Abstract
More than 50% of prostate cancers have undergone a genomic reorganization that juxtaposes the androgen-regulated promoter of TMPRSS2 and the protein coding parts of several ETS oncogenes. These gene fusions lead to prostate-specific and androgen-induced ETS expression and are associated with aggressive lesions, poor prognosis, and early-onset prostate cancer. In this study, we showed that an enhancer at 13 kb upstream of the TMPRSS2 transcription start site is crucial for the androgen regulation of the TMPRSS2 gene when tested in bacterial artificial chromosomal vectors. Within this enhancer, we identified the exact androgen receptor binding sequence. This newly identified androgen response element is situated next to two binding sites for the pioneer factor GATA2, which were identified by DNase I footprinting. Both the androgen response element and the GATA-2 binding sites are involved in the enhancer activity. Importantly, a single nucleotide polymorphism (rs8134378) within this androgen response element reduces binding and transactivation by the androgen receptor. The presence of this SNP might have implications on the expression and/or formation levels of TMPRSS2 fusions, because both have been shown to be influenced by androgens.
TMPRSS2 (transmembrane protease, serine 2) is a member of the type II transmembrane serine proteases, a family of 17 serine proteases that are characterized by a short cytoplasmic N-terminal domain, a single transmembrane domain, and an extracellular C-terminal domain containing the serine protease domain (1–3). TMPRSS2 is predominantly expressed in the luminal cells of the prostate epithelium, where its expression is positively regulated by androgens (4–7). Despite the high prostate-specific expression levels, the exact physiological function of TMPRSS2 in this tissue remains unclear.
Due to a genomic translocation present in at least 50% of prostate cancers (CaP), the androgen-regulated promoter of TMPRSS2 is fused to the coding sequences of ERG (v-ets erythroblastosis virus E26 oncogene-related gene). This results in androgen-regulated overexpression of ERG and explains why this oncogene is the most frequently overexpressed oncogene in CaP (8–13). Moreover, another 10% of the CaPs harbor a fusion between TMPRSS2 and ETV1, ETV4, or ETV5 (reviewed in [14, 15]). ERG, ETV1, ETV4, and ETV5 belong to the family of the proto-oncogenic ETS transcription factors, which are involved in many biological processes including cellular proliferation, differentiation, apoptosis, metastasis, and tissue remodeling (16, 17).
Most studies indicate that the presence of the TMPRSS2:ERG fusion in CaP is associated with a poorer clinical prognosis compared with TMPRSS2:ERG negative CaP, but there is still controversy over this point (for a review, see Ref. 18). Possibly, prognosis depends on the mechanism by which the fusion has arisen, which would implicate that further subclassification of TMPRSS2:ERG-positive tumors is necessary (19–28). Very recently, two independent studies showed that the ETS fusions are more frequent in early-onset CaP, which might be reflecting the negative consequences of this genetic alteration (29, 30). Furthermore, when patterns of tumor evolution were inferred from gene reorganization patterns called “chromoplexy”, Baca et al concluded that, for part of the CaPs analyzed, the TMPRSS2:ERG fusion could be one of the starting events (31). Moreover, the TMPRSS2:ERG fusion, either alone or in combination with prostate-specific antigen or prostate cancer antigen 3, has already proven its usefulness as a diagnostic urinary marker for men with aggressive CaP (32–36).
Despite its established involvement in CaP tumorigenesis, the exact mechanisms behind the androgen regulation of the TMPRSS2 gene have not been studied extensively. The first study that provided insight into this was based on ChIP-on-chip and described two androgen receptor (AR) binding sites (ARBSs) at 13 and 60 kb upstream of the TMPRSS2 transcription start site (TSS) (37). Subsequently published ChIP-seq datasets in LNCaP and VCaP cells, however, revealed evidence for additional ARBSs in intron 1, and at 12 and 17 kb upstream of the TSS (38–40). These binding sites were recently confirmed in AR ChIP-seq experiments on CaP biopsies (41). Furthermore, knockdown experiments demonstrated a role of GATA2 and Oct1 in the androgen-responsiveness of TMPRSS2 (37, 42). Here, we analyzed if and how TMPRSS2 expression is regulated via these described ARBSs. Furthermore, we studied the effect of genetic polymorphisms in one of the regulatory elements.
Materials and Methods
Materials
Restriction and other DNA-modifying enzymes were purchased from Invitrogen, MBI Fermentas, and Roche Applied Science. Oligonucleotides were purchased from Integrated DNA Technologies.
Plasmid constructs
The full-length human AR expression vector was created as described (43). The pCMV-β-gal expression vector was obtained from Stratagene. The pIRESluc plasmid was a kind gift from Charles A. O'Brien (University of Arkansas for Medical Sciences). The pGalK plasmid was provided by N. Copeland (National Cancer Institute). The luciferase reporter plasmids were constructed by using a pGL3 reporter vector driven by a minimal human E1B TATA box promoter (44, 45). To construct the ARBS plasmids, the desired TMPRSS2 fragments were copied from genomic DNA obtained from the LNCaP cell line by PCR amplification (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org) and cloned in the pGL3 reporter vector by using MluI and XhoI restriction sites. Mutations in the −13-kb ARBS luciferase reporter construct were introduced by site-directed mutagenesis with the appropriate primers (Supplemental Table 1) (as described in Ref. 46). The 4xARE pGL3 plasmids were created as previously described (47).
Cell culture
The COS-1 and LNCaP cells were purchased from the American Type Culture Collection. The VCaP cells were a kind gift from the Laboratory for Experimental Medicine and Endocrinology (KU Leuven) and were authenticated by short tandem repeat DNA profiling by Genetica.
COS-1 and VCaP cells were cultured in DMEM containing 1000 mg/L (COS-1) or 4500 mg/L (VCaP) glucose. The LNCaP cells were maintained in phenol red-free RPMI 1640 medium. All media were supplemented with 100 IU/mL penicillin, 0.1 mg/mL streptomycin, and 10% (v/v) heat-inactivated fetal calf serum (FCS).
Matrix calculation
Screening of the −13-kb and −60-kb ARBS for candidate AREs was done using the Regulatory Sequence Analysis Tool (48, 49) with the P cutoff at <.005 and a position-specific probability matrix (PSPM) obtained as previously described (50).
Transient transfections experiments
On day 1, VCaP and LNCaP cells were seeded in a 24-well plate at a density of 100 000 cells per well in the appropriate medium (see Cell culture) supplemented with 5% dextran-coated charcoal-stripped FCS. The next day, the VCaP cells were transfected with a mixture of 500 ng reporter plasmid, 25 ng pCMV-β-gal expression plasmid (as an internal control for transfection efficiency), 1.6 μL lipofectamine 2000, and 100 μL Opti-mem per well. For LNCaP cells, the same amount of DNA was added to a mixture of 1 μL X-tremeGENE HP and 25 μL Opti-mem. After overnight incubation, the medium was replaced and cells were stimulated with vehicle or 10 nM of the synthetic androgen methyltrienolone (R1881). Luciferase and β-galactosidase activities were measured as described previously (50). The relative luciferase activity and induction factors were calculated as described (47). The values shown are the averages of at least three independent experiments done in triplicate. The error bars are the SEM.
EMSA
The AR-DNA-binding domains (DBD) and the nuclear COS-1/VCAP extracts were prepared as previously described (44, 50, 51). For the EMSAs, double-stranded oligonucleotides described in Supplemental Table 1 were used. EMSAs were performed as described earlier (50). Specificity of the DNA-protein interaction was confirmed in competition experiments with a 1- or 10-fold excess of unlabeled double-stranded oligonucleotides representing consensus binding sites. The identity of the protein was confirmed by a supershift after preincubation with an antibody against AR (in-house polyclonal antiserum recognizing the first 20 residues of the human AR, used as described in Ref. 52), GATA2 (sc-9008), GATA3 (sc-9009), or Oct1 (ab15112). For the calculation of the dissociation constants (Kd), EMSAs were performed with increasing amounts of purified AR-DBD (0, 3.1, 6.3, 12.5, 25, 50, 100 ng). Next, the percentage of DBD-bound probe was calculated relative to the total amount of radioactivity and plotted against the concentrations of AR-DBD. The fit curve was generated by GraphPad Prism with allosteric Hill kinetics.
Quantitative real-time PCR
For the quantitative real-time PCR, 1 × 106 LNCaP cells were seeded in 6-cm Petri dishes in 5% dextran-coated charcoal-stripped medium. The next day, the cells were stimulated for 24 hours with vehicle or with 10 nM R1881. Total RNA was extracted from LNCaP cells using the RNeasy Mini Kit according to the manufacturer's protocols. After digestion with DNase I, cDNA was synthesized from 1 μg RNA using the RevertAid M-MuLV Reverse Transcriptase kit and random hexamer primers. For the cDNA quantification, the 7500 FastReal-Time PCR system was used with the Fast RT-PCR two-step protocol (2 minutes at 50°C, 20 seconds at 95°C, and 40 cycles of 3 seconds at 95°C and 30 seconds at 60°C). The PCR reaction mixtures contained 1× Platinum SYBR Green qPCR SuperMix-UDG, 0.15 μM of each primer (Supplemental Table 1) and 50 nM ROX Reference Dye in a final volume of 10 μL. If possible, primers were designed to hybridize to different exons, and generation of single amplicons was checked in melting curve assays.
