 3A). The time integral of Igating (Qmax) estimates the number of channels in the cell membrane and the ratio of Itail and Qmax reflects the relative channel open probability (Wei et al., 1994; Takahashi et al., 2004). Cotransfection of GFP-densin caused a significant increase in Qmax (50.8 ± 14.1 fC for Cav1.2 + eGFP, n = 9 vs 94.6 ± 17.5 fC for Cav1.2 + GFP-densin, n = 13, p = 0.02 by t test; Fig. 3B). The slope of the Itail–Qmax relationship was steeper (∼2-fold; Fig. 3C) in cells cotransfected with GFP-densin compared with cells transfected with Cav1.2 and eGFP. Therefore, densin enhances the number of functional channels as well as their open probability. To determine whether the effect of densin requires interaction with the channel, we deleted the densin-binding sequence within the NT (Cav1.2ΔB1a + eGFP). In cells transfected with this construct, GFP-densin did not affect Qmax (61.2.0 ± 10.4 fC for Cav1.2ΔB1a + eGFP, n = 14 vs 73.9 ± 5.9 fC for Cav1.2ΔB1a + GFP-densin, n = 14, p = 0.30 by t test; Fig. 3D,E) or the Itail–Qmax relationship (Fig. 3F). Similar results were obtained for Cav1.2ΔNT (data not shown). These results demonstrate that the interaction of densin with Cav1.2 is required for facilitation of channel function by densin.
Figure 3.
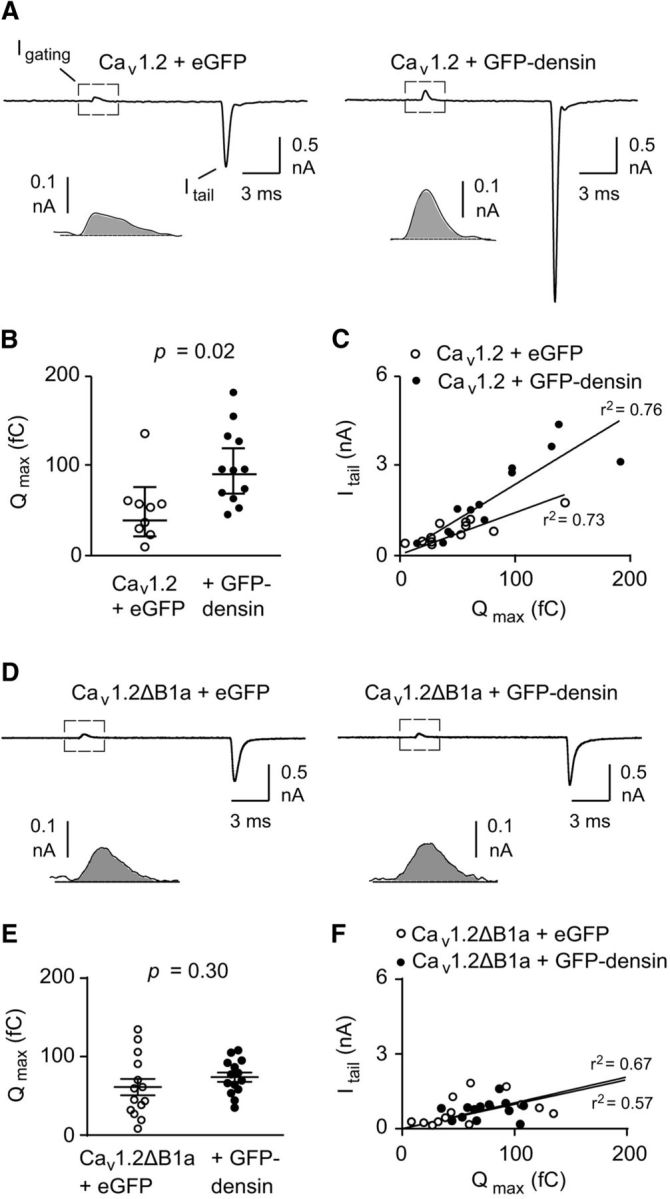
Densin increases the number of functional Cav1.2 channels and their relative open probability. A, Representative traces showing Igating and Itail measured at the reversal potential (+70 mV). Inset shows outward gating current on expanded timescale. Shaded area indicates Qmax. B, C, Dot plot of Qmax (B) and Itail versus Qmax (C) in cells cotransfected with Cav1.2 and eGFP (n = 9) or GFP-densin (n = 12). The fit by linear regression and coefficient of determination (r2) are shown. D–F, Same as in A–C except in cells transfected with Cav1.2ΔB1a and eGFP (n = 14) or GFP-densin (n = 14). p-values were determined by t test.
Densin promotes the forward trafficking of Cav1.2 channels
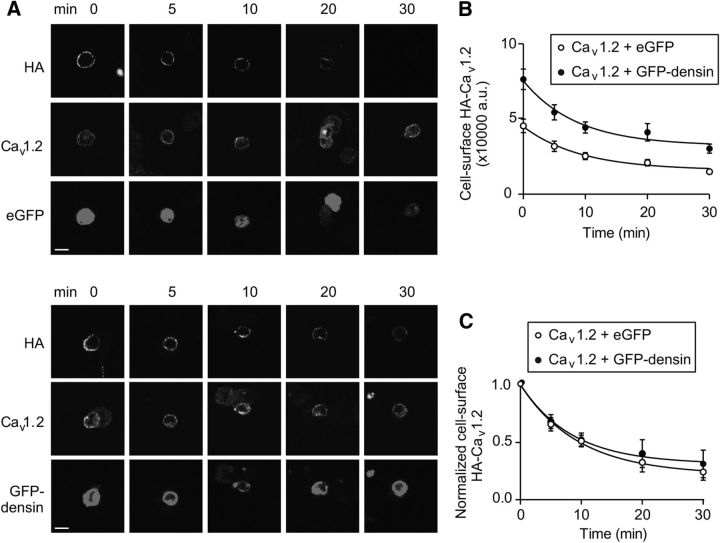
The effect of densin on the number of Cav1.2 channels could result from a larger amount of Cav1.2 protein in the plasma membrane either from reduced endocytosis and/or enhanced forward trafficking. To test this, we used Cav1.2 channels tagged with an external HA epitope (Cav1.2-HA; Altier et al., 2002) such that cell surface levels of channel could be measured by labeling with HA antibodies in living cells. After antibody labeling, HEK293T cells cotransfected with Cav1.2-HA and eGFP or GFP-densin were incubated in fresh medium for varying durations to allow for channel internalization (Fig. 4A). The level of cell surface Cav1.2-HA was significantly greater in cells cotransfected with GFP-densin than with eGFP at all time points (p < 0.0001 by ANOVA; Fig. 4B). However, exponential fits of the data normalized to Cav1.2 HA staining at the start of the experiment (time = 0 min) indicated no effect of densin on the speed of internalization of Cav1.2-HA (τ = 8.4 ± 0.7 min for GFP-densin, n = 62 cells, vs τ = 9.5 ± 0.8 min for eGFP, n = 60 cells, p = 0.22; Fig. 4C). These results confirm that densin increases the cell surface density of Cav1.2 channels, but not by inhibiting their endocytosis.
