Abstract
Structured lifestyle interventions can reduce diabetes incidence and cardiovascular disease (CVD) risk among persons with impaired glucose tolerance (IGT), but it is unclear whether they should be implemented among persons without IGT. We conducted a systematic review and meta-analyses to assess the effectiveness of lifestyle interventions on CVD risk among adults without IGT or diabetes. We systematically searched MEDLINE, EMBASE, CINAHL, Web of Science, the Cochrane Library, and PsychInfo databases, from inception to May 4, 2016. We selected randomized controlled trials of lifestyle interventions, involving physical activity (PA), dietary (D), or combined strategies (PA+D) with follow-up duration ≥12 months. We excluded all studies that included individuals with IGT, confirmed by 2-hours oral glucose tolerance test (75g), but included all other studies recruiting populations with different glycemic levels. We stratified studies by baseline glycemic levels: (1) low-range group with mean fasting plasma glucose (FPG) <5.5mmol/L or glycated hemoglobin (A1C) <5.5%, and (2) high-range group with FPG ≥5.5mmol/L or A1C ≥5.5%, and synthesized data using random-effects models. Primary outcomes in this review included systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), and triglycerides (TG). Totally 79 studies met inclusion criteria. Compared to usual care (UC), lifestyle interventions achieved significant improvements in SBP (-2.16mmHg[95%CI, -2.93, -1.39]), DBP (-1.83mmHg[-2.34, -1.31]), TC (-0.10mmol/L[-0.15, -0.05]), LDL-C (-0.09mmol/L[-0.13, -0.04]), HDL-C (0.03mmol/L[0.01, 0.04]), and TG (-0.08mmol/L[-0.14, -0.03]). Similar effects were observed among both low-and high-range study groups except for TC and TG. Similar effects also appeared in SBP and DBP categories regardless of follow-up duration. PA+D interventions had larger improvement effects on CVD risk factors than PA alone interventions. In adults without IGT or diabetes, lifestyle interventions resulted in significant improvements in SBP, DBP, TC, LDL-C, HDL-C, and TG, and might further reduce CVD risk.
Introduction
Cardiovascular disease (CVD) is the number one killer globally.[1] CVD is also the major cause of morbidity and mortality among persons with diabetes, and the largest contributor to health care costs associated with diabetes.[2,3] On the other hand, CVD and diabetes share similar risk factors such as unhealthy diet, physical inactivity, and obesity.[2–4] Previous studies have demonstrated that structured lifestyle interventions incorporating physical activity, diet, and behavior change strategies could prevent or delay type 2 diabetes incidence and reduce CVD risk factors.[5–7] However, these major prevention trials focused on populations with impaired glucose tolerance (IGT).[5–7] Although individuals with IGT are the priority target population because they lie at the higher end of the diabetes risk spectrum, populations without IGT but with other CVD risk factors may outnumber those with high diabetes risk and have the same urgent needs for risk reduction, as many RCT studies have indicated.[8–14] According to the American Diabetes Association’s (ADA) definitions of pre-diabetes (which includes impaired fasting glucose (IFG): 100-125mg/dL), about 60% of US individuals with pre-diabetes do not have IGT,[15] and according to the World Health Organization’s (WHO) definition of intermediate hyperglycemia (measured by fasting plasma glucose (FPG): 110-139mg/dL), about 70% of individuals with this condition do not have IGT.[16] Whether lifestyle interventions should be applied more broadly to the population at lower risk (i.e. those below the IGT threshold) to reduce CVD risk needs to be examined.
According to an American Heart Association (AHA) Special Report,[17] cardiovascular health is defined by 7 metrics, including health behaviors and health indicators as follows: smoking status, body mass index (BMI), physical activity (PA) levels, healthy diet scores, total cholesterol (TC), blood pressure (BP) level, and fasting plasma glucose level. To achieve the AHA ideal cardiovascular health promotion goal, each indicator must fall into certain ranges (e.g., FPG<100 mg/dL). This definition of cardiovascular health addresses health behaviors and health indicators related to both CVD and diabetes, and thus offer guidance for how to achieve improvements in preventing both CVD and diabetes at the same time.
Evidence regarding the effects of lifestyle intervention on CVD risk reduction has previously been systematically synthesized by examining 6 of the 7 CVD health indicators mentioned above, especially by examining the different stratum of BMI (e.g., moderate weight loss will reduce both diabetes and CVD risk among overweight or obese populations[5–7]), as indicated by the 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk.[18] However, how this evidence is aligned with the stratification of different glucose levels is still unclear. Lack of this information may prevent public health practitioners from fully understanding the role lifestyle interventions can play in reducing both diabetes and CVD risk among populations with varying risk levels. In contrast, a synthesis of evidence on the impact of lifestyle interventions among populations with different risk levels may help to inform decisions regarding the allocation of finite public health resources.
We conducted a systematic review to assess the aggregated impact of lifestyle interventions on glucose regulation and CVD risk factors among adults (age≥18 years) without IGT or diabetes. By conducting this review, we intend to answer the following research question: can lifestyle interventions similar to those found efficacious among populations with IGT achieve the same magnitude of improvement in CVD risk reduction among populations with lower diabetes risk? We also aimed to examine whether lifestyle interventions focused on diet, PA or their combination have varying impact on CVD risk reduction. To understand how to reach the comprehensive goal of preventing both CVD and diabetes, we also examined how the lifestyle interventional effect on CVD risk reduction is related to the effect sizes of glucose improvement and weight loss.
Materials and methods
Search strategy and selection criteria
We followed Cochrane Collaboration standards for a meta-analysis of randomized control trial (RCT) studies to develop our protocol.[19] We systemically searched MEDLINE, EMBASE, CINAHL, Web of Science, the Cochrane Library, and PsychInfo databases, from inception to May 4, 2016. Medical Subject Headings, text words, and search strategies are presented in our online-only supplements (S1 File). We examined reference lists of all included studies and relevant reviews for additional studies. We directly contacted authors to clarify data as needed.
We selected RCTs published in any language that examined lifestyle strategies involving PA and/or dietary (D) interventions, among adults (≥18 years) and with glycemic indicators and CVD risk factors reported as intervention outcomes (e.g., systolic blood pressure (SBP), diastolic blood pressure (DBP), TC, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), or triglycerides (TG)). Included studies investigated persons without IGT or diabetes. We excluded all studies that included individuals with IGT, confirmed by 2-hours oral glucose tolerance test (75g), but included all other studies recruiting populations with different glycemic levels. However, to examine whether there was heterogeneity of effect by baseline glycemia, we grouped all studies as: (1) low range glycemia group with mean fasting plasma glucose (FPG)<5.5mmol/L or mean glycated hemoglobin (A1C)<5.5% and (2) high range group with mean FPG≥5.5mmol/L or mean A1C≥5.5%. Data from the low and high range glycemic groups were analyzed separately. We only included interventions with a follow-up interval of at least 12 months.
Study selection and data extraction
Two reviewers independently reviewed each article title and abstract for inclusion. If any disagreement occurred between two reviewers, a third reviewed the item and consensus was reached through discussions.
We extracted data regarding demographic and intervention characteristics. Primary outcomes included SBP, DBP, TC, LDL-C, HDL-C, and TG. In our review, all interventions were classified as PA alone, D alone, or combined interventions (PA+D). PA interventions included any strategy used to promote physical activity levels using counseling, exercise prescription, and/or a supervised or unsupervised exercise program. D interventions included any strategy used to reduce or control calorie intake, e.g., very low-calorie diet (<800 kcal/d) or low-calorie diet (800 to 1500 kcal/d). Studies using combined PA and D strategies usually also employed behavioral modification strategies, including counseling, education, cognitive-behavioral therapy, or social support, as an intervention component.
Statistical analysis and quality assessment
We assessed study quality by examining potential selection, attrition, and detection bias.[19] We did not exclude any study that was considered poor quality (e.g., studies with attrition ≥30%). However, we conducted a sensitivity analysis to compare pooled effects between studies with potentially significant bias and those without. For example, for those studies with attrition ≥30%, their data were not used in our primary meta-analyses, but were used in our sensitivity analyses.
Among studies with similar intervention and comparison groups reporting a similar outcome of interest, we conducted meta-analyses to determine pooled effects. We calculated the mean difference between baseline and follow-up measures for the intervention (I) and comparison (C) groups (delta I and delta C) and the standard error of each difference. We used three strategies to estimate pooled effects: (1) stratified by baseline glucose levels (low range vs. high range); (2) stratified by the length of follow-up (12months vs. 13–23 months vs. ≥24 months); and (3) stratified by type of interventions (PA vs. D vs. PA+D).
We used DerSimonian and Laird random-effects models[20] to determine pooled effects. Effect size was defined by the mean difference between delta I and delta C divided by the standard deviation of the mean. We used meta-regression to determine whether various study-level characteristics (mean age, follow-up interval, duration of the intervention, number of intervention contacts, attrition, and year of publication) affected the between-group differences in SBP, DBP, TC, LDL-C, HDL-C, and TG, and we examined interaction terms for all models. We also used meta-regression analyses to examine the relationship between interventional effects on CVD risk reduction and interventional effects on diabetes risk reduction measured by the effect sizes of glucose improvement and weight loss. The meta-regression was conducted using SPSS (version 20.0, Armonk, NY: IBM Corp.). We used the chi-squared test to examine heterogeneity, and we used Cochrane Review Manager software (version 5.1; Copenhagen, Denmark) to calculate pooled effects.
If a comparison group in a study used a similar approach as the intervention group did, but only differed in dose, intensity, or frequency (e.g., diet plan A vs. diet plan B; or swimming vs. walking), we analyzed the effects of treatment in a single arm model to determine within-group changes (between post-intervention and pre-intervention in one arm) for both intervention and comparison group. These effects were also estimated by using the DerSimonian and Laird random-effect model. We did not, however, conduct any sensitivity analysis for these studies. Because this paper focused on the net lifestyle intervention effect (any lifestyle intervention vs. no intervention [e.g., usual care (UC)]), pooled effects from our single arm model are not reported in our results section, but are presented as an online supplementary table (Table C in S1 File).
Results
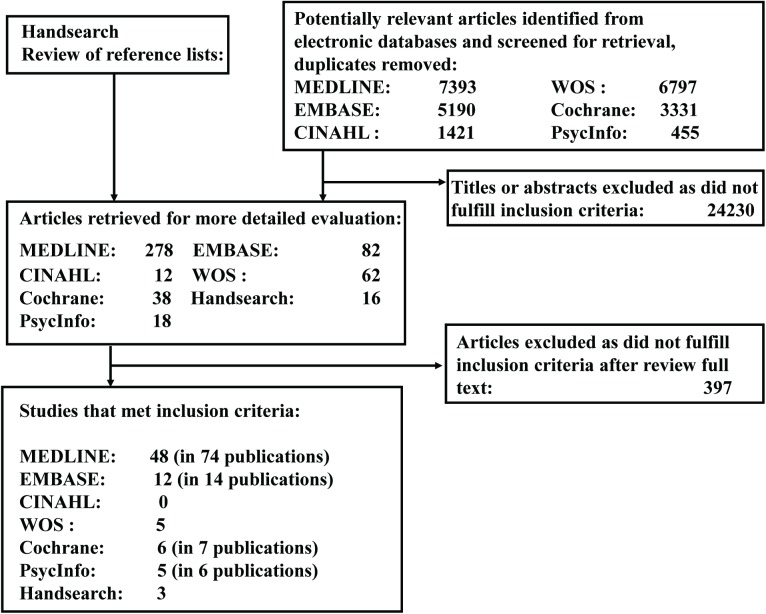
Seventy-nine studies[10,11,13,14, 21–95] and 30 companion publications[9,96–124] encompassing 15618 participants (Table 1: range, 20 to 1089) fulfilled the inclusion criteria (Fig 1). Follow-up time ranged from 12 to 54 months. The mean age of the participants was 50.6 years (range, 30.2 to 70.4 year), and mean BMI was 30.5 kg/m2 (range, 23.3 to 38.7 kg/m2). Mean baseline SBP, DBP, TC, LDL-C, HDL-C, and TG were 127.5 mmHg, 79.2 mmHg, 5.4 mmol/L, 3.3 mmol/L. 1.3 mmol/L, and 1.5 mmol/L, respectively. More studies took place in community settings than in clinics (58 vs. 21). Sampling methods varied, but most participants were recruited through screening programs. Attrition ranged from 0% to 60%, and in 16 studies,[21,34–36,45,60,62,66,69,74,76,78,81,82,86,94] attrition was 30% or more; longer follow-up resulted in higher attrition. Thirty-nine studies with mean FPG <5.5mmol/L or mean A1C <5.5% were classified as low range group, and 40 studies with mean FPG ≥5.5mmol/L or mean A1C ≥5.5% were classified as high range group.
Table 1. Characteristics of study participants.
