Abstract
Background
Candidemia is a life-threatening fungal infection and it can affect patients of all ages. Characterization of candidemia in the elderly is lacking.
Methods
We performed a retrospective study of adults (≥ 18 years) with candidemia diagnosed in our center in 2010–2015. Demographics, comorbidities, clinical and microbiologic characteristics, antifungal treatment and outcome were compared between older (≤65 years) and younger (>65 years) patients.
Results
Among 302 patients with candidemia identified during the study period, 188 (62%) belonged to the elderly group. Comorbidities were significantly more frequent in older patients and included chronic pulmonary diseases, cardiovascular diseases, diabetes mellitus, and chronic renal failure (p ranging from <0.0001 to 0.017). A significantly higher proportion of older patients had septic shock (p = 0.040) at the time of candidemia. Candida albicans accounted for 53% of isolates and there were no significant differences between patients’ age and Candida species. Thirty-day mortality was significantly higher in older (45%) than in younger (28%) patients (p = 0.003). Factors associated with a significant higher proportion of death in the elderly included older age (i.e.: old-old), being hospitalized in ICU rather than in other wards, suffering from chronic pulmonary diseases, the presence of septic shock, multiple organ failure, dialysis and being infected with C. glabrata (p ranging from <0.0001 to 0.034). On multivariate analysis septic shock (HR 1.744 [CI95% 1.049–2.898], p = 0.032) and multiple organ failure (HR 2.242 [CI95% 1.070–4.698], p = 0.032) were independently associated with a higher risk of death. The probability of 30-days survival of older patients was significantly reduced when compared to that of younger patients (p = 0.005) who did not receive any treatment. In the elderly, there was a trend toward higher MICs for fluconazole/C. albicans, fluconazole/C. glabrata, amphotericin B/C. albicans, and caspofungin/C. glabrata.
Conclusions
In our study, we found that elderly patients with Candida bloodstream infections are characterized by a high mortality rate. In particular, the lack of any antifungal therapy as well as the occurrence of septic shock increased significantly the overall mortality. Additionally, we found that there was a trend of higher MIC for specific drug/Candida combination.
Introduction
The yeast belonging to the genus Candida is the major causative agent of fungal infections in hospitalized patients and the global incidence of candidemia has increased more than fivefold in the last decade [1–7]. Although Candida albicans remains the most frequently isolated species, geographical differences are emerging in epidemiology between different countries demonstrating a shift towards non-albicans species [5–8].
Candidemia represents a major challenge among the healthcare-related infections due to its difficult diagnostic and therapeutic management [1, 2, 4]. Patients with candidemia have serious underlying diseases and prognosis varies according to several factors [1–7]. The crude mortality of candidemia is extremely high ranging from 36% to 63% depending on the patients population considered [1–13]. The elderly population, which is increasing worldwide, is particularly vulnerable to Candida infections. This can be due to several factors such as high frequency of comorbidities, aging-related physiological changes, polypharmacy, and high colonization rate [9–13].
As far, few data are available on the epidemiology and outcome of candidemia in the elderly.
Therefore, we performed a retrospective study of adults with candidemia diagnosed in our center in 2010–2015. Demographics, comorbidities, clinical and microbiologic characteristics, antifungal treatment and outcome were compared between older and younger patients.
Patients and methods
A retrospective observational study of all cases of candidemia was carried out from January 1, 2010 to December 31, 2015 in a single 980-bedded referral University Hospital in Ancona, Italy. A case of Candida bloodstream infection (BSI) was defined as a peripheral isolation of Candida species from blood culture in a patient with temporally related clinical signs and symptoms of infection.
All Candida BSIs were identified through the microbiological laboratory database. Data regarding demographic characteristics and clinical risk factors were collected from the patient’s medical records. Charlson comorbidity index for each patient was also calculated [14]. Appropriate antifungal therapy was considered when an appropriate drug (based on subsequent in vitro susceptibility testing results) with adequate dosage was started within 72 h from the first blood culture performed. Adequate dosage of an antifungal agent was defined according to IDSA 2009 guidelines [15]. Early central venous catheter (CVC) removal was defined a removal of the line within 48 h from drawing blood culture [16]. Mortality was calculated after 7 and 30 days from the occurrence of the episode of Candida BSI. To ascertain the outcome, we considered only those patients from which clinical information was included in the regional health surveillance system. Candida species were isolated from blood samples using BacT/ALERT (bioMérieux) and identified with standard techniques. Antifungal susceptibility testing was performed using the SensitreYeastOne colorimetric plate (Trek Diagnostic System) and MIC results of fluconazole, amphotericin B and caspofungin were interpreted according to latest species-specific clinical breakpoints (CBPs) as established by the Clinical and Laboratory Standards Institute (CLSI) [17–19]. The three drugs were selected since each of them is the representative of a specific class.
The present research has been performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendements. The Institutional Review Board of the Azienda Ospedaliero-Universitaria Ospeadali Riuniti Umberto I°-Lancisi-Salesi granted retrospective access to the data without need for individual informed consent. The consent was not given since the data were analyzed anonymously.
For analysis, patients were divided into two groups according to age: older group (>65 years) and younger group (≤65 years). Patients <18 years old were excluded from this study. Quantitative data were shown as the median with interquartile ranges (Q1–Q3). Qualitative variables were expressed as absolute and relative frequencies. Categorical variables were compared using the χ2 test, whereas Mann–Whitney U test or Fisher exact test were applied for continuous variables. Variables with a p ≤0.05 at the descriptive analysis were analyzed by Cox regression. Additionally, survival analysis of older vs younger patients was performed to estimate the 30-days cumulative survival probability according to either the lack of antifungal therapy or the presence of septic shock via Kaplan-Meier curves. Comparisons of curves were evaluated with the log-rank test. All statistical analyses were performed using the statistical package SPSS for Windows v. 20 (SPSS Inc., Chicago, IL, USA). A p value of ≤0.05 was considered to represent statistical significance and all statistical tests were two-tailed.