Figure 4.
Densin does not affect internalization of Cav1.2 in transfected HEK293T cells. A, Cells expressing Cav1.2-HA and eGFP (top) or GFP-densin (bottom) were incubated with HA antibodies to label cell surface channels and incubated for the indicated times (in minutes) to allow for channel internalization. The cells were then fixed and processed with Cav1.2 antibodies to label total channel protein. Scale bars, 10 μm. B, Cell surface HA fluorescence (arbitrary units, a.u.) was plotted against internalization time. Results are representative of three independent experiments. C, Same as B but HA intensity was normalized to that at time = 0 min. Smooth line represents fit with a single exponential equation.
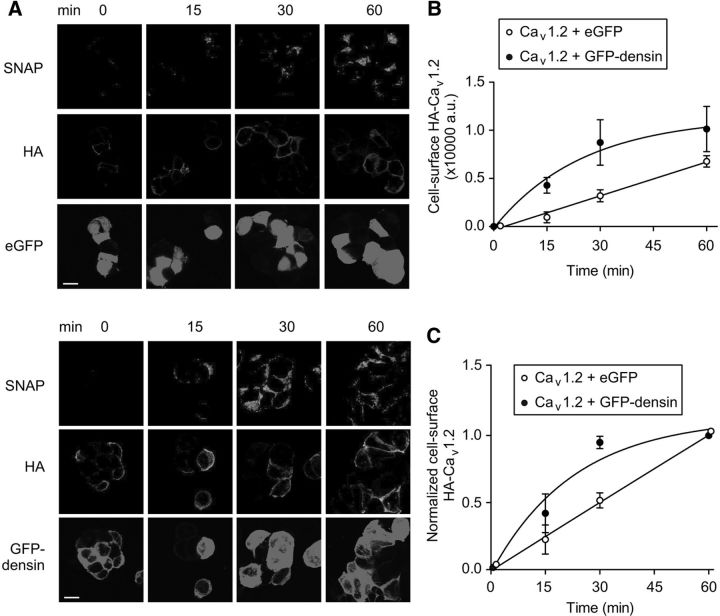
To determine whether densin enhances the trafficking of Cav1.2 to the cell surface, we generated Cav1.2 constructs containing the SNAP tag, an engineered variant of the DNA repair protein O6-alkylguanine-DNA-alkyltransferase that uses benzylguanine as a substrate (Keppler et al., 2004). SNAP-tagged Cav1.2-HA (Cav1.2-HA-SNAP) was cotransfected with eGFP or GFP-densin in HEK293T cells that were incubated first with benzylguanine to block existing channels. Subsequent incubation of the cells at 37°C for varying durations allowed for synthesis of new, unlabeled channels that were identified with a cell-permeant fluorescent derivative of benzylguanine (TMR-STAR; Fig. 5A). The cells were then processed for live labeling with HA antibodies and colocalized TMR-STAR and HA fluorescence was used to estimate the density of cell surface Cav1.2 channels. As in the endocytosis experiments, cotransfection with GFP-densin increased the amount of cell surface channels at most time points (Fig. 5B). When normalized to the last time point (60 min), the maximal density of cell surface HA-Cav1.2 was achieved within 30 min in cells cotransfected with GFP-densin compared with 1 h for cells cotransfected with eGFP (Fig. 5C). Therefore, densin increases the cell surface density of Cav1.2 channels by enhancing their trafficking to, rather than decreasing their removal from, the plasma membrane.
Figure 5.
Densin accelerates the forward trafficking of Cav1.2 in transfected HEK293T cells. A, Cells expressing Cav1.2-HA-SNAP and eGFP (top) or GFP-densin (bottom) were processed for TMR-STAR labeling of newly synthesized channels (see Materials and Methods). After incubation for the indicated times to allow forward trafficking of channels, cells were subjected to labeling with HA antibodies to mark cell surface channels. Scale bars, 10 μm. B, Cell surface TMR-STAR fluorescence (arbitrary units, a.u.) was plotted against forward trafficking time. Results are representative of three independent experiments. C, Same as B but TMR-STAR signal was normalized to that at time = 60 min. Smooth line represents fit with a single exponential equation.
Densin increases the cell surface levels of Cav1.2 in neurons
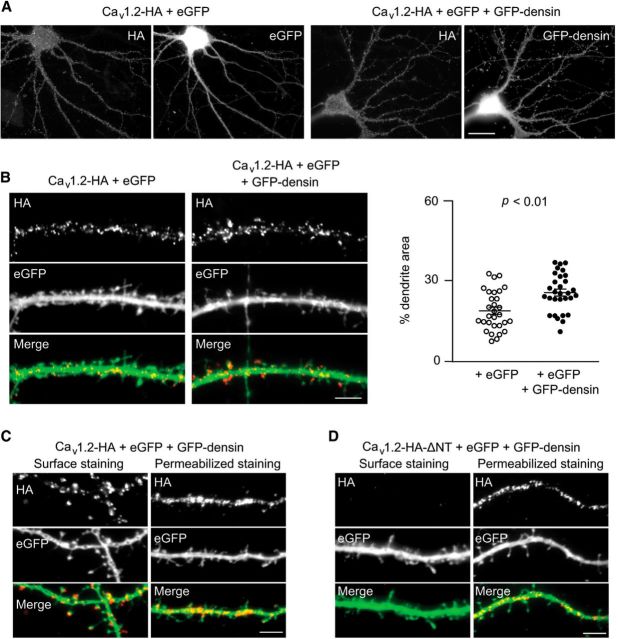
In neurons, Cav1.2 and densin are primarily localized in somatodendritic regions, particularly in dendritic spines (Apperson et al., 1996; Obermair et al., 2004; Tippens et al., 2008). To determine whether densin regulates the trafficking of Cav1.2 in neurons, we analyzed the distribution of cell surface Cav1.2-HA cotransfected with eGFP or GFP-densin in dendrites of hippocampal neurons in culture under nonpermeabilized conditions. We chose this approach rather than immunolabeling endogenously expressed Cav1.2 because Cav1.2 antibodies that are currently available recognize intracellular epitopes; the detergent permeabilization required for immunofluorescent detection would not restrict analysis to cell surface channels. Because densin is primarily localized at synapses of hippocampal neurons (Carlisle et al., 2011; Stanika et al., 2016), control and experimental groups were cotransfected with eGFP because the diffuse localization of eGFP in dendrites and dendritic spines facilitates the selection of dendritic regions for the analysis of cell surface channel clusters (Obermair et al., 2004). Consistent with previous studies (Obermair et al., 2004), punctate labeling for cell surface Cav1.2-HA channels was evident in the soma and dendrites. This distribution of Cav1.2-HA was qualitatively similar in neurons transfected with GFP-densin and eGFP alone (Fig. 6A). Consistent with our results in transfected HEK293T cells (Figs. 4, 5), quantitative analyses revealed that cotransfection with GFP-densin increased the dendritic area covered by cell surface clusters of Cav1.2-HA channels (Fig. 6B).