| Citation | Sample size | Length of follow-up (month) |
Age at BL (years) [mean (SD)] |
Sex (% female) |
Setting; Race/ethnicity |
BMI at BL (kg/m2) [mean (SD)] |
SBP/DBP at BL (mmHg) [mean (SD)] |
TC at BL (mmol/L) [mean (SD)] |
LDL/HDL at BL (mmol/L) [mean (SD)] |
TG at BL (mmol/L) [mean (SD)] |
Inclusion criteria | Sampling method | Attrition (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ackermann et al. 2008 | 92 | 12 | 58.3 (10.1) | 55.4 | Community Indianapolis IN 81.5% white, 12.0% black |
31.4 (4.9) | 132.5 (16.6)/ 81.5 (9.1) |
4.9 (1.0) | NR/ 1.2(0.4) |
NR | People with ADA risk score≥10 and casual capillary blood glucose (CCBG) of 110–199 mg/dl | Recruited from YMCA by a community-based screening | 32.6 |
| Almeida et al. 2011 | 53 | 12 | Range: 20–29: 12% 30–39: 26% ≥40: 52% |
18.9 | Clinic Sao Paulo Brazil |
23.3 (2.7) | 111.1 (11.6)/ 75.2 (7.3) |
4.8 (1.0) | 2.8 (0.8)/ 1.2 (0.3) |
1.5 (0.8) | Aged: 20-59yrs; without hyperlipidemia, hypertrygliceridemia, hyperglycemia, obesity, cancer, anabolic, or corticosteroid drugs use, or pregnancy | Recruited from a reference HIV clinic | 20.8 |
| Anderson et al. 2014 Craigie et al. 2011 |
329 | 12 | 63.6 (6.8) | 26.0 | Community Scotland UK 99.0% white |
30.7 (4.2) | 142.5 (17.8)/ 84 (10.0) |
5.1 (1.2) | 3.0 (1.1)/ 1.4 (0.4) |
1.7 (1.1) | Aged: 50-74yrs; BMI>25kg/m2; with polypectomy for adenoma, without pregnancy, DM | Recruited from a bowel screening program | 7.3 |
| Anderssen et al. 1996 & 1998 Jacobs et al. 2009 The ODES Investigators 1993 Torjesen et al. 1997 |
219 | 12 | 44.9 (2.5) | 9.6 | Community Oslo Norway |
28.8 (3.4) | 131.5 (12.4)/ 90.1 (8.1) |
6.3 (0.8) | NR/ 1.0 (0.2) |
2.3 (1.1) | BMI>24 kg/m2 DBP: 86–99 mmHg TC: 5.20–7.74 mmol/L HDL-C<1.2 mmol/L TG>1.4 mmol/L |
Recruited from a continuously ongoing screening program in Oslo | 4.6 |
| Arguin et al. 2012 | 25 | 12 | 60.5 (6.0) | 100.0 | Community Sherbrooke Quebec Canada |
Weight (SD) 79.6 (10.7) |
NR/ NR |
5.8 (0.7) | 3.5 (0.6)/ 1.5 (0.3) |
1.8 (0.9) | Sedentary obese postmenopausal women without: (1) abnormal fasting lipid profile (2) CVD (3) DM |
Using a computer-generated randomization list | 12.0 |
| Bazzano et al. 2014 | 148 | 12 | 46.8 (10.1) | 88.5 | Community New Orleans LA 45.3% white 51.4% black 2% Hispanic |
35.4 (4.2) | 122.6 (13.3)/ 78.4 (8.7) |
5.2 (1.1) | 3.2 (1.0)/ 1.4 (0.3) |
1.3 (0.8) | Obese people (BMI: 30–45 kg/m2) without DM and CVD | Recruited from community screenings and TV ads | 17.8 |
| Bo et al. 2007&2009 | 375 | 48 | 55.7 (5.7) | 58.2 | Community Asti Italy |
29.7 (4.4) | 142.1 (14.7)/ 88.0 (9.2) |
5.9 (1.1) | NR/ 1.4 (0.3) |
1.9 (0.9) | People with MetS defined by FPG>110 mg/dL, without DM and CVD | Recruited from a metabolic screening | 10.7 |
| Bouchonville et al. 2014 Villareal et al. 2011 |
107 | 12 | 69.7 (4.0) | 62.6 | Community St. Louis MO |
37.2 (5.0) | 134.7 (18.8)/ 73.0 (10.1) |
NR | NR/ 1.4 (0.4) |
1.6 (0.7) | Old (≥65yrs) and obese (≥30 kg/m2) people without DM | Recruited from ads | 13.0 |
| Brinkworth et al. 2004 | 58 | 12 | 50.2 (NR) | 77.6 | Community Adelaide Australia |
34.0 (NR) | 132.0 (13.9)/ 75.1 (10.7) |
5.6 (0.9) | 3.8 (0.9)/ 1.0 (0.3) |
1.9 (0.7) | Obese, hyperinsulinemic persons aged between 20 and 65yrs, insulin > 12 mu/l without DM | NR | 25.9 |
| Broekhuizen et al. 2012 | 340 | 12 | 45.3 (12.9) | 56.7 | Community Amsterdam The Netherland |
26.5 (5.0) | 124.5 (15.0)/ NR |
5.2 (1.3) | 3.6 (1.3)/ 1.2 (0.4) |
1.2 (0.6) | Aged: 18-70yrs, with familial hypercholesterolemia, a LDL-C level>75th percentile | Recruited from the national cascade screening program | 7.4 |
| Burke V, et al. 2007 & 2008 | 241 | 36 | 56.2 (7.3) | 55.6 | Community Perth Australia |
30.1 (2.7) | 126.5 (9.5)/ 76.5 (7.5) |
5.1 (0.9) | NR/ 1.3 (0.3) |
1.3 (0.7) | Overweight, age>40yrs persons using 1 or 2 drugs to treat HT >3 Months without DM, chronic renal failure, CVD | Recruited by media advertising | 16.2 |
| Burtscher et al. 2009&2012 | 36 | 12 | 57.5 (6.9) | 55.6 | Clinic Innsbruck Austria |
29.0 (3.9) | 191 0 (25.9)/ 91.6 (11.0) |
5.8 (1.0) | NR/ 1.4 (0.4) |
NR | Patients with IFG (FPG:100–125 mg/dl), aged: 40-65yrs; BMI>25 kg/m2, and without DM | Recruited from family physicians through screening | 0.0 |
| Chirinos 2016 | 120 | 12 | 51.7 (8.4) | 55.8 | Clinics Coral Gables FL 84.0% Hispanic 10.9% black |
NR | 125.2 (16.8)/ 79.3 (9.5) |
NR | NR/ 1.0 (0.2) |
2.4 (1.1) | Aged: 30-70yrs, obese adults with WC≥102 cm for males, 88 cm for females, TG≥ 150 mg/dl, HDL-C< 40 mg/dl for males, <50mg/dl for females, IFG≥100 mg/dl. | Recruited from low-income community clinics | 22.5 |
| Choo et al. 2014 | 110 | 12 | 43.1 (9.0) | 100.0 | Community Seoul South Korea |
28.5 (3.8) | 116.5 (13.1)/ NR |
5.5 (1.0) | 3.3 (0.9)/ 1.4 (0.3) |
1.5 (0.9) | Age: 18-65yrs; elevated waist circumference (≥85cm), abdominal obesity without DM and CVD | Recruited via poster, leaflet, telephone, and ads | 55.5 |
| Clifton et al. 2008 | 119 | 12 | 49.0 (9.0) | 100.0 | Community Adelaide Australia |
32.8 (3.5) | NR/ NR |
5.8 (1.1) | 3.9 (0.9)/ 1.3 (0.3) |
1.4 (0.6) | Women, aged: 20-65yrs, BMI:27-40kg/m2, without DM, or renal or liver disease | Recruited from public ads and screened | 33.6 |
| Cole et al. 2013 | 94 | 12 | 58.3 (9.6) | 46.0 | Community San Antonio TX 64.0% white, 17.0% black, 19.0% Hispanic |
30.8 (4.9) | 143.0 (17.0)/ 83.0 (10.0) |
5.0 (1.0) | 2.9 (0.9)/ 1.4 (0.4) |
1.8 (1.4) | Aged:18+yrs; without DM, but with pre-DM, by ADA defined IFG (100–125 mg/dL) | Recruited from a pre-DM education class | 31.0 |
| Coon et al. 1989 | 20 | 12 | 59.5 (7.5) | 0.0 | Community Baltimore MD |
29.0 (3.0) | NR/ NR |
4.6 (0.7) | 3.1 (0.7)/ 0.8 (0.2) |
1.5 (0.4) | Aged 45+yrs, healthy persons without DM | Recruited by ads | 0.0 |
| Cox et al 2006 & 2008 & 2010 | 116 | 12 | 55.5 (4.7) | 100.0 | Community Berth Western Australia |
26.4 (3.3) | NR/ NR |
5.2 (0.7) | 3.2 (0.7)/ 1.5 (0.3) |
1.1 (0.5) | Aged: 50-70yrs; BMI<34 kg/m2; non-smoker, with sedentary lifestyle, without DM | Recruited by ads. | 25.9 |
| Ditschuneit et al. 1999 & 2001 | 100 | 24 | 45.7 (10.6) | 79.0 | Clinics Ulm Germany |
33.4 (3.6) | 139 5 (14.5)/ 82.5 (6.0) |
5.9 (1.0) | NR/ 1.3 (0.4) |
2.2 (1.3) | Age>18yrs, BMI between 25 and 40 kg/m2 without endocrine disorders | Recruited by referring to the obesity clinics | 27.0 |
| Donnelly et al. 2000 | 22 | 18 | 51.5 (8.5) | 100.0 | Community Kearney NE |
31.2 (4.0) | 133.0 (16.1)/ 80.5 (9.2) |
4.9 (1.1) | NR/ 1.1 (0.3) |
NR | BMI>25 kg/m2, low aerobic capacity, at risk for continued weight gain | NR | 0.0 |
| Esposito et al. 2003 | 120 | 24 | 34.6 (5.0) | 100.0 | Clinic Naples Italy |
34.9 (2.4) | 123.5 (8.2)/ 85.0 (4.8) |
5.1 (0.6) | NR/ 1.2 (0.3) |
1.6 (0.6) | Obese premenopausal women, aged: 20-46yrs; without DM, IGT (140–200 mg/dl), CAD, pregnancy. OGTT confirmed | Recruited from an outpatient dept. | 6.7 |
| Esposito et al. 2004a | 110 | 24 | 43.3 (5.0) | 0.0 | Clinic Naples Italy |
36.7 (2.4) | 127.5 (7.6)/ 85.5 (3.9) |
5.5 (0.8) | NR/ 1.0 (0.3) |
1.9 (0.6) | Obese men with erectile dysfunction, aged:35-55yrs; without DM and IGT, OGTT confirmed | Recruited from an outpatient department list | 5.5 |
| Esposito et al. 2004b (JAMA v.292) & 2009 | 180 | 24 | 43.9 (6.2) | 45.0 | Clinic Naples Italy |
28.0 (3.3) | 135.0 (9.5)/ 85.5 (6.5) |
5.1 (0.9) | NR/ 1.1 (0.2) |
1.9 (0.6) | Sedentary people with MetS, FPG≥110 mg/dL, | Recruited from a screening program | 8.9 |
| Fatouros et al. 2005 | 50 | 12 | 70.4 (3.8) | 0.0 | Community Alexandroupolis Greece |
29.5 (3.3) | NR/ NR |
NR | NR/ NR |
NR | Inactive old men, nonsmoker, without DM, FPG≤7 mmol/L | Recruited from a volunteer database in local community | 0.0 |
| Fernandez et al. 2012 | 40 | 12 | 40.9 (13.5) | 67.5 | Community Leon Spain |
31.8 (2.4) | 124.8 (17.6)/ 78.5 (12.6) |
5.2 (0.9) | 3.1 (0.7)/ 1.4 (0.5) |
1.7 (1.0) | Aged: 18-70yrs; BMI: 28–35 kg/m2; without DM and pregnancy | Recruited from a clinic trial | 60.0 |
| Ferrara et al. 2012 | 188 | 24 | 56.4 (9.5) | 47.9 | Clinic Naples Italy |
29.2 (4.5) | 134.1 (16.0)/ 84.4 (10.6) |
5.1 (0.9) | 3.2 (0.9)/ 1.3 (0.3) |
1.5 (1.0) | People with HT | Recruited from an outpatient clinic | 0.0 |
| Fischer et al. 2016 | 163 | 12 | 46.4 (11.5) | 75.8 | Clinics Denver CO |
NR | 118.8 (14.1)/ NR |
NR | NR/ NR |
NR | Patients aged 18+yrs, with A1C: 5.7–6.4%; BMI: 25–50 kg/m2; without DM | Recruited from health centers | 5.7 |
| Fisher et al. 2012 | 97 | 12 | Range: 21–46 |
100.0 | Community Birmingham AL 53.6% black; 46.4% white |
28.0 (1.0) | NR/ NR |
NR | NR/ NR |
NR | Aged: 21-46yrs; BMI: 27–30 kg/m2; non-smoker, with sedentary lifestyle premenopausal women | Recruited from a previous parent study | 0.0 |
| Fogelholm et al. 2000 | 82 | 24 | Range: 30–45 |
100.0 | Community Tampere Finland |
34.0 (3.6) | 119.0 (10.0)/ 78.0 (7.0) |
5.0 (0.9) |
NR/ 1.2 (0.2) |
1.3 (0.5) | Aged: 30-45yrs, BMI: 30–45 kg/m2, physical inactive | Recruited by ads | 9.8 |
| Fonolla et al. 2009 | 297 | 12 | 46.0 (8.4) | 15.5 | Community Granada Spain |
28.8 (5.0) | 122.1 (15.2)/ 79.5 (9.0) |
5.6 (1.0) | 3.7 (1.0)/ 1.1 (0.3) |
1.6 (1.2) | People with moderate risk of CVD, without DM and pregnancy | Recruited from a screening program | 14.8 |
| Frank et al. 2005 | 173 | 12 | 60.7 (6.7) | 100.0 | Community Seattle Washington |
30.4 (3.9) | NR/ NR |
NR | NR/ NR |
1.4 (0.6) | Postmenopausal women, aged: 50-75yrs, sedentary at baseline BMI≥25 kg/m2 without DM, nonsmoker | Recruited through a combination of mailings and media placements | 1.7 |
| Groeneveld et al. 2008 & 2010 | 816 | 12 | 46.6 (9.0) | 0.0 | Community Amsterdam The Netherlands |
28.5 (3.5) | 142.9 (15.3)/ 88.8 (9.6) |
NR | NR/ 1.1 (0.2) |
NR | Male construction workers with elevated risk of CVD | Recruited from Periodical Health Screening | 27.6 |
| Heshka et al. 2003 | 423 | 24 | 44.5 (10.0) | 84.6 | Clinics NY, Medison, Baton Rouge, Boulder, Davis, Durham, Woodbury |
33.7 (3.6) | 122.0 (13.0)/ 79.0 (8.5) |
5.5 (1.0) | NR/ 1.3 (0.3) |
1.7 (1.0) | Aged: 18-65yrs; BMI: 27–40 kg/m2; with FPG<7.8 mmol/L, | Recruited from existing clinic records, or by ads | 27.0 |
| Imayama et al. 2013 Foster-Schubert et al. 2012 Mason et al. 2011&2013 |
439 | 12 | 58.0 (5.0) | 100.0 | Community Seattle WA 85.0% white |
30.9 (4.1) | NR/ NR |
NR | NR/ NR |
NR | Aged: 50-75yrs; BMI: ≥25 kg/m2; <100 min/w PA; postmenopausal; without DM; FPG<126 mg/dL | Recruited from mass mailing ads | 9.1 |
| Juul et al. 2016 | 127 | 12 | NR | 68.6 | Community Holstebro Denmark |
NR | 133.0 (14.1)/ 82.5 (8.5) |
5.3 (1.1) | 3.2 (0.9)/ 1.3 (0.3) |
NR | Aged<70yrs, FPG: 6.1–6.9 mmol/l; A1C: 6.0-<6.5% | Recruited from a referral | 15.0 |
| Kanaya et al. 2012 Delgadillo et al. 2010 |
238 | 12 | 56.5 (16.5) | 73.5 | Community Berkeley, Oakland, etc CA 22.5% white, 23.0% black, 37.0% Hispanic |
30.0 (5.7) | 127.2 (20.0)/ NR |
NR | 3.0 (1.1)/ 1.4 (0.4) |
1.6 (1.2) | Aged: 25+yrs; a capillary blood glucose:106–160 mg/dL, without DM | Recruited from a community-based education outreach | 12.2 |
| Kanaya et al. 2014 | 180 | 12 | 55.0 (7.0) | 72.0 | Clinics San Francisco, San Diego CA 65% white |
34.3 (6.7) | 124.0 (14.0)/ 72.5 (9.0) |
5.3 (1.0) | 3.2 (0.9)/ 1.3 (0.3) |
1.8 (0.8) | Aged: 21-65yrs; with MetS (FPG:100–125 mg/dL), HT, and underactive lifestyle (<150min/w of moderate intensity activity), without DM | Recruited by ads and flyers in community and clinical settings | 21.1 |
| Katula et al. 2010&2011&2013 | 301 | 24 | 57.9 (9.5) | 57.5 | Community Winston-Salem NC 73.8%white, 24.6%black |
32.7 (4.0) | NR/ NR |
NR | NR/ NR |
NR | Patients with pre-DM defined by FPG of 95–125 mg/dl and BMI of 25–39 kg/m2 and without DM and CVD | Recruited from mass mailing, community health fair or referrals | 12.6 |
| Kawano et al. 2009 | 217 | 17 | 60.9 (13.8) | 66.5 | Community Saga City Japan |
23.7 (4.4) | 127.5 (17.8)/ 72.3 (8.9) |
5.3 (0.9) | 3.1 (0.7)/ 1.5 (0.4) |
1.4 (0.8) | People with FPG: 100–140 mg/dL, or A1C: 5.5–6.0% | Recruited from health checkup | 27.2 |
| Keogh et al. 2007 | 36 | 12 | 48.6 (5.2) | 68.0 | Community Adelaide Australia |
32.9 (4.5) | 122.0 (10.8)/ 75.0 (3.6) |
5.5 (1.4) | 3.6 (1.4)/ 1.3 (0.4) |