Results
A total of 302 patients with candidemia were identified during the study period. There were 114 patients aging ≤ 65 years (median/IQR: 57/48-62 years) and 188 patients aging > 65 years (median/IQR: 76/72-80 years).
Demographic and clinical characteristics are reported in Table 1.
Table 1. Demographics and clinical characteristics of 302 patients with BSIs due to Candida species considered in this study.
| Characteristics | Age | |||
|---|---|---|---|---|
| All patients (n = 302) | ≤65 years (n = 114) | >65 years (n = 188) | p value a | |
| Male sex, n (%) | 194 (64) | 72 (63) | 122 (65) | 0.760 |
| Ward | ||||
| Internal Medicine, n (%) | 121 (40) | 52 (45) | 69 (37) | 0.296 |
| Surgery, n (%) | 76 (25) | 27 (24) | 49 (26) | |
| Intensive Care Unit, n (%) | 105 (35) | 35 (31) | 70 (37) | |
| Chronic pulmonary diseases, n (%) b | 47 (16) | 3 (1) | 44 (23) | <0.0001 |
| Haematological malignancy, n (%) | 10 (3) | 3 (3) | 7 (4) | 0.607 |
| Cardiovascular diseases, n (%) c | 162 (54) | 37 (32) | 125 (66) | <0.0001 |
| Neurological diseases, n (%) d | 49 (16) | 23 (20) | 26 (14) | 0.147 |
| Gastrointestinal diseases, n (%) e | 85 (28) | 35 (31) | 50 (27) | 0.441 |
| Diabetes mellitus, n (%) | 57 (19) | 11 (10) | 46 (24) | 0.001 |
| Solid tumors, n (%) | 96 (32) | 32 (28) | 63 (34) | 0.323 |
| Chronic renal failure, n (%) | 39 (13) | 8 (7) | 31 (16) | 0.017 |
| Charlson comorbidity index, median (IQR) f | 6 (5–7) | 4 (3–6) | 7 (5–8) | <0.0001 |
| Previous surgery (<30 days), n (%) | 160 (53) | 61 (54) | 99 (53) | 0.886 |
| Central venous catheter, n (%) | 267 (88) | 100 (88) | 167 (89) | 0.770 |
| Central venous catheter-related BSIs, n (%) g | 90 (30) | 35 (31) | 55 (29) | 0.789 |
| Central venous catheter-related BSIs with concomitant bacteriemia, n (%) | 34 (11) | 13 (11) | 21 (11) | 0.950 |
| Early central venous catheter removal, n (%) h | 155 (58) | 65 (65) | 90 (54) | 0.075 |
| Other devices, n (%) i | 275 (91) | 102 (89) | 173 (92) | 0.451 |
| Multiple organ failure, n (%) j | 18 (6) | 8 (7) | 10 (5) | 0.545 |
| Dialysis, n (%) | 23 (8) | 10 (9) | 13 (7) | 0.555 |
| Mechanical ventilation, n (%) | 17 (6) | 7 (6) | 10 (5) | 0.764 |
| Previous invasive procedures (<72 hours), n (%) jk | 96 (32) | 37 (32) | 59 (31) | 0.846 |
| Parenteral nutrition, n (%) | 208 (69) | 73 (64) | 135 (72) | 0.157 |
| Steroid therapy, n (%) | 82 (27) | 26 (23) | 56 (30) | 0.186 |
| Immunosuppressive therapy, n (%) kl | 27 (9) | 16 (14) | 11 (7) | 0.015 |
| Neutropenia, n (%) | 5 (2) | 2 (2) | 3 (2) | 0.916 |
| Septic shock, n (%) | 36 (12) | 8 (7) | 28 (15) | 0.040 |
| Candida species | ||||
| Candida albicans, n (%) | 160 (53) | 63 (55) | 97 (52) | 0.874 |
| Candida parapsilosis, n (%) | 69 (23) | 24 (21) | 45 (24) | |
| Candida tropicalis, n (%) | 33 (11) | 14 (12) | 19 (10) | |
| Candida glabrata, n (%) | 28 (9) | 9 (8) | 19 (10) | |
| Other Candida species, n (%) lm | 12 (4) | 4 (4) | 8 (4) | |
| Appropriate antifungal therapy, n (%) mn | 157 (52) | 60 (54) | 97 (51) | 0.861 |
| Primary antifungal therapy | ||||
| Azoles, n (%) | 156 (68) | 55 (64) | 101 (70) | 0.360 |
| Echinocandins, n (%) | 69 (30) | 30 (35) | 39 (27) | |
| Polyenes, n (%) | 5 (2) | 1 (1) | 4 (3) | |
| No treatment, n (%) | 72 (24) | 28 (25) | 44 (23) | 0.819 |
| Early mortality, n (%) no | 75 (25) | 23 (20) | 52 (28) | 0.144 |
| Late mortality, n (%) op | 42 (14) | 9 (8) | 33 (17) | 0.006 |
| Overall mortality, n (%) | 117 (39) | 32 (28) | 85 (45) | 0.003 |
a Comparisons between groups were performed using Chi-Square test or Fisher Exact Test when expected frequencies were less than five.
b Chronic pulmonary diseases include asthma, chronic bronchitis, emphysema and lung fibrosis.
c Cardiovascular diseases include heart failure, ischemic heart disease, endocarditis and arrhythmia.