Figure 6.
GFP-densin increases the cell surface levels of dendritic Cav1.2 channels in neurons. A, Mouse hippocampal neurons were transfected with Cav1.2-HA + eGFP together with or without GFP-densin and subjected to live labeling with HA antibodies to mark cell surface channels. Representative fluorescent images are shown. Scale bar, 20 μm. B, Left, Dendrites of neurons transfected as in A shown at higher magnification. Scale bar, 5 μm. Data are representative of at least two independent experiments (number of dendrites analyzed per experiment: 15). Right, Quantification of cell surface Cav1.2-HA in hippocampal neurons expressed as the percentage of the dendritic area (defined by eGFP fluorescence) occupied by HA fluorescence. Statistical significance was determined by Student's t test. C, D, Dendrites of neurons transfected with eGFP, GFP-densin, and Cav1.2-HA (C) or Cav1.2-HA-ΔNT (D). Neurons were subjected to live cell staining with HA antibodies (left) or HA-labeling for total channels after permeabilization (right). Scale bars, 5 μm.
To verify that densin binding to Cav1.2 was required for this effect, we initially analyzed neurons transfected with GFP-densin and Cav1.2-HA-ΔB1a, which does not interact functionally with densin in HEK293T cells (Figs. 2C, 3D–F). However, Cav1.2-HA-ΔB1a did not express at high levels in neurons (data not shown). Therefore, we turned to Cav1.2-HA-ΔNT, which lacks the entire NT domain and also does not interact with densin (Fig. 1F). Total levels of Cav1.2-HA-ΔNT measured by HA labeling under permeabilized conditions were not different from those of WT Cav1.2-HA, but we could not detect Cav1.2-HA-ΔNT at the cell surface when coexpressed with GFP-densin (Fig. 6C,D). Although we cannot rule out the possibility that the NT has additional roles in promoting insertion of Cav1.2 in the dendritic membrane, our results are consistent with the importance of the densin–Cav1.2 interaction in determining the postsynaptic levels of Cav1.2 in neurons.
Cav1 current density and signaling to the nucleus are weakened in neurons from densin KO mice
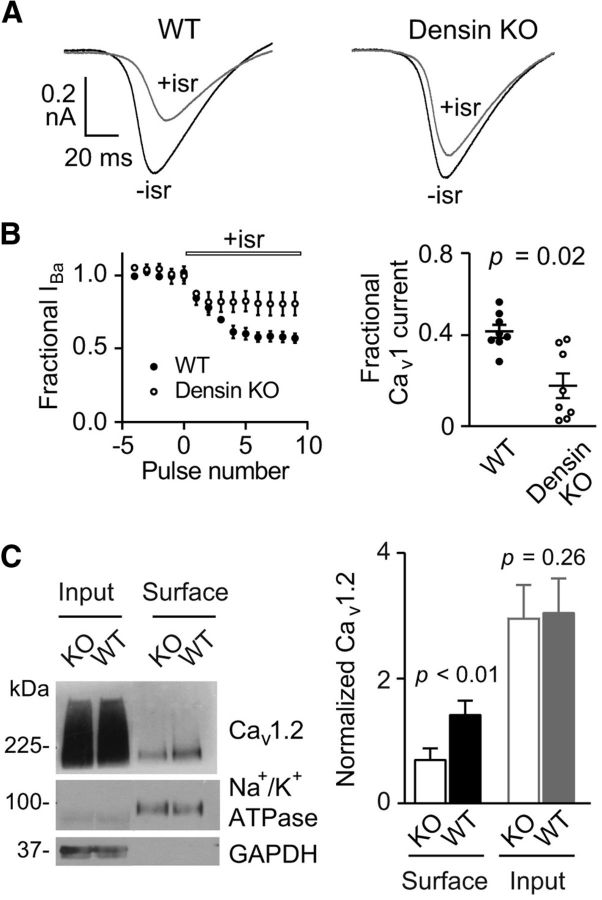
If densin supports the cell surface levels of Cav1.2 in neurons, then genetic inactivation of densin should cause a reduction in Cav1 current density and signaling in neurons. To test this, we used densin KO mice that were characterized previously (Carlisle et al., 2011). In whole-cell patch-clamp recordings, voltage ramps were used to evoke Ba2+ currents (IBa) in cortical neuron cultures obtained from WT and densin KO mice. Because multiple classes of Cav channels are expressed in these neurons (Lorenzon and Foehring, 1995), we isolated Cav1 currents pharmacologically by recording currents before and after bath perfusion of the Cav1 antagonist isradipine. Because Cav1.2 channels account for ∼80–90% of Cav1 channels in the brain (Sinnegger-Brauns et al., 2004), this strategy should largely reflect Cav1.2-mediated IBa. Using the isradipine-sensitive current as a measure of the Cav1 current, Cav1 channels accounted for ∼40% of peak IBa, which is similar to that reported in rat neocortical neurons (Lorenzon and Foehring, 1995). In recordings obtained from 3 separate neuronal cultures, the fractional Cav1 current was significantly greater (∼2-fold) in neurons from WT mice (0.42 ± 0.07; n = 8 neurons) than from densin KO mice (0.22 ± 0.05, n = 8 neurons, p < 0.02; Fig. 7A,B).
Figure 7.