DNase I footprinting
For the radiolabeling of the probe, the −13-kb ARBS luciferase reporter plasmid was cut with EcoRI, and a fill-in reaction with Klenow fragment in the presence of [α-32P] deoxythymidine triphosphate (dTTP) was performed. After purification with the Qiaquick PCR purification kit, the DNA fragment was cut with PstI, creating a ±400-bp fragment covering the −13-kb ARBS, which was then purified after separation on a nondenaturing 4% polyacrylamide gel (53). The DNase I footprinting experiment was essentially performed as described before (44). Specificity of the interaction was confirmed in competition experiments with a 1- or 10-fold excess of unlabeled double-stranded oligonucleotides representing consensus binding sites.
Bacterial artificial chromosome (BAC) reporter constructs
BAC clone RP11–671L10 was obtained from BACPAC Resource Center and introduced in SW102 cells as described (54). Sanger sequence analysis of the −13-kb ARBS in BAC clone RP11–671L10 revealed that it contained the variant A allele of the rs8134378 SNP. For the construction of the TMPRSS2-Luc-BAC rs8134378, a targeting construct was generated as described (54) using primers with 50 bp of homology with the TMPRSS2 3′ untranslated region and 20 bp homology with the IRES-Luciferase-Tk-Neomycin cassette from the pIRESluc plasmid (Supplemental Table 1). The amplified and SacI linearized DNA was introduced in RP11–617L10 using standard recombineering techniques and recombinants were selected as described previously (55, 56). Bacterial colonies were screened for correct recombination by colony PCR using primers flanking the recombination sites and the junctions were verified by sequence analysis. Integrity of the BACs was evaluated by matching the restriction enzyme digestion pattern with that of the parental BAC construct.
The TMPRSS2-Luc-BAC and Δ−13-kb ARBS-TMPRSS2-Luc-BAC vectors were constructed using galK-positive/negative selection (57). Briefly, the targeting construct for galK-positive selection was generated from SacI linearized pGalK plasmid by PCR amplification of the galK open reading frame, using primers that respectively contain 50 bp of homology with the sequences flanking TM-ARE2 or with the −13-kb ARBS (Supplemental Table 1). The PCR product was introduced into TMPRSS2-Luc-BAC rs8134378 by recombineering, which resulted in the substitution of TM-ARE2 rs8134378 or the −13-kb ARBS by the galK open reading frame. Recombinants were selected as described earlier (57).
For the construction of TMPRSS2-Luc-BAC, a PCR fragment of 156 bp was generated using the −13-kb ARBS pGL3 vector (which contains the reference G allele of rs8134378) (primers described in Supplemental Table 1). This PCR fragment was introduced in a Gal+ colony and recombinants were selected as described (57). For the construction of Δ−13-kb ARBS-TMPRSS2-Luc-BAC, a Gal+ colony was selected, in which the galK cassette was substituted by a double-stranded DNA oligonucleotide with homology to the regions flanking the −13-kb ARBS (Supplemental Table 1). Bacterial colonies were visible after 4 days and screened for correct recombination as described above.
Preparation of stable cell lines
BAC DNA was purified using the Qiaquick plasmid midi kit according to the manufacturer's guidelines. BAC DNA was digested with NotI and this was heat inactivated for 20 minutes at 80°C. Two micrograms of the linearized BAC DNA was introduced in LNCaP cells by electroporation using an Amaxa Nucleofector (program T09) according to the manufacturer's instructions. Four days after nucleofection, the medium was replaced with medium consisting of 50% v/v fresh 20% FCS medium, 50% v/v 20% FCS conditioned medium, and 12 μg/mL G418 (geneticin). Conditioned medium was retrieved after 2 to 3 days growth of LNCaP cells and filter sterilized. Every 3 or 4 days, the medium was replaced with fresh medium until colonies appeared (approximately after 25 days). Colonies were picked up and expanded in 50% v/v medium (20% FCS), 50% v/v conditioned medium (20% FCS), and 6 μg/mL G418.
For the analysis of the luciferase activity, stable LNCaP cells were seeded in a 96-well plate at a density of 25 × 103 cells per well in 5% dextran-coated charcoal-stripped medium. After 48 hours, the cells were stimulated with 10 nM R1881 or vehicle. Luciferase activities were measured 48 hours later as described previously (50). The values shown are the mean luciferase activities of at least seven independent clones, of which the luciferase activity was measured three times in triplicate. The error bars illustrate the SEM. Induction factors represent the average luciferase activity of hormone-stimulated cells relative to that of unstimulated cells.
Results
Enhancer activity of ARBSs in or near the TMPRSS2 gene
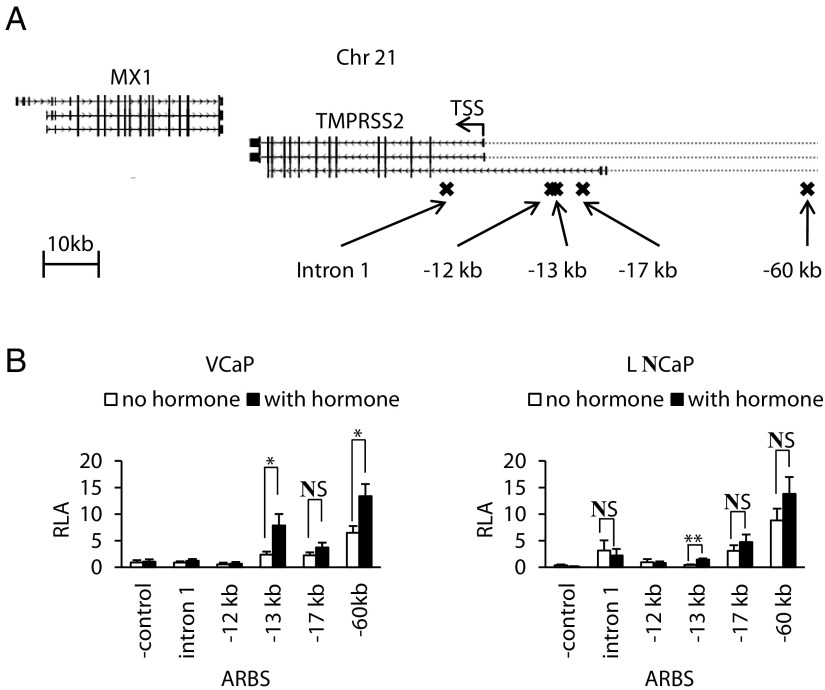
The ARBSs in intron 1 and at −12 kb, −13 kb, −17 kb, and −60 kb upstream of the TMPRSS2 TSS (Figure 1A) were subcloned as 0.4-kb fragments upstream of a luciferase reporter gene. The transcriptional response of these constructs to androgens was assessed in transiently transfected VCaP and LNCaP cells. As can be seen in Figure 1B, the −13-kb ARBS was androgen-responsive in both cell lines (fold induction of 3.2 ± 0.2 and 4.1 ± 1.0, respectively). Among the other ARBSs tested, the −17-kb and −60-kb ARBS increased basal activity of the reporter gene, but only the −60-kb ARBS conferred an androgen response when tested in VCaP cells (fold induction 2.2 ± 0.2).
Figure 1.
Enhancer activity of androgen receptor binding sites (ARBSs) in the TMPRSS2 locus. (A) Schematic representation of the human TMPRSS2 gene locus. Image generated by the University of California, Santa Cruz genome browser (85). RefSeq genes TMPRSS2 and MX1 are shown in gray. The transcription start site (TSS) and direction of transcription of TMPRSS2 is indicated with an arrow. Crosses indicate ARBSs obtained with ChIP-seq. (B) Functional analysis of the ARBSs in the TMPRSS2 gene for their enhancer activity. The indicated ARBSs were subcloned in a pGL3 promoter vector and transiently transfected in VCaP and LNCaP cells (as described in Matherials and Methods). The next day, the cells were stimulated for 24 hours with 10 nM methyltrienolone (R1881) or vehicle. Empty pGL3 served as a negative control. The data are presented as the mean relative luciferase activity (RLA) ± SEM in cellular extracts of at least four independent experiments performed in triplicate. *, P < .05; **, P < .005. NS, not significant, Student's t test.