d Neurological diseases include Parkinson’s disease, Alzheimer’s disease and paralysis.
e Gastrointestinal diseases include Crohn’s disease, ulcerative colitis, chronic pancreatitis and gallbladder stones.
f IQR, Interquartile range.
g A catheter-related candidemia was defined according to the guidelines of the infectious diseases society of America (IDSA: Mermel LA et al., Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America Clin. Infect. Dis. 2009; 49:1–45).
h Early central venous catheter removal was considered occurring within 48 h from blood cultures drawing.
i Other devices include urinary catheter, surgical drainage, cutaneous gastrostomy and tracheostomy tube.
j Multiple organ failure was defined as altered organ function in acutely ill patients involving two or more organ systems (Irwin RS and Rippe JM, Intensive Care Medicine, 7th Ed., 2011). Among younger patients there were: kidney and cardiovascular failures (n = 3), kidney and gastrointestinal [liver failure and gastrointestinal bleeding] failures (n = 2), respiratory and gastrointestinal failures (n = 2), respiratory and kidney and gastrointestinal failures (n = 1); among older patients there were: kidney and cardiovascular failures (n = 2), kidney and gastrointestinal [liver failure and gastrointestinal bleeding] failures (n = 2), respiratory and neurologic failures (n = 2), respiratory and cardiovascular failures (n = 1), respiratory and kidney failures (n = 1), kidney and neurologic failures (n = 1), respiratory and kidney and cardiovascular failures (n = 1).
k Previous invasive procedures include endoscopy and positioning of any device.
l Immunosuppressive therapy include calcineurin inhibitors and monoclonal antibodies.
m Other Candida species included Candida guilliermondii (n = 5), Candida krusei (n = 2), Candida lusitaniae (n = 2), Candida dubliniensis (n = 1), Candida pelliculosa (n = 1), and Candida utilis (n = 1).
n Appropriate antifungal therapy was considered when the appropriate drug with adequate dosage was started within 72 hours the first blood culture performed.
o Early mortality was considered as occurring within 7 days from the onset of candidemia.
p Late mortality was considered as occurring within 30 day from the onset of candidemia.
The majority of patients were hospitalized in medical wards (40%) followed by ICUs (35%) and surgical wards (25%). There were no significant differences between patients age and hospitalization wards. The following comorbidities were significantly more frequent in older patients: higher comorbidity index chronic pulmonary diseases (p <0.0001), cardiovascular diseases (p <0.0001), diabetes mellitus (p = 0.001), and chronic renal failure (p = 0.017). Comorbidity score (i.e.: Charlson comorbidity index) was significantly higher in older patients (p <0.0001). A significantly higher proportion of older patients had septic shock (p = 0.040) at the time of candidemia.
The majority of patients were infected with C. albicans (53%), followed by C. parapsilosis (23%), C. tropicalis (11%), C. glabrata (9%), and other Candida species (4%). There were no significant differences between patients age and Candida species.
Primary antifungal therapy consisted of azoles in the majority of patients (68%) followed by echinocandins (30%) and polyenes (2%). Of note, 72 patients (24%) did not receive any treatment. Overall mortality was 39% and it was significantly higher in older (85/188 [45%]) than in younger (32/114 [28%]) patients (p = 0.003).
Factors related to outcome of older patients are reported in Table 2.
Table 2. Outcome of 188 elderly patients with BSIs due to Candida species considered in this study.
| Characteristics | 30-day outcome | ||
|---|---|---|---|
| Survival (n = 103) | Death (n = 85) | p value a | |
| Age (years), median (IQR) b | 76 (72–80) | 78 (73–81) | 0.017 |
| Male sex, n (%) | 63(61) | 59 (69) | 0.238 |
| Ward | |||
| Internal Medicine, n (%) | 38 (37) | 31 (36) | <0.0001 |
| Surgery, n (%) | 40 (39) | 9 (11) | |
| Intensive Care Unit, n (%) | 25 (24) | 45 (53) | |
| Chronic pulmonary diseases, n (%) c | 18 (17) | 26 (31) | 0.034 |
| Haematological malignancy, n (%) | 4 (4) | 3 (4) | 1.000 |
| Cardiovascular diseases, n (%) d | 64 (62) | 61 (72) | 0.163 |
| Neurological diseases, n (%) e | 12 (12) | 14 (16) | 0.340 |
| Gastrointestinal diseases, n (%) f | 26 (25) | 24 (28) | 0.643 |
| Diabetes mellitus, n (%) | 23 (22) | 23 (27) | 0.452 |
| Solid tumors, n (%) | 32 (31) | 31 (36) | 0.434 |
| Chronic renal failure, n (%) | 13 (13) | 18 (21) | 0.115 |
| Charlson comorbidity index, median (IQR) b | 6 (5–7) | 7 (6–8) | <0.0001 |
| Previous surgery (<30 days), n (%) | 57 (55) | 42 (49) | 0.417 |
| Central venous catheter, n (%) | 92 (89) | 75 (88) | 0.814 |
| Central venous catheter-related BSIs, n (%) g | 32 (31) | 23 (27) | 0.547 |
| Central venous catheter-related BSIs with concomitant bacteriemia, n (%) | 13 (13) | 8 (9) | 0.486 |
| Early central venous catheter removal, n (%) h | 48 (47) | 35 (41) | 0.455 |
| Other devices, n (%) i | 93 (90) | 80 (94) | 0.335 |
| Multiple organ failure, n (%) j | 1 (1) | 9 (11) | 0.005 |
| Dialysis, n (%) | 3 (3) | 10 (12) | 0.021 |
| Mechanical ventilation, n (%) | 3 (3) | 7 (8) | 0.189 |
| Previous invasive procedures (<72 hours), n (%) k | 27 (26) | 32 (37) | 0.092 |
| Parenteral nutrition, n (%) | 73 (71) | 62 (73) | 0.753 |
| Steroid therapy, n (%) | 26 (25) | 30 (35) | 0.151 |
| Immunosuppressive therapy, n (%) l | 8 (8) | 3 (4) | 0.350 |
| Neutropenia, n (%) | 1 (1) | 2 (2) | 0.590 |
| Septic shock, n (%) | 6 (6) | 22 (26) | <0.0001 |
| Candida species | |||
| Candida albicans, n (%) | 49 (47) | 48 (57) | 0.029 |
| Candida parapsilosis, n (%) | 28 (27) | 17 (20) | |
| Candida tropicalis, n (%) | 13 (13) | 6 (7) | |
| Candida glabrata, n (%) | 6 (6) | 13 (15) | |
| Other Candida species, n (%) m | 7 (7) | 1 (1) | |
| Appropriate antifungal therapy n | 57 (55) | 40 (47) | 0.258 |
| Primary antifungal therapy | |||
| Azoles, n (%) | 62 (74) | 39 (65) | 0.312 |
| Echinocandins, n (%) | 19 (22) | 20 (33) | |
| Polyenes, n (%) | 3 (4) | 1 (2) | |
| No treatment, n (%) | 19 (18) | 25 (29) | 0.077 |
a Comparisons between groups were performed using Mann-Whitney test for quantitative variables and Chi-Square test (or Fisher Exact Test when expected frequencies were less than five) for qualitative variables.
