Abstract
Investigating the effects of a multifunctional microbial peptide possessing strong anti-inflammatory activity against pathogenic bacteria. The antimicrobial activity of the purified peptide (CSP32) against various multidrug-resistant as well as anaerobic pathogens was determined. Anti-inflammatory activity was determined by an enzyme-linked immunosorbent assay, western blotting, and RT-PCR in RAW 264.7 macrophages. Molecular weight and structural elucidation were performed by several analytical methods such as mass spectrometry and chemoinformatic analysis. CSP32, purified from newly isolated Bacillus sp. CS32, was active against methicillin-resistant Staphylococcus aureus, vancomycin-resistant S. aureus, vancomycin-resistant enterococci, and anaerobic pathogens Propionibacterium acne and Clostridium difficile. Furthermore, CSP32 showed strong inhibitory effects on lipopolysaccharide (LPS)-induced nitric oxide (NO) production and nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) expression in RAW 264.7 macrophages. At concentrations of 10, 50, and 100 μg/mL, CSP32 treatment attenuated LPS-induced expression of nuclear factor kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs) as well as other proinflammatory mediators such as tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and IL-1β. CSP32 potently inhibited translocation of NF-κB into the nucleus by suppressing degradation of IκB kinase (IκBα) and its phosphorylation, thereby causing NF-κB to remain inactive. CSP32 may be the first oligomer of bacitracin with anti-inflammatory properties.
Conclusion
CSP32 has stable characteristics and may find bio-industrial and therapeutic applications.
Introduction
Antimicrobial peptides (AMPs) are ubiquitously secreted by a broad range of microorganisms, including bacteria, to protect themselves from other microbes [1]. These peptides are composed of 10–40 amino acid residues and affect active components of the innate immune response. In addition, AMPs have been confirmed to kill Gram-negative and positive bacteria including clinically important pathogens, mycobacteria, protozoa, viruses, fungi, and cancer cells [2]. AMPs produced by microbes such as Bacillus spp. have major therapeutic applications [3]. Bacillus is a genus of Gram-positive, rod-shaped, endospore-forming bacteria widespread in the environment and can serve as obligate aerobes or facultative anaerobes. Bacillus has been widely used in the fermentation industry for the production of antibiotics and several extracellular enzymes [4, 5]. A large number of peptides with biological activities from this group has been reported and has become a center of attention for antimicrobial research [3, 6–14]. On the basis of their origin, these peptides are categorized into two classes: nonribosomally synthesized and ribosomally synthesized. The first group includes bacitracin, penicillin, and some others, containing nonprotein amino acids such as D-amino acids or hydroxyl amino acids and other amino acids that undergo extensive modification. The second group, which includes subtilin, undergoes posttranslational modification and proteolytic processing [10].
Inflammation is a part of the complex biological response of vascular tissues to harmful stimuli, such as pathogens, damaged cells, or irritants [15]. The inflammatory process is a protective response to infection, tissue injury, or noxious stimuli [16]. MAPKs translate extracellular signals into a variety of cellular processes, including cell differentiation, proliferation, survival, and apoptosis. NF-κB is one of the most vital transcription factors involved in transactivation of a variety of genes associated with regulation of immune function, cellular proliferation, inflammatory responses, and tumorigenesis. In the inflammatory process, activated inflammatory cells secrete increased amounts of nitric oxide (NO) and cytokines, such as interleukins IL-1β, IL-6, and tumor necrosis factor (TNF)-α [17]. NO is a major product, and its formation is controlled by NO synthases (e.g., iNOS). iNOS is highly expressed in macrophages, and its activation leads to organ destruction in some inflammatory and autoimmune diseases. During inflammation, macrophages play a central role in the management of many immunopathological phenomena, including the overproduction of proinflammatory cytokines and inflammatory mediators such as iNOS, COX-2, TNF- α, IL-1β, and IL-6 [17, 18].
The rapid development of antibiotic resistance has drawn many researchers’ attention to the search for new antibacterial agents. Nonsteroidal anti-inflammatory drugs (NSAIDs) have been used widely in our society for the treatment of acute and chronic inflammatory diseases. However, treatment with NSAIDs for a prolonged period is associated with a major side effect: gastrointestinal problems. Therefore, researchers have continued to show interest in screening of new biological compounds from various sources. For a long time, our research group has maintained interest in identification of new compounds that have both antibacterial and anti-inflammatory properties with low toxicity.
With the aim of characterizing a microbial peptide with therapeutic applications, an AMP-producing strain was isolated from traditional fermented Korean kimchi. The AMP was purified, biochemically characterized, and its structure was analyzed. The antimicrobial and anti-inflammatory activities of the pure compound were evaluated.
Materials and methods
Materials
Sepharose CL-6B and Sephadex G-50 were acquired from Pharmacia (Uppsala, Sweden). Trypsin, proteinase K, α-chymotrypsin, and lipase were purchased from Sigma (St. Louis, MO, USA). Lipopolysaccharide (LPS), dimethyl sulfoxide (DMSO), Griess reagent, and 3-(4,5-dimethylthiazol-2-2,5-diphenyltetrazolium bromide (MTT) were also purchased from Sigma. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and a penicillin-streptomycin solution were acquired from Invitrogen (Grand Island, NY, USA). A rabbit anti-mouse iNOS polyclonal antibody was purchased from Santa Cruz Biotech Inc. (Santa Cruz, CA, USA), and an anti-mouse COX-2 antibody from Cayman Co. Horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG antibody and anti-mouse IgG antibody were purchased from Cell Signaling Technology (Danvers, MA). Alkaline-phosphatase-conjugated donkey anti-mouse IgG antibody was purchased from Jackson Immunoresearch Laboratories Inc. Mouse TNF-α, IL-6, IL-1β, and an enzyme-linked immunosorbent assay (ELISA) kits were purchased from BD Biosciences (San Diego, CA, USA). All other chemicals and reagents were of analytical grade.