Androgen response elements (ARE) in the −13-kb and −60-kb ARBS
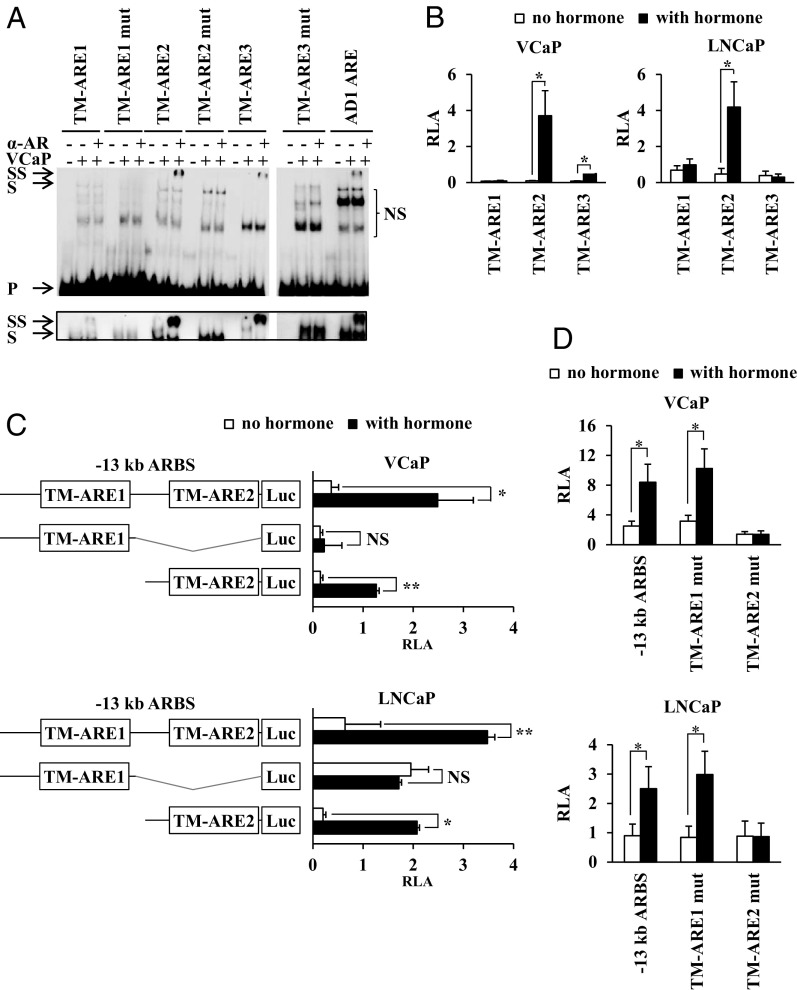
Using a PSPM (Supplemental Table 2) combined with the Regulatory Sequence Analysis Tool software with the P cutoff value set at <.005 (48, 50), two candidate AREs were indicated in the −13-kb ARBS (TM-ARE1 and TM-ARE2) and one ARE in the −60-kb ARBS (TM-ARE3) (Table 1). The in vitro affinity of the AR for these three candidate AREs was tested in EMSAs. As a positive control, the AD1 ARE from the ADAMTS1,A gene was used (37, 50). Figure 2A shows that the AR binds to TM-ARE2 and TM-ARE3, but only very weakly at best to TM-ARE1. Mutation of the bases known to be necessary for AR-ARE interaction in general, completely abolished AR binding to TM-ARE2 and TM-ARE3, while addition of an AR-specific antibody resulted in a supershift, confirming that the bound protein was indeed the AR. These EMSA results have been confirmed with nuclear extracts from COS-1 cells overexpressing the human AR (Supplemental Figure 1A). The following relative dissociation constants (Kd) of the AR-DBD for these candidate AREs were calculated from titration experiments (Supplemental Figure 1B) as described in Materials and Methods: 218 ± 43 nM for TM-ARE1, 51 ± 14 nM for TM-ARE2, and 60 ± 16 nM for TM-ARE3.
Table 1.
Sequences of the Predicted AREs in −13-kb and −60-kb ARBSs of the TMPRSS2 gene
| Location | Name | Sequence |
|---|---|---|
| −13-kb ARBS | TM-ARE1 | AGAGTGcacTGTCCT |
| TM-ARE1 mut | AGAGTTcacTTTCCT | |
| TM-ARE2 | AGTACCtgcCGTACC | |
| TM-ARE2 mut | AGTATCtgcCTTACC | |
| −60-kb ARBS | TM-ARE3 | GGAACGttgTGAAAC |
| TM-ARE3 mut | GGAAAGttgTAAAAC |
The two AR-interacting hexamers are indicated in capital letters. Mutations with respect to the wild-type sequence are depicted in bold.
Figure 2.
Analysis of the androgen response elements (AREs) of the −13-kb and −60-kb ARBS of the TMPRSS2 gene. (A) EMSA study of the affinity of the full-size AR in VCaP extracts for TM-ARE1, TM-ARE2, and TM-ARE3 (sequences described in Supplemental Table 1). The AD1 ARE from the ADAMTS1,A gene was used as a positive control (37, 50). VCaP cells were treated for 1 hour with 10 nM R1881 before preparation of nuclear extracts. Addition of nuclear extract (VCaP) or an AR-specific antibody (α-AR) to the 32P radiolabeled probes was indicated at the top with “−“ or “+.” Arrows indicate the positions of the unbound (P), shifted (S), and supershifted (SS) probes. NS, nonspecific binding. Inset, longer exposure (6 hours for the lower panel vs 2 hours for the upper panel) of the gel to visualize the shift caused by AR binding. (B, C, D) VCaP and LNCaP cells were transiently transfected as described in the legend of Figure 1B with pGL3 reporter constructs containing (B) four copies of TM-ARE1, TM-ARE2, or TM-ARE3, (C) the subfragments of the −13-kb ARBS depicted on the left, or (D) the −13-kb ARBS in which TM-ARE1 or TM-ARE2 were mutated. Data are presented as described in the legend of Figure 1B. *, P < .05; **, P < .005, Student's t test.
The functionality of the candidate AREs was tested in VCaP and LNCaP cells (Figure 2B). Consistent with the results obtained in the EMSAs, the TM-ARE1-based luciferase reporter was not androgen-responsive, whereas androgen stimulation of the TM-ARE2 construct resulted in a 44 ± 16-fold induction in VCaP and a 21 ± 7-fold induction in LNCaP cells. As expected from the transfection studies with the full size −60-kb ARBS, the TM-ARE3 construct was not androgen-responsive in LNCaP cells. In the VCaP cells, however, we observed a 6.5 ± 1.3-fold induction after androgen stimulation.
Minimal sequences necessary for androgen regulation of the −13-kb ARBS
The previous data suggest that the −13-kb ARBS is a control site for the androgen regulation of the TMPRSS2 gene. To delineate which sequences of the −13-kb ARBS are necessary and sufficient for its activity, we tested two subfragments of this ARBS for their transcriptional response to androgens. Figure 2C shows that only the fragment covering TM-ARE2 was androgen-responsive (fold induction: 7.6 ± 1.5 in VCaP and 8.0 ± 0.9 in LNCaP cells), which is comparable to the full-size −13-kb ARBS (fold induction: 6.5 ± 1.9 in VCaP and 6.8 ± 0.6 in LNCaP cells). Moreover, in the context of the −13-kb ARBS, the mutation of TM-ARE1 had no effect on the androgen response (fold induction: 3.3 ± 0.2 for TM-ARE1 mut vs 3.2 ± 0.2 for the full-size −13-kb ARBS in VCaP and 5.2 ± 1.2 vs 4.2 ± 1.0 in LNCaP cells), while mutation of TM-ARE2 completely abolished it (Figure 2D).