Cav1 currents and cell surface Cav1.2 protein levels are reduced in cortical neurons from densin KO mice. A, IBa was evoked by voltage ramps from −60 to +80 mV (0.5 ms/mV) from a holding voltage of −60 mV in cortical neurons from WT (n = 8 cells from 3 mice) and densin KO (n = 8 from 3 mice) mice before (−isr) and after (+isr) bath application of isradipine (5 μm). B, Measurement of the Cav1-mediated current. Left, Peak currents for data collected as in A were plotted for voltage ramps evoked every minute before and after application of isradipine. Right, Fractional Cav1 current represents the fraction of the total IBa that was blocked by isradipine. Statistical significance was determined by Student's t test. C, Biotinylated cell surface proteins in prefrontal cortex of WT and densin KO mice were isolated on streptavidin agarose and subjected to Western blotting. Left, Representative Western blot probed with antibodies against Cav1.2, Na+/K+ ATPase, and GAPDH. Lanes represent biotinylated proteins before (input; ∼10% of total amount used for pull-down) or after streptavidin pull-down (surface). Results are representative of four independent experiments, with one WT and one densin KO littermate used per experiment. Right, Densitometric analysis of biotinylated cell surface Cav1.2 channels. Signals corresponding to Cav1.2 were normalized to that for Na+/K+ ATPase (surface) or GAPDH (input). Results are from four independent experiments. Statistical significance was determined by Student's t test.
Although these results were consistent with a role for densin in enhancing the cell surface density of Cav1.2 channels, upregulation of non-Cav1 channels may have contributed to the decrease in the isradipine-sensitive current in densin KO neurons. Moreover, at the concentration used in our experiments, isradipine (5 μm) inhibits both Cav1.2 and Cav1.3 (Koschak et al., 2001). To confirm that decreased cell surface levels of Cav1.2 could explain the loss of Cav1 currents in densin KO neurons, we isolated cell surface proteins by biotinylation in brain slices and purification on streptavidin agarose followed by Western blotting with Cav1.2 antibodies. Although there was no difference in total Cav1.2 protein levels, there was ∼50% less cell surface Cav1.2 in densin KO than in WT brain slices (Fig. 7C). This reduction is similar to, and thus may account for, the decrease in the Cav1 current in densin KO neurons (Fig. 7A,B). These results confirm that densin is required to support the cell surface density of Cav1.2 channels in the brain.
Activation of postsynaptic Cav1 channels promotes local Ca2+ signaling events that support activity-dependent gene transcription through phosphorylation of CREB (Wheeler et al., 2012), a transcription factor involved in synaptic plasticity and learning and memory (Deisseroth et al., 2003). If densin controls the number of postsynaptic Cav1.2 channels, then depolarization-induced levels of phosphorylated CREB (pCREB) should be reduced in densin KO neurons. To test this prediction, we fixed and immunolabeled neurons with antibodies recognizing pCREB after depolarization with solution containing 40 mm K+, which strongly activates Cav1-mediated CREB phosphorylation (Wheeler et al., 2012). Although pCREB levels were not different between genotypes under basal conditions (i.e., with 5 mm K+), the net increase in pCREB upon depolarization was significantly less in neurons from densin KO compared with WT mice (Fig. 8). We conclude that densin promotes excitation–transcription coupling in neurons, likely through enhancing the cell surface levels of postsynaptic Cav1.2 channels.
Figure 8.
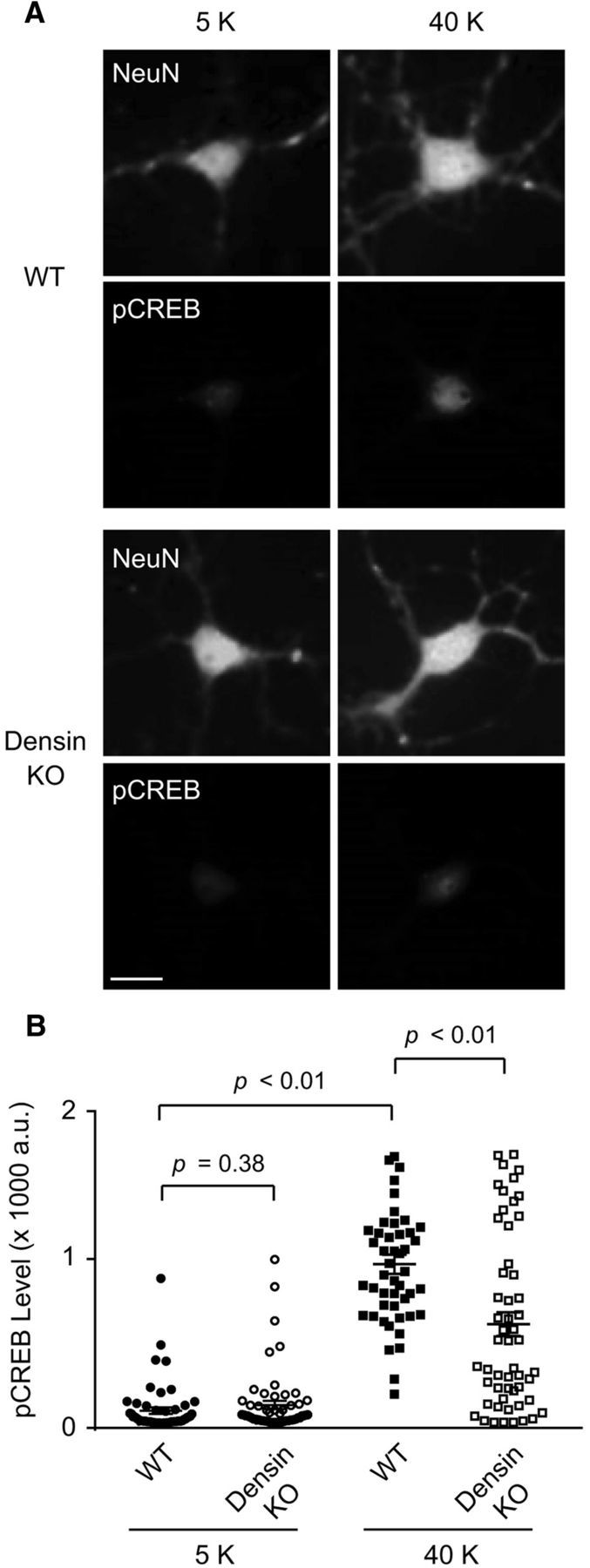
Excitation–transcription coupling is reduced in densin KO neurons. A, Epifluorescence images of cortical neurons from WT and densin KO mice exposed to 5 or 40 mm K+ for 3 min and processing for double-label immunofluorescence with antibodies against pCREB and the neuronal marker, NeuN. Images are representative of three independent experiments. B, Quantification of the intensity of pCREB fluorescence (arbitrary units, a.u.) in WT and densin KO cortical neurons (n = 50–58 neurons per condition from three different sets of cultures). Statistical significance was determined by Student's t test.