In vitro screening, isolation, and culture conditions for Bacillus sp. CS32
Korean fermented kimchi food samples were collected at various locations in Chonnam Province. The Korean food samples were resuspended in distilled water. After dilution, the samples were inoculated onto the surface of Bacillus isolation agar (MRS, Mueller Hinton) plates. The 16S ribosomal RNA (rRNA) sequence analysis was carried out according to Bergey’s Manual of Systemic Bacteriology as stated in our previous report [19]. All the non-ATCC strains are deposited in Korean collection for type culture (KCTC), which is belong to World Data Centre for Microorganisms (WDCM). AMP production by the strain was optimized by means of several carbon and nitrogen sources. The influence of various carbon sources on AMP production was determined using media supplemented with 1% yeast extract combined with 1% supplements such as glucose, mannitol, starch, lactose, fructose, sorbitol, sucrose, or maltose. Fermentation was carried out in 250-mL Erlenmeyer flasks containing 50 mL of a medium with constant shaking at 180 rpm. After that, the influence of the nitrogen source on peptide production was determined using a medium containing 1% glucose (best carbon source) combined with 1% supplements such as beef extract, malt extract, tryptone, yeast extract, oat meal, soytone, or peptone.
Purification of CSP32
Bacillus sp. CS32 was cultured for 60 h in the glucose–beef extract–peptone medium (1% glucose, 0.5% beef extract, and 0.5% peptone). The cells were separated by centrifugation (6000 × g rpm, 30 min, 4°C), and peptides were precipitated from the supernatant at 4°C overnight with ammonium sulfate of 60% saturation. The precipitate was collected by centrifugation (6000 × g rpm, 60 min, 4°C), resuspended in 10 mM Tris-HCl buffer (pH 8.0) and dialyzed using a 1-kDa cutoff membrane (Millipore). The crude extracts were applied to a Sepharose CL-6B column (2.2 × 116 cm) and next to a Sephadex G-50 column (1.5 × 70 cm), and eluted with the same buffer. Active fractions were pooled and concentrated using a YM1 amicon filter and stored at −20°C.
Analysis of molecular weight and N-terminal amino acid sequence
The molecular weight of the purified peptide was determined by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (tricine SDS-PAGE). For in situ detection of inhibitory activity, the gel was washed with 50 mM Tris-HCl buffer (pH 7.9) containing 2.5% Triton X-100 and overlaid with soft agar containing an indicator strain, Mycobacterium smegmatis ATCC 9341, (106 colony-forming units) and incubated overnight at 37°C. N-terminal amino acid sequencing was carried out in the Process 492 Amino Sequencer (Applied Biosystems, Foster City, CA) by the automated Edman degradation method.
Mass spectrometry (MS) and chemoinformatic analysis
The molecular mass of the peptide was evaluated by matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) MS. Furthermore, liquid chromatography with tandem mass spectrometry (LC-MS/MS) was performed on a triple-quadruple tandem mass spectrometer in positive electrospray ionization (ESI) mode as well. The product mass spectra were collected in the m/z range from 50 to 3000 Da.
Antimicrobial activity of CSP32
This activity in terms of minimal inhibitory concentration (MIC) was determined by dilution and disk diffusion methods as per the Clinical and Laboratory Standards Institute (CLSI) guidelines for antimicrobial susceptibility testing [20]. The examination of bacteria was conducted after incubation for 18 h at 37°C following inoculation of various test microorganisms, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant S. aureus (VRSA), or vancomycin-resistant enterococci (VRE). Control antibiotics were bacitracin and vancomycin.
Susceptibilities for Propionibacterium acnes KCTC5933 and Clostridium difficile KCTC2251 were determined using the NCCLS reference agar dilution procedure [21]. A colony from a 40 h culture was suspended in thioglycolate broth and adjusted a density equivalent to a McFarland 0.5 standard then inoculated onto Brucella agar (Difco, Becton Dickinson, Sparks MD21152, USA) supplemented with 5% lysed sheep blood, 5 mg/L haemin and 1 mg/L vitamin K. Afterwards, plates were inoculated with ~105 cfu per spot, using an antibiotic sensitivity replicator (H.I. Clements Pty Ltd., Sydney, Australia). All plates were incubated in an anaerobic cabinet (Don Whitley Scientific Ltd, West Yorkshire, England) for 48 h. Propionibacterium acnes and Clostridium difficile were used an anaerobic control bacteria. All antimicrobials were tested at dilutions, from 0.2 to 0.02 mg/L (CSP32) and 1 to 0.125 mg/L (Monensin).
Stability of the Bacillus sp. CS32-produced AMP
To analyze thermostability, CSP32 activity was evaluated by measuring residual activities after various treatments. After heat treatment (30–121°C, 10–60 min), the samples were tested for residual antimicrobial activity against indicator strain Micrococcus luteus ATCC 9341. Effects of pH on antimicrobial activity were estimated by varying the pH levels. Samples were diluted in the following buffers: citrate with Na2HPO4 (pH 3.0–7.0), Tris-HCl (pH 7.0–9.0), and NaHCO3 with NaOH (pH 10.0–11.0). After incubation for 1 h at room temperature, pH in the samples was adjusted to 7.0, and the samples were tested for antimicrobial activity against the indicator strain. The AMP was digested with various proteolytic enzymes at a final concentration of 1 mg/mL: lipase (50 mM Tris-HCl, pH 7.5), protease K (50 mM Tris-HCl, pH 7.5), α-chymotrypsin (50 mM Tris-HCl, pH 7.5), or trypsin (50 mM Tris-HCl, pH 8.0). After 1-h incubation at room temperature, the enzymes were boiled for 2 min for inactivation.
