Abstract
The higher organization of β-cells into spheroid structures termed islets of Langerhans is critical for the proper regulation of insulin secretion. Thus, rodent β-cells form a functional syncytium that integrates and propagates information encoded by secretagogues, producing a “gain-of-function” in hormone release through the generation of coordinated cell-cell activity. By contrast, human islets possess divergent topology, and this may have repercussions for the cell-cell communication pathways that mediate the population dynamics underlying the intraislet regulation of insulin secretion. This is pertinent for type 2 diabetes mellitus pathogenesis, and its study in rodent models, because environmental and genetic factors may converge on these processes in a species-specific manner to precipitate the defective insulin secretion associated with glucose intolerance. The aim of the present minireview is therefore to discuss the structural and functional underpinnings that influence insulin secretion from human islets, and the possibility that dyscoordination between individual β-cells may play an important role in some forms of type 2 diabetes mellitus.
The proper control of blood glucose levels requires the concerted activity of cells within the islets of Langerhans, small (∼50–500 μm) hormone-releasing micro-organs that are diffusely scattered throughout the pancreatic parenchyma. Dysregulation of insulin and glucagon secretion, together with increased peripheral resistance to circulating insulin, is a characteristic feature of the glucose intolerance associated with type 2 diabetes mellitus (T2DM), a disease state currently affecting approximately 8% of the adult population worldwide (1). Whereas the mechanisms controlling insulin secretion at the level of the single β-cell are well studied (2), whether and how single cells within an islet cooperate during activated insulin secretion is less well characterized, especially in human islets. Because phylogenetic differences exist in islet architecture and composition, as well as paracrine and autocrine regulation of cell function, the intraislet mechanisms that regulate insulin secretion may provide an enigmatic route through which the diabetogenic milieu contributes to T2DM. Focusing on studies in human islets, the aim of this minireview is to provide a synopsis of the structural and functional cell-cell signaling processes underlying insulin secretion in man.
Origins of electrical activity in human β-cells
Within individual β-cells, rising glucose levels enhance glycolytic and citrate cycle flux to increase the cytoplasmic ratio of ATP:ADP (3, 4); alternative fates for glucose (eg, anaerobic production of lactate) are suppressed (5, 6). This, in turn, leads to the closure of hyperpolarizing ATP-sensitive potassium (K+) channels (KATP) through binding of the pore-forming Kir6.2 subunits that, along with the regulatory, SUR1 subunits, form the characteristic octameric channel structure (4, 7, 8). The resultant depolarization of the plasma membrane opens voltage-dependent calcium (Ca2+)-channels, generating action potentials and mediating the extracellular Ca2+ influx that underlies Ca2+-dependent exocytosis of insulin-containing granules (2, 9). In human β-cells, the voltage gating of Ca2+ influx stems from T (Ca(V)3.2)-type Ca2+-channels that transiently operate from −55mV and possess a putative pacemaker function, and P/Q (Ca(V)2.1)- and L (Ca(V)1.3)-type Ca2+-channels that require higher activation voltages but contribute most conductance (10–12). Because glucose-stimulated insulin secretion (GSIS) persists in islets derived from donors harboring inactive KATP due to mutations in SUR1 (13), KATP-independent signals are thought to be important for potentiating the effects of the triggering (Ca2+) pathway on exocytosis. Although the nature of such signals is poorly defined in both rodent and human tissue (14), they usually, although not always (15), exhibit a degree of Ca2+ dependency (16, 17). In addition to Ca2+ currents, human β-cells are also characterized by a robust tetrodotoxin-sensitive sodium (Na+) conductance, which emanates from voltage-gated Na+ (Nav1.6/Nav1.7)-channels comprising a pore-dilating voltage sensor coupled to a Na+ selectivity filter (10, 18, 19). These channels appear to contribute to, rather than generate, action potential firing in human β-cells, as tetrodotoxin only lowers the peak action potential voltage (10).
As β-cell electrical activity is oscillatory in the presence of high glucose, mechanisms must exist to transiently repolarize the cell membrane. This is principally accomplished via K+ efflux along its electrochemical gradient due to the activation of big conductance Ca2+-activated K+ channels, with a contribution from small conductance Ca2+-activated K+ channels (10, 20). Due to their slow inactivation kinetics, the latter may play a role in generating bursting activity patterns by appropriately spacing the rapid action potentials detected in human β-cells (20).
β-Cell population dynamics in response to glucose
Patch clamp-based measurements of membrane potential cannot be extended to more than a few β-cells and, since imaging with voltage-sensitive dyes is still in its infancy, proxy measures must instead be used when assessing activity profiles at the multicellular (ie, intact islet) level. Because [Ca2+]i is the major determinant of insulin secretion and reflects β-cell electrical status, Ca2+ imaging can instead be used as a useful surrogate to monitor the organization of β-cell population activity following stimulation. Whereas inferences about the cell dynamics underlying islet function have historically been drawn from observations of synchrony between crudely subdivided islet regions, it is only recently that rapid confocal microscopy techniques have allowed β-cell behavior to be captured in situ with cell resolution from a large field of view. Indeed, we and others have pioneered the use of functional multicellular calcium imaging techniques, allied to mathematical algorithms capable of delineating cell-cell interactions, to allow identification and mapping of the coordinated endocrine cell subpopulations involved in hormone release (21–25).