Discussion
Our study provides new insights into the role of densin in regulating neuronal Ca2+ signaling. First, in contrast to its interaction with the C-terminal domain of Cav1.3, densin binds to the NT of Cav1.2 and increases the cell surface levels of Cav1.2 by promoting its forward trafficking in transfected cells. Second, densin supports the postsynaptic levels of Cav1.2 in neurons, which are significantly reduced in the absence of densin. Finally, densin is required for robust Cav1-mediated excitation-transcription coupling in neurons. These effects of densin may contribute to the role of Cav1.2 in synaptic plasticity and spatial memory, processes that are disrupted in densin KO mice (Carlisle et al., 2011). In addition to regulating Ca2+ and CaMKII-dependent facilitation Cav1.3 (Jenkins et al., 2010), densin also regulates the clustering of heterologously expressed Cav1.3 channels in dendrites and the stability of dendritic spines in hippocampal neurons in culture (Stanika et al., 2016). Together with these findings, our results reveal densin as a multifunctional regulator of neuronal Cav1 channels.
The inability of densin to bind to the C-terminal domain of Cav1.2 may seem unexpected given that this region contains a type 1 PDZ-binding sequence similar to that which binds densin in Cav1.3 (Jenkins et al., 2010). However, there appears to be some selectivity with which PDZ-domains associate with the Cav1 C termini. For example, the PDZ domains of Erbin and Shank interact with the C termini of Cav1.3, but not Cav1.2 (Zhang et al., 2005; Calin-Jageman et al., 2007). Although deletion of the Cav1.2 PDZ-binding site does not affect Cav1.2 trafficking in neurons (Di Biase et al., 2008) or cardiac myocytes (Yang et al., 2015), deletions within the NT domain increase the cell surface density and maximal open probability of Cav1.2 in Xenopus oocytes (Wei et al., 1996; Kanevsky and Dascal, 2006). Binding of densin to the NT domain may mimic the effect of these deletions because densin not only increases the number of functional channels, but also their relative open probability (Fig. 3). Deletion of a CaMKII-binding site in the Cav1.2 NT domain decreases cell surface Cav1.2 protein, but increases Cav1.2 current density in tsA-201 cells (Simms et al., 2015). Although the effect of densin in our transfected cell recordings did not require CaMKII (Fig. 2B), we cannot discount the possibility that densin modulates the interaction of CaMKII with neuronal Cav1.2 channels. CaMKII binds to the α1 and β subunit of Cav1.2, which facilitates channel opening (Hudmon et al., 2005; Koval et al., 2010). Densin may alter such interactions because it enhances CaMKII binding to α-actinin (Walikonis et al., 2001; Robison et al., 2005), but can modulate CaMKII phosphorylation of AMPA and NMDA receptors differentially (Jiao et al., 2011).
Although Cav1.2 undergoes activity-dependent endocytosis in neurons (Green et al., 2007; Hall et al., 2013), the basal turnover rate of Cav1.2 channels in dendritic spines is slow (>1 h) and their lateral mobility is restricted (Di Biase et al., 2011). The stability of postsynaptic Cav1.2 channels could be due to their interactions with a variety of proteins in dendritic spines. For example, α-actinin and AKAP79/150 maintain the localization (Hall et al., 2013) and activity (Oliveria et al., 2007) of Cav1.2 in spines, respectively. However, activation of NMDA receptors causes a redistribution of α-actinin and AKAP79/150 into the dendritic shaft without affecting the localization of Cav1.2 in the spine (Di Biase et al., 2008), suggesting that these proteins are not sufficient for stabilizing dendritic Cav1.2 channels. In addition to CaMKII and α-actinin (Strack et al., 2000; Walikonis et al., 2001), densin interacts with Shank (Quitsch et al., 2005), δ-catenin (Izawa et al., 2002), and spinophilin (Baucum et al., 2010). Densin may help to anchor Cav1.2 channels within a network of interacting proteins, the dissolution of which may cause the loss of cell surface Cav1.2 channels in brain slices and neurons from densin KO mice (Fig. 7). This mechanism may explain how densin also promotes the dendritic clustering Cav1.3 (Stanika et al., 2016) despite binding to a different site than Cav1.2 (Fig. 1C,E).
The intracellular accumulation of Cav1.2-HA-ΔNT in the dendritic shaft, as well as its exclusion from spines (Fig. 6D), is similar to that of Cav1.2 mutants deficient in interactions with the auxiliary β subunit (Obermair et al., 2010). Considering that the forward trafficking of SNAP-tagged Cav1.2 channels in HEK293T cells is accelerated by densin (Fig. 5), densin may play a similar role as the β subunit in facilitating the export of Cav1.2 channels from their sites of synthesis and insertion into the postsynaptic membrane. This effect of densin may be particularly important to replenish Cav1.2 channels endocytosed during high levels of neuronal excitation (Green et al., 2007; Hall et al., 2013). The NT of Cav1.2 contains binding sites for multiple proteins including calmodulin (Ivanina et al., 2000), CaMKII (Simms et al., 2015), and CaBP1 (Zhou et al., 2005). Therefore, the loss of Cav1.2-HA-ΔNT interactions with densin and potentially other proteins could have collectively contributed to the complete absence of cell surface labeling of these mutant channels in dendrites (Fig. 6D).
As one of the major CaMKII-binding proteins in postsynaptic density (Apperson et al., 1996; Strack et al., 2000; Walikonis et al., 2001; Carlisle et al., 2011; Lowenthal et al., 2015), densin may provide a local pool of CaMKII that is necessary for coupling Cav1 channels to the activation of CREB in the nucleus (Wheeler et al., 2008; Wheeler et al., 2012). Ca2+ influx through Cav1 channels is sensed by calmodulin, which binds to and causes autophosphorylation of CaMKII, followed by a series of events leading to translocation of calmodulin to the nucleus and phosphorylation of CREB (Ma et al., 2014). Consistent with a role for densin in positioning CaMKII close to Cav1-mediated Ca2+ signals, the level of autophosphorylated CaMKII is profoundly reduced (∼75%) in neurons from densin KO compared with WT mice (Carlisle et al., 2011). Therefore, densin may not only ensure a sufficient density of Cav1.2 channels, but also of associated CaMKII enzymes available for excitation–transcription coupling. This would explain the strong reductions in Cav1-mediated pCREB induction in densin KO neurons (Fig. 8) and upon shRNA knockdown of densin in hippocampal neurons (Wheeler et al., 2012).