Cell culture and assessment of cell viability
Cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously by Choi et al. (2016) [22]. For the cell viability assay, RAW 264.7 cells were seeded into a 96-well flat bottom microplate at a density of 2 × 104 cells per well and incubated at 37°C for 1 h. The cells were then treated with various concentrations (5–100μg/mL) of CSP32. After 24 h of incubation, the medium was removed and wash in PBS once followed by 100 μl of MTT (5 mg/ml in PBS) solution was added to each well, and the plate was incubated for another 1 h. Then 100 μl of DMSO (100%) was added to dissolve the formazan crystals. Absorbance values were then measured at 450 nm using a microplate reader (Victor3, PerkinElmer).
NO analysis and quantification of cytokine production
NO levels in the culture supernatants were measured by the Griess reaction. Cells (106 cells/mL) were seeded in 6-well plates and pretreated with the indicated concentrations of AMP for 30 min before stimulation with 1 μg/mL LPS for 24 h. The sample supernatants were mixed with an equal volume of the Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% naphthyl ethylene diamine dihydrochloride), and then incubated at room temperature for 10 min. The absorbance was measured at 550 nm on a microplate reader (Thermo Fisher Co.). The nitrite concentration was determined using a dilution of sodium nitrite as a standard.
An enzyme immunoassay of cytokines
The inhibitory effects of CSP32 on the production of proinflammatory cytokines—TNF-α, IL-1β, and IL-6—were determined by an enzyme-linked immune sorbent assay (ELISA). Cells were incubated with various concentrations of CSP32 (10–100 μg/mL) for 1 h followed by LPS (1 μg/mL) treatment for 24 h. The concentration of nitrite in the supernatant was calculated using a sodium nitrite standard curve. The supernatant was analyzed for TNF-α, IL-1β, and IL-6 by ELISAs using commercial kits (BD Biosciences) according to the manufacturer’s instructions.
Western blotting
Macrophages were incubated with or without LPS in the presence or absence of the AMPs. Cells were harvested, washed twice with ice-cold Tris-HCl-buffered saline (TBS), and resuspended in lysis buffer [100 mM Tris-HCl, 5 mM EDTA, 50 mM NaCl, 50 mM β- glycerophosphate, 50 mM NaF (sodium fluoride), 0.5% NP-40, 1% sodium deoxycholate, 0.1 mM sodium orthovanadate, and 1% phenylmethylsulfonyl fluoride (PMSF)]. The cytosolic fraction was obtained from the supernatant after 12000 × g rpm centrifugation at 4°C for 20 min. Samples (20 μg in terms of protein) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 10% gel and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% nonfat milk in TBS with Tween 20 (0.1%) for 2 h and then incubated with primary antibodies specific for ERK, phospho-ERK, JNK, phospho-JNK, p38 MAPK, phospho-p38 MAPK (all them from Cell Signaling Technology), NF-κB, IκB, and phospho-IκB (1:1000; Santa Cruz Biotechnology). To prove equal loading, the blots were analyzed for β-actin expression using an anti-β-actin antibody (Santa Cruz Biotechnology). After three washes with TBS–Tween 20, the membrane was hybridized with an HRP-conjugated secondary antibody for 1 h. The membranes were washed three times for 10 min and developed with the ECL Western Blotting Detection System (GE Healthcare, Incheon, Republic of Korea,). The immunoreactive proteins were detected by means of a LAS-3000 Luminescent image analyzer (Fuji Photo Film Co., Ltd., Tokyo, Japan).
RNA preparation and mRNA expression analysis by reverse-transcription (RT)-PCR
Total RNA from the AMP-treated cells was prepared using the RNAiso Reagent (Takara) and was stored at −80°C until use. For the detection of iNOS, COX-2, TNF-α, IL-Iβ, and IL-6, total RNA was extracted after stimulation and treatment. The mRNA expression levels of iNOS, COX-2, TNF-α, IL-Iβ, and IL-6 in the treated cells were compared to the expression levels in control cells. One microgram of RNA was reverse-transcribed into cDNA and used as a template for PCR amplification. The primer sequences are listed in Table 1. PCR was performed on a DNA Gene Cycler, under the following conditions: 40 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and primer extension at 72°C for 40 s. PCR products were analyzed on 1% agarose gels, and bands were visualized by ethidium bromide staining.
Table 1. Primers for RT-PCR.
| Primers | Primer sequences | |
|---|---|---|
| Forward | Reverse | |
| iNOS | 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ | 5′-GGCTGTCAGAGCCTCGTGGCTTTGG-3′ |
| COX-2 | 5′-CACTACATCCTGACCCACTT-3′ | 5′-ATGCTCCTGCTTGAGTATGT-3′ |
| TNF-α | 5′-TCTCATCAGTTCTATGGCCC-3′ | 5′-GGGAGTAGACAAGGTACAAC-3′ |
| IL-1β | 5′-TGGACGGACCCCAAAAGATG-3′ | 5′-AGAAGGTGCTCATGTCCTCA-3′ |
| IL-6 | 5′-GTTCTCTGGGAAATCGTGGA-3′ | 5′-TGTACTCCAGGTAGCTATGG-3′ |
| GAPDH | 5′-CACTCACGGCAAATTCAACGGCAC-3′ | 5′-GACTCCACGACATACTCAGCAC-3′ |
Statistical analysis
Student’s t-test and one- way multivariate analysis of variance (ANOVA) were used to analyze the data. Differences were considered significant when *p < 0.01.