1.6 (0.6) | Overweight or obese people, aged: 20-65yrs; BMI: 27–40 kg/m2; without DM, with FPG≤7.0mmol/L. | Recruited from newspaper ads | 30.6 |
| Lawton et al. 2009 | 1089 | 24 | 58.9 (6.9) | 100.0 | Clinics Wellington New Zealand |
29.2 (6.0) | 123.1 (17.5)/ 74.3 (9.3) |
6.1 (1.2) | NR/ 1.6 (0.5) |
NR | Physically inactive women, aged: 40-74yrs without medical condition | Recruited by invitation letters or practice register | 7.4 |
| Lim et al. 2010 | 113 | 12 | 47.0 (10.0) | 82.3 | Community Adelaide Australia |
32.0 (6.0) | 127.0 (12.6)/ 76.3 (10.2) |
5.6 (1.0) | 2.9 (1.7)/ 1.3 (0.3) |
1.6 (0.8) | Aged: 20-65yrs, BMI: 28–40 kg/m2, with at least one CVD risk factor, without DM | Recruited by ads | 38.9 |
| Lombard et al. 2010 | 250 | 12 | 40.4 (4.8) | 100.0 | Community Melbourne Australia |
27.8 (5.4) | NR/ NR |
4.9 (0.9) | 2.6 (0.8)/ 1.7 (0.4) |
1.0 (0.7) | Women with a child in schools without pregnancy and serious medical conditions | Recruited through an invitation attached to school newsletter | 14.0 |
| Ma et al. 2009&2013 | 241 | 15 | 52.9 (10.6) | 47.0 | Clinic San Francisco CA 78% white, 17% Asian |
32.0 (5.4) | 118.8 (11.7)/ 73.6 (8.3) |
4.9 (0.9) | 2.8 (0.8)/ 1.2 (0.3) |
1.9 (0.8) | Patients aged≥18yrs, BMI≥25 kg/m2, with pre-DM defined by FPG of 100–125 mg/dl, or MetS | Recruited from a single primary care clinic | 8.3 |
| Marrero et al. 2016 | 225 | 12 | 52.0 (11.0) | 84.4 | Community Indianapolis IN 64.5% white 25.3% black |
36.8 (7.2) | 130.2 (14.0)/ 81.4 (8.5) |
4.9 (0.9) | NR/ 1.2 (0.4) |
NR | Aged 18+yrs, BMI>24 kg/m2 (>/ = 23 kg/m2 for Asian); ADA risk score≥5; A1C: 5.7–6.5% | Recruited from a screening | 22.2 |
| Marsh et al. 2010 | 96 | 12 | 30.2 (5.2) | 100.0 | Clinic Sydney Australia |
34.5 (4.2) | NR/ NR |
4.8 (0.7) | 2.8 (0.7)/ 1.4 (0.7) |
1.3 (0.7) | Women, aged: 18-40yrs; BMI<25 kg/m2, with polycystic ovary syndrome, without pregnancy and DM | Recruited from a screening program | 49.0 |
| Mason et al. 2016 | 194 | 12 | 47.0 (12.7) | 78.0 | Community San Francisco CA 58.8% white 12.9% black 11.9% Hispanic |
35.5 (3.6) | NR/ NR |
NR | NR/ NR |
NR | Obese adults aged 18+yrs, with BMI: 30–45.9 kg/m2; WC>102 cm for males, >88 cm for females, without DM, confirmed by FPG<126 mg/dl | Recruited from community by newspaper ads. | 23.2 |
| McAuley et al. 2005&2006 | 93 | 12 | Range: 30–70 |
100.0 | Community Dunedin New Zealand |
35.7 (5.0) | 126.8 (13.0)/ 81.9 (10.0) |
5.8 (1.0) | 3.8 (0.8)/ 1.2 (0.3) |
1.9 (0.7) | Overweight women, aged: 30-70yrs; BMI:>27 kg/m2; without pregnancy | Recruited by local ads | 18.3 |
| Mellberg et al. 2014 | 70 | 24 | 59.9 (5.7) | 100.0 | Community Umea Sweden |
32.7 (3.5) | 139.5 (13.0)/ 83.0 (8.3) |
5.7 (1.1) | 3.8 (1.0)/ 1.4 (0.4) |
1.2 (0.6) | Postmenopausal non-smoking women, BMI≥27 kg/m2, without DM, FPG<7 mmol/L | Recruited by newspapers ads | 30.0 |
| Muto et al. 2001 | 326 | 18 | 42.5 (3.7) | 0.0 | Community Tokyo Japan |
24.7 (3.0) | 123.2 (15.6)/ 78.4 (12.1) |
5.5 (0.9) | NR/ 1.3 (0.4) |
2.3 (1.4) | Male workers with at least one abnormality, including FPG>100 mg/dL | Recruited from a building maintenance company | 7.4 |
| Narayan et al. 1998 | 95 | 12 | Range: 25–50 |
75.8 | Community Pima AZ |
Range: 20.2–59.9 |
Range: 90.0-176/ 48.0–98.0 |
Range: 2.1–6.1 |
NR/ NR |
Range: 0.3–3.6 |
Overweight/obese people, aged: 25-54yrs; BMI>25kg/m2, without DM, OGTT<7.8mmol/L | Recruited from an epidemiological study | 2.0 |
| Nilsson et al. 1992 | 94 | 12 | 55.0 (7.2) | NR | Community Dalby Sweden |
Weight (kg): 81.4 (11.6) |
145.0 (18.0)/ 84.3 (7.6) |
5.6 (0.8) | 3.9 (0.7)/ 0.9 (0.2) |
1.6 (0.7) | Patients with or without HT, but no DM | Recruited from a cross-sectional study | 8.5 |
| Nilsson et al 2001 | 113 | 18 | 49.7 (6.2) | 60.9 | Community Helsingborg Sweden |
27.8 (5.6) | 132.5 (18.0)/ 77.4 (9.7) |
5.8 (0.9) | 3.9 (0.9)/ 1.2 (0.3) |
1.3 (0.7) | Aged: 40-50yrs; with a cardiovascular risk score sum of ≥9 | Recruited from a screening program | 18.6 |
| Ockene et al. 2012 Merriam et al. 2009 |
312 | 12 | 52.0 (11.2) | 74.4 | Community Lawrence MA |
33.9 (5.6) | 128.7 (12.4)/ NR |
NR | NR/ 1.2 (0.3) |
NR | Age>25+yrs, BMI>24kg/m2, with risk for DM, but without DM | Recruited from the Greater Lawrence Family Health Center | 7.4 |
| Poston et al. 2006 | 250 | 12 | 41.0 (8.5) | 92.4 | Community Huston TX |
36.1 (3.1) | 121.5 (12.0)/ 72.3 (8.6) |
5.2 (1.0) | 3.1 (0.8)/ 1.4 (0.3) |
1.5 (0.8) | Overweight/obese people, aged: 25-55yrs; BMI: 27–40 kg/m2; without DM or pregnancy, FPG<7mmol/L, confirmed by OGTT | Recruited from a screening program | 45.6 |
| Potteiger et al. 2003 & 2002 | 66 | 16 | NR | 57.6 | Community Denver CO |
Range: 25–34.9 |
NR/ NR |
NR | NR/ NR |
NR | Sedentary people without DM and heart disease | Recruited from the Midwest Exercise Trial | 10.1 |
| Reid et al. 2014 | 426 | 12 | 51.5 (11.6) | 61.3 | Clinic Ottawa Canada 95.3% white |
29.4 (5.7) | 121.1 (16.1)/ 76.5 (9.5) |
5.2 (1.0) | 3.3 (0.9)/ 1.3 (0.4) |
1.3 (0.8) | Obese people with coronary risk, without DM, pregnancy, FPG<7 mmol/L | Recruited from a care cardiac center by ads and flyers | 25.8 |
| Rossner et al. 1997 | 93 | 12 | 41.0 (NR) | 67.7 | Clinics Stockhlom Sweden |
38.7 (4.5) | 136.3 (16.9)/ 86.5 (12.2) |
5.7 (0.9) | NR/ NR |
1.9 (1.0) | Obese people with BMI> 30 kg/m2, without DM | Recruited from hospital waiting list | 38.7 |
| Ryttig et al. 1997 | 81 | 28 | 42.5 (10) | 54.3 | Clinics Stockhlom Sweden |
37.7 (4.6) | 136.2 (17.3)/ 85.3 (9.9) |
5.7 (1.0) | NR/ 1.1 (0.2) |
2.0 (1.2) | Obese people, aged: 21-64yrs; BMI:>30 kg/m2; without DM and pregnancy | Recruited from hospital waiting list | 4.9 |
| Sartorelli et al. 2005 | 104 | 12 | 45.5 (9.1) | 79.8 | Community Sao Paulo Brazil |
28.7 (2.5) | 116.6 (17.6)/ 77.5 (18.3) |
5.3 (1.2) | 3.6 (1.1)/ 1.2 (0.4) |
1.6 (0.9) | Overweight or obese people, aged: 30-65yrs; BMI: 24–35 kg/m2; without DM, or pregnancy | Recruited from a screening of high-risk group for DM | 31.7 |
| Sattin et al. 2016 | 604 | 12 | 46.5 (10.9) | 83.0 | Community Augusta GA |
35.7 (7.3) | 130.5 (16.6)/ 82.6 (9.7) |
NR | NR/ NR |
NR | African Americans aged: 20-64yrs; BMI≥25 kg/m2; without DM, confirmed by FPG<126 mg/dl | Recruited from church | 0.0 |
| Simkin-Silverman et al. 1995 & 1998 & 2003 Kuller et al. 2001 & 2006&2012 |
535 | 54 | 47.0 (1.0) | 100.0 | Community Allegheny PN 92.0% white |
25.1 (3.3) | 110.0 (12.8)/ 68.0 (8.2) |
4.9 (0.6) | 3.0 (0.6)/ 1.5 (0.3) |
0.9 (0.5) | Premenopausal women, aged: 44-50yrs; BMI: 20–34 kg/m2; FPG<7.8mmol/L | Recruited from the Women's Healthy Lifestyle Project | 2.8 |
| Siu et al. 2015 | 182 | 12 | 56.0 (9.1) | 74.2 | Community Hong Kong China |
NR | 133.8 (16.8)/ 82.4 (9.8) |
NR | NR/ 1.2 (0.3) |
2.2 (1.8) | Aged: 18-94yrs; with MetS by 1) WC: 90 cm for males, 80 cm for females; 2) SBP>130 mmHg, DBP>85 mmHg; 3) FPG>/ = 5.5 mmol/l; 4) TG>1.7 mmol/l; 5) HDL-C<40 mmol/l for males, 50 mmol/l for females | Recruited from a screening | 35.7 |
| Staten et al. 2004 | 361 | 12 | 57.2 (4.8) | 100.0 | Community Tucson AZ 100% Hispanics |
29.5 (5.3) | 124.8 (16.7)/ 74.1 (9.6) |
5.6 (1.3) | NR/ NR |
NR | Uninsured Hispanic women, aged≥50yrs, | Recruited from clinic registration | 33.4 |
| Stefanick et al. 1998 | 377 | 12 | 52.1 (7.3) | 47.7 | Community Palo Alto CA |
26.7 (3.0) | 115.5 (12.8)/ 73.2 (7.4) |
6.2 (0.6) | 4.2 (0.5)/ 1.2 (0.2) |
1.8 (0.8) | Postmenopausal women, aged: 45-64yrs; men aged:30-64yrs; without DM, FPG<7.8mmol/L, OGTT confirmed | Recruited from the Diet and Exercise for Elevated Risk Trial | 27.0 |
| Tapsell et al. 2014 | 120 | 12 | 48.9 (9.3) | 75.0 | Community Wollongong Australia |
30.0 (2.7) | NR/ NR |
5.2 (0.9) | 3.2 (0.8)/ NR |
NR | Healthy adults aged 18-65yrs, BMI: 25–35 kg/m2, without DM | Recruited by ads in the local media | 22.5 |
| ter Bogt et al. 2009 | 457 | 12 | 56.1 (7.8) | 57.9 | Community Bilthoven The Netherlands |
29.6 (3.4) | 145.5 (17.0)/ 86.5 (8.9) |
5.6 (1.0) | 3.5 (0.9)/ 1.4 (0.4) |
NR | Overweight or obese people, aged: 40-70yrs; BMI: 25–40 kg/m2; with HT or dyslipidemia, without DM | Recruited from a screening program | 9.0 |
| Thompson et al. 2005 | 90 | 12 | 41.4 (8.9) | 85.6 | Clinic Knoxville TN |
34.8 (3.1) | NR/ NR |
5.0 (0.9) | 3.1 (0.9)/ 1.1 (0.3) |
1.8 (1.2) | Obese people, aged: 25-70yrs; BMI: 30–40 kg/m2; without DM or pregnancy | Recruited from ad posters | 13.3 |
| Tsai et al. 2010 | 50 | 12 | 49.4 (11.9) | 88.0 | Clinic Philadelphia PA 81% black; 19% white |
36.5 (6.0) | 129.4 (12.2)/ 80.7 (8.2) |
4.9 (0.9) | 3.0 (0.9)/ 1.4 (0.3) |
1.1 (0.7) | Overweight or obese people with BMI: 27–50 kg/m2, without serious psychiatric illness | Recruited from flyers, and referrals from PCPs | 6.0 |
| Vainionpaa et al. 2007 | 120 | 12 | Range: 35–40 |
100.0 | Community Oulu Finland |
25.3 (4.6) | NR/ NR |
5.3 (0.9) | 3.2 (0.8)/ 1.7 (0.4) |
1.0 (0.5) | Women with age: 35-40yrs, without chronic disease | Recruited from the National Population Register of Finland | 33.3 |
| Vetter et al. 2013 Wadden et al. 2011 |
390 | 24 | 51.5 (11.5) | 79.7 | Clinic Philadelphia PA 59% white, 38.5%black |
38.5 (4.7) | 121.4 (16.3)/ 76.2 (10.4) |
4.6 (1.0) | 2.9 (0.8)/ 1.1 (0.3) |
1.3 (0.7) | Aged: 21+yrs; BMI: 30–50 kg/m2; with MetS (FPG≥110mg/dL); without cardiovascular events | Recruited from primary care practices | 13.8 |
| von Thiele Schwarz et al. 2008 | 195 | 12 | 46.6 (10.8) | 100.0 | Community Stockholm Sweden |
NR | 114.0 (16.9)/ 79.1 (11.6) |
5.2 (1.0) | 2.9 (0.8)/ 1.8 (0.4) |
1.0 (0.6) | Working age women without DM and pregnancy | Recruited from a public dental health care organization | 9.2 |
| Watanabe et al. 2003 | 173 | 12 | 55.1 (7.1) | 0.0 | Community Tokyo Japan |
24.4 (2.9) | 121.7 (14.4)/ 76.9 (10.5) |
5.2 (0.9) | NR/ 1.4 (0.4) |
1.4 (0.8) | Male workers with risk for DM, aged:35-70yrs; OGTT confirmed | Recruited from annual check-up list | 9.8 |
| Weinstock et al. 1998 | 45 | 23 | 43.3 (7.4) | 100.0 | Community Syracuse NY |
35.9 (6.0) | NR/ NR |
NR | NR/ NR |
NR | Women without DM, CAD, and pregnancy | Recruited from a cohort study | 0.0 |
| Weiss et al. 2006 | 48 | 12 | 56.8 (3.0) | 63.2 | Community St. Louis MO |
27.3 (2.1) | NR/ NR |
NR | NR/ NR |
NR | Sedentary people, aged: 50-60yrs; BMI:23.5–29.9kg/m2; non-smoker without DM. FPG<7mmol/L, OGTT confirmed | Recruited from a screening program | 4.2 |
| Wing et al. 1995 | 202 | 18 | 37.4 (5.3) | 48.1 | Community Pittsburgh PA |
30.9 (2.1) | 111.7 (10.7)/ 71.8 (8.1) |
5.0 (0.8) | NR/ 1.2 (0.2) |
1.2 (0.7) | Aged: 25-45yrs; 13.6–31.8 kg above ideal body weight, without serious disease | Recruited from newspaper or radio ads | 21.3 |
| Wing et al. 1998 | 154 | 24 | 45.7 (4.4) | 79.0 | Community Pittsburgh PA |
35.9 (4.3) | 116.7 (14.9)/ 74.8 (10.1) |
5.0 (0.8) | 3.1 (0.8)/ 1.2 (0.3) |
NR | Overweight people, aged:40-55yrs; with diabetic parents | Recruited from newspaper ads | 22.0 |
| Wycherley et al. 2012 | 123 | 12 | 50.8 (9.3) | 0.0 | Clinic Adelaide Australia |
33.0 (3.9) | 135.1 (12.5)/ 84.0 (10.7) |
5.2 (0.9) | 3.2 (0.8)/ 1.3 (0.4) |
1.7 (0.7) | Overweight or obese males, aged: 20-65yrs; BMI: 27–40 kg/m2, without DM | Recruited by a screening program | 44.7 |
| Yeh et al. 2016 | 60 | 12 | 58.9 (10.9) | 56.7 | Community New York 100% Asian |
26.1 (2.4) | 126.9 (16.1)/ 78.4 (9.6) |
4.8 (1.0) | 2.8 (0.9)/ 1.4 (0.3) |
1.4 (0.7) | Patients with pre-DM defined by A1C: 5.7–6.4% and BMI>/ = 24kg/m2 | Recruited from hospital record | 3.3 |
| Mean (SD) | 50.6 (8.7) | 30.5 (4.6) | 127.5 (15.2)/ 79.2 (9.3) |
5.4 (1.0) | 3.3 (0.9)/ 1.3 (0.3) |
1.5 (0.9) | |||||||
| Total Range |
15618 20–1089 |
12–54 |
0–100 | 23.3–38.7 | 0–60.0 |
Abbreviations: BG: blood glucose; BL: baseline; BMI: body mass index; CAD: coronary Artery Disease; CVD: cardiovascular disease; DBP: diastolic blood pressure; DM: diabetes mellitus; FBG: fasting blood glucose; FPG: fasting plasma glucose; HDL-C: high density cholesterol; HT: hypertension; IGT: impaired glucose tolerance; LDL-C: low density cholesterol; MetS: metabolic syndrome; min/w: minutes/week; NR: not reported; OGTT: oral glucose tolerance test; PG: plasma glucose; SD: standard deviation; TC: total cholesterol; TG: triglycerides.