Our results suggest a potential mechanism to account for the overlap in the behavioral phenotypes of densin KO mice and those in mice with targeted deletion of Cav1.2. Compared with the respective control mice, anxiety-like behavior is significantly greater in densin KO mice (Carlisle et al., 2011) and mice lacking Cav1.2 expression in the prefrontal cortex (Lee et al., 2012). Anxiety disorders are common in patients with schizophrenia (Achim et al., 2011) and other neuropsychiatric disorders linked to polymorphisms in the CACNA1C gene encoding Cav1.2 (Heyes et al., 2015). It is tempting to speculate that the reduced abundance of functional Cav1.2 channels may contribute to the increased anxiety-like behavior and other endophenotypes of schizophrenia in densin KO mice (Carlisle et al., 2011). Indeed, molecular analyses of schizophrenia-associated polymorphisms in an intronic region of CACNA1C suggest the potential for altered levels of CACNA1C expression in patients harboring these variants (Eckart et al., 2016). Therefore, densin KO mice may serve as an appropriate mouse model with which to study how loss of function of Cav1.2 leads to cellular and behavioral anomalies underlying mental illness.
Footnotes
This work was supported by the National Institutes of Health (Grants DC009433 and NS084190 to A.L.; Grants NS17660 and NS 028710 to M.B.K.; and Grant MH063232 to R.J.C.), a Carver Research Program of Excellence Award to A.L.; the American Heart Association (Grant 14PRE18420020 to X.W.); the Austrian Science Fund (Grants P24079 and F4415 to G.J.O.); the G. and B. Moore Foundation (M.B.K.), and the Howard Hughes Medical Institute (M.B.K.). We thank J. Hell for providing cDNAs and M. Joiner and other members of the Lee and Colbran laboratories for valuable input. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding agencies.
The authors declare no competing financial interests.
References
- Achim AM, Maziade M, Raymond E, Olivier D, Mérette C, Roy MA (2011) How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull 37:811–821. 10.1093/schbul/sbp148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Dubel SJ, Barrère C, Jarvis SE, Stotz SC, Spaetgens RL, Scott JD, Cornet V, De Waard M, Zamponi GW, Nargeot J, Bourinet E (2002) Trafficking of L-type calcium channels mediated by the postsynaptic scaffolding protein AKAP79. J Biol Chem 277:33598–33603. 10.1074/jbc.M202476200 [DOI] [PubMed] [Google Scholar]
- Apperson ML, Moon IS, Kennedy MB (1996) Characterization of densin-180, a new brain-specific synaptic protein of the O-sialoglycoprotein family. J Neurosci 16:6839–6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucum AJ 2nd, Jalan-Sakrikar N, Jiao Y, Gustin RM, Carmody LC, Tabb DL, Ham AJ, Colbran RJ (2010) Identification and validation of novel spinophilin-associated proteins in rodent striatum using an enhanced ex vivo shotgun proteomics approach. Mol Cell Proteomics 9:1243–1259. 10.1074/mcp.M900387-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin-Jageman I, Lee A (2008) Cav1 L-type Ca2+ channel signaling complexes in neurons. J Neurochem 105:573–583. 10.1111/j.1471-4159.2008.05286.x [DOI] [PubMed] [Google Scholar]
- Calin-Jageman I, Yu K, Hall RA, Mei L, Lee A (2007) Erbin enhances voltage-dependent facilitation of Cav1.3 Ca2+ channels through relief of an autoinhibitory domain in the Cav1.3 α1 subunit. J Neurosci 27:1374–1385. 10.1523/JNEUROSCI.5191-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle HJ, Luong TN, Medina-Marino A, Schenker L, Khorosheva E, Indersmitten T, Gunapala KM, Steele AD, O'Dell TJ, Patterson PH, Kennedy MB (2011) Deletion of densin-180 results in abnormal behaviors associated with mental illness and reduces mGluR5 and DISC1 in the postsynaptic density fraction. J Neurosci 31:16194–16207. 10.1523/JNEUROSCI.5877-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NC, Nagano N, Kuenzi FM, Jarolimek W, Huber I, Walter D, Wietzorrek G, Boyce S, Kullmann DM, Striessnig J, Seabrook GR (2003) Neurological phenotype and synaptic function in mice lacking the CaV1.3 alpha subunit of neuronal L-type voltage-dependent Ca2+ channels. Neuroscience 120:435–442. 10.1016/S0306-4522(03)00329-4 [DOI] [PubMed] [Google Scholar]
- Cox B, Emili A (2006) Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat Protoc 1:1872–1878. 10.1038/nprot.2006.273 [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium (2013) Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379. 10.1016/S0140-6736(12)62129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Mermelstein PG, Xia H, Tsien RW (2003) Signaling from synapse to nucleus: the logic behind the mechanisms. Curr Opin Neurobiol 13:354–365. 10.1016/S0959-4388(03)00076-X [DOI] [PubMed] [Google Scholar]
- Di Biase V, Obermair GJ, Szabo Z, Altier C, Sanguesa J, Bourinet E, Flucher BE (2008) Stable membrane expression of postsynaptic CaV1.2 calcium channel clusters is independent of interactions with AKAP79/150 and PDZ proteins. J Neurosci 28:13845–13855. 10.1523/JNEUROSCI.3213-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase V, Tuluc P, Campiglio M, Obermair GJ, Heine M, Flucher BE (2011) Surface traffic of dendritic CaV1.2 calcium channels in hippocampal neurons. J Neurosci 31:13682–13694. 10.1523/JNEUROSCI.2300-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckart N, Song Q, Yang R, Wang R, Zhu H, McCallion AS, Avramopoulos D (2016) Functional characterization of schizophrenia-associated variation in CACNA1C. PLoS One 11:e0157086. 10.1371/journal.pone.0157086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher BE, Andrews SB, Fleischer S, Marks AR, Caswell A, Powell JA (1993) Triad formation: organization and function of the sarcoplasmic reticulum calcium release channel and triadin in normal and dysgenic muscle in vitro. J Cell Biol 123:1161–1174. 10.1083/jcb.123.5.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EM, Barrett CF, Bultynck G, Shamah SM, Dolmetsch RE (2007) The tumor suppressor eIF3e mediates calcium-dependent internalization of the L-Type calcium channel Cav1.2. Neuron 55:615–632. 10.1016/j.neuron.2007.