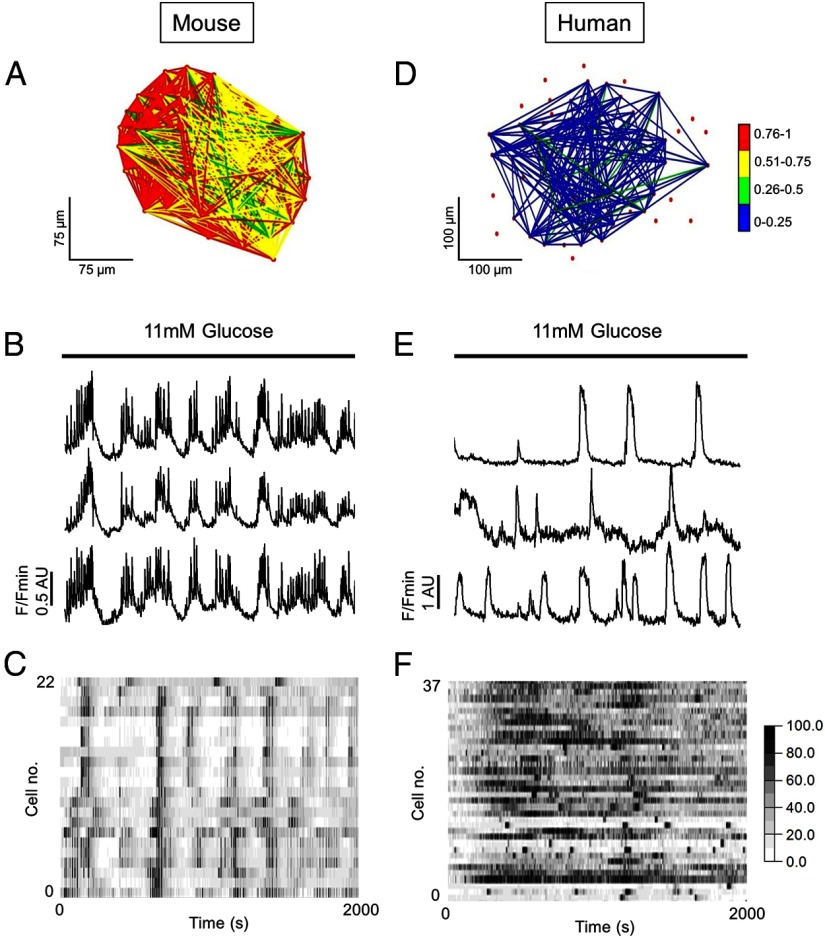
Using these approaches, it has been shown that, in response to increasing glucose concentrations, β-cells within mouse islets form highly coordinated small world networks (Figure 1A) that rapidly (millisecond) propagate the rhythmic oscillations in cytosolic free Ca2+ widely acknowledged to underlie pulsatile insulin release (25–31) (Figure 1, B and C). Cell-cell interactivity is likely to generate these activity profiles, as dissociated β-cells mount variable responses to stimulus, secrete less hormone in response to glucose, and possess diminished (pro)insulin biosynthesis (32–36). By contrast, in response to the same stimulus, β-cells within human islets occupy a sparsely connected and poorly correlated random network (Figure 1D) that supports apparently chaotic rises in cystosolic Ca2+, with synchrony detectable only between small cell clusters (37, 38) (Figure 1, E and F). This is not simply due to the relative paucity of β-cells in human vs rodent islets, because the number of correlated links per cell is lower in the former species.
Figure 1.
Glucose-stimulated β-cell dynamics in mouse vs human islets. Islets were loaded with the Ca2+-indicator fluo-2 for 30 minutes before being subjected to functional multicellular calcium imaging (fMCI) at the indicated glucose concentrations using a Nipkow spinning disk confocal (Zeiss M200 Axiovert coupled to a Yokogawa CSU10; λex = 491 nm; λex = 525 nm). Following image acquisition, glucose-responsive cells, here assumed to represent β-cells, were manually triaged before extraction of intensity over time traces. Correlations were determined by iterative pair-wise comparison of cell activity profiles using the Pearson R statistic, and a weighted functional connectivity (FC) map constructed on the basis of number, strength, and location (x-y) of correlated cell pairs within islets (see Refs. 21, 22, 57). Significance was calculated against the expected t-distribution of independent R-values assuming an equal sample size (P < .05). A, FC map showing the location and strength (color-coded; 0 [blue] = lowest, 1 [red] = highest) of correlated links between β-cells in an intact mouse islet during glucose stimulation. B, The network topology in panel A constitutes a functional syncytium that supports coordinated Ca2+-oscillations in response to elevated glucose. Representative traces from 3 individual 11 mM glucose-responsive cells are shown, here assumed to represent β-cells. C, A heat map showing signal intensity as a function of color depicts episodes of synchronous activity throughout the imaged population. D, As for panel A but β-cells within an intact human islet form a weakly correlated and poorly connected network. E, The human β-cell population responds to elevated glucose in a more stochastic manner (right panel) than their rodent counterpart. F, As for panel C. These data are previously unpublished. AU, arbitrary units.
Nonetheless, the extent of GSIS (as measured as a fold-change vs basal secretion) is similar in rodents and humans (39), implying that adaptations to population dynamics may reflect key evolutionary differences in islet structure-function relationships, as opposed to hormone release per se. Despite the consequences of this for T2DM pathogenesis, gaps remain in our knowledge regarding the intraislet regulation of β-cell function in man, namely: the structural basis for activity dynamics, the nature of the intercellular communication pathways that provoke stochasticity, the role of nonsugar secretagogues in coaxing coordinated activity, and how highly organized hormone pulses can emerge from randomness.
Structural basis for insulin secretion
Compared with their murine counterparts, human islets at first glance appear to possess a markedly different topology. When viewed as a 2-dimensional optical slice, β-cells are randomly intermingled with α- and other cells and do not conform to the classic β-cell core/α-cell mantle arrangement associated with rodent islets (38, 40, 41). However, when imaged in 3 dimensions using reconstruction of serial immunostained sections, α-cells were found to be present only in the core due to their arrangement along interiorly pervading vessels (42). Human islets might therefore best be described as flattened mouse islets, possessing a higher degree of structural complexity due to a unique tertiary folding step. Such architecture promotes heterologous contact between β- and α-cells, while preserving homologous contacts between β-cells (42), and emanates from the unusually high proportion of α-cells contained within human islets (∼40% representing ∼1:1.25 ratio with β-cells) (38, 40, 41). Hence, in mouse islets, the distance between any 2 β-cells is minimized by the core arrangement, whereas in human islets, the folded arrangement greatly exaggerates average path length but maximizes β-α-cell contacts. This would be expected to decrease the efficiency of information exchanges between distant β-cells and may contribute to the observation that only small clusters of human β-cells are able to display coordinated activity (37). Importantly, the concept that blood flow is largely arranged in the order β→α→δ cell, as originally suggested by histologic studies in rat islets (43) and functional analyses in dogs (44), is also believed to be the case in human islets (45). Consequently, and notwithstanding tight heterotypic cellular interactions (see below), direct roles for α-cell-secretory products in the global control of insulin release would appear to be unlikely.
Intercellular transmission of electrical signals
Rodent β-cells form a functional syncytium due to gap junction (GJ) linkages that facilitate ionic and metabolic coupling. These membrane channels, composed of connexin 36 protein (Cx36, encoded by GJD2), exhibit high-charge selectivity and pass only cations in addition to low molecular weight cytosolic signaling molecules such as nucleotides (eg, ATP and cAMP) (46, 47). Because GJs electrotonically couple neighboring cells, they play a critical role in recruiting and entraining β-cells during glucose stimulation, and this requires a 3-dimensional scaffold to be maximally effective (28). Thus, islets lacking Cx36 are unable to mount coordinated Ca2+ responses to glucose (28, 29, 48), leading to impaired pulsatile insulin secretion both in vitro and in vivo (30), and intercellular signal transfer is severely abrogated in β-cell monolayers (49). Moreover, GJ function can be modulated by cell activity status, constituting a further level of exocytosis regulation by allowing dynamic engagement of cells to insulin release as a function of demand (50, 51). As well as promoting insulin release, GJs also limit basal hormone output by preventing spontaneous Ca2+ rises through effects on coupling impedance, cellular heterogeneity, and transfer of hypopolarizing current between coupled cells (52, 53).