b IQR, Interquartile range.
c Chronic pulmonary diseases include asthma, chronic bronchitis, emphysema and lung fibrosis.
d Cardiovascular diseases include heart failure, ischemic heart disease, endocarditis and arrhythmia.
e Neurological diseases include Parkinson’s disease, Alzheimer’s disease and paralysis.
f Gastrointestinal diseases include Crohn’s disease, ulcerative colitis, chronic pancreatitis and gallbladder stones.
g A catheter-related candidemia was defined according to the guidelines of the infectious diseases society of America (IDSA: Mermel LA et al., Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America Clin. Infect. Dis. 2009; 49:1–45).
h Early central venous catheter removal was considered occurring within 48 h from blood cultures drawing.
i Other devices include urinary catheter, surgical drainage, cutaneous gastrostomy and tracheostomy tube.
j Multiple organ failure was defined as altered organ function in acutely ill patients involving two or more organ systems (Irwin RS and Rippe JM, Intensive Care Medicine, 7th Ed., 2011). There were: kidney and cardiovascular failures (n = 2), kidney and gastrointestinal [liver failure and gastrointestinal bleeding] failures (n = 2), respiratory and neurologic failures (n = 2), respiratory and cardiovascular failures (n = 1), respiratory and kidney failures (n = 1), kidney and neurologic failures (n = 1), respiratory and kidney and cardiovascular failures (n = 1).
k Previous invasive procedures include endoscopy and positioning of any device.
l Immunosuppressive therapy include corticosteroids, calcineurin inhibitors and monoclonal antibodies.
m Other Candida species included Candida guilliermondii (n = 4), Candida krusei (n = 1), Candida lusitaniae (n = 2), and Candida utilis (n = 1).
n Appropriate antifungal therapy was considered when the appropriate drug with adequate dosage was started within 72 hours the first blood culture performed.
The specific risk factors significantly more common among older patients who died within 30 days were: older age (p = 0.017), being hospitalized in ICU rather than in other wards (p <0.0001), suffering from chronic pulmonary diseases (p = 0.034), higher comorbidity index (p <0.0001), the presence of septic shock (p <0.0001), multiple organ failure (p = 0.005), dialysis (p = 0.021), and being infected with C. glabrata (p = 0.029). The following variables were included in the Cox regression analysis: age, ward, the presence of chronic pulmonary disease, comorbidity index, septic shock, dialysis, and Candida species. On multivariate analysis septic shock (HR 1.744 [CI95% 1.049–2.898], p = 0.032) and multiple organ failure (HR 2.242 [CI95% 1.070–4.698], p = 0.032) were independently associated with a higher risk of death. Factors related to outcome of younger patients are reported in Table 3.
Table 3. Outcome of 114 younger patients with BSIs due to Candida species considered in this study.