Results
Identification of strain CS32
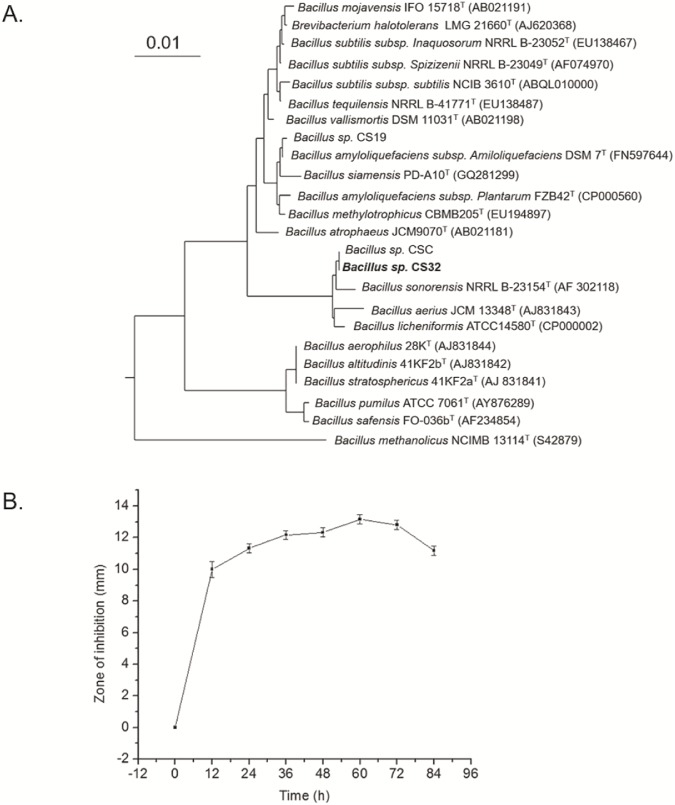
Microbial strains capable of producing AMPs were isolated from traditional Korean fermented kimchi. Morphological and biochemical characteristics showed that strain CS32 is a Bacillus species. Furthermore, the 16S rRNA sequence of the local isolate was compared with the sequences of closely related Bacillus species. Computer-assisted RNA searches in a bacterial database revealed that the 16S rRNA sequence of CS32 was 99.392% identical to Bacillus licheniformis. The phylogenetic tree based on the sequence alignment is presented in Fig 1A.
Fig 1.
(A) A neighbor-joining tree based on nearly complete 16S rRNA gene sequences showing relations between strain CS32 and related members of the genus Bacillus. The percentages at the nodes are the levels of bootstrap support based on neighbor-joining analyses of 1000 resampled datasets. The sequence of Bacillus licheniformis ATCC 14580(T) served as an outgroup. The scale bar: 0.01 nucleotide substitutions per position. (B) Production of CSP32 and the growth-inhibitory activity against Mycobacterium smegmatis ATCC 9341. The antibacterial activity is maximal at 60 h. The zone of inhibition against Mycobacterium smegmatis ATCC 9341 at every 12 h is shown. Cultivation was carried out in 250-mL flasks with 50 mL of a medium, at pH 7 and 37°C, with shaking at 180 rpm.
Purification, molecular weight and N-terminal sequence analysis
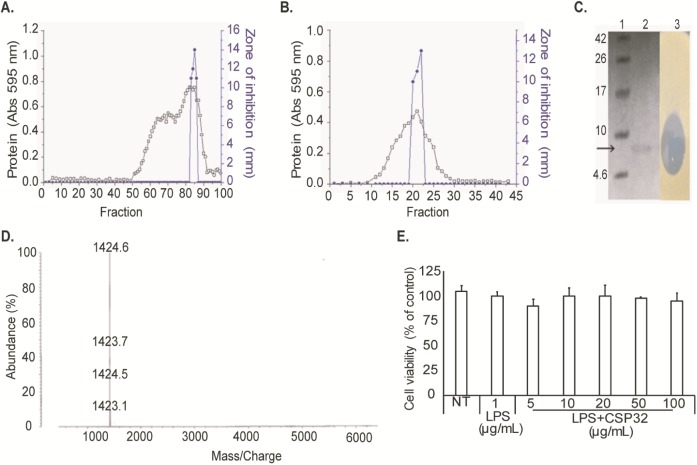
The CSP32, from 60 h cultured cell-free supernatant (Fig 1B), was purified to homogeneity by a two-step procedure (Fig 2A and 2B). Tricine SDS-PAGE and an assay of in situ inhibitory activity (Fig 2C) confirmed the homogeneity and activity of the purified AMP. In the MALDI-TOF-MS analysis (Fig 2D), CSP32 appeared as a single peak with the molecular mass of 5697.9 Da which confirmed the presence of single compound. The first 12 amino acid residues of the N terminus of CSP32 were found to be APLEIXXIFHDN. The sequence was compared with related bacitracin derivatives presented in Table 2.
Fig 2.
(A) Gel filtration chromatography on a Sepharose CL-6B column (2.2 cm × 116 cm). The proteins were eluted at a flow rate of 5 mL/min. (B) Gel filtration chromatography on a Sephadex G-50 column (1.5 cm × 70 cm). The proteins were eluted at a flow rate of 1 mL/min. Determination of the molecular weight. (C) Tricine SDS-PAGE and activity staining of CSP32. Tricine SDS-PAGE: Lane 1, protein molecular weight markers with the corresponding value in kDa on the left; Lane 2, purified CSP32; Lane 3, activity staining (in situ). (D) MALDI-TOF-MS. (E) Cell viability was measured after 24-h incubation. Survival rates were tested by an MTT assay in RAW 264.7 cells. The cells were incubated in the presence or absence of 5–100 μg/mL CSP32 for 24 h. Each bar shows mean ± SD of three independent experiments performed in triplicate.