Compared with their murine counterparts, little is known about how GJs contribute to human islet function. Previous studies have shown that human β-cells are similarly coupled by GJs composed of Cx36, with a potential contribution from Cx26 (54), and that expression levels of Cx36 are nonlinearly correlated with insulin levels in islets isolated from control and T2DM subjects (55). Down-regulation of GJ signaling may therefore be central to glucose homeostasis in man, although the exact underlying mechanisms remain unexplored. It is unlikely that GJs contribute meaningfully to the islet dynamics involved in glucose-stimulated Ca2+ oscillations, because silencing of Cx36 in human tissue was unable to significantly impact correlated cell-cell activity (56). Instead, human β-cell-β-cell coupling may serve a more important role in ensuring coordinated responses to other endogenous insulin secretagogues such as glucagon-like peptide-1 (GLP-1), the effects of which are markedly inhibited by Cx36 silencing (56) (see below).
Since GJ coupling tends to be restricted to close neighbors, as assessed using measures of impedance, it is not clear how Cx36 contributes to interactions between distant cells, especially in rodent islets that house a β-cell syncytium. Potential and nonmutually exclusive explanations include the existence of 3-dimensionally coupled cell chains located outside of the field of view (23, 57), dynamic gating of GJ pores by small signaling molecules such as cAMP and ATP (58, 59), interactions with other cell types including non-Cx36-expressing α-cells that may regionally influence β-cell electrical activity via secretion of paracrine factors (60), neural transmission of signals (61) (see below), or putative pacemaker β-cells that may display unusually high Cx36 levels and regenerative activity. To further delineate the existence of these mechanisms, tools such as caged compounds and cell-targeted optogenes (62) will be required to harness more precise spatiotemporal control over β-cells.
Paracrine cell-cell signaling processes
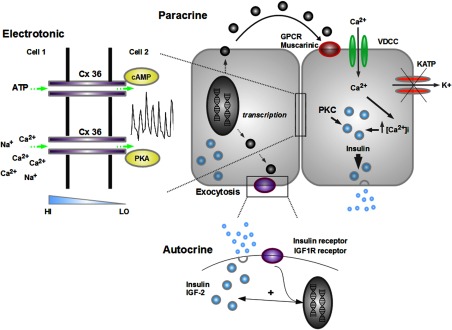
Endocrine cells within islets secrete a wide range of diffusible chemical messengers which, via binding to cognate receptors, are able to evoke biological effects in neighboring cells (see Table 1 and Figure 2). These so-called paracrine factors may play a number of different roles, mediating diverse processes ranging from cell survival and proliferation to hormone secretion (60, 63). In particular, the structural arrangement of human islets is likely to reflect the predominance of a paracrine mode of cell-cell communication and may have arisen as a consequence of altered innervation patterns (61). Thus, the orientation (ie, close contacts) between α- and β-cells constitutes a unique paracrine route of β-cell regulation whereby vesicular acetylcholine transporter-expressing α-cells secrete the neurotransmitter acetylcholine (ACh). During transition from high to low glucose, ACh is locally released into the islet matrix and primes β-cells to subsequent challenge with glucose, amplifying insulin release (64). Although intriguing, this hypothesis is intrinsically paradoxical given that release of ACh from the α-cells is expected to be stimulated under conditions (notably hypoglycemia) in which insulin secretion is usually suppressed.
Table 1.
Summary of the Chemical Messengers So Far Implicated in Human β-Cell Function
| Messenger | Mode | Receptor | Action | References |
|---|---|---|---|---|
| ACh | Paracrine | M3 | Increases Ca2+-dependent exocytosis via IP3 generation | |
| Increases Ca2+-sensitivity of exocytosis via PKC | 64 | |||
| Somatostatin | Paracrine | SSTR 2 and 3 | Activation of G protein-gated inward rectifier K+ channels (GIRK) | |
| Hyperpolarization and decreased hormone release | 65 | |||
| Insulin | Autocrine | IR A and B | Engagement of PI3K reduces pre-prepropinsulin gene expression | |
| Suppressed hormone release | 72, 73 | |||
| ATP | Autocrine | P2X | Opening of the P2X channel pore allowing influx of Ca2+ and Na+ | |
| Depolarization and exocytosis | 79, 81 | |||
| GABA | Autocrine | GABA(A) and GABA (B) | GABA(A) stimulate hormone release | |
| GABA(B) suppress hormone release | 83, 84 | |||
| Dopamine | Autocrine | D2R | Gi-coupled receptor leading to inhibition of adenylate cyclase | |
| Suppressed hormone release | 85, 86, 88 |
D2R, dopamine 2 receptor; IP3, inositol triphosphate; IR, insulin receptor; M3, muscarinic acetylcholine receptor M3; PI3K, phosphatidyl phosphate 3 kinase; PKC, protein kinase C; SSTR, somatostatin receptor.
Figure 2.
Schematic depicting cell-cell signaling modalities present within human islets. Chemical messengers act on the cell of origin (autocrine) or close neighbors (paracrine), and increase both insulin transcription and secretion. GJs composed of Cx36 provide electrotonic coupling between adjacent cells and also facilitate passage of small molecular weight molecules such as adenine nucleotides along their diffusion gradients (shown in blue). GJ transcription, phosphorylation status, and function can all be modulated by a range of intracellular signals including protein kinase A (PKA) and cAMP (figure adapted from Ref. 57). HI, high; KATP, ATP-sensitive K+ channel; LO, low; PKC, protein kinase C; VDCC, voltage-dependent calcium channel.
The δ cell minority within the islet is also likely to participate in the paracrine regulation of β-cell function, and this may again be encouraged by the presence of heterotypic contacts between the 2 cell types in human tissue (38). Somatostatin, the prototypic δ-cell-secretory product, binds somatostatin receptor subtypes 2 and 3, leading to hyperpolarization and suppressed GSIS from human islets (65). These observations are in line with those from somatostatin knockout mouse models that present with elevated circulating insulin levels and GSIS (66), albeit with differences in receptor subtype activation (67, 68).
Autocrine control of β-cell function
By contrast to paracrine signaling, autocrine factors act upon the cell of origin in self-sustaining regulatory circuits (see Table 1 and Figure 2). In the most classical feed-forward mechanism, extensively described in the mouse model, insulin binds to cognate receptors on the β-cell surface, reinforcing activation of the transcriptional machinery to boost preproinsulin gene expression; this has also been shown to be a feature of human β-cells (69–72). Evidence for a positive role for insulin in the control of β-cell exocytosis was also obtained in vivo in man using B28 Asp insulin that could be immunologically distinguished from the endogenous hormone (73). Nevertheless, others have very recently questioned this hypothesis, positing that high local concentrations of insulin are likely to lead to insulin (and IGF-I) receptor down-regulation from the β-cell surface (74). In our view, high local concentrations of insulin may be achieved at the mouth of the secretory granule fusion pore during the stimulation of release events and can be visualized using total internal reflection of fluorescence microscopy (75). However the relative paucity of cargo release across the cell surface (dozens of events per minute during stimulation with high glucose (75, 76)), together with the near-complete absence of such events at low glucose concentrations from healthy β-cells, suggests that at physiological rates of blood flow even these high insulin concentrations may quickly dissipate, making local signaling through plasma membrane-located insulin receptors perfectly plausible. Dysregulated insulin release or impaired intraislet blood flow in T2DM may mean, however, that β-cell insulin/IGF-I receptors are down-regulated, leading to impaired insulin signaling (ie, β-cell insulin resistance). This may eventually impair insulin production to exacerbate the disease.