| Characteristics | 30-day outcome | ||
|---|---|---|---|
| Survival (n = 82) | Death (n = 32) | p value a | |
| Age (years), median (IQR) b | 57 (44–61) | 58 (51–63) | 0.067 |
| Male sex, n (%) | 48 (59) | 32 (75) | 0.101 |
| Ward | |||
| Internal Medicine, n (%) | 40 (49) | 12 (38) | 0.168 |
| Surgery, n (%) | 21 (26) | 6 (19) | |
| Intensive Care Unit, n (%) | 21 (26) | 14 (44) | |
| Chronic pulmonary diseases, n (%) c | 1 (1) | 2 (6) | 0.189 |
| Haematological malignancy, n (%) | 1 (1) | 2 (6) | 0.189 |
| Cardiovascular diseases, n (%) d | 28 (34) | 9 (28) | 0.537 |
| Neurological diseases, n (%) e | 18 (22) | 5 (16) | 0.605 |
| Gastrointestinal diseases, n (%) f | 26 (32) | 9 (28) | 0.709 |
| Diabetes mellitus, n (%) | 6 (7) | 5 (16) | 0.286 |
| Solid tumors, n (%) | 21 (26) | 11 (34) | 0.349 |
| Chronic renal failure, n (%) | 6 (7) | 2 (6) | 1.000 |
| Charlson comorbidity index, median (IQR) b | 4 (3–5) | 5 (4–6) | <0.0001 |
| Previous surgery (<30 days), n (%) | 45 (55) | 16 (50) | 0.638 |
| Central venous catheter, n (%) | 71 (87) | 29 (91) | 0.554 |
| Central venous catheter-related BSIs, n (%) g | 28 (34) | 7 (22) | 0.260 |
| Central venous catheter-related BSIs with concomitant bacteriemia, n (%) | 11 (13) | 2 (6) | 0.279 |
| Early central venous catheter removal, n (%) h | 40 (49) | 16 (50) | 0.906 |
| Other devices, n (%) i | 73 (89) | 29 (90) | 0.860 |
| Multiple organ failure, n (%) j | 6 (7) | 2 (6) | 1.000 |
| Dialysis, n (%) | 6 (7) | 4 (1) | 0.463 |
| Mechanical ventilation, n (%) | 3 (4) | 4 (1) | 0.095 |
| Previous invasive procedures (<72 hours), n (%) k | 25 (30) | 12 (38) | 0.472 |
| Parenteral nutrition, n (%) | 47 (57) | 26 (81) | 0.016 |
| Steroid therapy, n (%) | 22 (27) | 4 (13) | 0.137 |
| Immunosuppressive therapy, n (%) l | 12 (15) | 4 (13) | 1.000 |
| Neutropenia, n (%) | 0 (0) | 2 (6) | 0.077 |
| Septic shock, n (%) | 1 (1) | 7 (22) | 0.0005 |
| Candida species | |||
| Candida albicans, n (%) | 42 (51) | 21 (66) | 0.418 |
| Candida parapsilosis, n (%) | 19 (23) | 5 (16) | |
| Candida tropicalis, n (%) | 12 (15) | 2 (6) | |
| Candida glabrata, n (%) | 7 (9) | 2 (6) | |
| Other Candida species, n (%) m | 2 (2) | 2 (6) | |
| Appropriate antifungal therapy n | 43 (52) | 17 (53) | 0.947 |
| Primary antifungal therapy | |||
| Azoles, n (%) | 38 (63) | 17 (65) | 0.800 |
| Echinocandins, n (%) | 21 (35) | 9 (35) | |
| Polyenes, n (%) | 1 (2) | 0 (0) | |
| No treatment, n (%) | 22 (27) | 6 (19) | 0.367 |
a Comparisons between groups were performed using Mann-Whitney test for quantitative variables and Chi-Square test (or Fisher Exact Test when expected frequencies were less than five) for qualitative variables.
b IQR, Interquartile range.
c Chronic pulmonary diseases include asthma, chronic bronchitis, emphysema and lung fibrosis.
d Cardiovascular diseases include heart failure, ischemic heart disease, endocarditis and arrhythmia.
e Neurological diseases include Parkinson’s disease, Alzheimer’s disease and paralysis.
f Gastrointestinal diseases include Crohn’s disease, ulcerative colitis, chronic pancreatitis and gallbladder stones.
g A catheter-related candidemia was defined according to the guidelines of the infectious diseases society of America (IDSA: Mermel LA et al., Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America Clin. Infect. Dis. 2009; 49:1–45).
h Early central venous catheter removal was considered occurring within 48 h from blood cultures drawing.
i Other devices include urinary catheter, surgical drainage, cutaneous gastrostomy and tracheostomy tube.
j Multiple organ failure was defined as altered organ function in acutely ill patients involving two or more organ systems (Irwin RS and Rippe JM, Intensive Care Medicine, 7th Ed., 2011). There were: kidney and cardiovascular failures (n = 3), kidney and gastrointestinal [liver failure and gastrointestinal bleeding] failures (n = 2), respiratory and gastrointestinal failures (n = 2), respiratory and kidney and gastrointestinal failures (n = 1).
k Previous invasive procedures include endoscopy and positioning of any device.
l Immunosuppressive therapy include corticosteroids, calcineurin inhibitors and monoclonal antibodies.
m Other Candida species included Candida dubliniensis (n = 1), Candida guilliermondii (n = 1), Candida krusei (n = 1), and Candida pelliculosa (n = 1).
n Appropriate antifungal therapy was considered when the appropriate drug with adequate dosage was started within 72 hours the first blood culture performed.
The specific risk factors significantly more common among younger patients who died within 30 days were: higher comorbidity index (p < 0.0001), parenteral nutrition (p = 0.016) and the presence of septic shock (p = 0.0005). These variables were included in the Cox regression analysis. Again, only the presence of septic shock resulted independently associated with a significant higher risk of death (HR 3.304 [CI95% 1.422–7.677], p = 0.005). Because septic shock had such profound adverse prognostic impact on candidemia regardless patients’ age, we analyzed the occurrence of septic shock in patients according to appropriate therapy (9% [14/157]), no appropriate therapy (18% [13/73]), and no therapy (13% [9/72] and we did not find any statistical difference (p = 0.150).