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory FD, Bryan KE, Pangršič T, Calin-Jageman IE, Moser T, Lee A (2011) Harmonin inhibits presynaptic Cav1.3 Ca2+ channels in mouse inner hair cells. Nat Neurosci 14:1109–1111. 10.1038/nn.2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory FD, Pangrsic T, Calin-Jageman IE, Moser T, Lee A (2013) Harmonin enhances voltage-dependent facilitation of Cav1.3 channels and synchronous exocytosis in mouse inner hair cells. J Physiol 591:3253–3269. 10.1113/jphysiol.2013.254367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DD, Dai S, Tseng PY, Malik Z, Nguyen M, Matt L, Schnizler K, Shephard A, Mohapatra DP, Tsuruta F, Dolmetsch RE, Christel CJ, Lee A, Burette A, Weinberg RJ, Hell JW (2013) Competition between alpha-actinin and Ca2+-calmodulin controls surface retention of the L-type Ca2+ channel Cav1.2. Neuron 78:483–497. 10.1016/j.neuron.2013.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes S, Pratt WS, Rees E, Dahimene S, Ferron L, Owen MJ, Dolphin AC (2015) Genetic disruption of voltage-gated calcium channels in psychiatric and neurological disorders. Prog Neurobiol 134:36–54. 10.1016/j.pneurobio.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS (2005) CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol 171:537–547. 10.1083/jcb.200505155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanina T, Blumenstein Y, Shistik E, Barzilai R, Dascal N (2000) Modulation of L-type Ca2+ channels by Gβγ and calmodulin via interactions with N and C termini of alpha 1C. J Biol Chem 275:39846–39854. 10.1074/jbc.M005881200 [DOI] [PubMed] [Google Scholar]
- Izawa I, Nishizawa M, Ohtakara K, Inagaki M (2002) Densin-180 interacts with delta-catenin/neural plakophilin-related armadillo repeat protein at synapses. J Biol Chem 277:5345–5350. 10.1074/jbc.M110052200 [DOI] [PubMed] [Google Scholar]
- Jenkins MA, Christel CJ, Jiao Y, Abiria S, Kim KY, Usachev YM, Obermair GJ, Colbran RJ, Lee A (2010) Ca2+-dependent facilitation of Cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II. J Neurosci 30:5125–5135. 10.1523/JNEUROSCI.4367-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Robison AJ, Bass MA, Colbran RJ (2008) Developmentally regulated alternative splicing of densin modulates protein-protein interaction and subcellular localization. J Neurochem 105:1746–1760. 10.1111/j.1471-4159.2008.05280.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Jalan-Sakrikar N, Robison AJ, Baucum AJ 2nd, Bass MA, Colbran RJ (2011) Characterization of a central Ca2+/calmodulin-dependent protein kinase IIalpha/beta binding domain in densin that selectively modulates glutamate receptor subunit phosphorylation. J Biol Chem 286:24806–24818. 10.1074/jbc.M110.216010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanevsky N, Dascal N (2006) Regulation of maximal open probability is a separable function of Ca(v)beta subunit in L-type Ca2+ channel, dependent on NH2 terminus of alpha1C (Ca(v)1.2alpha). J Gen Physiol 128:15–36. 10.1085/jgp.200609485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler A, Pick H, Arrivoli C, Vogel H, Johnsson K (2004) Labeling of fusion proteins with synthetic fluorophores in live cells. Proc Natl Acad Sci U S A 101:9955–9959. 10.1073/pnas.0401923101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J (2001) α1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem 276:22100–22106. 10.1074/jbc.M101469200 [DOI] [PubMed] [Google Scholar]
- Koval OM, Guan X, Wu Y, Joiner ML, Gao Z, Chen B, Grumbach IM, Luczak ED, Colbran RJ, Song LS, Hund TJ, Mohler PJ, Anderson ME (2010) CaV1.2 beta-subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proc Natl Acad Sci U S A 107:4996–5000. 10.1073/pnas.0913760107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurschner C, Yuzaki M (1999) Neuronal interleukin-16 (NIL-16): a dual function PDZ domain protein. J Neurosci 19:7770–7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurschner C, Mermelstein PG, Holden WT, Surmeier DJ (1998) CIPP, a novel multivalent PDZ domain protein, selectively interacts with Kir4.0 family members, NMDA receptor subunits, neurexins, and neuroligins. Mol Cell Neurosci 11:161–172. 10.1006/mcne.1998.0679 [DOI] [PubMed] [Google Scholar]
- Lacinova L, Moosmang S, Langwieser N, Hofmann F, Kleppisch T (2008) Cav1.2 calcium channels modulate the spiking pattern of hippocampal pyramidal cells. Life Sci 82:41–49. 10.1016/j.lfs.2007.10.009 [DOI] [PubMed] [Google Scholar]
- Lee AS, Ra S, Rajadhyaksha AM, Britt JK, De Jesus-Cortes H, Gonzales KL, Lee A, Moosmang S, Hofmann F, Pieper AA, Rajadhyaksha AM (2012) Forebrain elimination of cacna1c mediates anxiety-like behavior in mice. Mol Psychiatry 17:1054–1055. 10.1038/mp.2012.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D, Allen SE, Toro CP (2013) Control of neuronal voltage-gated calcium ion channels from RNA to protein. Trends Neurosci 36:598–609. 10.1016/j.tins.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzon NM, Foehring RC (1995) Characterization of pharmacologically identified voltage-gated calcium channel currents in acutely isolated rat neocortical neurons. II. Postnatal development. J Neurophysiol 73:1443–1451. [DOI] [PubMed] [Google Scholar]
- Lowenthal MS, Markey SP, Dosemeci A (2015) Quantitative mass spectrometry measurements reveal stoichiometry of principal postsynaptic density proteins. J Proteome Res 14:2528–2538. 10.1021/acs.jproteome.5b00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Groth RD, Cohen SM, Emery JF, Li B, Hoedt E, Zhang G, Neubert TA, Tsien RW (2014) gammaCaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 159:281–294. 10.1016/j.cell.2014.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Müller J, Stiess M, Marais E, Schulla V, Lacinova L, Goebbels S, Nave KA, Storm DR, Hofmann F, Kleppisch T (2005) Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci 25:9883–9892. 10.1523/JNEUROSCI.1531-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermair GJ, Szabo Z, Bourinet E, Flucher BE (2004) Differential targeting of the L-type Ca2+ channel alpha 1C (Cav1.2) to synaptic and extrasynaptic compartments in hippocampal neurons. Eur J Neurosci 19:2109–2122. 