Interestingly, evidence from mouse islets has suggested that IGF-II, cosecreted with insulin and able to act via IGF-I receptors, may be important for mediating the antiapoptotic properties of GLP-1 (77). Whether this signaling cascade is also important in human β-cells, in which long-term effects of GLP-1 on islet survival and growth have been more difficult to demonstrate, or is involved in the acute effects of the incretin on insulin secretion (Figure 3), remains to be seen. Indirect evidence for a role of IGF-II in man is however provided by the observation that polymorphic variants at IGF2BP, which encodes IGF-2 mRNA-binding protein, are associated with increased T2DM risk (78).
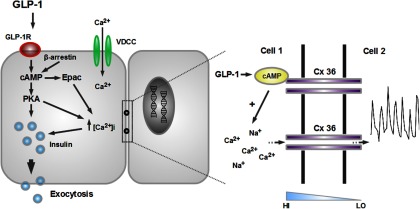
Figure 3.
Incretins evoke coordinated activity in a subnetwork of human β-cells to influence insulin release. At permissive glucose concentrations, GLP-1 binding to the GLP-1R leads to stimulation of adenylate cyclase activity and generation of cAMP, as well as engagement of a β-arrestin scaffold. By interacting with downstream partners such as exchange protein activated by cAMP (Epac) and protein kinase A (PKA), GLP-1 potentiates insulin release via effects on Ca2+-influx and Ca2+-sensitivity of exocytosis. Coordinated intercellular transmission of GLP-1-derived signals is achieved by GJ coupling, and GLP-1 may modulate this in a state-dependent manner via its cAMP-raising effects (blue ramp; diffusion gradient) (figure adapted from Ref. 57). GLP-1R, GLP-1 receptor; HI, high; LO, low.
Along similar lines, ATP coreleased from insulin-containing granules has been shown to amplify glucose-stimulated insulin secretion from human islets. Upon elevation of the sugar, ATP binds P2X3 receptors, increasing the magnitude of unidentified inward currents and leading to depolarization and Ca2+ influx (79–81). This purinergic positive feedback loop is hypothesized to improve the sensitivity and amplitude of β-cell responses in a context-dependent manner (81, 82). γ-Aminobutyric acid (GABA) (GABA)ergic signaling, which is vital for inhibitory neural circuits in the central nervous system, exerts a dual control over insulin secretion. Human β-cells are rich in GAD65, the enzyme that catalyzes the formation of GABA from glutamate, and activation of GABA(A) and GABA(B) receptors is thought to stimulate and inhibit insulin secretion, respectively, through effects on Cl− currents and membrane potential (83, 84). Most recently, the catecholamine dopamine, which is packaged into granules by vesicular monoamine transporter 2, has been shown to suppress GSIS by binding Gi-coupled dopamine 2 receptors (85, 86). This is likely to involve dampened KATP-independent signal input, because dopamine 2 receptors are linked to inhibition of adenylate cyclase and cAMP in other endocrine tissues (87). Dopamine may also act in a paracrine manner due to some expression in pancreatic polypeptide-cells located within islets toward the head of the pancreas (88).
Spatiotemporal regulation of chemical messenger action
Whereas the effects of paracrine messengers on human tissue are well studied at the single cell or insulin-release levels, practically nothing is known about how such regulatory loops dictate population dynamics. Most of these molecules diffuse stochastically between neigboring cells by Brownian motion (89), and it is difficult to envisage how this could account for the complex spatiotemporal activity patterns observed in human islets, unless in the rare cases where active transport mechanisms or GJ-mediated transfers are involved (57). Plausible explanations include human islet architecture that promotes formation of structural signaling motifs due to the preferential arrangement of β-cell clusters in proximity to paracrine-secreting cell types, as well as the existence of discrete diffusion corridors that facilitate timely delivery of paracrine factors to neighboring and maybe even distant cells in a nongraded manner. Indeed, in silico modeling of in vitro experiments in which laminar flow was manipulated using microfluidics has demonstrated that responses to paracrine stimulation depend on local cell topology, flow rate, and position in the flow field (90).
By contrast, autocrine factors tend to be coreleased with insulin, and it can be inferred from Ca2+-imaging studies that this is likely to be a stochastic process in human but not mouse islets if exocytosis were to reflect its Ca2+ dependency (29, 38, 56). Therefore, in adult tissues with low proliferation rates such as islets, autocrine signaling may serve to set the global output gain rather than influencing population dynamics/cell fate. Alternatively, cells may display pacemaker properties should they host regenerative autocrine signals as a consequence of their inherent electrical rhythmicity coupled to neural supply (91).
Incretins orchestrate coordinated islet function
The incretins glucagon-like peptide 1 (GLP-1) and gastric inhibitory peptide (GIP) are released from enteroendocrine cells in response to food transit and act to potentiate GSIS. Both GLP-1 and GIP bind G-protein/β-arrestin-coupled receptors, which increase insulin exocytosis through elevation of cAMP and interactions with downstream pathways including protein kinase A and exchange protein activated by cAMP (Figure 3) (92–97). Moreover, cAMP has been shown to gate GJ patency in intact but not dissociated islets (58, 98); therefore, it is conceivable that incretins may also influence β-cell population function. Because humans generally consume meals in 3–4 sittings, as opposed to mice that graze throughout darkness (65), it can be hypothesized that incretins may provide a convenient means to entrain insulin release to peak tissue demand in man.
Using in situ imaging approaches, we have recently demonstrated that GIP and GLP-1 stimulate synchronous Ca2+ rises in intact human islets, recruiting a highly coordinated subnetwork (∼50%) of glucose-responsive β-cells to augment insulin release. GJs convey the spatiotemporally precise propagation of information, because GLP-1-derived signals become asynchronous in the presence of Cx36 blockade/silencing (56). This action of incretin has relevance for T2DM, because “lipotoxicity” (99), which may exist under these conditions, disrupts GLP-1-induced connectivity in a GJ-dependent manner, and donor body mass index, which is strongly linked to elevated disease risk (100, 101), is negatively correlated with coordinated subpopulation responses. Providing evidence that defective β-cell incretin signaling contributes to insulin-secretory dysregulation in vivo is the observation that both GIP and GLP-1-stimulated insulin secretion are severely attenuated in T2DM subjects independently of raised glucose levels (102–106), and GLP-1 responses in islets from an individual with T2DM were noted to be severely impaired (our unpublished observations). Indeed, the synthesis and release of GLP-1 from human α-cells has recently been demonstrated in T2DM islets (107) and may represent a response, albeit through presently unknown mechanisms, to impaired incretin signaling in the β-cell.