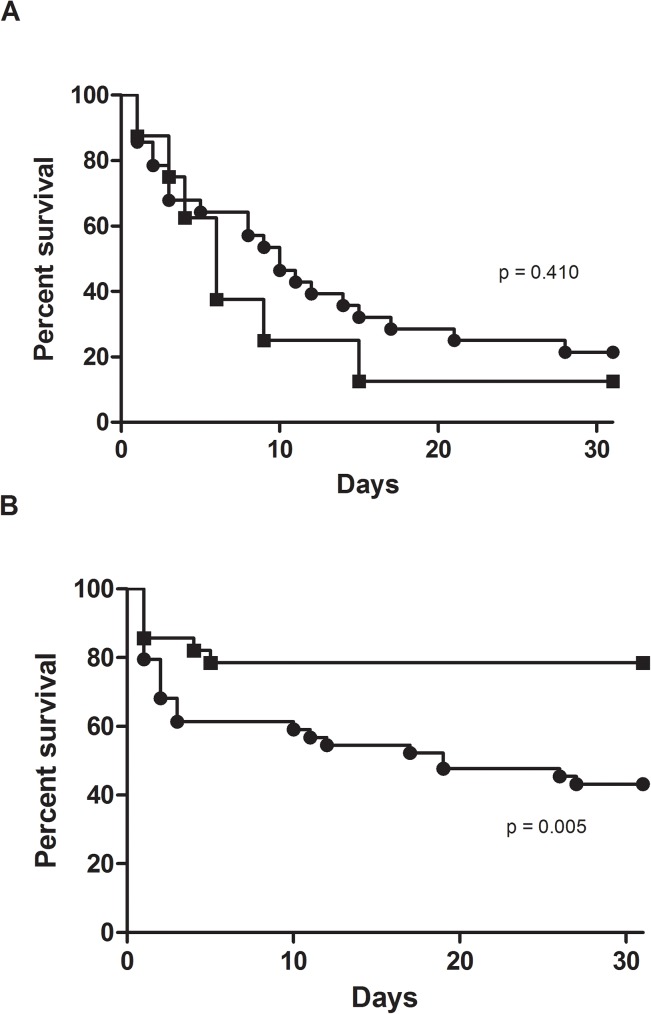
We further analyzed the probability of 30-days survival of older vs younger patients suffering from septic shock and we did not find any statistically significance (Fig 1A; p = 0.410).
Fig 1.
30-days survival of older (circle) vs younger (squares) patients with candidemia with septic shock (A) and without any antifungal therapy (B).
Since there was a high proportion of candidemic patients who did not received any treatment, we analyzed the impact of lack of antifungal therapy on survival. As shown in Fig 1B, the probability of 30-days survival of older patients was significantly reduced when compared to that of younger patients (p = 0.005) who did not receive any treatment.
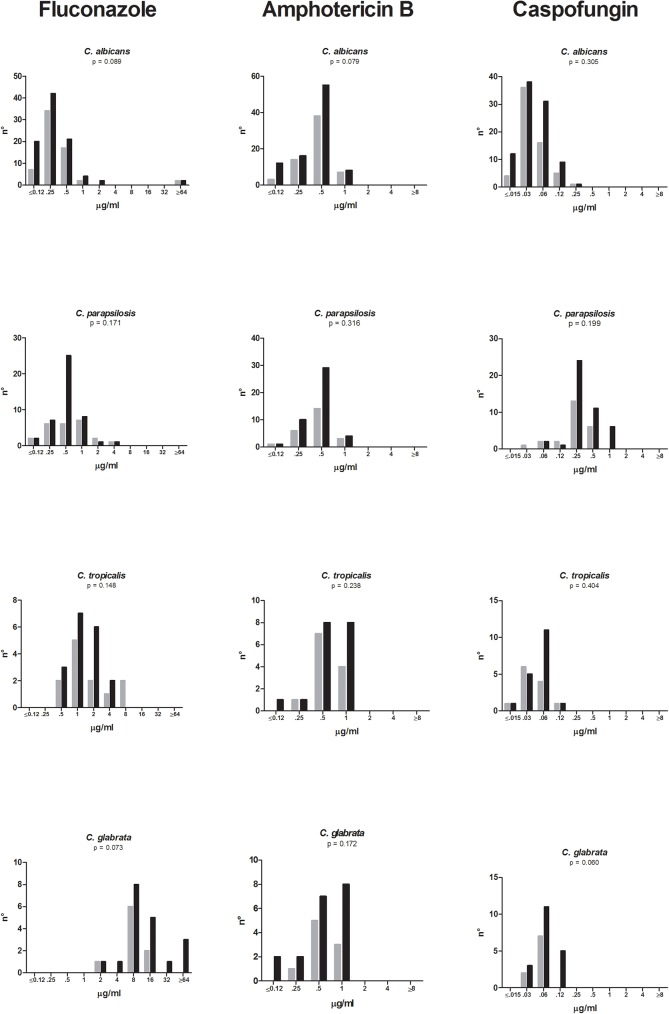
Fig 2 depicts antifungal susceptibility patterns of fluconazole, caspofungin and amphotericin B against the isolates belonging to the four most common Candida species. MICs results were available for 96% of the isolated yeasts. Fluconazole resistance accounted for 3%, 6%, 11%, and 0% of isolates of C. albicans (4/160), C. tropicalis (2/33), C. glabrata (3/28), and C. parapsilosis (0/69), respectively. All isolates were susceptible to amphotericin B (WT phenotype) and to caspofungin. Although in the elderly there was a trend toward higher MICs for fluconazole/C. albicans, fluconazole/C. glabrata, amphotericin B/C. albicans, and caspofungin/C. glabrata, a statistically significant difference was not reached. There were 21/188 (11%) of older patients vs 9/114 (8%) of younger patients exposed to fluconazole before developing candidemia (p = 0.356).
Fig 2.
Fluconazole (A), amphotericin B (B), and caspofungin (C) MICs for clinical isolates of Candida spp. recovered from younger (gray bars) and older (black bars) patients.
