Abstract
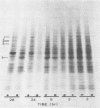
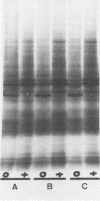
We have investigated the relationship between cell proliferation and protein synthetic capacity in a cytokinin-requiring strain of cultured soybean cells (Glycine max [L.] Merr. cv. Sodifuri, of cotyledonary origin) in suspension culture. When transferred to a defined medium lacking cytokinin, very little cell division or cell enlargement took place over the course of a 6-day culture period. Cells transferred to medium of the same composition, but containing 0.5 μm zeatin, exhibited rapid initial growth, with maximum mitotic activity occurring after 24 hours in culture, and a doubling of the cell population within the first 36 hours of the culture period. The polyribosomal RNA content of the cells decreased over the course of the first 24 hours of the growth cycle while the polyribosome to monoribosome (P/M) ratio increased. The increase in the P/M ratio was greater in the cytokinin-treated cells. This apparent relationship between cytokinin-induced cell proliferation and polyribosome formation was examined further. Polyribosome formation was stimulated when zeatin was added directly to cell populations which had been cultured for 24 hours in medium lacking a cytokinin. Transfer to fresh medium alone also stimulated polyribosome formation, whether this medium contained a cytokinin or not. The magnitude of transfer-induced polyribosome formation depended upon the initial cell density (number of cells/ml of medium). Regardless of the initial cell density and independent of the P/M ratios attained, the cytokinin-treated cell populations divided while the cytokinin-deprived cell populations did not. In vivo labeling with [35S]methionine and slab gel electrophoretic separation of sodium dodecyl sulfate derivatives of the labeled polypeptides demonstrated qualitative changes in the spectrum of proteins synthesized by the cytokinin-treated cells. These qualitative changes were independent of the cell density (and hence, independent of the P/M ratio) but they preceded cytokinin-induced cell division.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers L., Poulson R. Protein Synthesis in Cotyledons of Pisum sativum L: I. Changes in Cell-Free Amino Acid Incorporation Capacity during Seed Development and Maturation. Plant Physiol. 1972 Apr;49(4):476–481. doi: 10.1104/pp.49.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen M. D., Whitehead E. I., Kenefick D. G. Requirement for extraction of polyribosomes from barley tissue. Plant Physiol. 1972 May;49(5):733–739. doi: 10.1104/pp.49.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B. J., Turnock G. Synthesis and processing of ribosomal RNA in cultured plant cells. Eur J Biochem. 1973 Aug 17;37(2):367–376. doi: 10.1111/j.1432-1033.1973.tb02996.x. [DOI] [PubMed] [Google Scholar]
- Davies E., Larkins B. A., Knight R. H. Polyribosomes from peas: an improved method for their isolation in the absence of ribonuclease inhibitors. Plant Physiol. 1972 Nov;50(5):581–584. doi: 10.1104/pp.50.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. W., Cocking E. C. Protein synthesis in tomato-fruit locule tissue. Incorporation of amino acids into protein by aseptic cell-free systems. Biochem J. 1967 Jul;104(1):23–33. doi: 10.1042/bj1040023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. F., Kende H. Comparative Studies on Nitrate Reductase in Agrostemma githago Induced by Nitrate and Benzyladenine. Plant Physiol. 1974 Dec;54(6):821–825. doi: 10.1104/pp.54.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosket D. E., Torrey J. G. Hormonal control of cell proliferation and xylem differentiation in cultured tissues of Glycine max var. Biloxi. Plant Physiol. 1969 Jun;44(6):871–880. doi: 10.1104/pp.44.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R., Stanley W. M., Jr An economical procedure for the preparation of L-( 35 S)methionine of high specific activity. Anal Biochem. 1972 Jun;47(2):505–513. doi: 10.1016/0003-2697(72)90145-5. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J. Genetic control of the cell division cycle in yeast. Science. 1974 Jan 11;183(4120):46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Sequential function of gene products relative to DNA synthesis in the yeast cell cycle. J Mol Biol. 1976 Jul 15;104(4):803–817. doi: 10.1016/0022-2836(76)90183-2. [DOI] [PubMed] [Google Scholar]