Therefore, incretins are powerful regulators of the islet dynamics underlying insulin release in man, and this may be targeted by the diabetic milieu to exacerbate T2DM (see Figure 3 for a schematic). Such observations are pertinent in light of the increasing use of GLP-1 mimetics and dipeptidyl peptidase 4 inhibitors for amelioration of glucose intolerance, because the full therapeutic insulin-raising potential of these agents may not be realized in high body mass index individuals due to the presence of defective intraislet communications. It remains to be seen whether other endogenous regulators of β-cell function similarly improve GSIS by boosting intercellular signaling.
The genome-wide association studies (GWAS) era and intraislet communication
GWAS have now identified approximately 100 single-nucleotide polymorphisms, in 70 distinct genomic loci, which exaggerate the risk of developing glucose intolerance and T2DM. Whereas the intracellular mechanisms underlying the effects of variants at the loci with the greatest effect on disease risk (eg, those encoding TCF7L2 and SLC30A8/ZnT8) are slowly being teased apart (108–111), practically nothing is known about whether these and other genes impact β-cell population function. Indirectly suggesting that this may be the case are the observations that TCF2L7 and ZnT8 knockdown lead to aberrant islet Ca2+/Zn2+-handling (108, 109), and cell-cell communication is dependent on fine regulation of both ions (112, 113). Moreover, the gene encoding Cx36 is located at a chromosome locus (15q14) that confers increased disease susceptibility (55), and gene-gene and gene-environment interactions may be important determinants of multigenic disorders like T2DM (114, 115). Lastly, single-nucleotide polymorphisms are genome wide, and their localization to β-cells is not absolute meaning that permissive intercellular communications with α- and other cell types may also be affected in carriers. Therefore, the involvement of GWAS genes in β-cell cross talk would add a new facet to the mechanisms responsible for tipping the odds ratio (OR) in favor of developing T2DM in subjects harboring common risk variants.
Extrapolating activity patterns to pulsatile hormone release
In both mice and humans, pulsatile insulin release is most effective at evoking downstream actions in target tissues and, unsurprisingly, impaired insulin pulsatility is a hallmark of T2DM (116, 117). However, it is unclear how two divergent glucose-stimulated β-cell population activity signatures, coordinated in mice and random in humans, can result in the same endpoint. One possibility is that islet electrical oscillations and Ca2+ dynamics may reflect the mechanisms that sculpt GSIS in vivo. Indeed, islets are highly vascularized structures, and interactions between endocrine cells and capillaries may determine the fate of secreted insulin. It has been shown in other endocrine tissues that liberated hormone collects in the perivascular space and remains there for longer than the theoretical clearance rate calculated on the basis of molecular weight (118–120). Therefore, gatekeeping mechanisms present in the organ microenvironment, and potentially involving glucose-stimulated alterations to blood flow (120, 121), may translate noisy and stochastic cell dynamics into discrete and large insulin pulses by rate-limiting hormone release. Alternatively, glucose transport from the blood into the interstitial space (∼5–6 minutes in man; (122)) may homogenize stochastic human β-cell population dynamics by differentially activating islet regions depending on proximity to the capillary meshwork. Supporting a role for glucose delivery dynamics in population function is the observation that rodent islets are insufficiently coupled to allow global propagation of oscillations following local exposure to high glucose (123). In any case, involvement of the pancreatic circulation would certainly fit with the observation that dysregulation of islet vascularization and blood flow are major contributors to glucose intolerance and T2DM (124, 125). It should be noted, however, that incretin-stimulated insulin secretion relies on driving high levels of coordinated activity throughout β-cells within human islets. Hence, amplifying secretagogues such as GLP-1 and GIP may be less reliant on fine control by the vasculature to improve insulin-secretory burst mass (126), and may instead serve to increase the size of the hormone pool ready for transit into the vasculature.
Summary
It is becoming increasingly apparent that divergence in the structural and functional architecture of islets has repercussions for the intraislet regulation of hormone secretion. Indeed, human islets possess a high degree of heterotypic cell-cell contacts, and this impacts how the embedded endocrine populations communicate to organize their hormone-releasing activities. Dysregulation of local secretory circuits may therefore contribute to islet decompensation and the progression from impaired fasting glucose to frank diabetes. We further suggest that islet dynamics represent an important target for environmental factors, such as excess free fatty acids or inflammatory cytokines, as well as for genes affecting disease risk, each of which has the potential to perturb β-cell function and hence glucose homeostasis. On the basis of these observations, further studies are warranted to define the exact mechanisms that govern and pace β-cell activity within human islets, both from healthy donors and those suffering from T2DM.
Acknowledgments
This work was supported by a Diabetes United Kingdom R.D. Lawrence Research Fellowship (12/0004431) (to D.J.H.) and Wellcome Trust Senior Investigator (WT098424AIA), Medical Research Council Programme (MR/J0003042/1), Diabetes United Kingdom Project Grant (11/0004210) and Royal Society Wolfson Research Merit Awards (to G.A.R.). The work leading to this publication has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 155005 (IMIDIA), resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007–2013) and EFPIA companies' in kind contribution (to G.A.R.).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by a Diabetes United Kingdom R.D. Lawrence Research Fellowship (12/0004431) (to D.J.H.) and Wellcome Trust Senior Investigator (WT098424AIA), Medical Research Council Programme (MR/J0003042/1), Diabetes United Kingdom Project Grant (11/0004210) and Royal Society Wolfson Research Merit Awards (to G.A.R.). The work leading to this publication has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 155005 (IMIDIA), resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007–2013) and EFPIA companies' in kind contribution (to G.A.R.).
Footnotes
- Cx36
- connexin 36
- GABA
- γ-aminobutyric acid
- GIP
- glucose-dependent insulinotropic polypeptide
- GJ
- gap junction
- GLP-1
- glucagon-like peptide-1
- GSIS
- glucose-stimulated insulin secretion
- GWAS
- genome-wide association studies
- T2DM
- type 2 diabetes mellitus.