Fig 1. Study flow diagram.
CINAHL, Cumulative Index to Nursing and Allied Health Literature EMBASE, Excerpta Medica Database MEDLINE, Medical Literature Analysis and Retrieval System Online PsycInfo, Psychological Information Database WOS, Web of Science.
We observed considerable heterogeneity in the treatments provided to both intervention and comparison groups (Tables A&B in S1 File). In 29 studies, a similar approach was used in both intervention and control groups: data from these studies were synthesized by a single arm model, and are presented in Table C in S1 File as an online supplement. In the other 50 studies, UC was used in the control group. In the 50 studies that compared an intervention to UC, 38 had two arms, 5 studies[49,64,87,88,91] had 3 arms, and 7 studies[13,24,28,44,54,62,93] had 4 arms (e.g., PA, D, PA+D and control arm). The randomization procedure was described in 48 studies (Table B in S1 File). In 29 studies, allocation concealment was adequately reported. Meta-regression analyses indicated that there was no significant interaction between the between-group change in FPG and all study-level characteristics, such as mean age, publication date, the length of F/U, number of contacts, attrition, and their interaction terms. An Egger’s plot demonstrated a symmetrical shape distribution (except for two outliers) which is consistent with no publication bias.
Changes in CVD risk factors
In 57 studies or study arms comparing interventions to UC with attrition <30%, the pooled effect estimate from all studies demonstrated that compared to UC, all lifestyle interventions, including PA, D, or PA+D interventions, achieved significant improvements in SBP (-2.05mmHg [95%CI, -2.81, -1.28]), DBP (-1.65mmHg [-2.16, -1.14]), TC (-0.09mmol/L [-0.14, -0.04]), LDL-C (-0.08mmol/L [-0.13, -0.03]), HDL-C (0.03mmol/L [0.01, 0.04]), and TG (-0.08mmol/L [-0.14, -0.03]) (Table 2). When including the 15 studies with attrition ≥30% in the sensitivity analysis, we observed similar effects. The remaining results are limited to studies with attrition <30%.
Table 2. Lifestyle interventional effect: Meta-analyses results.
| SBP (mmHg) | DBP (mmHg) | TC (mmol/L) | LDL-C (mmol/L) | HDL-C (mmol/L) | TG (mmol/L) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies (sample size) |
Pooled effect mean (effect size) (95% CI) |
Hetero- Geneity p value |
Studies (sample size) |
Pooled effect mean (effect size) (95% CI) |
Hetero- Geneity p value |
Studies (sample size) |
Pooled effect mean (effect size) (95% CI) |
Hetero- Geneity p value |
Studies (sample size) |
Pooled effect mean (effect size) (95% CI) |
Hetero- Geneity p value |
Studies (sample size) |
Pooled effect mean (effect size) (95% CI) |
Hetero- Geneity p value |
Studies (sample size) |
Pooled effect mean (effect size) (95% CI) |
Hetero- Geneity p value |
|
| LI vs UC (all studies*) |
42 (8331) |
-2.05 (0.06) (-2.81, -1.28) |
<0.01 | 39 (7631) |
-1.65 (0.07) (-2.16, -1.14) |
<0.01 | 36 (6925) |
-0.09 (0.04) (-0.14, -0.04) |
<0.01 | 27 (4563) |
-0.08 (0.05) (-0.13, -0.03) |
<0.01 | 43 (8414) |
0.03 (0.03) (0.01, 0.04) |
<0.01 | 38 (5926) |
-0.08 (0.03) (-0.14, -0.03) |
<0.01 |
| LI vs UC (all studiesƚ) |
50 (9053) |
-2.13 (0.04) (-2.88, -1.38) |
<0.01 | 46 (8261) |
-1.57 (0.06) (-2.07, -1.07) |
<0.01 | 44 (7541) |
-0.11 (0.05) (-0.16, -0.06) |
<0.01 | 34 (5087) |
-0.09 (0.04) (-0.15, -0.04) |
<0.01 | 52 (9212) |
0.03 (0.03) (0.01, 0.04) |
<0.01 | 46 (6632) |
-0.08 (0.04) (-0.13, -0.03) |
<0.01 |
| LI vs UC (Group 1ⱡ) |
17 (3492) |
-0.95 (0.04) (-1.75, -0.15) |
0.02 | 15 (2949) |
-1.40 (0.06) (-2.24, -0.56) |
<0.01 | 16 (2904) |
-0.06 (0.03) (-0.13, 0.01) |
<0.01 | 15 (3065) |
-0.08 (0.05) (-0.14, -0.02) |
<0.01 | 19 (3770) |
0.01 (0.03) (0.00, 0.03) |
0.06 | 19 (3240) |
-0.04 (0.02) (-0.10, 0.02) |
0.19 |
| LI vs UC (Group 2§) |
25 (4839) |
-2.89 (0.08) (-3.95, -1.83) |
<0.01 | 24 (4682) |
-1.83 (0.08) (-2.50, -1.17) |
<0.01 | 20 (4021) |
-0.12 (0.06) (-0.18, -0.05) |
<0.01 | 12 (1498) |
-0.10 (0.06) (-0.18, -0.01) |
0.02 | 24 (4644) |
0.04 (0.06) (0.02, 0.06) |
<0.01 | 20 (2686) |
-0.12 (0.05) (-0.21, -0.04) |
<0.01 |
| LI vs UC (F/U = 12m) |
34 (6616) |
-2.07 (0.05) (-3.19, -0.95) |
<0.01 | 31 (5916) |
-1.62 (0.06) (-2.29, -0.95) |
<0.01 | 29 (5813) |
-0.06 (0.04) (-0.10, -0.01) |
<0.01 | 23 (3643) |
-0.08 (0.05) (-0.13, -0.02) |
<0.01 | 33 (6782) |
0.02 (0.05) (0.01, 0.03) |
<0.01 | 27 (3959) |
-0.08 (0.04) (-0.14, -0.03) |
<0.01 |
| LI vs UC (F/U = 13-23m) |
6 (1418) |
-1.73 (0.08) (-2.80, -0.65) |
0.98 | 6 (1436) |
-1.25 (0.08) (-2.02, -0.48) |
0.60 | 6 (974) |
-0.19 (0.17) (-0.26, -0.11) |
0.46 | 5 (1033) |
-0.12 (0.10) (-0.19, -0.05) |
0.36 | 7 (1494) |
0.00 (0.0) (-0.03, 0.03) |
0.37 | 7 (1494) |
-0.08 (0.03) (-0.21, 0.05) |
<0.01 |
| LI vs UC (F/U≥24m) |
14 (3123) |
-1.58 (0.05) (-2.71, -0.45) |
<0.01 | 14 (3122) |
-1.36 (0.05) (-2.30, -0.41) |
<0.01 | 13 (2788) |
-0.07 (0.03) (-0.17, 0.03) |
<0.01 | 5 (543) |
0.06 (0.04) (-0.07, 0.20) |
0.39 | 14 (3122) |
0.05 (0.06) (0.02, 0.08) |
<0.01 | 13 (2034) |
-0.08 (0.03) (-0.19, 0.03) |
<0.01 |
| PA vs UC | 7 (1466) |
-0.72 (0.03) (-1.89, 0.44) |
0.22 | 7 (1465) |
-1.12 (0.05) (-2.34, 0.10) |
0.22 | 6 (1429) |
-0.02 (0.01) (-0.09, 0.06) |
0.76 | 3 (256) |
-0.03 (0.02) (-0.18, 0.12) |
0.91 | 7 (1463) |
0.01 (0.02) (-0.02, 0.04) |
0.10 | 6 (375) |
-0.10 (0.08) (-0.22, 0.02) |
0.48 |
| D vs UC | 4 (263) |
-1.45 (0.07) (-3.83, 0.94) |
0.23 | 4 (263) |
-2.28 (0.16) (-4.07, -0.49) |
0.74 | 3 (228) |
-0.17 (0.13) (-0.34, -0.01) |
0.89 | 3 (228) |
-0.14 (0.11) (-0.30, 0.02) |
0.99 | 4 (263) |
0.00 (0.00) (-0.04, 0.04) |
0.78 | 4 (263) |
-0.15 (0.07) (-0.41, 0.10) |
0.14 |
| PA+D vs UC | 31 (6602) |
-2.29 (0.06) (-3.19, -1.40) |
<0.01 | 28 (5903) |
-1.66 (0.07) (-2.24, -1.09) |
<0.01 | 27 (5268) |
-0.10 (0.05) (-0.16, -0.05) |
<0.01 | 21 (4079) |
-0.08 (0.04) (-0.14, -0.02) |
<0.01 | 32 (6688) |
0.03 (0.07) (0.02, 0.05) |
<0.01 | 29 (5288) |
-0.07 (0.03) (-0.13, -0.01) |
0.02 |
Abbreviations: D: dietary; DBP: diastolic blood pressure; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; LI: lifestyle intervention; m: month; NA: not applicable; PA: physical activity; SBP: systolic blood pressure; TC: total cholesterol; TG: triglycerides; UC: usual care; vs: versus
* All studies with attrition <30%.
ƚ All studies with attrition <30% plus studies with attrition ≥30%.
ǂ All studies with attrition <30% and participants with FPG<5.5 mmol/L or A1C <5.5%.
§ All studies with attrition <30% and participants with FPG≥5.5 mmol/L or A1C≥5.5%.
Comparison according to participant baseline glycemic level
In the 39 studies among persons with low range glycemic level, lifestyle interventions were associated with significantly improved SBP (-0.95mmHg [-1.75, -0.15]), DBP (-1.40mmHg [-2.24, -0.56]), LDL-C (-0.08mmol/L [-0.14, -0.02]), and HDL-C (0.01mmol/L [0.00, 0.03])), except for TC (-0.06mmol/L [-0.13, 0.01]) and TG (-0.04mmol/L [-0.10, 0.02). In the 40 studies among persons with high range glycemic level, lifestyle interventions significantly improved most CVD risk indicators, and the improvements were more substantial: SBP (-2.89mmHg [-3.95, -1.83]), DBP (-1.83mmHg [-2.50, -1.17]), TC (-0.12mmol/L [-0.18, -0.05]), LDL-C (-0.10mmol/L [-0.18, -0.01]), HDL-C (0.04mmol/L [0.02, 0.06]), and TG (-0.12mmol/L [-0.21, -0.04]).