Table 2. Comparative analysis of N-terminal amino acid sequences.
| Sl. No. | peptide | amino acid residues | theoretical MW (Da) | identity (%) | monomer sequence |
|---|---|---|---|---|---|
| 1 | CSP32 | A-P-L-E-I-X-X-I-F-H-D-N | 1390.98 | 100 | current study |
| 2 | Bacitracin A1 | I-C-L-E-I-K-O-I-F-H-D-N | 1581.89 | 66.7 | Position 1: Ile, position 2: Cys |
| 3 | Bacitracin A2 | I-C-L-E-I-K-O-I-F-H-D-N | 1581.89 | 66.7 | 1: D-aIle, 2: Cys |
| 4 | Bacitracin D1 | V-C-L-E-I-K-O-V-F-H-D-N | 1553.84 | 66.7 | 2: Cys |
| 5 | Bacitracin H1 | V-C-L-E-I-K-O-I-F-H-D-N | 1567.87 | 66.7 | 2: dhCys |
| 6 | Bacitracin F | I-C-L-E-I-K-O-I-F-H-D-N | 1581.89 | 66.7 | 1: Ile, 2: dhCys |
| 7 | Bacitracin B3 | I-C-L-E-I-K-O-V-F-H-D-N | 1567.87 | 58.3 | 2: CYS |
| 8 | Bacitracin I1 | V-C-L-E-I-K-O-V-F-H-D-N | 1553.84 | 58.3 | 2: dhCys |
| 9 | Bacitracin I2 | V-C-L-E-V-K-O-I-F-H-D-N | 1553.84 | 58.3 | 2: dhCys |
| 10 | Bacitracin H2 | I-C-L-E-I-K-O-V-F-H-D-N | 1567.87 | 58.3 | 2: dhCys |
| 11 | Bacitracin H3 | I-C-L-E-V-K-O-I-F-H-D-N | 1567.87 | 58.3 | 2: dhCys |
| 12 | Bacitracin B2 | I-C-L-E-V-K-O-I-F-H-D-N | 1567.87 | 58.3 | 2: Cys |
| 13 | Bacitracin B1 | V-C-L-E-I-K-O-I-F-H-D-N | 1567.87 | 58.3 | 2: Cys |
| 14 | Bacitracin D2 | V-C-L-E-V-K-O-I-F-H-D-N | 1553.84 | 58.3 | 2: Cys |
| 15 | Bacitracin I3 | I-C-L-E-V-K-O-V-F-H-D-N | 1553.84 | 50.0 | 2: dhCys |
| 16 | Bacitracin E | V-C-L-E-V-K-O-V-F-H-D-N | 1539.81 | 50.0 | - |
| 17 | Bacitracin C | I-C-L-E-V-K-O-V-F-H-D-N | 1553.84 | 50.0 | 2: Cys |
The effect of CSP32 on viability of RAW 264.7 cells
RAW 264.7 cells were incubated with various concentrations of CSP32 for 24 h and cell viability was assessed by the MTT assay. As shown in Fig 2E, CSP32 did not exert cytotoxicity toward RAW 264.7 cells in the range of 5–100 μg/mL. Therefore, CSP32 was used at or below 100 μg/mL in the further experiments.
Structure elucidation
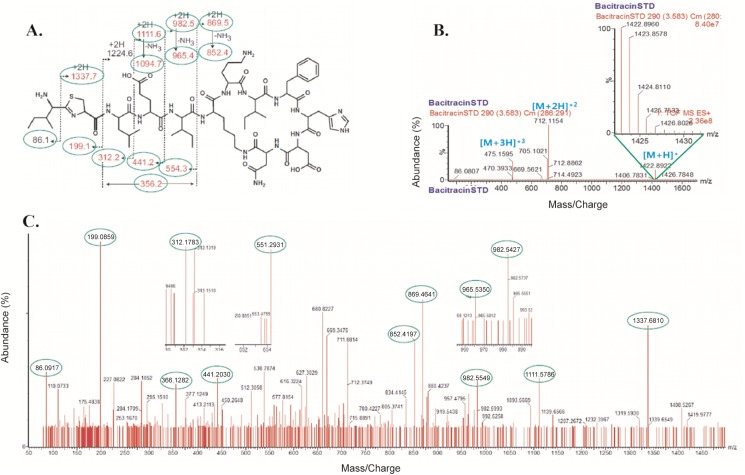
The MS result of CSP32 matched with Bacitracin ~1422 Da with several subunits (up to 11). A robust and efficient HR-QTOF ESI/MS analysis revealed the existence of a complex of 1–11 monomers of bacitracin, a nonribosomal peptide (Table 3), in CSP32. MS/MS analysis of CSP32 and peptide sequencing (analysis of the MS fragmentation pattern) showed that the MS/MS molecular pattern was identical to that of bacitracin A (Fig 3A–3C).
Table 3. Comparison of MS data with predicted molecular weight of each subunit.