References
- 1. International Diabetes Federation. IDF Diabetes Atlas, 5th ed Brussels, Belgium: International Diabetes Federation; 2011. [Google Scholar]
- 2. Rutter GA. Nutrient-secretion coupling in the pancreatic islet β-cell: recent advances. Mol Aspects Med. 2001;22:247–284. [DOI] [PubMed] [Google Scholar]
- 3. Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2:163–214. [DOI] [PubMed] [Google Scholar]
- 4. Tarasov AI, Semplici F, Ravier MA, Bellomo EA, Pullen TJ, Gilon P, Sekler I, Rizzuto R, Rutter GA. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic β-cells. PloS One. 2012;7:e39722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pullen TJ, Rutter GA. When less is more: the forbidden fruits of gene repression in the adult β-cell. Diabetes Obes Metab. 2013;15:503–512. [DOI] [PubMed] [Google Scholar]
- 6. Sekine N, Cirulli V, Regazzi R, et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic β-cells. Potential role in nutrient sensing. J Biol Chem. 1994;269:4895–4902. [PubMed] [Google Scholar]
- 7. Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Nature. 1984;312:446–448. [DOI] [PubMed] [Google Scholar]
- 8. Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984;311:271–273. [DOI] [PubMed] [Google Scholar]
- 9. Rutter GA. Visualising insulin secretion. The Minkowski Lecture 2004. Diabetologia. 2004;47:1861–1872. [DOI] [PubMed] [Google Scholar]
- 10. Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, Johnson PR, Rorsman P. Voltage-gated ion channels in human pancreatic β-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. [DOI] [PubMed] [Google Scholar]
- 11. Islam MS, ed. The Islets of Langerhans. 1st ed The Netherlands: Springer; 2010 [Google Scholar]
- 12. Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–179. [DOI] [PubMed] [Google Scholar]
- 13. Straub SG, Cosgrove KE, Ammälä C, et al. Hyperinsulinism of infancy: the regulated release of insulin by KATP channel-independent pathways. Diabetes. 2001;50:329–339. [DOI] [PubMed] [Google Scholar]
- 14. Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52:739–751. [DOI] [PubMed] [Google Scholar]
- 15. Komatsu M, Schermerhorn T, Aizawa T, Sharp GW. Glucose stimulation of insulin release in the absence of extracellular Ca2+ and in the absence of any increase in intracellular Ca2+ in rat pancreatic islets. Proc Natl Acad Sci USA. 1995;92:10728–10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. [DOI] [PubMed] [Google Scholar]
- 17. Henquin JC. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in β-cells. Diabetes Res Clin Pract. 2011;93(Suppl. 1):S27–S31. [DOI] [PubMed] [Google Scholar]
- 18. Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barnett DW, Pressel DM, Misler S. Voltage-dependent Na+ and Ca2+ currents in human pancreatic islet β-cells: evidence for roles in the generation of action potentials and insulin secretion. Pflugers Arch. 1995;431:272–282. [DOI] [PubMed] [Google Scholar]
- 20. Jacobson DA, Mendez F, Thompson M, Torres J, Cochet O, Philipson LH. Calcium-activated and voltage-gated potassium channels of the pancreatic islet impart distinct and complementary roles during secretagogue induced electrical responses. J Physiol. 2010;588:3525–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanchez-Cardenas C, Fontanaud P, He Z, et al. Pituitary growth hormone network responses are sexually dimorphic and regulated by gonadal steroids in adulthood. Proc Natl Acad Sci USA. 2010;107:21878–21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hodson DJ, Molino F, Fontanaud P, Bonnefont X, Mollard P. Investigating and modelling pituitary endocrine network function. J Neuroendocrinol. 2010;22:1217–1225. [DOI] [PubMed] [Google Scholar]
- 23. Hodson DJ, Schaeffer M, Romano N, et al. Existence of long-lasting experience-dependent plasticity in endocrine cell networks. Nat Commun. 2012;3:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mollard P, Hodson DJ, Lafont C, Rizzoti K, Drouin J. A tridimensional view of pituitary development and function. Trends Endocrinol Metab. 2012;23:261–269. [DOI] [PubMed] [Google Scholar]
- 25. Stozer A, Gosak M, Dolensek J, et al. Functional connectivity in islets of Langerhans from mouse pancreas tissue slices. PLoS Comp Biol. 2013;9:e1002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Santos RM, Rosario LM, Nadal A, Garcia-Sancho J, Soria B, Valdeolmillos M. Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflugers Arch. 1991;418:417–422. [DOI] [PubMed] [Google Scholar]
- 27. Gilon P, Henquin JC. Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic B-cell. J Biol Chem. 1992;267:20713–20720. [PubMed] [Google Scholar]
- 28. Rocheleau JV, Remedi MS, Granada B, et al. Critical role of gap junction coupled KATP channel activity for regulated insulin secretion. PLoS Biol. 2006;4:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benninger RK, Zhang M, Head WS, Satin LS, Piston DW. Gap junction coupling and calcium waves in the pancreatic islet. Biophys J. 2008;95:5048–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Head WS, Orseth ML, Nunemaker CS, Satin LS, Piston DW, Benninger RK. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes. 2012;61:1700–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stozer A, Dolensek J, Rupnik MS. Glucose-stimulated calcium dynamics in islets of Langerhans in acute mouse pancreas tissue slices. PloS One. 2013;8:e54638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salomon D, Meda P. Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells. Exp Cell Res. 1986;162:507–520. [DOI] [PubMed] [Google Scholar]
- 33. Bosco D, Meda P. Actively synthesizing β-cells secrete preferentially after glucose stimulation. Endocrinology. 1991;129:3157–3166. [DOI] [PubMed] [Google Scholar]
- 34. Kinard TA, de Vries G, Sherman A, Satin LS. Modulation of the bursting properties of single mouse pancreatic β-cells by artificial conductances. Biophys J. 1999;76:1423–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang M, Goforth P, Bertram R, Sherman A, Satin L. The Ca2+ dynamics of isolated mouse β-cells and islets: implications for mathematical models. Biophys J. 2003;84:2852–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bavamian S, Klee P, Britan A, et al. Islet-cell-to-cell communication as basis for normal insulin secretion. Diabetes Obes Metab. 2007;9(Suppl 2):118–132. [DOI] [PubMed] [Google Scholar]
- 37. Martín F, Soria B. Glucose-induced [Ca2+]i oscillations in single human pancreatic islets. Cell Calcium. 1996;20:409–414. [DOI] [PubMed] [Google Scholar]
- 38. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. MacDonald MJ, Longacre MJ, Stoker SW, Kendrick M, Thonpho A, Brown LJ, Hasan NM, Jitrapakdee S, Fukao T, Hanson MS, Fernandez LA, Odorico J. Differences between human and rodent pancreatic islets low pyruvate carboxylase, ATP citrate lyase, and pyruvate carboxylation and high glucose-stimulated acetoacetate in human pancreatic islets. J Biol Chem. 2011;286:18383–18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. [DOI] [PubMed] [Google Scholar]
- 41. Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bosco D, Armanet M, Morel P, et al. Unique arrangement of α- and β-cells in human islets of Langerhans. Diabetes. 2010;59:1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982;31:883–889. [DOI] [PubMed] [Google Scholar]