Comparison according to intervention modality
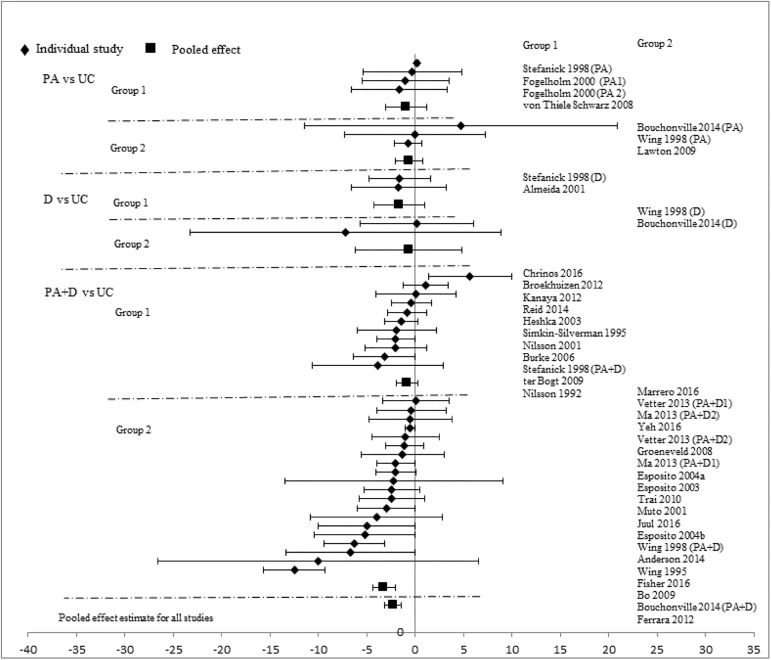
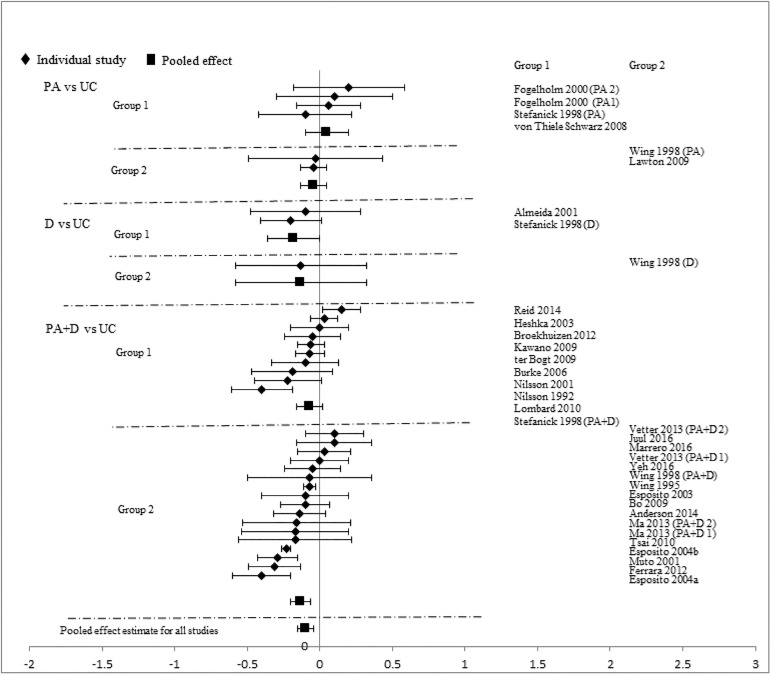
Analyses stratified by intervention types showed that PA+D vs UC achieved the best incremental improvements in SBP (-2.29mmHg [-3.19, -1.40]), DBP (-1.66mmHg [-2.24, -1.09]), TC (-0.10mmol/L [-0.16, -0.05]), LDL-C (-0.08mmol/L [-0.14, -0.02]), HDL-C (0.03mmol/L [0.02, 0.05]), and TG (-0.07mmol/L [-0.13, -0.01]). D vs UC showed significant improvements in two categories: DBP (-2.28mmHg [-4.07, -0.49]), TC (-0.17mmol/L[-0.34, -0.01]); improvements in other measures did not reach statistical significance. Improvements with PA vs UC did not reach statistical significance in any category: SBP (-0.72mmHg [-1.89, 0.44]), DBP (-1.12mmHg [-2.34, 0.10]), TC (-0.02mmol/L [-0.09, 0.06]), LDL-C (-0.03mmol/L [-0.18, 0.12]), HDL-C (0.01mmol/L [-0.02, 0.04]), and TG (-0.10mmol/L [-0.22, 0.02]). Pooled effects of CVD risk reduction are presented in Figs 2–7.
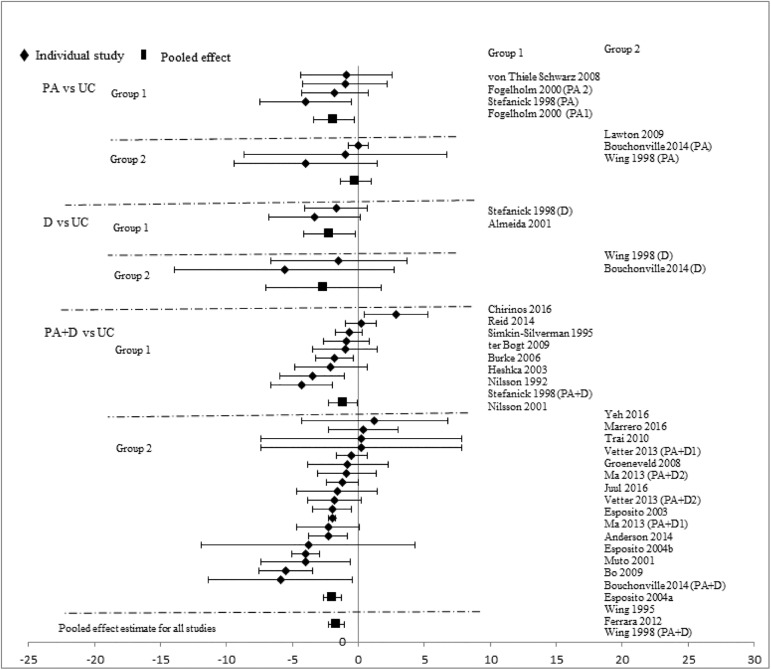
Fig 2. changes in systolic blood pressure in the intervention versus usual care groups (mmHg).
Group 1: low-range glycemic group (FPG<5.5mmol/L or A1C <5.5%). Group 2: high-range glycemic group (FPG ≥5.5mmol/L or A1C ≥5.5%). D, diet, PA, physical activity, UC, usual care, vs, versus.
Fig 7. Changes in triglycerides in the intervention versus usual care groups (mmol/L).
Group 1: low-range glycemic group (FPG<5.5mmol/L or A1C <5.5%). Group 2: high-range glycemic group (FPG ≥5.5mmol/L or A1C ≥5.5%). D, diet, PA, physical activity, UC, usual care, vs, versus.
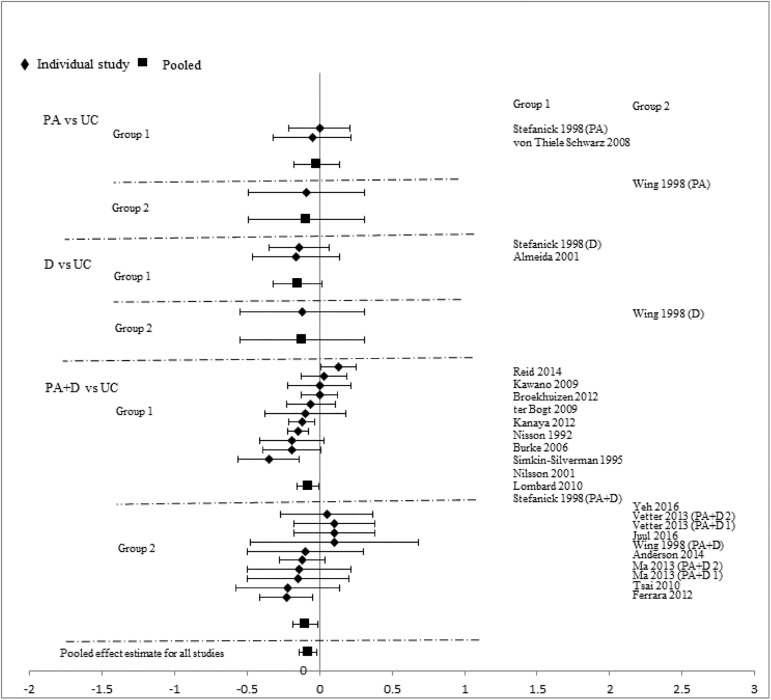
Fig 3. changes in diastolic blood pressure in the intervention versus usual care groups (mmHg).
Group 1: low-range glycemic group (FPG<5.5mmol/L or A1C <5.5%). Group 2: high-range glycemic group (FPG ≥5.5mmol/L or A1C ≥5.5%). D, diet, PA, physical activity, UC, usual care, vs, versus.
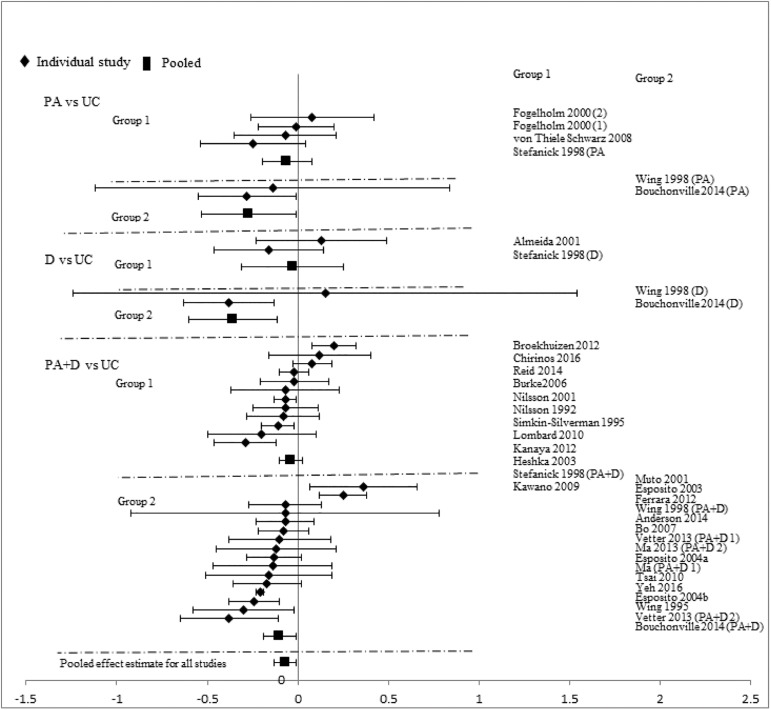
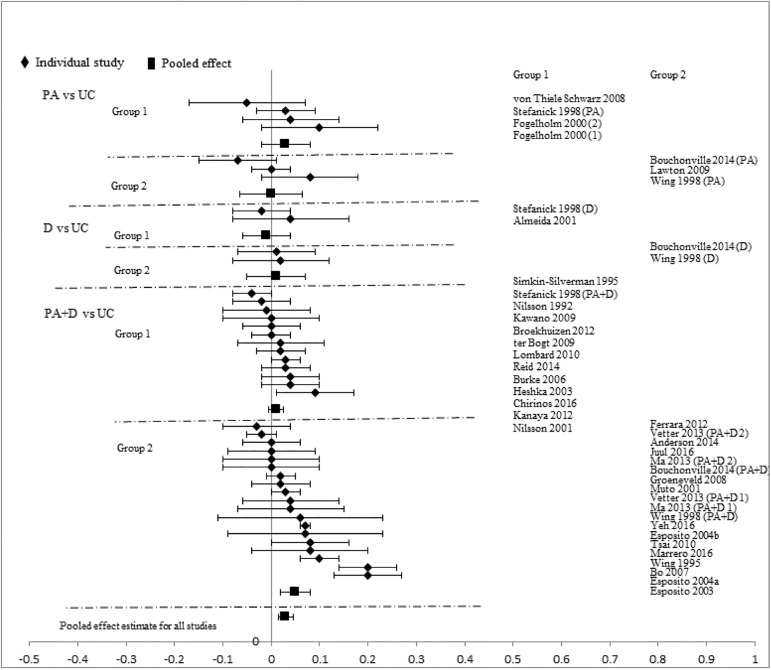
Fig 4. changes in total cholesterol in the intervention versus usual care groups (mmol/L).
Group 1: low-range glycemic group (FPG<5.5mmol/L or A1C <5.5%). Group 2: high-range glycemic group (FPG ≥5.5mmol/L or A1C ≥5.5%). D, diet, PA, physical activity, UC, usual care, vs, versus.
Fig 5. changes in low density lipoprotein cholesterol in the intervention versus usual care groups (mmol/L).
Group 1: low-range glycemic group (FPG<5.5mmol/L or A1C <5.5%). Group 2: high-range glycemic group (FPG ≥5.5mmol/L or A1C ≥5.5%). D, diet, PA, physical activity, UC, usual care, vs, versus.
Fig 6. changes in high density lipoprotein cholesterol in the intervention versus usual care groups (mmol/L).
Group 1: low-range glycemic group (FPG<5.5mmol/L or A1C <5.5%). Group 2: high-range glycemic group (FPG ≥5.5mmol/L or A1C ≥5.5%). D, diet, PA, physical activity. UC, usual care, vs, versus.
Comparison according to length of follow-up
In 34 studies or study arms with 12 months of follow-up, lifestyle interventions significantly improved all CVD risk factors: SBP (-2.07mmHg [-3.19, -0.95]), DBP (-1.62mmHg [-2.29, -0.95]), TC (-0.06mmol/L [-0.10, -0.01]), LDL-C (-0.08mmol/L [-0.13, -0.02]), HDL-C (0.02mmol/L [0.01, 0.03]), and TG (-0.08mmol/L [-0.14, -0.03]). For 7 studies or study arms with 13–23 months of follow-up, significant improvements were observed in four CVD risk factors: SBP (-1.73mmHg [-2.80, -0.65]), DBP (-1.25mmHg [-2.02, -0.48]), TC (-0.19mmol/L [-0.26, -0.11]), and LDL-C (-0.12mmol/L [-0.19, -0.05]). When the follow-up was ≥24 months (n = 14), significant improvements remained visible only for: SBP (-1.58mmHg [-2.71, -0.45]), DBP (-1.36mmHg [-2.30, -0.41]), and HDL-C (0.05mmol/L [0.02, 0.08]).
Correlation between interventional effects on CVD risk reduction and glucose change and weight loss effect sizes
Findings from meta-regression analyses demonstrated that except for LDL-C category, Pearson’s correlation, r between CVD risk reduction effect sizes and glucose effect sizes ranged from 0.73 to 0.83 in SBP, DBP, TC, HDL-C, and TG, but r between CVD risk reduction effect sizes and baseline FPG were very low, only ranging from 0.26 to 0.44 in SBP, DBP, TC, HDL-C, and TG. The r between CVD risk reduction effect sizes and weight followed the same patterns: except for LDL-C category, r between CVD risk reduction effect sizes and weight loss effect sizes ranged from 0.51 to 0.75 in SBP, DBP, TC, HDL-C, and TG, but r between CVD risk reduction effect sizes and baseline weight were very low, only ranging from 0.02 to 0.30 in SBP, DBP, TC, HDL-C, and TG. Compared to weight loss, glucose response is a better indicator of the CVD risk factor response because the glucose response has a stronger correlation with the CVD risk factor response as r ranges showed above (Table 3).
Table 3. Correlation between CVD Risk Reduction and FPG and Weight.
| CVD risk reduction | R | |||
|---|---|---|---|---|
| Effect size | Baseline FPG | FPG effect size | Baseline weight | Weight loss effect size |
| SBP | 0.32 | 0.752 | 0.068 | 0.506 |
| DPB | 0.259 | 0.728 | 0.023 | 0.58 |
| TC | 0.301 | 0.827 | 0.127 | 0.75 |
| LDL-C | 0.186 | 0.117 | 0.196 | 0.18 |
| HDL-C | 0.437 | 0.82 | 0.301 | 0.708 |
| TG | 0.38 | 0.82 | 0.172 | 0.707 |
Abbreviations: CVD: cardiovascular disease; DBP: diastolic blood pressure; FPG: fasting plasma glucose; HDL-C: high density cholesterol; LDL-C: low density cholesterol; SBP: systolic blood pressure; TC: total cholesterol; TG: triglycerides
Discussion
In this review of the effectiveness of lifestyle interventions on the reduction of CVD risk factors among adults with low glycemic levels (below the IGT threshold), we found that lifestyle interventions, including physical activity, diet, and behavioral modification, can significantly improve CVD risk profiles, including SBP, DBP, TC, LDL-C, HDL-C, and TG. When stratified by glycemic levels, we found similar intervention effects between studies of participants with low vs high-range glycemic levels, except for TC and TG. Greater improvements were observed among studies with 12 months of follow-up than those with longer follow up, such that only SBP, DBP, and HDL-C improvements were sustained after 24 months. Studies that used a combined strategy of PA and D had the strongest effect on improving CVD profiles, followed by studies using D interventions only; studies only using a PA intervention strategy had the weakest effect. We have previously reported that multi-faceted interventions combining PA and D are effective in improving glucose regulation in populations with average low-range and high-range glucose levels.[125] The results of the present analyses suggest the effect of such interventions also applies to traditional biologic CVD risk factors.