| Subunit no | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MW X unit no | 1421.7540 | 2843.5080 | 4265.2620 | 5687.0160 | 7108.7700 | 8530.5240 | 9952.2780 | 11374.0320 | 12795.7860 | 14217.5400 | 15639.2940 |
| 1421.7540 | 2843.5080 | 4265.1894 | 5687.0004 | 7108.7504 | 8530.4875 | 9952.3705 | 11374.9940 | 12795.7555 | 14217.5000 | 15639.5010 | |
| 1422.7567 | 2844.5112 | 4266.1544 | 5688.0012 | 7109.7520 | 8531.4920 | 9953.3330 | 11375.9935 | 12796.8480 | 14218.9380 | 15641.3730 | |
| 1423.7597 | 2845.5134 | 4267.1876 | 5689.0125 | 7110.7780 | 8532.5310 | 9954.3035 | 11377.0030 | 12797.8370 | 14220.3040 | 15642.2640 | |
| 1424.7615 | 2846.5202 | 4268.1876 | 5690.0178 | 7111.7648 | 8533.5055 | 9955.2755 | 11378.0345 | 12798.8245 | 14221.4610 | 15642.9816 | |
| 1425.7600 | 2847.5194 | 4269.1832 | 5691.0183 | 7112.7676 | 8534.5250 | 9956.2780 | 11379.0225 | 12799.8475 | 14222.5820 | 15644.2674 | |
| MS data | 1426.7638 | 2848.5144 | 4270.1958 | 5692.0143 | 7173.7696 | 8535.5270 | 9957.2820 | 11380.0415 | 12800.7530 | 14223.5890 | 15645.1848 |
| 1427.7617 | 2849.5230 | 4271.1768 | 5693.0234 | 7114.7636 | 8536.5390 | 9958.2815 | 11381.0290 | 12801.8360 | 15646.3302 | ||
| 1428.7636 | 2850.5250 | 4272.2246 | 5694.0237 | 7115.7656 | 8537.5115 | 9959.2770 | 11382.0645 | 12802.8200 | 15647.2176 | ||
| 1429.7592 | 2851.5200 | 5695.0125 | 7116.7712 | 8538.5200 | 9960.2960 | 11383.0520 | 12803.7915 | 15648.3324 | |||
| 1430.7570 | 2852.5114 | 5696.0238 | 7118.7848 | 8539.5195 | 9961.2915 | 11384.0550 | 12804.8265 | 15649.2642 | |||
| 1431.7538 | 2853.5276 | 5697.0195 | 7119.7876 | 8540.5480 | 9963.2830 | 11384.9965 | 12805.8315 | 15650.2926 | |||
| 1432.7393 | 9964.3160 | 11387.0445 | 12806.7895 | 15651.3954 | |||||||
| 11388.0590 | 12807.8030 | 15652.3416 | |||||||||
| 12809.9095 | 15653.2998 | ||||||||||
| 15654.4032 | |||||||||||
| 15655.4178 | |||||||||||
| 15656.2356 |
Fig 3. Chemoinformatic analysis of bacitracin A.
(A) Standard structure of bacitracin A; (B) fragmentation patterns and percentage of abundance of standard bacitracin A and (C) of CSP32.
Antimicrobial activity of CSP32
As shown in Table 4, CSP32 showed antimicrobial activity against Gram-positive but not Gram-negative bacteria. Moreover, CSP32 exerted an antagonistic effect against multidrug-resistant pathogens such as M. luteus, MRSA, VRSA, and VRE, which was similar or in some cases stronger than that of bacitracin or vancomycin.
Table 4. Minimum inhibitory concentration of CSP32 against various pathogens.
| Test organisms | MIC (μg/mL) | ||
|---|---|---|---|
| CSP32 | Bacitracin | Vancomycin | |
| Alcaligenes faecalis ATCC 1004 | >80 | >80 | >80 |
| Salmonella typhimurium KCTC 1925 | >80 | >80 | >80 |
| Escherichia coli KCTC 1923 | >80 | >80 | >80 |
| Pseudomonas aeruginosa KCTC 1637 | >80 | >80 | >80 |
| Micrococcus luteus ATCC 9341 | 0.156 | >80 | 0.156 |
| Mycobacterium smegmatis ATCC 9341 | >80 | >80 | 0.3125 |
| Enterococcus faecalis ATCC 29212 | 5 | 5 | 2.5 |
| Bacillus subtilis ATCC 6633 | >80 | 40 | >80 |
| Listeria monocytogenes KCTC3569 | 10 | 0.3125 | 0.156 |
| Staphylococcus aureus KCTC 1928 | 5 | 40 | 0.3125 |
| MRSA 639E | 2.5 | 40 | 0.3125 |
| MRSA 4–5 | 5 | 1.25 | 0.3125 |
| MRSA 5–3 | 2.5 | 1.25 | 0.3125 |
| MRSA U4 | 40 | 1.25 | 0.3125 |
| MRSA S3 | 10 | 1.25 | 0.3125 |
| MRSA P3 | 40 | 1.25 | 0.625 |
| MSRA S1 | 40 | 1.25 | 0.625 |
| VRSA | 40 | >80 | >80 |
| VRE 2 | 10 | 0.3125 | >80 |
| VRE3 | >80 | 20 | >80 |
| VRE4 | 10 | 5 | >80 |
| VRE5 | 10 | 1.25 | >80 |
| VRE6 | >80 | >80 | >80 |
| VRE82 | 80 | 40 | >80 |
| VRE89 | >80 | 40 | >80 |
Furthermore, CSP32 showed potent antimicrobial activity against anaerobic pathogens P. acne and C. difficile. When we compared its activity with monensin, CSP32 exerted manyfold stronger activity than that of monensin against those pathogens (Table 5).
Table 5. Antimicrobial activity of CSP32 and Monensin against anaerobic pathogenic microorganisms.
| Concentration (mg/mL) | Propionibacterium acnes (mm) |
Clostridium difficile (mm) |
|
|---|---|---|---|
| CSP32 | 0.2 | 23.33±0.58 | 19.00±0.50 |
| 0.1 | 19.00±0.50 | 17.17±0.29 | |
| 0.04 | 17.33±0.58 | 15.17±0.29 | |
| 0.02 | 12.83±0.29 | 13.17±0.58 | |
| Monensin | 1 | 17.17±0.29 | 17.33±0.29 |
| 0.5 | 17.33±0.58 | 15.17±0.29 | |
| 0.25 | 15.17±0.29 | 13.00±0.50 | |
| 0.125 | 13.00±0.50 | 13.17±0.29 |
Results are presented as means ± standard deviation (n = 3).
Thermal, pH, and proteolytic resistance of CSP32
The effects of temperature and pH on stability of CSP32 are shown in Table 6. These data indicate that CSP32 was almost completely stable up to 90°C and at pH 5.0–12.0. Its activity decreased sharply at or above 121°C. To test the effect of proteolytic enzymes, CSP32 was incubated with lipase, proteinase K, α-chymotrypsin, and trypsin agar diffusion assay. Residual activity in the presence of lipase, proteinase K, α-chymotrypsin or trypsin was 98.3%, 95.7%, 94.3%, and 100%, respectively, as compared to no-treatment control (set to 100%) (data not provided). Results are presented as means ± standard deviation (n = 3).