- 44. Samols E, Stagner JI, Ewart RB, Marks V. The order of islet microvascular cellular perfusion is B—-A—-D in the perfused rat pancreas. J Clin Invest. 1988;82:350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stagner JI, Samols E. The vascular order of islet cellular perfusion in the human pancreas. Diabetes. 1992;41:93–97. [DOI] [PubMed] [Google Scholar]
- 46. Meda P, Amherdt M, Perrelet A, Orci L. Metabolic coupling between cultured pancreatic b-cells. Exp Cell Res. 1981;133:421–430. [DOI] [PubMed] [Google Scholar]
- 47. Charpantier E, Cancela J, Meda P. β cells preferentially exchange cationic molecules via connexin 36 gap junction channels. Diabe tologia. 2007;50:2332–2341. [DOI] [PubMed] [Google Scholar]
- 48. Ravier MA, Güldenagel M, Charollais A, et al. Loss of connexin36 channels alters β-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes. 2005;54:1798–1807. [DOI] [PubMed] [Google Scholar]
- 49. Hauge-Evans AC, Squires PE, Persaud SJ, Jones PM. Pancreatic β-cell-to-β-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes. 1999;48:1402–1408. [DOI] [PubMed] [Google Scholar]
- 50. Meda P, Perrelet A, Orci L. Increase of gap junctions between pancreatic B-cells during stimulation of insulin secretion. J Cell Biol. 1979;82:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meda P, Michaels RL, Halban PA, Orci L, Sheridan JD. In vivo modulation of gap junctions and dye coupling between B-cells of the intact pancreatic islet. Diabetes. 1983;32:858–868. [DOI] [PubMed] [Google Scholar]
- 52. Speier S, Gjinovci A, Charollais A, Meda P, Rupnik M. Cx36-mediated coupling reduces β-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes. 2007;56:1078–1086. [DOI] [PubMed] [Google Scholar]
- 53. Benninger RK, Head WS, Zhang M, Satin LS, Piston DW. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J Physiol. 2011;589:5453–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pfeffer F, Koczan D, Adam U, et al. Expression of connexin26 in islets of Langerhans is associated with impaired glucose tolerance in patients with pancreatic adenocarcinoma. Pancreas. 2004;29:284–290. [DOI] [PubMed] [Google Scholar]
- 55. Serre-Beinier V, Bosco D, Zulianello L, et al. Cx36 makes channels coupling human pancreatic β-cells, and correlates with insulin expression. Hum Mol Genet. 2009;18:428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hodson DJ, Mitchell RK, Bellomo EA, et al. Lipotoxicity disrupts incretin-regulated human β cell connectivity. J Clin Invest. 2013;123:4182–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hodson DJ, Romanò N, Schaeffer M, et al. Coordination of calcium signals by pituitary endocrine cells in situ. Cell Calcium. 2012;51:222–230. [DOI] [PubMed] [Google Scholar]
- 58. Mears D, Sheppard NF Jr., Atwater I, Rojas E. Magnitude and modulation of pancreatic β-cell gap junction electrical conductance in situ. J Membr Biol. 1995;146:163–176. [DOI] [PubMed] [Google Scholar]
- 59. Vera B, Sánchez-Abarca LI, Bolaños JP, Medina JM. Inhibition of astrocyte gap junctional communication by ATP depletion is reversed by calcium sequestration. FEBS Lett. 1996;392:225–228. [DOI] [PubMed] [Google Scholar]
- 60. Caicedo A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin Cell Dev Biol. 2013;24:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Deisseroth K. Optogenetics. Nat Methods. 2011;8:26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang YH, Szabat M, Bragagnini C, et al. Paracrine signalling loops in adult human and mouse pancreatic islets: netrins modulate β cell apoptosis signalling via dependence receptors. Diabe tologia. 2011;54:828–842. [DOI] [PubMed] [Google Scholar]
- 64. Rodriguez-Diaz R, Dando R, Jacques-Silva MC, et al. α Cells secrete acetylcholine as a non-neuronal paracrine signal priming β cell function in humans. Nat Med. 2011;17:888–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kailey B, van de Bunt M, Cheley S, et al. SSTR2 is the functionally dominant somatostatin receptor in human pancreatic β- and α-cells. Am J Physiol Endocrinol Metab. 2012;303:E1107–E1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hauge-Evans AC, King AJ, Carmignac D, et al. Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009;58:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141:111–117. [DOI] [PubMed] [Google Scholar]
- 68. Rutter GA. Regulating glucagon secretion: somatostatin in the spotlight. Diabetes. 2009;58:299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Leibiger IB, Leibiger B, Moede T, Berggren PO. Exocytosis of insulin promotes insulin gene transcription via the insulin receptor/PI-3 kinase/p70 s6 kinase and CaM kinase pathways. Mol Cell. 1998;1:933–938. [DOI] [PubMed] [Google Scholar]
- 70. Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. [DOI] [PubMed] [Google Scholar]
- 71. Da Silva Xavier G, Qian Q, Cullen PJ, Rutter GA. Distinct roles for insulin and insulin-like growth factor-1 receptors in pancreatic β-cell glucose sensing revealed by RNA silencing. Biochem J. 2004;377:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Muller D, Huang GC, Amiel S, Jones PM, Persaud SJ. Identification of insulin signaling elements in human β-cells: autocrine regulation of insulin gene expression. Diabetes. 2006;55:2835–2842. [DOI] [PubMed] [Google Scholar]
- 73. Bouche C, Lopez X, Fleischman A, et al. Insulin enhances glucose-stimulated insulin secretion in healthy humans. Proc Natl Acad Sci USA. 2010;107:4770–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rhodes CJ, White MF, Leahy JL, Kahn SE. Direct autocrine action of insulin on β-cells: does it make physiological sense? Diabetes. 2013;62:2157–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tsuboi T, Rutter GA. Multiple forms of “kiss-and-run” exocytosis revealed by evanescent wave microscopy. Curr Biol. 2003;13:563–567. [DOI] [PubMed] [Google Scholar]
- 76. Rorsman P, Renström E. Insulin granule dynamics in pancreatic β cells. Diabetologia. 2003;46:1029–1045. [DOI] [PubMed] [Google Scholar]
- 77. Cornu M, Yang JY, Jaccard E, Poussin C, Widmann C, Thorens B. Glucagon-like peptide-1 protects β-cells against apoptosis by increasing the activity of an IGF-2/IGF-1 receptor autocrine loop. Diabetes. 2009;58:1816–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fernandez-Alvarez J, Hillaire-Buys D, Loubatières-Mariani MM, Gomis R, Petit P. P2 receptor agonists stimulate insulin release from human pancreatic islets. Pancreas. 2001;22:69–71. [DOI] [PubMed] [Google Scholar]
- 80. Silva AM, Rodrigues RJ, Tomé AR, et al. Electrophysiological and immunocytochemical evidence for P2X purinergic receptors in pancreatic β cells. Pancreas. 2008;36:279–283. [DOI] [PubMed] [Google Scholar]
- 81. Jacques-Silva MC, Correa-Medina M, Cabrera O, et al. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic β cell. Proc Natl Acad Sci USA. 2010;107:6465–6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shvartsman SY, Hagan MP, Yacoub A, Dent P, Wiley HS, Lauffenburger DA. Autocrine loops with positive feedback enable context-dependent cell signaling. Am J Physiol Cell Physiol. 2002;282:C545–C559. [DOI] [PubMed] [Google Scholar]
- 83. Braun M, Ramracheya R, Bengtsson M, et al. γ-Aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic β-cells. Diabetes. 2010;59:1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Taneera J, Jin Z, Jin Y, et al. gamma-Aminobutyric acid (GABA) signalling in human pancreatic islets is altered in type 2 diabetes. Diabetologia. 2012;55:1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Simpson N, Maffei A, Freeby M, et al. Dopamine-mediated autocrine inhibitory circuit regulating human insulin secretion in vitro. Mol Endocrinol. 2012;26:1757–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ustione A, Piston DW, Harris PE. Minireview: dopaminergic regulation of insulin secretion from the pancreatic islet. Mol Endocrinol. 2013;27:1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–763. [DOI] [PubMed] [Google Scholar]