- INGLE J., KEY J. L., HOLM R. E. DEMONSTRATION AND CHARACTERIZATION OF A DNA-LIKE RNA IN EXCISED PLANT TISSUE. J Mol Biol. 1965 Apr;11:730–746. doi: 10.1016/s0022-2836(65)80031-6. [DOI] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanneau J. P. Protein synthesis requirement for the cytokinin effect upon tobacco cell division. Exp Cell Res. 1975 Mar 1;91(1):184–190. doi: 10.1016/0014-4827(75)90156-1. [DOI] [PubMed] [Google Scholar]
- Kasten F. H., Lala R. The Feulgen reaction after glutaraldehyde fixation. Stain Technol. 1975 May;50(3):197–201. doi: 10.3109/10520297509117057. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Luttges M. W. Electrophoretic separation of nervous system proteins on exponential gradient polyacrylamide gels. J Neurochem. 1975 May;24(5):1077–1079. doi: 10.1111/j.1471-4159.1975.tb03680.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Davies E. Polyribosomes from Peas: V. An Attempt to Characterize the Total Free and Membrane-bound Polysomal Population. Plant Physiol. 1975 Apr;55(4):749–756. doi: 10.1104/pp.55.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Dyer J. A. Caution in the interpretation of plant ribosome studies. Biochem J. 1974 Oct;144(1):165–167. doi: 10.1042/bj1440165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G., Abrams M. A factor mediating interaction of kinins with the genetic material. Biochim Biophys Acta. 1970 Feb 18;199(2):511–518. doi: 10.1016/0005-2787(70)90093-6. [DOI] [PubMed] [Google Scholar]
- Payne P. I., Loening U. E. RNA breakdown accompanying the isolation of pea root microsomes. An analysis by polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1970 Nov 12;224(1):128–135. doi: 10.1016/0005-2787(70)90626-x. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Ramagopal S., Hsiao T. C. Polyribosomes from maize leaves. Isolation at high pH and amino acid incorporation. Biochim Biophys Acta. 1973 Mar 28;299(3):460–467. doi: 10.1016/0005-2787(73)90270-0. [DOI] [PubMed] [Google Scholar]
- Rogers M. E., Loening U. E., Fraser R. S. Ribosomal RNA precursors in plants. J Mol Biol. 1970 May 14;49(3):681–692. doi: 10.1016/0022-2836(70)90291-3. [DOI] [PubMed] [Google Scholar]
- Rudland P. S. Control of translation in cultured cells: continued synthesis and accumulation of messenger RNA in nondividing cultures. Proc Natl Acad Sci U S A. 1974 Mar;71(3):750–754. doi: 10.1073/pnas.71.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short K. C., Tepfer D. A., Fosket D. E. Regulation of polyribosome formation and cell division in cultured soybean cells by cytokinin. J Cell Sci. 1974 Jun;15(1):75–87. doi: 10.1242/jcs.15.1.75. [DOI] [PubMed] [Google Scholar]
- Sussex I., Clutter M., Walbot V. Benzyladenine reversal of abscisic Acid inhibition of growth and RNA synthesis in germinating bean axes. Plant Physiol. 1975 Nov;56(5):575–578. doi: 10.1104/pp.56.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Key J. L. Correlation between Polyribosome Level and the Ability to Induce Nitrate Reductase in Dark-grown Corn Seedlings. Plant Physiol. 1971 Nov;48(5):617–620. doi: 10.1104/pp.48.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. Control of the Protein Turnover Rates in Lemna minor. Plant Physiol. 1972 Jan;49(1):47–51. doi: 10.1104/pp.49.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Marcus A. Activation of Protein Synthesis upon Dilution of an Arachis Cell Culture from the Stationary Phase. Plant Physiol. 1974 Jan;53(1):83–87. doi: 10.1104/pp.53.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Marcus A. Regulation of RNA synthesis in plant cell culture: delayed synthesis of ribosomal RNA during transition from the stationary phase to active growth. Dev Biol. 1973 Jan;30(1):104–114. doi: 10.1016/0012-1606(73)90050-x. [DOI] [PubMed] [Google Scholar]