Lifestyle interventional effects on CVD risk reduction observed in our studies among people without IGT or diabetes are consistent with those from the main trials of diabetes prevention among persons with IGT. For example, the US Diabetes Prevention Program (DPP) Study among people with IGT reported improvements in CVD profiles for all categories as measured by the mean differences between lifestyle intervention and placebo groups. The magnitude of improvements in CVD profiles in the DPP[126] in 1-year follow-up are consistent with those from our review (DPP vs this review: SBP, -2.50 vs -2.07 mmHg; DBP, -2.71 vs -1.62 mmol/L; TC, -0.06 vs -0.06 mmol/L; LDL-C, -0.02 vs -0.08 mmol/L; HDL-C, 0.01 vs 0.02 mmol/L; TG, -0.18 vs. -0.08 mmol/L, respectively). This comparison is also true for other major diabetes prevention trials (e.g., Finish Diabetes Prevention Study).[127]
Our findings may have important implications for decision makers in the areas of both diabetes and CVD primary prevention. Our meta-regression analyses indicated that the magnitude of improvements in CVD risk profiles is less correlated with baseline glucose level, but highly correlated with the effect sizes of glucose improvement. Meanwhile, the meta-regression analyses also indicated that the magnitude of improvements in CVD risk profiles is less correlated with baseline body weight, but highly correlated with the effect sizes of weight loss. We thus conclude that lifestyle interventions may provide important benefits across the full distribution of glycemic levels and body weight, including populations with glycemic levels below the IGT threshold, for both the low and high ranges of baseline FPG, and for populations with normal weight but with CVD risk factors. However, economic factors as well as the effectiveness of interventions influence decisions regarding the types of interventions provided to individuals with glycemic levels below the IGT threshold.[128,129] The cost-effectiveness of lifestyle interventions that can simultaneously reduce diabetes and CVD risk among individuals with glycemic levels below the IGT threshold should be examined.
Our findings demonstrate that lifestyle interventions, compared to UC, achieved improvement in both diabetes prevention and CVD risk reduction, and these improvements were not only statistically significant, but also have clinical relevance. Previous studies indicated that each 0.03 mmol/L increase in HDL-C is associated with the reduction of coronary heart disease risk by 2–3%,[130] and each 5 mmHg reduction in SBP and 2 mmHg reduction in DBP reduce stroke risk by 13% and 11.5%, respectively.[131] According to an epidemiology study, a 1% decrease in total cholesterol leads to a decrease in the incidence of coronary events by 2%.[132] One study also indicated that weight loss improved CVD profiles because each kilogram change in body weight was related to the change in the risk of coronary heart disease by 3.1%.[133]
Given that lifestyle intervention program participants in our reviewed studies usually achieved improvements in CVD across a full spectrum of outcomes simultaneously, the overall combined benefits brought by lifestyle interventions could be amplified. An estimation of overall effect on CVD risk would be helpful for our understanding the importance of interventional impact. Unfortunately, although there are several models available for CVD risk calculation (e.g., Framingham Risk Score,[134] and the ACC/AHA CVD risk calculator[135]), we are not aware of any available estimation model by which we can calculate the overall combined effect of changes of different individual risk factor. Further research and validation test, therefore, maybe needed for creating this model. If this kind model is available in the future, we can apply this model to our meta-analytic findings to estimate the overall combined effect of changes of different individual risk factor. For example, if a population, through lifestyle and behavior changes, achieved CVD risk reductions as much as showed in our meta-analyses, we can estimate the overall health benefits (e.g., how many CVD events can be prevented in the future). Despite this unavailability, the improvement in glucose regulation[125] coupled with our findings regarding the improvement in CVD risk reduction suggested that lifestyle interventions can achieve a comprehensive improvement goal as stated in AHA Special Report[17] of preventing CVD and diabetes simultaneously among persons with lower diabetes risk.
Strong evidence shows that PA programs have important independent effects on non-insulin-mediated glucose transport, markers of inflammation, insulin resistance, blood pressure, lipid profile, fitness, and improved lean-to-fat mass ratio.[136] Our findings suggest that these effects were more likely observed in studies using multi-component interventions, including PA, calorie restriction, and behavioral support but less so for PA-only interventions. This finding may be related to methodological shortcomings in exercise-only interventions such as low adherence, insufficient exercise volume or length of intervention. Previous studies suggest that it may take up to 2 years for a previously sedentary obese individual to attain enough volume of exercise to effectively reduce CVD risk factors, and individuals in unverified, out-patient interventions are less likely to engage in the prescribed amount of exercise.[137,138] However, we previously reported that exercise-only interventions in our included studies significantly reduced FPG and body weight[125] which in turn further prevented diabetes. Since PA-related improvements in glucose regulation and weight loss can lead to reductions in CVD risk profiles, potential indirect benefits should be taken into account when interpreting our findings.
Unhealthy lifestyle factors are related to the atherosclerotic process and these long-term exposures lead to the clinical manifestations of cardiovascular events.[139] A previous study also indicated that lifestyle changes, only in the long-term, are likely to lead to CVD risk factor reduction.[30] Our findings demonstrate that the effects of lifestyle changes on the reduction in CVD risk factors reached their highest point at 12 months of follow-up, then gradually decreased over time. This may reflect the fact that the longer-term intervention may be more effective on reducing CVD risks only if participates remain highly adherent to the intended interventions, which is seldom observed. It could be also true that using CVD mortality, rather than CVD risk reduction alone, to measure the long-term effect of lifestyle changes on CVD is more appropriate as the extended legacy findings of the Chinese Da Qing Study indicated.[140]
Because we used a comprehensive search strategy including all major medical databases, we found a large number of eligible studies. Pooled effects based on a large sample size provide more robust findings than those from any single study. Our review has some limitations as well. First, lifestyle interventions were used in heterogeneous settings, among different populations of varying ages, health status, and race/ethnicity background. While the main components of the lifestyle interventions were generally PA and D, each of the strategies had its own requirements in type, dose, intensity, and frequency. UC also had varying definitions among different comparison groups. Heterogeneity across studies was also reflected in the length of intervention, duration and follow-up, and number of sessions. However, our meta-regression analyses found no interactions between the between-group change in glycemic indicators and study-level characteristics. We also stratified our data syntheses by glycemic level, length of follow-up, and type of interventions, taking the heterogeneity among included studies into account. Second, although we stratified by level of glycemic risk at the study level, there was considerable heterogeneity within studies, and the nature of aggregated data prevented individual level classification by glucose level. As a result, there was likely considerable overlap in participant characteristics between low range and high range glycemic groups in our study, which may introduce some misclassification bias. Misclassification bias could be also introduced by usage of both FPG and A1C in our review to identify population with low glycemic risks. Although a previous study indicated that the agreement between FPG and A1C is high,[141] they are not equal with each other.[142] Because of this misclassification bias, some individuals identified as with low glycemic risks could actually have glucose metabolism abnormalities. Audiences need to be cautious when interpreting our findings.
Conclusions
Our review is the first comprehensive examination of the impact of lifestyle interventions on risk for progression of dysglycemia and CVD risk reduction among persons below the IGT threshold. This systematic review suggests that lifestyle change is critical to both CVD risk reduction and diabetes prevention across the full spectrum of risk, complementing the major trials of diabetes prevention that focused on persons with IGT. This review also provides supportive evidences for designing strategies aimed at reducing CVD burden as delineated in the AHA Strategic Impact Goal through 2020 and Beyond.[17] Our findings demonstrated that among adults without IGT or diabetes, PA and D interventions, especially combined can significantly improve SBP, DBP, TC, LHL-C, HDL-C, and TG, in addition to glucose regulation and weight loss, and that these risk reductions may further prevent CVD events.
Supporting information
Appendix A. Protocol-Study Protocol with Search Strategy.
Appendix B. PRISMA Checklist- Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist.
Table A. Intervention Characteristics.
Table B. Quality Assessment.
Table C. Lifestyle Interventional Effect: Meta-analyses Results in A Single Arm Model.
Table D. Intervention effect on FPG and percent weight: meta-analyses results.
(DOCX)
Acknowledgments
This study was supported by the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Data Availability
Instructions for accessing studies reported on in this study are available in supporting information.
Funding Statement
This study was supported by the Centers for Disease Control and Prevention in the form of salaries for XZ, HMD, BS, GI, WT, SG, BB, PC, IGQ, CDJ, JMD, JS, LSG, EWG.
References
- 1.World Health Organization. Cardiovascular Disease (CVDs). available from http://www.who.int/cardiovascular_diseases/en
- 2.American Diabetes Association. Cardiovascular disease and risk management. Diabetes Care. 2015;38: S49–S57. doi: 10.2337/dc15-S011 [DOI] [PubMed] [Google Scholar]
- 3.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3: 105–113. pii: S2213-8587(14)70219-0. doi: 10.1016/S2213-8587(14)70219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care. 2014;37: S14–S80. doi: 10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346: 393–403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20: 537–544. [DOI] [PubMed] [Google Scholar]
- 7.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344: 1343–1350. doi: 10.1056/NEJM200105033441801 [DOI] [PubMed] [Google Scholar]
- 8.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336: 1117–1124. doi: 10.1056/NEJM199704173361601 [DOI] [PubMed] [Google Scholar]
- 9.Burke V, Mansour J, Beilin LJ, Mori TA. Long-term follow-up of participants in a health promotion program for treated hypertensives (ADAPT). Nutr Metab Cardiovasc Dis. 2008;18: 198–206. doi: 10.1016/j.numecd.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 10.Nilsson PM, Klasson EB, Nyberg P. Life-style intervention at the worksite—Reduction of cardiovascular risk factors in a randomized study. Scandinavian Journal of Work, Environment and Health. 2001;27: 57–62. [DOI] [PubMed] [Google Scholar]
- 11.Reid RD, McDonnell LA, Riley DL, Mark AE, Mosca L, Beaton L, et al. Effect of an intervention to improve the cardiovascular health of family members of patients with coronary artery disease: a randomized trial. CMAJ. 2014;186: 23–30. Pii: cmaj.130550. doi: 10.1503/cmaj.130550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simkinsilverman L, Wing RR, Hansen DH, Klem ML, Pasagianmacaulay A, Meilahn EN, et al. Prevention of cardiovascular risk factor elevations in healthy premenopausal women. Prev Med. 1995;24: 509–517. [DOI] [PubMed] [Google Scholar]
- 13.Stefanick ML, Mackey S, Sheehan M, Ellsworth N, Haskell WL, Wood PD. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N Engl J Med. 1998;339: 12–20. doi: 10.1056/NEJM199807023390103 [DOI] [PubMed] [Google Scholar]
- 14.ter Bogt NC, Bemelmans WJ, Beltman FW, Broer J, Smit AJ, van der Meer K. Preventing weight gain: One-year results of a randomized lifestyle intervention. Am J Prev Med. 2009;37: 270–277. doi: 10.1016/j.amepre.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 15.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32: 287–294. pii: dc08-1296. doi: 10.2337/dc08-1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The DECODE study group. Is fasting glucose sufficient to define diabetes? Epidemiological data from 20 European studies. Diabetologia. 1999;42: 647–654. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, van HL, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121: 586–613. pii: CIRCULATIONAHA.109.192703. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 18.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston MN, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129: S76–S99. pii: 01.cir.0000437740.48606.d1. doi: 10.1161/01.cir.0000437740.48606.d1 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT Green SE. Cochrane Handbook for Systematic Reviews of Interventions. [Version 5.1.0 [updated March 2011]]. 2011. The Cochrane Collaboration. 2012.