Activity of the −13-kb ARBS in a chromatin context
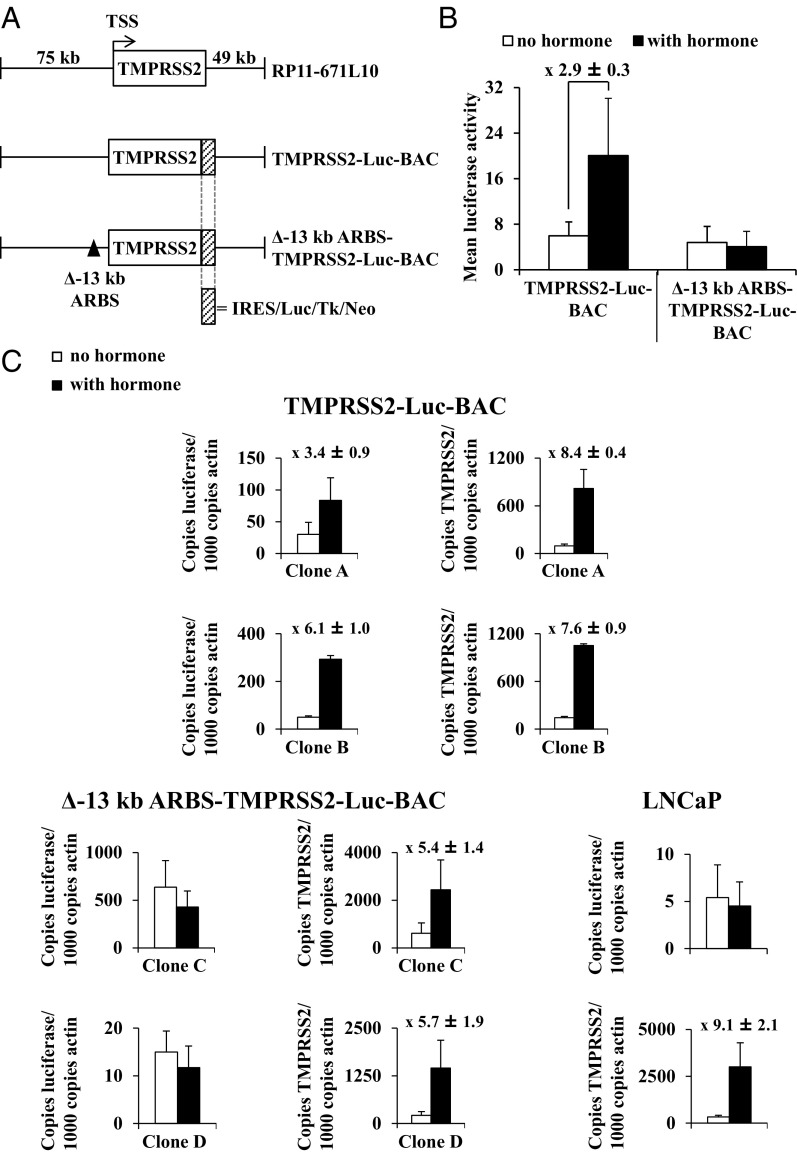
To study the activity of the −13-kb ARBS in a more native context, a BAC reporter vector was generated, which contained the complete TMPRSS2 gene with 74.784-kb upstream and 49.286-kb downstream sequence (Figure 3A). The 3′ part of this BAC also encodes MX1, but this gene is not androgen-regulated or expressed in LNCaP cells (Helsen C, unpublished RNA-seq data). The 5′ part of this BAC covers all the ARBSs described in Figure 1A. A luciferase encoding cassette was introduced immediately downstream of the stop codon of TMPRSS2 (creating the TMPRSS2-Luc-BAC construct) (Figure 3A). This BAC reporter was integrated in the chromatin of LNCaP cells and the luciferase activity in response to androgen stimulation of 16 independent LNCaP clones was tested. Seven of these 16 colonies (44%) were responsive to androgens, with an average fold induction of 2.9 ± 0.3 (Figure 3B). To analyze the individual contribution of the −13-kb ARBS on TMPRSS2 expression further, a sequence of 418 bp, which exactly overlaps with the −13-kb ARBS that was tested in the transient transfections (Figures 1B, 2C and D), was deleted in the BAC reporter vector, creating Δ−13-kb ARBS-TMPRSS2-Luc-BAC (Figure 3A). The luciferase activity of 10 stable Δ−13-kb ARBS-TMPRSS2-Luc-BAC LNCaP clones was tested, but none of these clones was androgen-responsive (Figure 3B).
Figure 3.
Characterization of the −13-kb ARBS in a chromatin context. (A) Schematic overview of bacterial artificial chromosome (BAC) clone RP11–671L10 and schematic presentation of the two TMPRSS2-Luc-BAC constructs. The TSS and direction of transcription is indicated with an arrow; deletion of the −13-kb ARBS is indicated with a black triangle. IRES, internal ribosomal entry site; Luc, luciferase reporter gene; Neo, neomycine resistance gene; Tk, thymidine kinase promoter. (B) The luciferase activity in cellular extracts of stable TMPRSS2-Luc-BAC (n = 7) and Δ−13-kb ARBS-TMPRSS2-Luc-BAC LNCaP clones (n = 10) was measured three times in triplicate 48 hours after stimulation with 10 nM R1881 or vehicle. The data are presented as the mean luciferase activities ± SEM of these clones. The average luciferase induction ± SEM of the seven stable TMPRSS2-Luc-BAC clones is given. (C) Quantitative real-time PCR analysis of luciferase and TMPRSS2 mRNA levels in stable TMPRSS2-Luc-BAC (clone A and B) and Δ−13-kb ARBS-TMPRSS2-Luc-BAC LNCaP clones (clone C and D) and in wild-type LNCaP cells. The cells were stimulated for 24 hours with vehicle or with 10 nM R1881 before extracting RNA. Gene expression values are expressed relative to levels of actin RNA and are presented as means ± SEM of at least two experiments performed in triplicate. For each clone, the fold induction was calculated by taking the ratio of the mean luciferase activity in extracts of stimulated cells over that of unstimulated cells (measured in triplicate). The mean fold induction of all the clones ± SEM is given above the charts.
To compare the luciferase transcript levels of the stable LNCaP cells with their endogenous TMPRSS2 transcript levels, a quantitative real-time PCR analysis was performed on two of each of the stable clones. As depicted in Figure 3C, in both the stable TMPRSS2-Luc-BAC (clone A and B) and the Δ−13-kb ARBS-TMPRSS2-Luc-BAC (clone C and D) clones, we observed an androgen-induced increase in TMPRSS2 mRNA levels, which was comparable with the wild-type LNCaP cells, indicating that none of the tested clones have lost their androgen regulation. On androgen stimulation, luciferase transcript levels in the TMPRSS2-Luc-BAC clones increased, whereas this was not the case for the Δ−13-kb ARBS-TMPRSS2-Luc-BAC clones and the wild-type LNCaP cells.
Binding of Oct1 and GATA2 at −13-kb ARBS
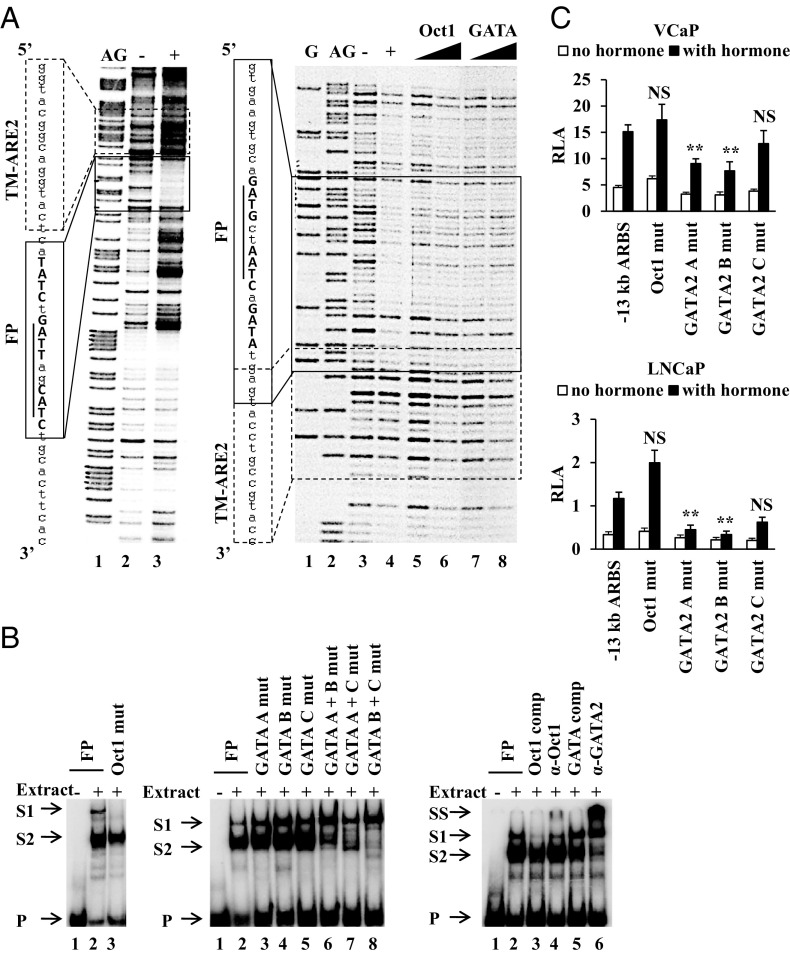
To map binding sites for transcription factors in the −13-kb ARBS, the DNA fragment was subjected to a DNase I footprint assay with nuclear extracts from VCaP cells. In the sense strand, immediately downstream of TM-ARE2, a clear DNase I protected region of 17 nt flanked by hypersensitive sites was present (Figure 4A, left panel). In the antisense strand on the other hand, a somewhat larger (30 nt) but fainter footprint that partially overlaps with the 5′ end of TM-ARE2 was detected (Figure 4A, right panel, and Table 2). In silico scanning of this 30-bp fragment for transcription factor motifs listed in the TRANSFAC and JASPAR databases (58, 59) identified one putative binding site for Oct1 and three putative binding sites for GATA factors. Competition experiments with oligonucleotides representing Oct1 (Figure 4A, right panel, lanes 5 and 6) or GATA high-affinity binding sites (Figure 4A, right panel, lanes 7 and 8) resulted in a loss of the footprint, suggesting protein binding was specific and which was tested further in EMSA.
Figure 4.