Table 6. Thermo and pH-stability of CSP32.
| Temperature | Time | Residual activity (%) |
|---|---|---|
| None | 100 | |
| 30°C | 10 min | 100 |
| 30 min | 100±0.28 | |
| 60 min | 100±0.34 | |
| 50°C | 10 min | 100±0.94 |
| 30 min | 98.40±0.39 | |
| 60 min | 98.07±0.36 | |
| 70°C | 10 min | 98.07±0.41 |
| 30 min | 98.00±0.41 | |
| 60 min | 98.07±0.39 | |
| 90°C | 10 min | 97.73±1.04 |
| 30 min | 95.93±0.05 | |
| 60 min | 95.93±0.05 | |
| 121°C | 15 min | 65.40±0.17 |
| pH | ||
| pH 2 | 61.33±1.04 | |
| pH 3 | 63.00±1.04 | |
| pH 4 | 89.60±3.44 | |
| pH 5 | 95.87±2.29 | |
| pH 6 | 100±0.50 | |
| pH 7 | 100 | |
| pH 8 | 100±0.20 | |
| pH 9 | 100±1.15 | |
| pH 10 | 98±3.38 | |
| pH 11 | 91.33±0.06 | |
| pH 12 | 91.77±0.06 |
Results are presented as means ± standard deviation (n = 3)
Inhibition of NO production and iNOS, COX-2 protein expression in LPS-stimulated RAW 264.7 macrophage cells
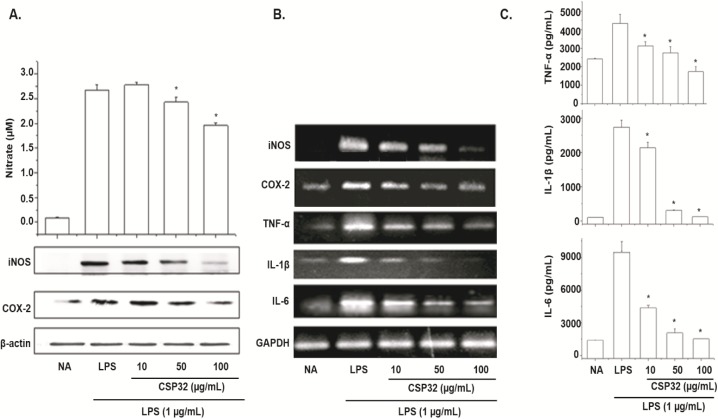
To assess the effect of CSP32 on NO production by LPS-stimulated RAW 264.7 cells, we measured NO concentration in the culture medium by the Griess method [23]. As shown in Fig 4A, LPS treatment significantly increased NO production as compared to the untreated cells. Treatment of cells with CSP32 at 10, 50, or 100 μg/mL attenuated the LPS-induced production of NO to a statistically significant extent.
Fig 4. Effects of CSP32 on NO production and expression of inflammation-associated proteins in LPS-stimulated RAW 264.7 macrophage cells.
The cells were treated with LPS (1 μg/mL) in the presence of various concentrations of CSP32 (10, 50, or 100 μg/mL) at 37°C for 24 h. (A) The amounts of NO were determined using the Griess reagent in the culture medium (Top panel). Equal amounts of cell lysates were resolved on SDS polyacrylamide gels, transferred to PVDF membranes, and probed with antibodies against iNOS and COX-2. β-Actin served as an internal control for western blot analysis (bottom panel). Effects of CSP32 on LPS-induced mRNA expression of iNOS, COX-2, TNF-α, IL-1β, and IL-6. (B) After LPS treatment for 2–12 h, the levels of iNOS, COX-2, TNF-α, IL-1β, and IL-6 mRNA were determined by RT-PCR. GAPDH served as an internal control for RT-PCR assays. Suppressive effects of CSP32 on TNF-α, IL-1β, and IL-6 production in LPS-stimulated RAW 264.7 macrophage cells. (C) The cells were incubated with the indicated concentrations of CSP32 for 30 min before treatment with LPS (1 μg/mL) for 24 h. After incubation for 24 h, the supernatant was collected, and the amounts of these proinflammatory cytokines were measured by ELISAs. Results represent the mean±S.D of three independent experiments performed in triplicate. * p<0.01; compared to LPS alone.
In an attempt to determine whether the inhibition of NO production by CSP32 was related to downregulation of iNOS and COX-2, we next examined the protein expression levels by western blotting. As shown in Fig 4A, both iNOS and COX-2 protein levels increased in the LPS-stimulated cells when compared to the controls. CSP32 treatment significantly attenuated this induction in a dose-dependent manner. The suppression of iNOS and COX-2 protein expression correlated well with that of NO production.
Effects of CSP32 on NO production and expression of inflammation-associated proteins in LPS-stimulated RAW 264.7 macrophage cells
The cells were treated with LPS (1 μg/mL) in the presence of various concentrations of CSP32 (10, 50, or 100 μg/mL) at 37°C for 24 h. (A) The amounts of NO were determined using the Griess reagent in the culture medium (Top panel). Equal amounts of cell lysates were resolved on SDS polyacrylamide gels, transferred to PVDF membranes, and probed with antibodies against iNOS and COX-2. β-Actin served as an internal control for western blot analysis (bottom panel). Effects of CSP32 on LPS-induced mRNA expression of iNOS, COX-2, TNF-α, IL-1β, and IL-6. (B) After LPS treatment for 2–12 h, the levels of iNOS, COX-2, TNF-α, IL-1β, and IL-6 mRNA were determined by RT-PCR. GAPDH served as an internal control for RT-PCR assays. Suppressive effects of CSP32 on TNF-α, IL-1β, and IL-6 production in LPS-stimulated RAW 264.7 macrophage cells. (C) The cells were incubated with the indicated concentrations of CSP32 for 30 min before treatment with LPS (1 μg/mL) for 24 h. After incubation for 24 h, the supernatant was collected, and the amounts of these proinflammatory cytokines were measured by ELISAs. Results represent the mean±S.D of three independent experiments performed in triplicate. * p<0.01; compared to LPS alone.