- 88. Freeby M, Ichise M, Harris PE. Vesicular monoamine transporter, type 2 (vmat2) expression as it compares to insulin and pancreatic polypeptide in the head, body and tail of the human pancreas. Islets. 2012;4:393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Batsilas L, Berezhkovskii AM, Shvartsman SY. Stochastic model of autocrine and paracrine signals in cell culture assays. Biophys J. 2003;85:3659–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moledina F, Clarke G, Oskooei A, Onishi K, Günther A, Zandstra PW. Predictive microfluidic control of regulatory ligand trajectories in individual pluripotent cells. Proc Natl Acad Sci USA. 2012;109:3264–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ebert SN, Taylor DG. Catecholamines and development of cardiac pacemaking: an intrinsically intimate relationship. Cardiovasc Res. 2006;72:364–374. [DOI] [PubMed] [Google Scholar]
- 92. Kashima Y, Miki T, Shibasaki T, et al. Critical role of cAMP-GEFII–Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276:46046–46053. [DOI] [PubMed] [Google Scholar]
- 93. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. [DOI] [PubMed] [Google Scholar]
- 94. Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu JC, Olefsky JM. β-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic β cells. Proc Natl Acad Sci USA. 2008;105:6614–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Quoyer J, Longuet C, Broca C, et al. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a β-arrestin 1-mediated ERK1/2 activation in pancreatic β-cells. J Biol Chem. 2010;285:1989–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Invest. 2010;1:8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Leech CA, Dzhura I, Chepurny OG, et al. Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic β cells. Prog Biophys Mol Biol. 2011;107:236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pérez-Armendariz M, Roy C, Spray DC, Bennett MV. Biophysical properties of gap junctions between freshly dispersed pairs of mouse pancreatic β cells. Biophys J. 1991;59:76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rutter GA, Parton LE. The β-cell in type 2 diabetes and in obesity. Front Horm Res. 2008;36:118–134. [DOI] [PubMed] [Google Scholar]
- 100. Prentki M, Nolan CJ. Islet β cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12:75–80. [DOI] [PubMed] [Google Scholar]
- 102. Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on β-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52:380–386. [DOI] [PubMed] [Google Scholar]
- 103. Knop FK, Aaboe K, Vilsboll T, Madsbad S, Krarup T, Holst JJ. Reduced incretin effect in obese subjects with normal glucose tolerance as compared to lean control subjects. Diabetologia. 2008;51:S258–S258. [Google Scholar]
- 104. Højberg PV, Vilsbøll T, Rabøl R, et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199–207. [DOI] [PubMed] [Google Scholar]
- 105. Holst JJ, Knop FK, Vilsboll T, Krarup T, Madsbad S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 2011;34(Suppl. 2):S251–S257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Knop FK, Aaboe K, Vilsboll T, et al. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes Metab. 2012;14:500–510. [DOI] [PubMed] [Google Scholar]
- 107. Marchetti P, Lupi R, Bugliani M, et al. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia. 2012;55:3262–3272. [DOI] [PubMed] [Google Scholar]
- 108. da Silva Xavier G, Loder MK, McDonald A, et al. TCF7L2 regulates late events in insulin secretion from pancreatic islet β-cells. Diabetes. 2009;58:894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nicolson TJ, Bellomo EA, Wijesekara N, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. da Silva Xavier G, Mondragon A, Sun G, et al. Abnormal glucose tolerance and insulin secretion in pancreas-specific Tcf7l2-null mice. Diabe tologia. 2012;55:2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. da Silva Xavier G, Bellomo EA, McGinty JA, French PM, Rutter GA. Animal models of GWAS-identified type 2 diabetes genes. J Diabetes Res. 2013;2013:906590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lurtz MM, Louis CF. Intracellular calcium regulation of connexin43. Am J Physiol Cell Physiol. 2007;293:C1806–C1813. [DOI] [PubMed] [Google Scholar]
- 113. Sun Z, Zhang DQ, McMahon DG. Zinc modulation of hemi-gap-junction channel currents in retinal horizontal cells. J Neurophysiol. 2009;101:1774–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Neuman RJ, Wasson J, Atzmon G, et al. Gene-gene interactions lead to higher risk for development of type 2 diabetes in an Ashkenazi Jewish population. PloS One. 2010;5:e9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Franks PW, Pearson E, Florez JC. Gene-environment and gene-treatment interactions in type 2 diabetes: progress, pitfalls, and prospects. Diabetes Care. 2013;36:1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121:2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wahren J, Kallas Å. Loss of pulsatile insulin secretion: a factor in the pathogenesis of type 2 diabetes? Diabetes. 2012;61:2228–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lafont C, Desarménien MG, Cassou M, et al. Cellular in vivo imaging reveals coordinated regulation of pituitary microcirculation and GH cell network function. Proc Natl Acad Sci USA. 2010;107:4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Schaeffer M, Hodson DJ, Lafont C, Mollard P. Functional importance of blood flow dynamics and partial oxygen pressure in the anterior pituitary. Eur J Neurosci. 2010;32:2087–2095. [DOI] [PubMed] [Google Scholar]
- 120. Schaeffer M, Hodson DJ, Lafont C, Mollard P. Endocrine cells and blood vessels work in tandem to generate hormone pulses. J Mol Endocrinol. 2011;47:R59–R66. [DOI] [PubMed] [Google Scholar]
- 121. Nyman LR, Ford E, Powers AC, Piston DW. Glucose-dependent blood flow dynamics in murine pancreatic islets in vivo. Am J Physiol Endocrinol Metab. 2010;298:E807–E814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Basu A, Dube S, Slama M, et al. Time lag of glucose from intravascular to interstitial compartment in humans [published online September 5, 2013]. Diabetes. doi:10.2337/db13-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rocheleau JV, Walker GM, Head WS, McGuinness OP, Piston DW. Microfluidic glucose stimulation reveals limited coordination of intracellular Ca2+ activity oscillations in pancreatic islets. Proc Natl Acad Sci USA. 2004;101:12899–12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Svensson AM, Ostenson CG, Jansson L. Age-induced changes in pancreatic islet blood flow: evidence for an impaired regulation in diabetic GK rats. Am J Physiol Endocrinol Metab. 2000;279:E1139–E1144. [DOI] [PubMed] [Google Scholar]
- 125. Li X, Zhang L, Meshinchi S, et al. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes. 2006;55:2965–2973. [DOI] [PubMed] [Google Scholar]
- 126. Pørksen N, Munn S, Steers J, Veldhuis JD, Butler PC. Effects of glucose ingestion versus infusion on pulsatile insulin secretion. The incretin effect is achieved by amplification of insulin secretory burst mass. Diabetes. 1996;45:1317–1323. [DOI] [PubMed] [Google Scholar]