Characterization of transcription factors that bind the −13-kb ARBS. (A) DNase I footprint of the −13-kb ARBS. A DNA fragment covering the −13-kb ARBS was 32P radiolabeled at the 3′ end of the sense (left panel) or antisense (right panel) strand and incubated without (−) or with (+) nuclear extract from VCaP cells before treatment with DNase I. Lanes G and AG are Maxam-Gilbert degradations of the probes (86). The protected sequences and its position are indicated with a box called FP. Underlined/bold + capital: predicted Oct1/GATA binding sites. Sequence and position of TM-ARE2 are indicated with a dashed box. Right panel, lanes 5/6 and 7/8: increasing amount of unlabeled Oct1 or GATA consensus binding sites were added before nuclease digestion. (B) EMSA with nuclear extracts from VCaP cells were performed with 32P-radiolabeled probes described in Supplemental Table 1 (name of the probe is indicated at the top). Addition of nuclear extract (−/+), 10-fold molar excess of cold competitor consensus binding sites for Oct1 (Oct1 comp), GATA (GATA comp), antibody specific for Oct1 (α-Oct1), or GATA2 (α-GATA2) is indicated at the top. Arrows indicate the positions of the unbound (P), shifted (S1 and S2), or supershifted (SS) probes. (C) For the functional analysis, VCaP and LNCaP cells were transiently transfected as described in the legend of Figure 1B with a pGL3 promoter vector containing the −13-kb ARBS in which the indicated transcription factor binding sites were mutated (same mutations as depicted in Table 2). The data are presented as described in the legend of Figure 1B. **, P < .01; NS, not significant. One-way ANOVA test followed by a Dunnett's posttest in comparison with the control group (−13-kb ARBS, stimulated).
Table 2.
Sequence of the Region in the −13-kb ARBS Protected From DNase I Digestion (FP) and Mutations Affecting Oct1 and GATA Binding
| Name | Sequence |
|---|---|
| FP | GTGAAGTGCAGATGCTAATCAGATATGAGT |
| Oct1 mut | GTGAAGTGCAGATGCCCATCAGATATGAGT |
| GATA A mut | GTGAAGTGCAAATGCTAATCAGATATGAGT |
| GATA B mut | GTGAAGTGCAGATGCTAATTAGATATGAGT |
| GATA C mut | GTGAAGTGCAGATGCTAATCAACCATGAGT |
| GATA A + B mut | GTGAAGTGCAAATGCTAATTAGATATGAGT |
| GATA A + C mut | GTGAAGTGCAAATGCTAATCAACCATGAGT |
| GATA B + C mut | GTGAAGTGCAGATGCTAATTAACCATGAGT |
Mutations with respect to the wild-type sequence affecting the predicted Oct1 or GATA binding sites are underlined.
EMSAs were performed with the sequence protected against DNase I cleavage in the antisense strand (Supplemental Table 1). This probe was shifted to two positions by VCaP nuclear extracts, indicating formation of two different protein-DNA complexes (Figure 4B). Mutation of the predicted Oct1 binding site resulted in the disappearance of the highest band, while the second shifted band disappeared when at least two of three GATA binding sites were mutated, suggesting cooperative GATA binding at these sites (Figure 4B). Competition experiments showed that addition of Oct1 high-affinity oligonucleotides reduced the upper band, but did not prevent the lower band formation. Competition with a GATA high-affinity binding site reduced the lower band, but did not prevent the formation of the upper band (Figure 4B). Finally, by the addition of specific antibodies against Oct1, GATA2, or GATA3, we identified the interacting proteins as Oct1 and GATA2 (Figure 4B and data not shown).
To determine the functional role of Oct1 and GATA2, their respective binding sites were mutated (same mutation as described in Table 2) in the −13-kb ARBS reporter construct. Transient transfections in VCaP and LNCaP cells revealed that Oct1 binding is not essential for androgen induction under these conditions (Figure 4C). Mutation of the GATA2 A or B binding sites resulted in a significant reduction (P < .05) of the androgen response in both the VCaP and the LNCaP cells (Figure 4C), whereas mutation of the GATA2 C binding site had no effect.
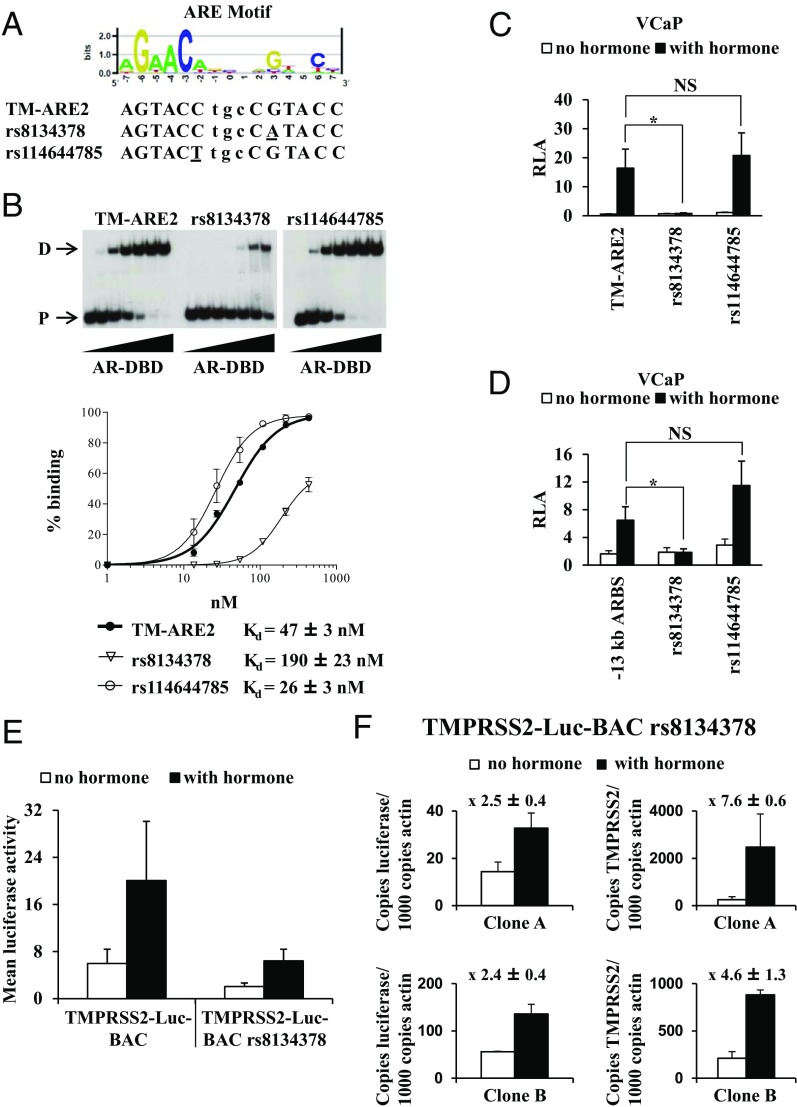
Functional polymorphisms in the −13-kb ARBS
The dbSNP database of the National Center for Biotechnology Information indicates that the rs8134378 SNP maps to position 3 of TM-ARE2, whereas rs114644785 maps to position −2 of TM-ARE2. A comparison of the variant sequences with the ARE consensus motif indicates that rs8134378 maps to an important AR-DNA contact site, whereas rs114644785 maps to a less important position (Figure 5A). EMSAs with the AR-DBD or with nuclear extracts from VCaP cells or COS-1 cells that overexpress the human AR indicated that the presence of the variant A allele of rs8134378 indeed severely impaired AR binding, while AR binding to the probe containing the variant T allele of the rs114644785 SNP was comparable with the reference probe (Figure 5B and Supplemental Figure 1, A and B). The relative dissociation constants (Kd) of the AR-DBD for the elements were calculated to be 47 ± 3 nM for TM-ARE2, 190 ± 23 nM for TM-ARE2 rs8134378, and 26 ± 3 nM for TM-ARE2 rs114644785 (Figure 5B).
Figure 5.
Single-nucleotide polymorphisms modulate AR binding in the −13-kb ARBS. (A) Sequence logo of androgen response elements (ARE) was created on http://weblogo.berkeley.edu/logo.cgi (87) and was based on the position-specific probability matrix (PSPM) shown in Supplemental Table 2. This PSPM was obtained as described (50) from a list of published AREs that were characterized in EMSA and transient transfections. The sequence of the reference and variants of TM-ARE2 sequences are given (variations are underlined). (B) Comparative EMSA with increasing amounts (0; 3.125; 6.25; 12.5; 25; 50 and100 ng) of isolated AR-DNA-binding domains (AR-DBD) for TM-ARE2 and its variants (sequences described in Supplemental Table 1). D, dimeric binding; P, free probe. Binding curves plotting the percentage of DBD-bound probe against the concentration of AR-DBD. The percentage of DBD-bound probe was calculated relative to the total amount of radioactivity in each lane. Data are presented as the mean ± SE from at least two independent experiments. The best fit curve was generated by GraphPad Prism with allosteric Hill kinetics and the apparent dissociation constants (Kd) were calculated as described in Materials and Methods. (C) VCaP cells were transiently transfected with the indicated reporter constructs, containing the reference sequence or the variant alleles of rs8134378 and rs114644785. (D) Rs8134378 or rs114644785 were introduced in the −13-kb ARBS pGL3 reporter vector and analyzed as in (C). (C and D) Transfections were performed as described in the legend of Figure 1B. The data are presented as described in the legend of Figure 1B. *, P < .05; Student's t test, compared with reference allele. (E) The luciferase activity in cellular extracts of stable TMPRSS2-Luc-BAC (n = 7) and TMPRSS2-Luc-BAC LNCaP rs8134378 clones (n = 7) was measured three times in triplicate 48 hours after stimulation with 10 nM R1881 or vehicle. The data are presented as the mean luciferase activities ± SEM of these clones. (F) Quantitative real-time PCR analysis of luciferase and TMPRSS2 mRNA levels in two stable TMPRSS2-Luc-BAC rs8134378 clones (clone A and B). The cells were stimulated for 24 hours with vehicle or with 10 nM R1881 before extracting RNA. Gene expression values are expressed as described in the legend of Figure 3C. Fold induction ± SEM is given above the charts.