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1954;7: 177–188. [DOI] [PubMed] [Google Scholar]
- 21.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med. 2008;35: 357–363. doi: 10.1016/j.amepre.2008.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almeida LB, Segurado AC, Duran ACF, Jaime PC. Impact of a nutritional counseling program on prevention of HAART-related metabolic and morphologic abnormalities. AIDS Care—Psychological and Socio-Medical Aspects of AIDS/HIV. 2011;23: 755–763. [DOI] [PubMed] [Google Scholar]
- 23.Anderson AS, Craigie AM, Caswell S, Treweek S, Stead M, Macleod M, et al. The impact of a bodyweight and physical activity intervention (BeWEL) initiated through a national colorectal cancer screening programme: randomised controlled trial. BMJ. 2014;348: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderssen SA, Hjermann I, Urdal P, Torjesen PA, Holme I. Improved carbohydrate metabolism after physical training and dietary intervention in individuals with the "atherothrombogenic syndrome'. Oslo Diet and Exercise Study (ODES). A randomized trial. J Intern Med. 1996;240: 203–209. [DOI] [PubMed] [Google Scholar]
- 25.Arguin H. Short- and long-term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women: a pilot study. Menopause. 2012;19: 870–876. doi: 10.1097/gme.0b013e318250a287 [DOI] [PubMed] [Google Scholar]
- 26.Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, et al. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med. 2014;161: 309–318. pii: 1900694. doi: 10.7326/M14-0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bo S, Ciccone G, Baldi C, Benini L, Dusio F, Forastiere G, et al. Effectiveness of a lifestyle intervention on metabolic syndrome. A randomized controlled trial. J Gen Intern Med. 2007;22: 1695–1703. doi: 10.1007/s11606-007-0399-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouchonville M, Armamento-Villareal R, Shah K, Napoli N, Sinacore DR, Qualls C, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes (Lond). 2014;38: 423–431. pii: ijo2013122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinkworth GD, Noakes M, Keogh JB, Luscombe ND, Wittert GA, Clifton PM. Long-term effects of a high-protein, low-carbohydrate diet on weight control and cardiovascular risk markers in obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 2004;28: 661–670. doi: 10.1038/sj.ijo.0802617 [DOI] [PubMed] [Google Scholar]
- 30.Broekhuizen K, van Poppel MN, Koppes LL, Kindt I, Brug J, van Mechelen W. No significant improvement of cardiovascular disease risk indicators by a lifestyle intervention in people with familial hypercholesterolemia compared to usual care: results of a randomised controlled trial. BMC Res Notes. 2012;5: 181–189. doi: 10.1186/1756-0500-5-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke V, Beilin LJ, Cutt HE, Mansour J, Williams A, Mori TA. A lifestyle program for treated hypertensives improved health-related behaviors and cardiovascular risk factors, a randomized controlled trial. J Clin Epidemiol. 2007;60: 133–141. doi: 10.1016/j.jclinepi.2006.05.012 [DOI] [PubMed] [Google Scholar]
- 32.Burtscher M, Gatterer H, Kunczicky H, Brandstatter E, Ulmer H. Supervised exercise in patients with impaired fasting glucose: impact on exercise capacity. Clin J Sport Med. 2009;19: 394–398. doi: 10.1097/JSM.0b013e3181b8b6dc [DOI] [PubMed] [Google Scholar]
- 33.Chirinos DA, Goldberg RB, Llabre MM, Gellman M, Gutt M, McCalla J, et al. Lifestyle modification and weight reduction among low-income patients with the metabolic syndrome: the CHARMS randomized controlled trial. J Behav Med. 2016;39: 483–492. pii: 10.1007/s10865-016-9721-2. doi: 10.1007/s10865-016-9721-2 [DOI] [PubMed] [Google Scholar]
- 34.Choo J, Lee J, Cho JH, Burke LE, Sekikawa A, Jae SY. Effects of weight management by exercise modes on markers of subclinical atherosclerosis and cardiometabolic profile among women with abdominal obesity: a randomized controlled trial. BMC Cardiovasc Disord. 2014;14: 82 pii: 1471-2261-14-82. doi: 10.1186/1471-2261-14-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clifton PM, Keogh JB, Noakes M. Long-term effects of a high-protein weight-loss diet. Am J Clin Nutr. 2008;87: 23–29. [DOI] [PubMed] [Google Scholar]
- 36.Cole RE, Boyer KM, Spanbauer SM, Sprague D, Bingham M. Effectiveness of prediabetes nutrition shared medical appointments: prevention of diabetes. Diabetes Educ. 2013;39: 344–353. pii: 0145721713484812. doi: 10.1177/0145721713484812 [DOI] [PubMed] [Google Scholar]
- 37.Coon PJ, Bleecker ER, Drinkwater DT, Meyers DA, Goldberg AP. Effects of body composition and exercise capacity on glucose tolerance, insulin, and lipoprotein lipids in healthy older men: a cross-sectional and longitudinal intervention study. Metabolism. 1989;38: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 38.Cox KL, Burke V, Beilin LJ, Puddey IB. A comparison of the effects of swimming and walking on body weight, fat distribution, lipids, glucose, and insulin in older women-the Sedentary Women Exercise Adherence Trial 2. Metabolism: Clinical and Experimental. 2010;59: 1562–1573. [DOI] [PubMed] [Google Scholar]
- 39.Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr. 1999;69: 198–204. [DOI] [PubMed] [Google Scholar]
- 40.Donnelly JE, Jacobsen DJ, Heelan KS, Seip R, Smith S. The effects of 18 months of intermittent vs. continuous exercise on aerobic capacity, body weight and composition, and metabolic fitness in previously sedentary, moderately obese females. Int J Obes Relat Metab Disord. 2000;24: 566–572. [DOI] [PubMed] [Google Scholar]
- 41.Esposito K, Marfella R, Ciotola M, Palo C, Giugliano F, Giugliano G, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292: 1440–1446. doi: 10.1001/jama.292.12.1440 [DOI] [PubMed] [Google Scholar]
- 42.Esposito K, Giugliano F, Di PC, Giugliano G, Marfella R, D'Andrea F, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291: 2978–2984. pii: 291/24/2978. doi: 10.1001/jama.291.24.2978 [DOI] [PubMed] [Google Scholar]
- 43.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized trial. JAMA. 2003;289: 1799–1804. doi: 10.1001/jama.289.14.1799 [DOI] [PubMed] [Google Scholar]
- 44.Fatouros IG, Tournis S, Leontsini D, Jamurtas AZ, Sxina M, Thomakos P, et al. Leptin and adiponectin responses in overweight inactive elderly following resistance training and detraining are intensity related. J Clin Endocrinol Metab. 2005;90: 5970–5977. doi: 10.1210/jc.2005-0261 [DOI] [PubMed] [Google Scholar]
- 45.Fernandez AC, Casariego AV, Rodriguez IC, Pomar MDB. One-year effectiveness of two hypocaloric diets with different protein/carbohydrate ratios in weight loss and insulin resistance. Nutr Hosp. 2012;27: 2093–2101. doi: 10.3305/nh.2012.27.6.6133 [DOI] [PubMed] [Google Scholar]
- 46.Ferrara AL, Pacioni D, Di Fronzo V, Russo BF, Staiano L, Speranza E, et al. Lifestyle Educational Program Strongly Increases Compliance to Nonpharmacologic Intervention in Hypertensive Patients: A 2-Year Follow-Up Study. J Clin Hypertens (Greenwich). 2012;14: 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer HH, Fischer IP, Pereira RI, Furniss AL, Rozwadowski JM, Moore SL, et al. Text Message Support for Weight Loss in Patients With Prediabetes: A Randomized Clinical Trial. Diabetes Care. 2016;39: 1364–1370. pii: dc15-2137. doi: 10.2337/dc15-2137 [DOI] [PubMed] [Google Scholar]
- 48.Fisher G, Hunter GR, Gower BA. Aerobic exercise training conserves insulin sensitivity for 1 yr following weight loss in overweight women. J Appl Physiol. 2012;112: 688–693. doi: 10.1152/japplphysiol.00843.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fogelholm M, Kukkonen-Harjula K, Nenonen A, Pasanen M. Effects of walking training on weight maintenance after a very-low-energy diet in premenopausal obese women: a randomized controlled trial. Arch Intern Med. 2000;160: 2177–2184. [DOI] [PubMed] [Google Scholar]
- 50.Fonollá J, López-Huertas E, Machado FJ, Molina D, Alvarez I, Mármol E, et al. Milk enriched with "healthy fatty acids" improves cardiovascular risk markers and nutritional status in human volunteers. Nutrition (Burbank, Los Angeles County, Calif). 2009;25: 408–414. [DOI] [PubMed] [Google Scholar]
- 51.Frank LL, Sorensen BE, Yasui Y, Tworoger SS, Schwartz RS, Ulrich CM, et al. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obes Res. 2005;13: 615–625. doi: 10.1038/oby.2005.66 [DOI] [PubMed] [Google Scholar]
- 52.Groeneveld IF, Proper KI, van der Beek AJ, van Mechelen W. Sustained body weight reduction by an individual-based lifestyle intervention for workers in the construction industry at risk for cardiovascular disease: results of a randomized controlled trial. Prev Med. 2010;51: 240–246. doi: 10.1016/j.ypmed.2010.07.021 [DOI] [PubMed] [Google Scholar]
- 53.Heshka S, Anderson JW, Atkinson RL, Greenway FL, Hill JO, Phinney SD, et al. Weight Loss with Self-help Compared with a Structured Commercial Program: A Randomized Trial. JAMA. 2003;289: 1792–1798. doi: 10.1001/jama.289.14.1792 [DOI] [PubMed] [Google Scholar]
- 54.Imayama I, Alfano CM, Mason C, Wang C, Duggan C, Campbell KL, et al. Weight and metabolic effects of dietary weight loss and exercise interventions in postmenopausal antidepressant medication users and non-users: a randomized controlled trial. Prev Med. 200357: 525–532. pii: S0091-7435(13)00230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juul L, Andersen VJ, Arnoldsen J, Maindal HT. Effectiveness of a brief theory-based health promotion intervention among adults at high risk of type 2 diabetes: One-year results from a randomised trial in a community setting. Prim Care Diabetes. 2016;10: 111–120. pii: S1751-9918(15)00099-6. doi: 10.1016/j.pcd.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 56.Kanaya AM, Santoyo-Olsson J, Gregorich S, Grossman M, Moore T, Stewart AL. The Live Well, Be Well study: a community-based, translational lifestyle program to lower diabetes risk factors in ethnic minority and lower-socioeconomic status adults. Am J Public Health. 2012;102: 1551–1558. doi: 10.2105/AJPH.2011.300456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanaya AM, Araneta MR, Pawlowsky SB, Barrett-Connor E, Grady D, Vittinghoff E, et al. Restorative yoga and metabolic risk factors: the Practicing Restorative Yoga vs. Stretching for the Metabolic Syndrome (PRYSMS) randomized trial. J Diabetes Complications. 2014;28: 406–412. pii: S1056-8727(13)00327-9. doi: 10.1016/j.jdiacomp.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katula JA, Vitolins MZ, Morgan TM, Lawlor MS, Blackwell CS, Isom SP, et al. The Healthy Living Partnerships to Prevent Diabetes study: 2-year outcomes of a randomized controlled trial. Am J Prev Med. 2013;44: S324–S332. pii: S0749-3797(13)00023-8. doi: 10.1016/j.amepre.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawano M, Shono N, Yoshimura T, Yamaguchi M, Hirano T, Hisatomi A. Improved cardio-respiratory fitness correlates with changes in the number and size of small dense LDL: Randomized controlled trial with exercise training and dietary instruction. Intern Med. 2009;48: 25–32. [DOI] [PubMed] [Google Scholar]
- 60.Keogh JB, Brinkworth GD, Clifton PM. Effects of weight loss on a low-carbohydrate diet on flow-mediated dilatation, adhesion molecules and adiponectin. Br J Nutr. 2007;98: 852–859. doi: 10.1017/S0007114507747815 [DOI] [PubMed] [Google Scholar]
- 61.Lawton BA, Rose SB, Raina Elley C, Dowell AC, Fenton A, Moyes SA. Exercise on prescription for women aged 40–74 recruited through primary care: two year randomised controlled trial. BJSM online. 2009;43: 120–126. [PubMed] [Google Scholar]
- 62.Lim SS, Noakes M, Keogh JB, Clifton PM. Long-term effects of a low carbohydrate, low fat or high unsaturated fat diet compared to a no-intervention control. Nutr Metab Cardiovasc Dis. 2010;20: 599–607. doi: 10.1016/j.numecd.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 63.Lombard C, Deeks A, Jolley D, Ball K, Teede H. A low intensity, community based lifestyle programme to prevent weight gain in women with young children: cluster randomised controlled trial. BMJ. 2010;41: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma J, King AC, Wilson SR, Xiao L, Stafford RS. Evaluation of lifestyle interventions to treat elevated cardiometabolic risk in primary care (E-LITE): a randomized controlled trial. BMC Fam Pract. 2009;10: 71–82. doi: 10.1186/1471-2296-10-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marrero DG, Palmer KN, Phillips EO, Miller-Kovach K, Foster GD, Saha CK. Comparison of Commercial and Self-Initiated Weight Loss Programs in People With Prediabetes: A Randomized Control Trial. Am J Public Health. 2016;106: 949–956. doi: 10.2105/AJPH.2015.303035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marsh KA, Steinbeck KS, Atkinson FS, Petocz P, Brand-Miller JC. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr. 2010;92: 83–92. doi: 10.3945/ajcn.2010.29261 [DOI] [PubMed] [Google Scholar]
- 67.Mason AE, Epel ES, Kristeller J, Moran PJ, Dallman M, Lustig RH, et al. Effects of a mindfulness-based intervention on mindful eating, sweets consumption, and fasting glucose levels in obese adults: data from the SHINE randomized controlled trial. J Behav Med. 2016;39: 201–213. pii: 10.1007/s10865-015-9692-8. doi: 10.1007/s10865-015-9692-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McAuley KA, Smith KJ, Taylor RW, McLay RT, Williams SM, Mann JI. Long-term effects of popular dietary approaches on weight loss and features of insulin resistance. International journal of obesity. 2006;30: 342–349. doi: 10.1038/sj.ijo.0803075 [DOI] [PubMed] [Google Scholar]
- 69.Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, Larsson C, et al. Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68: 350–357. pii: ejcn2013290. doi: 10.1038/ejcn.2013.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muto T, Yamauchi K. Evaluation of a multicomponent workplace health promotion program conducted in Japan for improving employees' cardiovascular disease risk factors. Prev Med. 2001;33: 571–577. doi: 10.1006/pmed.2001.0923 [DOI] [PubMed] [Google Scholar]
- 71.Narayan KM, Hoskin M, Kozak D, Kriska AM, Hanson RL, Pettitt DJ, et al. Randomized clinical trial of lifestyle interventions in Pima Indians: a pilot study. Diabet Med. 1998;15: 66–72. doi: 10.1002/(SICI)1096-9136(199801)15:1<66::AID-DIA515>3.0.CO;2-A [DOI] [PubMed] [Google Scholar]
- 72.Nilsson PM, Lindholm LH, Schersten BF. Life style changes improve insulin resistance in hyperinsulinaemic subjects: a one-year intervention study of hypertensives and normotensives in Dalby. J Hypertens. 1992;10: 1071–1078. [PubMed] [Google Scholar]
- 73.Ockene IS, Tellez TL, Rosal MC, Reed GW, Mordes J, Merriam PA, et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health. 2012;102: 336–342. doi: 10.2105/AJPH.2011.300357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poston WSC, Haddock CK, Pinkston MM, Pace P, Reeves RS, Karkoc N, et al. Evaluation of a primary care-oriented brief counselling intervention for obesity with and without orlistat. Journal of Intern Med. 2006;260: 388–398. [DOI] [PubMed] [Google Scholar]
- 75.Potteiger JA, Jacobsen DJ, Donnelly JE, Hill JO, Midwest Exercise T. Glucose and insulin responses following 16 months of exercise training in overweight adults: the Midwest Exercise Trial. Metabolism. 2003;52: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 76.Rossner S, Flaten H. VLCD versus LCD in long-term treatment of obesity. Int J Obes Relat Metab Disord. 1997;21: 22–26. [DOI] [PubMed] [Google Scholar]
- 77.Ryttig KR, Flaten H, Rossner S. Long-term effects of a very low calorie diet (Nutrilett) in obesity treatment. A prospective, randomized, comparison between VLCD and a hypocaloric diet+behavior modification and their combination. Int J Obes Relat Metab Disord. 1997;21: 574–579. [DOI] [PubMed] [Google Scholar]
- 78.Sartorelli DS, Sciarra EC, Franco LJ, Cardoso MA. Beneficial effects of short-term nutritional counselling at the primary health-care level among Brazilian adults. Public Health Nutr. 2005;8: 820–825. [DOI] [PubMed] [Google Scholar]