Discussion
In order to investigate the outcome predictors of candidemia in the elderly, we evaluated a database of patients with candidemia diagnosed over a six years period in our hospital and compared clinical and microbiologic characteristics in elderly versus younger patients. Data from the literature often report older age as an independent factor for mortality in patients with candidemia [2, 5–7, 9, 11, 12]. In our cohort, elderly patients (>65 years) represented 62% of the entire study group (188/302). This proportion, which is somewhat higher than those reported by other studies, makes our population particularly suitable to be investigated in this regard.
In our study, we found that elderly patients with Candida bloodstream infections are characterized by a high mortality rate. In particular, the lack of any antifungal therapy as well as the occurrence of septic shock increased significantly the overall mortality. Additionally, we found that there was a trend of higher MIC for specific drug/Candida combination.
We confirmed that the overall mortality in the elderly was significantly higher than that observed in younger patients (45% vs 28%). Similarly, two recent studies showed that 30-day mortality increased from 17% to 32% and from 45% to 70% in younger and older patients, respectively [9, 11]. This wide variation of mortality among studies can be due to several reasons such as the definition of elderly, the proportion of immunocompromised patients included (i.e.: hematologic), the type of antifungal utilized for primary therapy, the interval between blood drawing and initiation of drug, the variable CVC management. In particular, in our series there were only 10 hematologic patients (3 in younger and 7 in older groups) and neutropenia was found only in five patients.
As expected, we found a significant higher proportion of older patients suffering from specific comorbidities, such as chronic pulmonary diseases, diabetes mellitus, chronic renal failure and cardiovascular diseases. While the occurrence of the first three types of diseases was consistent with that reported in the literature (ranging from 16% to 24%), cardiovascular diseases accounted for 66% of our older patients. This figure is higher than that observed in other studies as far [9, 11]. Although septic shock was significantly more frequent in elderly than younger patients, its occurrence resulted to be an independent factor for mortality in both groups [20, 21]. Interestingly, we found that multiple organ failure (i.e.: altered organ function in acutely ill patients involving two or more organ systems) was an independent factor for higher risk of mortality only in older patients.
Although we found that timely antifungal therapy and early source control (i.e., removal of central lines), were not significantly associated with a better survival, both characteristics were proportionally higher in elderly patients who survived.
As reported in other studies, we found a high proportion of patients not receiving any antifungal drug (24% in our series) [11]. To see whether the lack of antifungal therapy would affect the prognosis of candidemia in the elderly, we performed Kaplan Meyer analysis and showed that there was a significantly higher proportion of elderly patients no treated who died within 30 days with respect to younger group. Although it’s hard to explain why a high proportion of patients in our study was not treated for Candida infection, we do exclude that this can be due by a decision of family / doctor / organizational factor, since the Italian health system provides any kind of therapeutic approach regardless of patients’ age thereby making any comparison with the results of other studies very difficult [11].
Unlike other reports, we did not find any difference between patients’ age and Candida species, thus reinforcing the importance of knowing the local epidemiology. Several studies from United States and Europe have recently demonstrated that older patients have increased risk of candidemia due to C. glabrata [1, 4, 8, 22, 23]. In contrast, an association between C. tropicalis candidemia and elderly patients has been reported in Brazil, Saudi Arabia, Taiwan and Singapore [24–27]. The most common isolated Candida species found in our study were represented by C. albicans followed by C. parapsilosis, regardless of patients’ age. This rank order is in line with that previously reported in studies of candidemia conducted in Italy [6, 7, 12]. Previous exposure to azoles or high proportion of specific underlying diseases, like malignancies, might explain these microbiologic differences.
It’s interesting to note that in our old patients group infections due to C. glabrata were characterized by a significant higher proportion of death with respect to other Candida species. This characteristic, however, was lost in multivariate analysis.
In this study, we also evaluated in vitro susceptibility patterns of fluconazole, amphotericin B and caspofungin against our collection of Candida clinical isolates. The three drugs were selected since each of them is the representative of a specific class. First, we found that occurrence of antifungal resistance was found only for fluconazole vs C. albicans (3%), C. tropicalis (6%), and C. glabrata (10%). Second, we observed a trend towards higher MICs for specific combinations drug/Candida strains isolated from the elderly. This was particularly evident, although not statistically significant, for fluconazole. This finding can be due to the fact that elderly patients, being more susceptible to various infections, are often exposed to several antibiotics and antifungal agents thus determining a selection of less susceptible isolates [10]. In reviewing our database we found that 11% (21/188) of older patients vs 8% (9/114) of younger patients were exposed to fluconazole before developing candidemia. Of note, we observed a shift to higher caspofungin MICs for C. glabrata isolates from the elderly. Although this finding again was not significant and the overall number of isolates was quite low (9/younger, 19/older), this phenomenon deserves not to be underestimated. Lately, an increase of reduced susceptibility to echinocandins among fluconazole-resistant isolates of C. glabrata has been documented [28, 29].
The present study have some limitations. First, being a retrospective study encompassing several departments and medical disciplines over a six years period, there was not a univocal management of each individual case. Second, although we have made every attempt to collect and analyze as many as clinical data as possible to reveal useful information for the patients management, some variables could not be explored because of missing data. Finally, since our data come from a single center experience, our findings may not be relevant to other patients populations.