Luciferase assays in VCaP cells with reporter constructs containing the sequences analyzed in the EMSAs demonstrated that the variant A allele of rs8134378 impedes AR functioning, whereas there was no significant difference in androgen-responsiveness for the variant T allele of rs114644785 (Figure 5C). In the −13-kb ARBS luciferase reporter context, the androgen stimulation was disrupted by the A allele of rs8134378, whereas the T allele of rs114644785 had no effect (Figure 5D).
Next, the effect of the rs8134378 SNP on the expression of the complete TMPRSS2 gene was tested in the context of the TMPRSS2-Luc-BAC (designated TMPRSS2-Luc-BAC rs8134378). This construct was stably integrated in the chromatin of LNCaP cells and the luciferase activity of 24 independent clones was tested, of which seven clones were responsive to androgens (29%). In Figure 5E, the mean luciferase activities with and without androgen stimulation of these seven clones were compared with the mean luciferase activities of seven stable TMPRSS2-Luc-BAC clones (also described in Figure 2B). Although not significant, there was a tendency that both the basal luciferase activity and the androgen-induced luciferase activity were lower in the stable TMPRSS2-Luc-BAC rs8134378 clones. No significant difference in fold induction between both constructs was observed. It should also be noted that 7 of 16 stable TMPRSS2-Luc-BAC clones were androgen- responsive vs 7 of 24 TMPRSS2-Luc-BAC rs8134378 SNP clones. In addition, luciferase mRNA levels in two stable TMPRSS2-Luc-BAC rs8134378 clones were compared with their endogenous TMPRSS2 mRNA levels (Figure 5F). The transcript levels of both TMPRSS2 and luciferase increased on androgen stimulation, although luciferase transcript levels were much lower than those for TMPRSS2.
It should be noted that the original BAC clone RP11-671L10 contains the minor A variant of the rs8134378 SNP. For the construction of TMPRSS2-Luc-BAC, we replaced the A allele with the reference G allele. This construct was used in Figure 3. The transfections studies (Figures 1 and 2) and footprinting experiments (Figure 4) were all performed with sequences containing the reference G allele. The BAC with the minor A variant (TMPRSS2-Luc-BAC rs8134378) was used in Figure 5.
Discussion
In approximately 50% of the CaPs, the TMPRSS2 gene is involved in a genomic reorganization with the complete or partial protein-coding parts of the ETS oncogenes (8–11, 14, 15). Here, we report on the transcription factors and their exact binding sites that are involved in the transcription control of the TMPRSS2 gene and hence also in that of its oncogene fusion partners. Wang et al first described two ARBSs at 13 and 60 kb upstream of the TMPRSS2 TSS (37). A compilation of subsequently published ChIP-seq datasets in LNCaP cells as well as in CaP biopsies revealed evidence for additional ARBSs in or near TMPRSS2: in intron 1, and at 12 and 17 kb upstream of the TSS (38–41). We first tested the enhancer properties of each of these ARBSs, and their capacity to confer androgen-responsiveness to a luciferase reporter gene. Surprisingly, only the −13-kb ARBS showed a significant androgen response in both VCaP and LNCaP cells. The −60-kb ARBS had the highest basal enhancer activity in both of the cell lines, but this enhancer was only androgen-responsive in the VCaP cells. Possibly, the high basal activity of this enhancer in LNCaP cells obscures the androgen response.
Despite the presence of an AR ChIP-seq signal in intron 1 and at 12 and 17 kb upstream of the TMPRSS2 TSS, none of these ARBSs were androgen-responsive in the VCaP or LNCaP cells. This could be due to the limitations of a transient transfection assay (like choice of the cell line, use of artificial promoters, and lack of chromatin) and therefore a role of these ARBSs in the androgen regulation of TMPRSS2 cannot be excluded. Nevertheless, our results clearly indicated that the isolated −13-kb ARBS can confer androgen-responsiveness to a reporter gene in transient transfections and therefore we decided to focus more on this ARBS.
To analyze the role of this ARBS in a chromatin environment further, a TMPRSS2-BAC-Luciferase construct covering the complete TMPRSS2 gene and all the known ARBSs described in Figure 1 was generated. Androgen stimulation of stable LNCaP cells, expressing this construct, resulted in increased luciferase activity in 44% (7/16) of the tested clones. Luciferase transcript levels were more variable among the different clones, compared with the endogenous TMPRSS2 transcript levels, which probably reflect effects of the integration site. These data indicate that the androgen-regulated TMPRSS2 expression is recapitulated on the BAC construct, but that this system suffers from a high background, because not all the tested colonies were androgen-responsive. We subsequently deleted the sequence that exactly overlaps with the −13-kb ARBS tested in the transient transfections from the TMPRSS2-BAC-Luciferase construct and introduced the resulting reporter vector in the chromatin of LNCaP cells. None of the 10 tested LNCaP clones expressed androgen-responsive luciferase, strongly indicating that the −13-kb ARBS is crucial for the androgen regulation of TMPRSS2.
Initial in silico analyses pointed out a candidate ARE in the −13-kb ARBS, which corresponds to TM-ARE1 in the current study (37, 50). In a comparative study, this TM-ARE1 motif displayed some binding to overexpressed AR but low affinity for the isolated AR-DBD (50). In our EMSAs with VCaP or COS-1 extracts and with purified AR-DBDs, we detected very weak binding at best. Moreover, a −13-kb ARBS fragment lacking TM-ARE1 was still androgen-responsive, and when this ARE was mutated in the −13-kb ARBS pGL3-based vector, the construct was still androgen-responsive, indicating that TM-ARE1 is not necessary for the androgen-responsiveness of the −13-kb ARBS. We subsequently screened this ARBS for the presence of other candidate AREs with an extended PSPM (Supplemental Table 2) and thus identified a second putative ARE, called TM-ARE2. Surprisingly, this element was much more active than TM-ARE1 in EMSA as well as in transient transfections and the mutation of TM-ARE2 in the −13-kb ARBS completely abolished the androgen-responsiveness of the −13-kb ARBS reporter. This indicates that TM-ARE2 is crucial for the androgen regulation of this construct. It also illustrates that in silico searches for AREs are only as good as the PSPM that is used.
Because the AR is known to cooperate with other transcription factors (eg, [37, 60, 61]), we searched for binding sites for other factors in the −13-kb ARBS. In vitro DNase I footprinting and EMSA identified a protein binding site next to TM-ARE2 covering three putative GATA2 binding sites, two of which were necessary for the androgen-responsiveness of the −13-kb ARBS in transient transfections. The close location of these binding sites to TM-ARE2 suggest that there must be extensive protein-protein interactions or alternating DNA binding events happening between AR and GATA2 (62). The presence of functional GATA2 binding sites that flank AREs have also been observed in other enhancers, as well as by in silico analyses of ARBS (37, 42, 63–65). Importantly, it has been shown that GATA2 functions as a pioneering factor for AR binding (37, 42). Indeed, knock-down of GATA2 in LNCaP cells attenuated the recruitment of the AR (and PolII) at the −13-kb ARBS. Our transient transfection data are in accordance with this, because they show cooperativity of GATA2 and AR, even in the absence of chromatin. The DNase I footprinting data also pointed at an Oct1 binding site, which was confirmed by EMSA. A specific mutation of this Oct1 binding site had no effect on the androgen enhancer activity of the −13-kb ARBS in transient transfections. However, Wang et al showed that AR interacts with Oct1 and that knock-down of Oct1 decreased PolII loading on the −13-kb ARBS (37), suggesting that this binding site only becomes important in the context of chromatin. Notably, no AR footprint covering TM-ARE2 was visible, but this can be due to the lower sensitivity of DNase I footprinting as compared with EMSA (66). Although in EMSA only a small percentage of the radiolabeled probe has to bind protein to yield a shift in a DNase I footprinting experiment, most of the DNA molecules must be bound to obtain a visible footprint (66). The EMSA data in Figure 2A indicate that there is insufficient AR-binding to obtain a footprint. Although footprints can be obtained with isolated AR-DBDs (eg, [67–72]), few groups have succeeded in obtaining in vitro footprints with full-size AR (73–76).
Variation in gene expression in and between populations is often associated with altered transcription factor binding due to genetic polymorphisms (77, 78). Furthermore, epidemiological studies between different populations suggest that there is in part a genetic component in the etiology of CaP (79–81). This knowledge prompted us to search the dbSNP database for the presence of genetic variations in the −13-kb ARBS. Strikingly, TM-ARE2 is prone to genetic variation in ∼10% of the population (MAF:A = 0.088/192). The variant A allele of this rs8134378 SNP severely affected the androgen-responsiveness when tested in oligonucleotide constructs and in the −13-kb ARBS construct. Moreover, it nearly abolished in vitro AR and AR-DBD binding to TM-ARE2. In the context of the TMPRSS2-Luc-BAC construct, we observed only a tendency that both the basal and the androgen-induced luciferase levels are lower in the stable clones containing the minor A-variant of the SNP. These data suggest that in a more natural context, the effects of the SNP might be more subtle. It should be noted, however, that only one in two of the stable TMPRSS2-Luc-BAC clones and one in three of the TMPRSS2-Luc-BAC rs8134378 SNP clones were androgen-responsive, indicating that this system is not robust enough to make major conclusions on the role of the SNP in a chromatin context.