- 79.Sattin RW, Williams LB, Dias J, Garvin JT, Marion L, Joshua TV, et al. Community Trial of a Faith-Based Lifestyle Intervention to Prevent Diabetes Among African-Americans. J Community Health. 2016;41: 87–96. pii: 10.1007/s10900-015-0071-8. doi: 10.1007/s10900-015-0071-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simkin-Silverman LR, Wing RR, Boraz MA, Kuller LH. Lifestyle intervention can prevent weight gain during menopause: results from a 5-year randomized clinical trial. Ann Behav Med. 2003;26: 212–220. [DOI] [PubMed] [Google Scholar]
- 81.Siu P, Yu A, Benzie I, Woo J. Effects of 1-year yoga on cardiovascular risk factors in middle-aged and older adults with metabolic syndrome: a randomized trial. Diabetology & Metabolic Syndrome. 2015;7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Staten LK, Gregory-Mercado KY, Ranger-Moore J, Will JC, Giuliano AR, Ford ES, et al. Provider counseling, health education, and community health workers: The Arizona WISEWOMAN project. Journal of Womens Health. 2004;13: 547–556. [DOI] [PubMed] [Google Scholar]
- 83.Tapsell LC, Batterham MJ, Thorne RL, O'Shea JE, Grafenauer SJ, Probst YC. Weight loss effects from vegetable intake: a 12-month randomised controlled trial. Eur J Clin Nutr. 2014;68: 778–785. pii: ejcn201439. doi: 10.1038/ejcn.2014.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thompson WG, Rostad Holdman N, Janzow DJ, Slezak JM, Morris KL, Zemel MB. Effect of energy-reduced diets high in dairy products and fiber on weight loss in obese adults. Obes Res. 2005;13: 1344–1353. doi: 10.1038/oby.2005.163 [DOI] [PubMed] [Google Scholar]
- 85.Tsai AG, Wadden TA, Rogers MA, Day SC, Moore RH, Islam BJ. A primary care intervention for weight loss: Results of a randomized controlled pilot study. Obesity (Silver Spring). 2010;18: 1614–1618. [DOI] [PubMed] [Google Scholar]
- 86.Vainionpaa A, Korpelainen R, Kaikkonen H, Knip M, Leppaluoto J, Jamsa T. Effect of impact exercise on physical performance and cardiovascular risk factors. Med Sci Sports Exerc. 2007;39: 756–763. doi: 10.1249/mss.0b013e318031c039 [DOI] [PubMed] [Google Scholar]
- 87.Vetter ML, Wadden TA, Chittams J, Diewald LK, Panigrahi E, Volger S, et al. Effect of lifestyle intervention on cardiometabolic risk factors: results of the POWER-UP trial. Int J Obes (Lond). 2013;37(Suppl 1): S19–S24. pii: ijo201392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.von Thiele Schwarz U, Lindfors P, Lundberg U. Health-related effects of worksite interventions involving physical exercise and reduced workhours. Scandinavian Journal of Work, Environment and Health. 2008;34: 179–188. [DOI] [PubMed] [Google Scholar]
- 89.Watanabe M, Yamaoka K, Yokotsuka M, Tango T. Randomized controlled trial of a new dietary education program to prevent type 2 diabetes in a high-risk group of Japanese male workers. Diabetes Care. 2003;26: 3209–3214. [DOI] [PubMed] [Google Scholar]
- 90.Weinstock RS, Dai H, Wadden TA. Diet and exercise in the treatment of obesity: effects of 3 interventions on insulin resistance. Arch Intern Med. 1998;158: 2477–2483. [DOI] [PubMed] [Google Scholar]
- 91.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84: 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wing RR, Jeffery RW. Effect of modest weight loss on changes in cardiovascular risk factors: Are there differences between men and women or between weight loss and maintenance? Int J Obes (Lond). 1995;19: 67–73. [PubMed] [Google Scholar]
- 93.Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care. 1998;21: 350–359. [DOI] [PubMed] [Google Scholar]
- 94.Wycherley TP, Brinkworth GD, Clifton PM, Noakes M. Comparison of the effects of 52 weeks weight loss with either a high-protein or high-carbohydrate diet on body composition and cardiometabolic risk factors in overweight and obese males. Nutr Diabetes. 2012;2: e40–e47. pii: nutd201211. doi: 10.1038/nutd.2012.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yeh MC, Heo M, Suchday S, Wong A, Poon E, Liu G, et al. Translation of the Diabetes Prevention Program for diabetes risk reduction in Chinese immigrants in New York City. Diabet Med. 2016;33: 547–551. doi: 10.1111/dme.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anderssen SA, Holme I, Urdal P, Hjermann I. Associations between central obesity and indexes of hemostatic, carbohydrate and lipid metabolism. Results of a 1-year intervention from the Oslo Diet and Exercise Study. Scand J Med Sci Sports. 1998;8: 109–115. [DOI] [PubMed] [Google Scholar]
- 97.Bo S, Gambino R, Ciccone G, Rosato R, Milanesio N, Villois P, et al. Effects of TCF7L2 polymorphisms on glucose values after a lifestyle intervention. Am J Clin Nutr. 2009;90: 1502–1508. doi: 10.3945/ajcn.2009.28379 [DOI] [PubMed] [Google Scholar]
- 98.Burtscher M, Gatterer H, Dunnwald T, Pesta D, Faulhaber M, Netzer N, et al. Effects of supervised exercise on gamma-glutamyl transferase levels in patients with isolated impaired fasting glucose and those with impaired fasting glucose plus impaired glucose tolerance. Exp Clin Endocrinol Diabetes. 2012;120: 445–450. doi: 10.1055/s-0032-1311642 [DOI] [PubMed] [Google Scholar]
- 99.Cox KL, Burke V, Beilin LJ, Grove JR, Blanksby BA, Puddey IB. Blood pressure rise with swimming versus walking in older women: the Sedentary Women Exercise Adherence Trial 2 (SWEAT 2). J Hypertens. 2006;24: 307–314. pii: 00004872-200602000-00017. doi: 10.1097/01.hjh.0000200514.25571.20 [DOI] [PubMed] [Google Scholar]
- 100.Cox KL, Burke V, Beilin LJ, Derbyshire AJ, Grove JR, Blanksby BA, et al. Short and long-term adherence to swimming and walking programs in older women—the Sedentary Women Exercise Adherence Trial (SWEAT 2). Prev Med. 2008;46: 511–517. pii: S0091-7435(08)00048-0. doi: 10.1016/j.ypmed.2008.01.010 [DOI] [PubMed] [Google Scholar]
- 101.Craigie AM, Caswell S, Paterson C, Treweek S, Belch JJ, Daly F, et al. Study protocol for BeWEL: the impact of a BodyWEight and physicaL activity intervention on adults at risk of developing colorectal adenomas. BMC Public Health. 2011;1: 184–191. pii: 1471-2458-11-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Delgadillo AT, Grossman M, Santoyo-Olsson J, Gallegos-Jackson E, Kanaya AM, Stewart AL. Description of an academic community partnership lifestyle program for lower income minority adults at risk for diabetes. Diabetes Educ. 2010;36: 640–650. pii: 0145721710374368. doi: 10.1177/0145721710374368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ditschuneit HH, Flechtner-Mors M. Value of structured meals for weight management: risk factors and long-term weight maintenance. Obes Res. 2001;9(Suppl 4): 284S–289S. [DOI] [PubMed] [Google Scholar]
- 104.Esposito K, Ciotola M, Giugliano F, Maiorino MI, Autorino R, De SM, et al. Effects of intensive lifestyle changes on erectile dysfunction in men. J Sex Med. 2009;6: 243–250. pii: JSM1030. doi: 10.1111/j.1743-6109.2008.01030.x [DOI] [PubMed] [Google Scholar]
- 105.Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring). 2012;20: 1628–1638. pii: oby201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Groeneveld IF, Proper KI, van der Beek AJ, van Duivenbooden C, van Mechelen W. Design of a RCT evaluating the (cost-) effectiveness of a lifestyle intervention for male construction workers at risk for cardiovascular disease: the health under construction study. BMC Public Health. 2008;8: 1–12. doi: 10.1186/1471-2458-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jacobs DR Jr., Sluik D, Rokling-Andersen MH, Anderssen SA, Drevon CA. Association of 1-y changes in diet pattern with cardiovascular disease risk factors and adipokines: results from the 1-y randomized Oslo Diet and Exercise Study. Am J Clin Nutr. 2009;89: 509–517. doi: 10.3945/ajcn.2008.26371 [DOI] [PubMed] [Google Scholar]
- 108.Katula JA, Vitolins MZ, Rosenberger EL, Blackwell C, Espeland MA, Lawlor MS, et al. Healthy Living Partnerships to Prevent Diabetes (HELP PD): design and methods. Contemp Clin Trials. 2010;31: 71–81. doi: 10.1016/j.cct.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Katula JA, Vitolins MZ, Rosenberger EL, Blackwell CS, Morgan TM, Lawlor MS, et al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care. 2011;34: 1451–1457. doi: 10.2337/dc10-2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuller LH, Simkin-Silverman LR, Wing RR, Meilahn EN, Ives DG. Women's Healthy Lifestyle Project: A randomized clinical trial: results at 54 months. Circulation. 2001;103: 32–37. [DOI] [PubMed] [Google Scholar]
- 111.Kuller LH, Kinzel LS, Pettee KK, Kriska AM, Simkin-Silverman LR, Conroy MB, et al. Lifestyle Intervention and Coronary Heart Disease Risk Factor Changes over 18 Months in Postmenopausal Women: The Women On the Move through Activity and Nutrition (WOMAN Study) Clinical Trial. J Womens Health (Larchmt). 2006;15: 962–974. [DOI] [PubMed] [Google Scholar]
- 112.Kuller LH, Gabriel KK, Kinzel LS, Underwood DA, Conroy MB, Chang Y, et al. The Women on the Move Through Activity and Nutrition (WOMAN) Study: Final 48-month results. Obesity (Silver Spring). 2012;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173: 113–121. Pii: 1485081. doi: 10.1001/2013.jamainternmed.987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mason C, Foster-Schubert KE, Imayama I, Kong A, Xiao L, Bain C, et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41: 366–375. doi: 10.1016/j.amepre.2011.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mason C, Risques RA, Xiao L, Duggan CR, Imayama I, Campbell KL, et al. Independent and combined effects of dietary weight loss and exercise on leukocyte telomere length in postmenopausal women. Obesity (Silver Spring). 2013;21: E549–E554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McAuley KA, Hopkins CM, Smith KJ, McLay RT, Williams SM, Taylor RW, et al. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia. 2005;48: 8–16. doi: 10.1007/s00125-004-1603-4 [DOI] [PubMed] [Google Scholar]
- 117.Merriam PA, Tellez TL, Rosal MC, Olendzki BC, Ma Y, Pagoto SL, et al. Methodology of a diabetes prevention translational research project utilizing a community-academic partnership for implementation in an underserved Latino community. BMC Med Res Methodol. 2009;9: 20–28. pii: 1471-2288-9-20. doi: 10.1186/1471-2288-9-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Potteiger JA, Jacobsen DJ, Donnelly JE. A comparison of methods for analyzing glucose and insulin areas under the curve following nine months of exercise in overweight adults. Int J Obes Relat Metab Disord. 2002;26: 87–89. doi: 10.1038/sj.ijo.0801839 [DOI] [PubMed] [Google Scholar]
- 119.Simkin-Silverman LR WRBMMEKL. Maintenance of cardiovascular risk factor changes among middle-aged women in a lifestyle intervention trial. Women's Health. 1998;4: 255–271. [PubMed] [Google Scholar]
- 120.Simkin-Silverman L, Wing RR, Hansen DH, Klem ML, Pasagian-Macaulay AP, Meilahn EN, et al. Prevention of cardiovascular risk factor elevations in healthy premenopausal women. Prev Med. 1995;24. [DOI] [PubMed] [Google Scholar]
- 121.The ODES investigators. The Oslo Diet and Exercise Study (ODES): design and objectives. Control Clin Trials. 1993;14: 229–243. [DOI] [PubMed] [Google Scholar]
- 122.Torjesen PA, Birkeland KI, Anderssen SA, Hjermann I, Holme I, Urdal P. Lifestyle changes may reverse development of the insulin resistance syndrome. The Oslo Diet and Exercise Study: a randomized trial. Diabetes Care. 1997;20: 26–31. [DOI] [PubMed] [Google Scholar]
- 123.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364: 1218–1229. doi: 10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365: 1969–1979. doi: 10.1056/NEJMoa1109220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang X, Imperatore G, Thomas W, Cheng Y, Lobelo F, Norris K, et al. Effect of lifestyle interventions on glucose regulation among adults without impaired glucose tolerance or diabetes: A systematic review and meta-analysis. Diab Res Clin Pract. 2017;123:149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Diabetes Prevention Program Research Group. Impact of intensive lifestyle and Metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care. 2005;28: 888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eriksson J, Lindstrom J, Valle T, Aunola S, Hamalainen H, Ilanne-Parikka P, et al. Prevention of Type II diabetes in subjects with impaired glucose tolerance: the Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999;42. [DOI] [PubMed] [Google Scholar]
- 128.Zhuo X, Zhang P, Selvin E, Hoerger TJ, Ackermann RT, Li R, et al. Alternative HbA1c cutoffs to identify high-risk adults for diabetes prevention: a cost-effectiveness perspective. Am J Prev Med. 2012;42: 374–381. pii: S0749-3797(12)00026-8. doi: 10.1016/j.amepre.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 129.Zhuo X, Zhang P, Kahn HS, Gregg EW. Cost-effectiveness of alternative thresholds of the fasting plasma glucose test to identify the target population for type 2 diabetes prevention in adults aged ≥45 years. Diabetes Care. 2013;36: 3992–3998. pii: dc13-0497. doi: 10.2337/dc13-0497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Toth PP. High-density lipoprotein as a therapeutic target: clinical evidence and treatment strategies. Am J Cardiol. 2005;96: 50K–58K. pii: S0002-9149(05)01372-X. doi: 10.1016/j.amjcard.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 131.Reboldi G, Gentile G, Angeli F, Ambrosio G, Mancia G, Verdecchia P. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29: 1253–1269. doi: 10.1097/HJH.0b013e3283469976 [DOI] [PubMed] [Google Scholar]
- 132.Fager G, Wiklund O. Cholesterol reduction and clinical benefit. Are there limits to our expectations? Arterioscler Thromb Vasc Biol. 1997;17: 3527–3533. [DOI] [PubMed] [Google Scholar]
- 133.Willett WC, Manson JE, Stampfer MJ, Colditz GA, Rosner B, Speizer FE, et al. Weight, weight change, and coronary heart disease in women. Risk within the 'normal' weight range. JAMA. 1995;273: 461–465. [DOI] [PubMed] [Google Scholar]
- 134.Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D’Agostino RB, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: The Framingham Heart Study. JAMA. 2003;290(8):1049–1056. doi: 10.1001/jama.290.8.1049 [DOI] [PubMed] [Google Scholar]
- 135.Goff DC Jr, Lloyd-Jones DM, Bennett G, O’Donnell CJ, Coady S, Robinson J, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63: 2935–2959. doi: 10.1016/j.jacc.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology (Bethesda). 2013;28: 330–358. pii: 28/5/330. [DOI] [PubMed] [Google Scholar]
- 137.Marcus BH, Williams DM, Dubbert PM, Sallis JF, King AC, Yancey AK, et al. Physical activity intervention studies: what we know and what we need to know: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity); Council on Cardiovascular Disease in the Young; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation. 2006;114: 2739–2752. pii: CIRCULATIONAHA.106.179683. doi: 10.1161/CIRCULATIONAHA.106.179683 [DOI] [PubMed] [Google Scholar]
- 138.Thompson PD. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23: 1319–1321. pii: 23/8/1319. doi: 10.1161/01.ATV.0000087143.33998.F2 [DOI] [PubMed] [Google Scholar]
- 139.Haskell WL. Cardiovascular disease prevention and lifestyle interventions: effectiveness and efficacy. J Cardiovasc Nurs. 2003;18: 245–255. [DOI] [PubMed] [Google Scholar]
- 140.Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2: 474–480. pii: S2213-8587(14)70057-9. doi: 10.1016/S2213-8587(14)70057-9 [DOI] [PubMed] [Google Scholar]
- 141.Mayega RW, Guwatudde D, Makumbi FE, Nakwagala FN, Peterson S, Tomson G, et al. Comparison of fasting plasma glucose and haemoglobin A1c point-of-care tests in screening for diabetes and abnormal glucose regulation in a rural low income setting. Diab Res Clin Pract. 2014;1 0 4:1 1 2–1 2 0. [DOI] [PubMed] [Google Scholar]
- 142.Kim C, Herman W, Cheung NW, Gunderson E, Richardson C. Comparison of hemoglobin A1c with fasting plasma glucose and 2-h postchallenge glucose for risk stratification among women with recent gestational diabetes mellitus. Diabetes Care 2011;34:1949–1951. doi: 10.2337/dc11-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Protocol-Study Protocol with Search Strategy.
Appendix B. PRISMA Checklist- Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist.
Table A. Intervention Characteristics.
Table B. Quality Assessment.
Table C. Lifestyle Interventional Effect: Meta-analyses Results in A Single Arm Model.
Table D. Intervention effect on FPG and percent weight: meta-analyses results.
(DOCX)
Data Availability Statement
Instructions for accessing studies reported on in this study are available in supporting information.