10.1111/j.0953-816X.2004.03272.x [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Schlick B, Di Biase V, Subramanyam P, Gebhart M, Baumgartner S, Flucher BE (2010) Reciprocal interactions regulate targeting of calcium channel beta subunits and membrane expression of alpha1 subunits in cultured hippocampal neurons. J Biol Chem 285:5776–5791. 10.1074/jbc.M109.044271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Dell'Acqua ML, Sather WA (2007) AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron 55:261–275. 10.1016/j.neuron.2007.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ (2005) G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci 25:1050–1062. 10.1523/JNEUROSCI.3327-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinggera A, Striessnig J (2016) Cav 1.3 (CACNA1D) L-type Ca2+ channel dysfunction in CNS disorders. J Physiol 594:5839–5849. 10.1113/JP270672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitsch A, Berhörster K, Liew CW, Richter D, Kreienkamp HJ (2005) Postsynaptic shank antagonizes dendrite branching induced by the leucine-rich repeat protein Densin-180. J Neurosci 25:479–487. 10.1523/JNEUROSCI.2699-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Matthies HJ, Owens WA, Sathananthan V, Christianson NS, Kennedy JP, Lindsley CW, Daws LC, Galli A (2010) Insulin reveals Akt signaling as a novel regulator of norepinephrine transporter trafficking and norepinephrine homeostasis. J Neurosci 30:11305–11316. 10.1523/JNEUROSCI.0126-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Bass MA, Jiao Y, MacMillan LB, Carmody LC, Bartlett RK, Colbran RJ (2005) Multivalent interactions of calcium/calmodulin-dependent protein kinase II with the postsynaptic density proteins NR2B, densin-180, and alpha-actinin-2. J Biol Chem 280:35329–35336. 10.1074/jbc.M502191200 [DOI] [PubMed] [Google Scholar]
- Simms BA, Zamponi GW (2014) Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron 82:24–45. 10.1016/j.neuron.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Simms BA, Souza IA, Rehak R, Zamponi GW (2015) The Cav1.2 N terminus contains a CaM kinase site that modulates channel trafficking and function. Pflugers Arch 467:677–686. 10.1007/s00424-014-1538-7 [DOI] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renström E, Wietzorrek G, Berjukov S, Cavalli M, Walter D, Koschak A, Waldschütz R, Hering S, Bova S, Rorsman P, Pongs O, Singewald N, Striessnig J (2004) Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca2+ channels. J Clin Invest 113:1430–1439. 10.1172/JCI20208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanika R, Campiglio M, Pinggera A, Lee A, Striessnig J, Flucher BE, Obermair GJ (2016) Splice variants of the CaV1.3 L-type calcium channel regulate dendritic spine morphology. Sci Rep 6:34528. 10.1038/srep34528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Robison AJ, Bass MA, Colbran RJ (2000) Association of calcium/calmodulin-dependent kinase II with developmentally regulated splice variants of the postsynaptic density protein densin-180. J Biol Chem 275:25061–25064. 10.1074/jbc.C000319200 [DOI] [PubMed] [Google Scholar]
- Takahashi SX, Miriyala J, Colecraft HM (2004) Membrane-associated guanylate kinase-like properties of beta-subunits required for modulation of voltage-dependent Ca2+ channels. Proc Natl Acad Sci U S A 101:7193–7198. 10.1073/pnas.0306665101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippens AL, Pare JF, Langwieser N, Moosmang S, Milner TA, Smith Y, Lee A (2008) Ultrastructural evidence for pre- and post-synaptic localization of Cav1.2 L-type Ca2+ channels in the rat hippocampus. J Comp Neurol 506:569–583. 10.1002/cne.21567 [DOI] [PubMed] [Google Scholar]
- Walikonis RS, Oguni A, Khorosheva EM, Jeng CJ, Asuncion FJ, Kennedy MB (2001) Densin-180 forms a ternary complex with the a-subunit of Ca2+/calmodulin-dependent protein kinase II and a-actinin. J Neurosci 21:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Neely A, Lacerda AE, Olcese R, Stefani E, Perez-Reyes E, Birnbaumer L (1994) Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac α1 subunit. J Biol Chem 269:1635–1640. [PubMed] [Google Scholar]
- Wei X, Neely A, Olcese R, Lang W, Stefani E, Birnbaumer L (1996) Increase in Ca2+ channel expression by deletions at the amino terminus of the cardiac alpha 1C subunit. Receptors Channels 4:205–215. [PubMed] [Google Scholar]
- Weick JP, Groth RD, Isaksen AL, Mermelstein PG (2003) Interactions with PDZ proteins are required for L-type calcium channels to activate cAMP response element-binding protein-dependent gene expression. J Neurosci 23:3446–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DG, Barrett CF, Groth RD, Safa P, Tsien RW (2008) CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J Cell Biol 183:849–863. 10.1083/jcb.200805048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DG, Groth RD, Ma H, Barrett CF, Owen SF, Safa P, Tsien RW (2012) Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca2+ signaling to control CREB-dependent gene expression. Cell 149:1112–1124. 10.1016/j.cell.2012.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG (2008) Conditional forebrain deletion of the L-type calcium channel Ca V 1.2 disrupts remote spatial memories in mice. Learn Mem 15:1–5. 10.1101/lm.773208 [DOI] [PubMed] [Google Scholar]
- Yang L, Katchman A, Weinberg RL, Abrams J, Samad T, Wan E, Pitt GS, Marx SO (2015) The PDZ motif of the alpha1C subunit is not required for surface trafficking and adrenergic modulation of CaV1.2 channel in the heart. J Biol Chem 290:2166–2174. 10.1074/jbc.M114.602508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Maximov A, Fu Y, Xu F, Tang TS, Tkatch T, Surmeier DJ, Bezprozvanny I (2005) Association of CaV1.3 L-type calcium channels with Shank. J Neurosci 25:1037–1049. 10.1523/JNEUROSCI.4554-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Kim SA, Kirk EA, Tippens AL, Sun H, Haeseleer F, Lee A (2004) Ca2+-binding protein-1 facilitates and forms a postsynaptic complex with Cav1.2 (L-type) Ca2+ channels. J Neurosci 24:4698–4708. 10.1523/JNEUROSCI.5523-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Yu K, McCoy KL, Lee A (2005) Molecular mechanism for divergent regulation of Cav1.2 Ca2+ channels by calmodulin and Ca2+-binding protein-1. J Biol Chem 280:29612–29619. 10.1074/jbc.M504167200 [DOI] [PubMed] [Google Scholar]