The genetic variation in androgen-responsiveness of TMPRSS2 might have clinical relevance. Indeed, TMPRSS2:ETS fusion-positive CaPs, in which the expression of the ETS transcription factor is regulated via the minor allele of TM-ARE2, might have lower ERG expression and hence be less aggressive. Alternatively, it was shown that androgen stimulation induces the physical proximity of the TMPRRS2 and ERG gene and promotes co-recruitment of the AR and TOP2B to TMPRSS2:ERG genomic breakpoints in LNCaP cells (82, 83). Concomitant genetic insults result in the generation of the TMPRRS2:ETS fusions and the start of chromoplexy (31, 84). Hence, it is conceivable that the less responsive A variant of rs8134378 would decrease the risk of the formation of the gene fusion. Comparative SNP frequency analyses of fusion-positive vs -negative CaPs should reveal whether this SNP has any clinical relevance and might lead to a much needed further subclassification of TMPRSS2:ERG-positive cancers.
In conclusion, we provided evidence that the −13-kb ARBS is necessary for the androgen regulation of the TMPRSS2 gene. We identified an ARE to which the AR binds with high affinity (TM-ARE2) and characterized functional binding sites for GATA2. This now allows detailed analyses of the role of each binding event in the androgen regulation of this gene. Importantly, we identified a SNP that reduces binding and transactivation of the AR via TM-ARE2. It is tempting to speculate that this SNP has clinical relevance, but this requires further evaluation.
Acknowledgments
The authors thank Charles A. O'Brien (University of Arkansas for Medical Sciences) for providing the pIRESluc plasmid. The authors are very grateful to Rita Bollen and Hilde De Bruyn for their excellent technical assistance and to their colleagues of the Molecular Endocrinology Laboratory for helpful discussions.
This study was financially supported by the Agency for Innovation by Science and Technology in Flanders (IWT), by Grant OT/09/035 from the Katholieke Universiteit Leuven, and Grant numbers G.0684.12 and G.0830.13 of the Fund for Scientific Research Flanders, Belgium (FWO-Vlaanderen). V.D. and M.L. are holders of an FWO doctoral fellowship. S.J. is holder of a grant from the Klinisch Onderzoeksfonds (KOF) from UZ Leuven.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This study was financially supported by the Agency for Innovation by Science and Technology in Flanders (IWT), by Grant OT/09/035 from the Katholieke Universiteit Leuven, and Grant numbers G.0684.12 and G.0830.13 of the Fund for Scientific Research Flanders, Belgium (FWO-Vlaanderen). V.D. and M.L. are holders of an FWO doctoral fellowship. S.J. is holder of a grant from the Klinisch Onderzoeksfonds (KOF) from UZ Leuven.
Footnotes
- AR
- androgen receptor
- ARBS
- AR binding sites
- BAC
- bacterial artificial chromosome
- CaP
- prostate cancer
- DBD
- DNA-binding domains
- FCS
- fetal calf serum
- PSPM
- position-specific probability matrix
- TSS
- transcription start site.
References
- 1. Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem. 2009;284:23177–23181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hooper JD, Scarman AL, Clarke BE, Normyle JF, Antalis TM. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur J Biochem. 2000;267:6931–6937. [DOI] [PubMed] [Google Scholar]
- 3. Paoloni-Giacobino A, Chen H, Peitsch MC, Rossier C, Antonarakis SE. Cloning of the TMPRSS2 gene, which encodes a novel serine protease with transmembrane, LDLRA, and SRCR domains and maps to 21q22.3. Genomics. 1997;44:309–320. [DOI] [PubMed] [Google Scholar]
- 4. Afar DE, Vivanco I, Hubert RS, et al. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res. 2001;61:1686–1692. [PubMed] [Google Scholar]
- 5. Lin B, Ferguson C, White JT, et al. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59:4180–4184. [PubMed] [Google Scholar]
- 6. Lucas JM, True L, Hawley S, et al. The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. J Pathol. 2008;215:118–125. [DOI] [PubMed] [Google Scholar]
- 7. Jacquinet E, Rao NV, Rao GV, Zhengming W, Albertine KH, Hoidal JR. Cloning and characterization of the cDNA and gene for human epitheliasin. Eur J Biochem. 2001;268:2687–2699. [DOI] [PubMed] [Google Scholar]
- 8. Mosquera JM, Mehra R, Regan MM, et al. Prevalence of TMPRSS2-ERG fusion prostate cancer among men undergoing prostate biopsy in the United States. Clin Cancer Res. 2009;15:4706–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Esgueva R, Perner S, LaFargue C, et al. Prevalence of TMPRSS2-ERG and SLC45A3-ERG gene fusions in a large prostatectomy cohort. Mod Pathol. 2010;23:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark J, Merson S, Jhavar S, et al. Diversity of TMPRSS2-ERG fusion transcripts in the human prostate. Oncogene. 2007;26:2667–2673. [DOI] [PubMed] [Google Scholar]
- 11. Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. [DOI] [PubMed] [Google Scholar]
- 12. Liu H, Shi J, Wilkerson M, Yang XJ, Lin F. Immunohistochemical evaluation of ERG expression in various benign and malignant tissues. Ann Clin Lab Sci. 2013;43:3–9. [PubMed] [Google Scholar]
- 13. Petrovics G, Liu A, Shaheduzzaman S, et al. Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene. 2005;24:3847–3852. [DOI] [PubMed] [Google Scholar]
- 14. Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29:3659–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. [DOI] [PubMed] [Google Scholar]
- 17. Seth A, Watson DK. ETS transcription factors and their emerging roles in human cancer. Eur J Cancer. 2005;41:2462–2478. [DOI] [PubMed] [Google Scholar]
- 18. St John J, Powell K, Conley-Lacomb MK, Chinni SR. TMPRSS2-ERG fusion gene expression in prostate tumor cells and its clinical and biological significance in prostate cancer progression. J Cancer Sci Ther. 2012;4:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. FitzGerald LM, Agalliu I, Johnson K, et al. Association of TMPRSS2-ERG gene fusion with clinical characteristics and outcomes: results from a population-based study of prostate cancer. BMC Cancer. 2008;8:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gopalan A, Leversha MA, Satagopan JM, et al. TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res. 2009;69:1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Attard G, Clark J, Ambroisine L, et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoshimoto M, Joshua AM, Cunha IW, et al. Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod Pathol. 2008;21:1451–1460. [DOI] [PubMed] [Google Scholar]
- 23. Mehra R, Tomlins SA, Yu J, et al. Characterization of TMPRSS2-ETS gene aberrations in androgen-independent metastatic prostate cancer. Cancer Res. 2008;68:3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perner S, Svensson MA, Hossain RR, et al. ERG rearrangement metastasis patterns in locally advanced prostate cancer. Urology. 2010;75:762–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hu Y, Dobi A, Sreenath T, et al. Delineation of TMPRSS2-ERG splice variants in prostate cancer. Clin Cancer Res. 2008;14:4719–4725. [DOI] [PubMed] [Google Scholar]
- 26. Wang J, Cai Y, Ren C, Ittmann M. Expression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancer. Cancer Res. 2006;66:8347–8351. [DOI] [PubMed] [Google Scholar]
- 27. Wang J, Cai Y, Yu W, Ren C, Spencer DM, Ittmann M. Pleiotropic biological activities of alternatively spliced TMPRSS2/ERG fusion gene transcripts. Cancer Res. 2008;68:8516–8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zammarchi F, Boutsalis G, Cartegni L. 5′ UTR control of native ERG and of Tmprss2:ERG variants activity in prostate cancer. PLoS One. 2013;8:e49721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weischenfeldt J, Simon R, Feuerbach L, et al. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell. 2013;23:159–170. [DOI] [PubMed] [Google Scholar]
- 30. Schaefer G, Mosquera JM, Ramoner R, et al. Distinct ERG rearrangement prevalence in prostate cancer: higher frequency in young age and in low PSA prostate cancer. Prostate Cancer Prostatic Dis. 2013;16(2):132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baca SC, Prandi D, Lawrence MS, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153:666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laxman B, Morris DS, Yu J, Siddiqui J, Cao J, Mehra R, Lonigro RJ, Tsodikov A, Wei JT, Tomlins SA, Chinnaiyan AM. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res. 2008;68:645–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tomlins SA, Aubin SM, Siddiqui J, et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci Transl Med. 2011;3:94ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leyten GH, Hessels D, Jannink SA, et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer [published online November 15, 2012]. Eur Urol. 2012. doi: 10.1016/j.eururo.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 35. Nguyen PN, Violette P, Chan S, et al. A panel of TMPRSS2:ERG fusion transcript markers for urine-based prostate cancer detection with high specificity and sensitivity. Eur Urol. 2011;59:407–414. [DOI] [PubMed] [Google Scholar]
- 36. Hessels D, Smit FP, Verhaegh GW, Witjes JA, Cornel EB, Schalken JA. Detection of TMPRSS2-ERG fusion transcripts and prostate cancer antigen 3 in urinary sediments may improve diagnosis of prostate cancer. Clin Cancer Res. 2007;13:5103–5108. [DOI] [PubMed] [Google Scholar]
- 37. Wang Q, Li W, Liu XS, et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27:380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sahu B, Laakso M, Ovaska K, et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 2011;30:3962–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu J, Mani RS, Cao Q, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Massie CE, Lynch A, Ramos-Montoya A, et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011;30:2719–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharma NL, Massie CE, Ramos-Montoya A, et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell. 2013;23:35–47. [DOI] [PubMed] [Google Scholar]
- 42. Andreu-Vieyra C, Lai J, Berman BP, et al. Dynamic nucleosome-depleted regions at androgen receptor enhancers in the absence of ligand in prostate cancer cells. Mol Cell Biol. 2011;31:4648–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Green S, Issemann I, Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988;16:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verrijdt G, Schauwaers K, Haelens A, Rombauts W, Claessens F. Functional interplay between two response elements with distinct binding characteristics dictates androgen specificity of the mouse sex-limited protein enhancer. J Biol Chem. 2002;277:35191–35201. [DOI] [PubMed] [Google Scholar]
- 45. Moehren U, Denayer S, Podvinec M, Verrijdt G, Claessens F. Identification of androgen-selective androgen-response elements in the human aquaporin-5 and Rad9 genes. Biochem J. 2008;411:679–686. [DOI] [PubMed] [Google Scholar]
- 46. http://www.chem.agilent.com/Library/usermanuals/Public/200518.pdf. Accessed April 1, 2013.