In conclusion, candidemia in the elderly has become an important clinical problem accounting for a great proportion of patients with this invasive fungal disease. Since it is characterized by a poor prognosis, the identification of host- (i.e.: specific comorbidities) and healthcare-related risk factors (i.e.: lack of antifungal therapy) are crucial to prevent a negative outcome.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Diekema DJ, Messer SA, Brueggemann AB, Coffman SL, Doern GV, Herwaldt LA, et al. (2002) Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J Clin Microbiol 40:1298–1302. doi: 10.1128/JCM.40.4.1298-1302.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, et al. (2003) A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis 37:634–643. doi: 10.1086/376906 [DOI] [PubMed] [Google Scholar]
- 3.Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, Faure O, et al. (2004) Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis 23: 317–322. doi: 10.1007/s10096-004-1103-y [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nucci M, Queiros-Telles F, Tobon AM, Restrepo A, Colombo AL. (2010) Epidemiology of opportunistic fungal infections in Latin America. Clin Infect Dis 51:887–895. [DOI] [PubMed] [Google Scholar]
- 6.Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M, Viscoli C. (2011) Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One 6: e24198 doi: 10.1371/journal.pone.0024198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barchiesi F, Orsetti E, Gesuita R, Skrami E, Manso E, Candidemia Study Group. (2016) Epidemiology, clinical characteristics, and outcome of candidemia in a tertiary referral center in Italy from 2010 to 2014. Infection 44:205–213. doi: 10.1007/s15010-015-0845-z [DOI] [PubMed] [Google Scholar]
- 8.Arendrup MC, Dzajic E, Jensen RH, Johansen HK, Kjaeldgaard P, Knudsen JD, et al. (2013) Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect 19:E343–353. doi: 10.1111/1469-0691.12212 [DOI] [PubMed] [Google Scholar]
- 9.Guimarães T, Nucci M, Mendonça JS, Martinez R, Brito LR, Silva N, et al. (2012) Epidemiology and predictors of a poor outcome in elderly patients with candidemia. Int J Infect Dis 16(6):e442–447. doi: 10.1016/j.ijid.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 10.Flevari A, Theodorakopoulou M, Velegraki A, Armaganidis A, Dimopoulos G. (2013) Treatment of invasive candidiasis in the elderly: a review. Clin Interv Aging 8:1199–1208. doi: 10.2147/CIA.S39120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Liu N, Yin M, Han H, Yue J, Zhang F, et al. (2014) The epidemiology, antifungal use and risk factors of death in elderly patients with candidemia: a multicentre retrospective study. BMC Infect Dis. 25;14:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luzzati R, Cavinato S, Deiana ML, Rosin C, Maurel C, Borelli M. (2015) Epidemiology and outcome of nosocomial candidemia in elderly patients admitted prevalently in medical wards. Aging Clin Exp Res 27:131–137. doi: 10.1007/s40520-014-0251-x [DOI] [PubMed] [Google Scholar]
- 13.Luzzati R, Merelli M, Ansaldi F, Rosin C, Azzini A, Cavinato S, et al. (2016) Nosocomial candidemia in patients admitted to medicine wards compared to other wards: a multicentre study. Infection First Online: 11 July 2016 [DOI] [PubMed] [Google Scholar]
- 14.Charlson M, Szatrowski TP, Peterson J, Gold J. (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 15.Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, et al. (2009) Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535. doi: 10.1086/596757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, et al. (2012) Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 54:1110–1122. doi: 10.1093/cid/cis021 [DOI] [PubMed] [Google Scholar]
- 17.Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, et al. (2011) Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat 14:164–176. doi: 10.1016/j.drup.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. (2012) Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement. CLSI document M27-S4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Pfaller MA, Diekema DJ. (2012) Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol 50:2846–2856. doi: 10.1128/JCM.00937-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. (2012) Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis 54:1739–46. doi: 10.1093/cid/cis305 [DOI] [PubMed] [Google Scholar]
- 21.Bassetti M, Righi E, Ansaldi F, Merelli M, Trucchi C, De Pascale G, et al. , (2014) A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med 40:839–845. doi: 10.1007/s00134-014-3310-z [DOI] [PubMed] [Google Scholar]
- 22.Blot S, Vandewoude K, Hoste E, Poelaert J, Colardyn F. (2001) Outcome in critically ill patients with candidal fungaemia: Candida albicans vs. Candida glabrata. J Hosp Infect 47:308–313. doi: 10.1053/jhin.2000.0918 [DOI] [PubMed] [Google Scholar]
- 23.Hesstvedt L, Gaustad P, Andersen CT, Haarr E, Hannula R, Haukland HH, et al. (2015) Twenty-two years of candidaemia surveillance: results from a Norwegian national study. Clin Microbiol Infect 21:938–945 doi: 10.1016/j.cmi.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 24.Nucci M, Colombo AL. (2007) Candidemia due to Candida tropicalis: clinical, epidemiologic, and microbiologic characteristics of 188 episodes occurring in tertiary care hospitals. Diagn Microbiol Infect Dis 58:77–82. doi: 10.1016/j.diagmicrobio.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 25.Al-Jasser AM, Elkhizzi NA (2004) Distribution of Candida species among bloodstream isolates. Saudi Med J 25:566–569. [PubMed] [Google Scholar]
- 26.Cheng YR, Lin LC, Young TG, Liu CE, Chen CH, Tsay RW. (2006) Risk factors for candidemia-related mortality at a medical center in central Taiwan. J Microbiol Immunol Infect 39:155–161. [PubMed] [Google Scholar]
- 27.Chai YAL, Wang Y, Khoo AL, Chan FY, Chow C, Kumarasinghe G, et al. (2007) Predominance of Candida tropicalis bloodstream infections in a Singapore teaching hospital. Med Mycol 45:435–439 doi: 10.1080/13693780701385868 [DOI] [PubMed] [Google Scholar]
- 28.Alexander BD, Johnson MD, Pfeiffer CD, Jiménez-Ortigosa C, Catania J, Booker R, et al. (2013) Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arendrup MC. (2014) Update on antifungal resistance in Aspergillus and Candida. Clin Microbiol Infect 20:42–48. doi: 10.1111/1469-0691.12513 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.