- 47. Haelens A, Verrijdt G, Callewaert L, Peeters B, Rombauts W, Claessens F. Androgen-receptor-specific DNA binding to an element in the first exon of the human secretory component gene. Biochem J. 2001;353:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thomas-Chollier M, Sand O, Turatsinze JV, et al. RSAT: regulatory sequence analysis tools. Nucleic Acids Res. 2008;36:W119–W127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. http://rsat.ulb.ac.be/rsat/. Accessed April 1, 2013.
- 50. Denayer S, Helsen C, Thorrez L, Haelens A, Claessens F. The rules of DNA recognition by the androgen receptor. Mol Endocrinol. 2010;24:898–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schoenmakers E, Alen P, Verrijdt G, et al. Differential DNA binding by the androgen and glucocorticoid receptors involves the second Zn-finger and a C-terminal extension of the DNA-binding domains. Biochem J. 1999;341(Pt. 3):515–521. [PMC free article] [PubMed] [Google Scholar]
- 52. Schauwaers K, De Gendt K, Saunders PT, et al. Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc Natl Acad Sci USA. 2007;104:4961–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sambrook J, Russell DW. Isolation of DNA fragments from polyacrylamide gels by the crush and soak method. CSH Protoc. 2006;2006(1). [DOI] [PubMed] [Google Scholar]
- 54. Fu Q, Manolagas SC, O'Brien CA. Parathyroid hormone controls receptor activator of NF-κB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol. 2006;26:6453–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Swaminathan S, Sharan SK. Bacterial artificial chromosome engineering. Methods Mol Biol. 2004;256:89–106. [DOI] [PubMed] [Google Scholar]
- 56. Zella LA, Meyer MB, Nerenz RD, Lee SM, Martowicz ML, Pike JW. Multifunctional enhancers regulate mouse and human vitamin D receptor gene transcription. Mol Endocrinol. 2010;24:128–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Portales-Casamar E, Thongjuea S, Kwon AT, et al. JASPAR 2010: the greatly expanded open-access database of transcription factor binding profiles. Nucleic Acids Res. 2010;38:D105–D110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Matys V, Kel-Margoulis OV, Fricke E, et al. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 2006;34:D108–D110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Claessens F, Verrijdt G, Schoenmakers E, et al. Selective DNA binding by the androgen receptor as a mechanism for hormone-specific gene regulation. J Steroid Biochem Mol Biol. 2001;76:23–30. [DOI] [PubMed] [Google Scholar]
- 61. Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim S, Broströmer E, Xing D, et al. Probing allostery through DNA. Science. 2013;339:816–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Masuda K, Werner T, Maheshwari S, et al. Androgen receptor binding sites identified by a GREF_GATA model. J Mol Biol. 2005;353:763–771. [DOI] [PubMed] [Google Scholar]
- 64. Perez-Stable CM, Pozas A, Roos BA. A role for GATA transcription factors in the androgen regulation of the prostate-specific antigen gene enhancer. Mol Cell Endocrinol. 2000;167:43–53. [DOI] [PubMed] [Google Scholar]
- 65. Bhardwaj A, Song HW, Beildeck M, et al. DNA demethylation-dependent AR recruitment and GATA factors drive Rhox5 homeobox gene transcription in the epididymis. Mol Endocrinol. 2012;26:538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Carey MF, Peterson CL, Smale ST. Experimental strategies for the identification of DNA-binding proteins. Cold Spring Harb Protoc. 2012;2012:18–33. [DOI] [PubMed] [Google Scholar]
- 67. Celis L, Claessens F, Peeters B, Heyns W, Verhoeven G, Rombauts W. Proteins interacting with an androgen-responsive unit in the C3(1) gene intron. Mol Cell Endocrinol. 1993;94:165–172. [DOI] [PubMed] [Google Scholar]
- 68. Claessens F, Celis L, De Vos P, et al. Intronic androgen response elements of prostatic binding protein genes. Biochem Biophys Res Commun. 1993;191:688–694. [DOI] [PubMed] [Google Scholar]
- 69. De Vos P, Claessens F, Winderickx J, et al. Interaction of androgen response elements with the DNA-binding domain of the rat androgen receptor expressed in Escherichia coli. J Biol Chem. 1991;266:3439–3443. [PubMed] [Google Scholar]
- 70. Lareyre JJ, Reid K, Nelson C, et al. Characterization of an androgen-specific response region within the 5′ flanking region of the murine epididymal retinoic acid binding protein gene. Biol Reprod. 2000;63:1881–1892. [DOI] [PubMed] [Google Scholar]
- 71. Maffey AH, Ishibashi T, He C, et al. Probasin promoter assembles into a strongly positioned nucleosome that permits androgen receptor binding. Mol Cell Endocrinol. 2007;268:10–19. [DOI] [PubMed] [Google Scholar]
- 72. Rundlett SE, Miesfeld RL. Quantitative differences in androgen and glucocorticoid receptor DNA binding properties contribute to receptor-selective transcriptional regulation. Mol Cell Endocrinol. 1995;109:1–10. [DOI] [PubMed] [Google Scholar]
- 73. Burgos-Trinidad M, Youngblood GL, Maroto MR, Scheller A, Robins DM, Payne AH. Repression of cAMP-induced expression of the mouse P450 17 α-hydroxylase/C17–20 lyase gene (Cyp17) by androgens. Mol Endocrinol. 1997;11:87–96. [DOI] [PubMed] [Google Scholar]
- 74. Kallio PJ, Palvimo JJ, Mehto M, Jänne OA. Analysis of androgen receptor-DNA interactions with receptor proteins produced in insect cells. J Biol Chem. 1994;269:11514–11522. [PubMed] [Google Scholar]
- 75. Scheller A, Hughes E, Golden KL, Robins DM. Multiple receptor domains interact to permit, or restrict, androgen-specific gene activation. J Biol Chem. 1998;273:24216–24222. [DOI] [PubMed] [Google Scholar]
- 76. Scheller A, Scheinman RI, Thompson E, Scarlett CO, Robins DM. Contextual dependence of steroid receptor function on an androgen-responsive enhancer. Mol Cell Endocrinol. 1996;121:75–86. [DOI] [PubMed] [Google Scholar]
- 77. Kasowski M, Grubert F, Heffelfinger C, et al. Variation in transcription factor binding among humans. Science. 2010;328:232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rockman MV, Wray GA. Abundant raw material for cis-regulatory evolution in humans. Mol Biol Evol. 2002;19:1991–2004. [DOI] [PubMed] [Google Scholar]
- 79. Eeles RA, Kote-Jarai Z, Al Olama AA, et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet. 2009;41:1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. [DOI] [PubMed] [Google Scholar]
- 81. Schaid DJ. The complex genetic epidemiology of prostate cancer. Hum Mol Genet. 2004;13(Spec. No.):R103–R121. [DOI] [PubMed] [Google Scholar]
- 82. Haffner MC, Aryee MJ, Toubaji A, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010;42:668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lin C, Yang L, Tanasa B, et al. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell. 2009;139:1069–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mani RS, Tomlins SA, Callahan K, et al. Induced chromosomal proximity and gene fusions in prostate cancer. Science. 2009;326:1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. http://genome.ucsc.edu/cgi-bin/hgGateway. Accessed April 1, 2013.
- 86. Maxam AM, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:499–560. [DOI] [PubMed] [Google Scholar]
- 87. Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]