Inhibition of mRNA expression of iNOS, COX-2, TNF-α, IL-1β, and IL-6 in LPS-stimulated RAW 264.7 macrophage cells
To determine whether the above effect on NO production was related to changes in the levels of iNOS and COX-2, we measured the expression of iNOS and COX-2 mRNA by RT-PCR (primers are shown in Table 1). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to normalize gene expression data. In the cells treated with LPS, the AMP significantly attenuated iNOS and COX-2 mRNA upregulation in a dose-dependent manner. We also examined mRNA expression of several proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6. LPS treatment increased TNF-α, IL-1β, and IL-6 mRNA levels and treatment of cells with CSP32 dose-dependently attenuated the LPS-enhanced expression (Fig 4B).
Inhibitory effects of CSP32 on the production of proinflammatory cytokines in LPS-stimulated RAW 264.7 macrophage cells
LPS-stimulated macrophages promote inflammation by secreting proinflammatory cytokines such as TNF-α, IL-1β, and IL-6. To test whether CSP32 affects cytokine production in LPS-stimulated RAW 264.7 cells, we measured TNF-α, IL-1β, and IL-6 concentrations in culture supernatants by ELISAs. LPS treatment significantly increased TNF-α, IL-1β, and IL-6 production as compared to the untreated cells. As shown in Fig 4C, the LPS-induced release of cytokines was significantly attenuated by CSP32 in a dose-dependent manner. These results revealed that CSP32 blocked LPS-induced proinflammatory mediators such as TNF-α, IL-1β, and IL-6 in macrophages. These results suggest CSP32 as a potential anti-inflammatory agent.
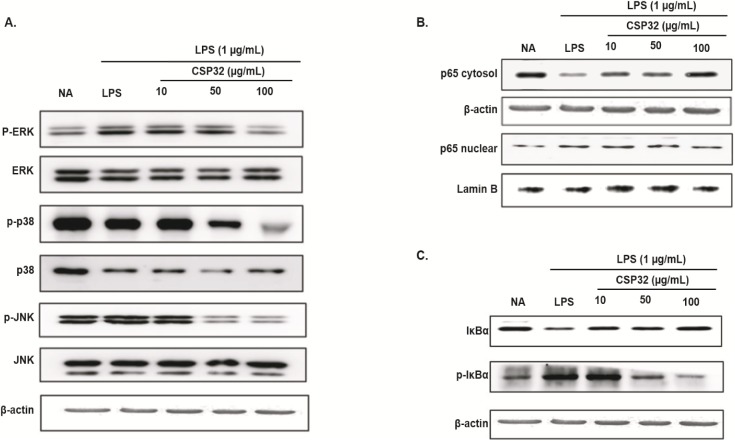
Effects of CSP32 on LPS-induced phosphorylation of MAPKs
As shown in Fig 5A, phosphorylation of ERK, JNK, and p38 MAPKs increased significantly when the cells were treated with LPS (1 μg/mL) for 1 h. Phosphorylation decreased in a dose-dependent manner by the treatment with CSP32 (10–100 μg/mL). In contrast, the amounts of unphosphorylated ERK, JNK, and p38 MAPKs were unaffected by the treatment with LPS or CSP32.
Fig 5.
(A) Effects of CSP32 on phosphorylation of MAPKs (ERK, JNK, and p38) in the LPS-stimulated RAW 264.7 cells. Effects of CSP32 on LPS-stimulated NF-κB expression, to be precise, inhibition of translocation into the nucleus (B) and inhibition of IκBα phosphorylation and degradation (C). The cells were separately treated with the indicated concentrations of CSP32 for 1 h prior to the addition of 1 μg/mL LPS, and the cells were further incubated for 24 h.
Effects of CSP32 on NF-κB translocation and on phosphorylation of IκBα in LPS-stimulated RAW 264.7 macrophage cells
To assess the effects of CSP32 on LPS-induced NF-κB activation, we examined the translocation of NF-κB into the nucleus using western blot analysis. As shown in Fig 5B, cytoplasmic levels of p65 decreased in response to LPS treatment. The LPS-induced changes in the levels of p65 in the nucleus and cytoplasm were attenuated in macrophages treated with CSP32. Furthermore, the level of p-IκBα that was increased by LPS was attenuated by CSP32 treatment (dose dependently), and at the same time, the total level of IκBα in the cytoplasm increased (dose dependently) as shown in Fig 5C. This finding suggests that an increase of CSP32 dose enhanced the ability of IκBα to maintain NF-κB in an inactive form in the cytoplasm.
Discussion
The primary aim of this study was to isolate, identify, and characterize a potent substance that has antimicrobial and anti-inflammatory properties from a local fermented food source (kimchi). Microbial strains capable of producing AMPs were isolated from Korean traditional food kimchi. Strain CS32 was selected from the various screened strains because of its well-pronounced antimicrobial activity. Based on morphological and physiological parameters and 16S rRNA gene sequences, the strain was identified as Bacillus sp. CS32.
This strain shares the highest 16S rRNA homology (99.392%) with B. licheniformis, which was reported to produce the antibacterial agent bacitracin. The AMP (designated as CSP32) produced by the strain in the optimal medium was purified to homogeneity and was found to have a molecular mass of ~5700 Da (~5.7 kDa). The first 12 amino acid residues of CSP32 are APLEIXXIFHDN. The molecular mass, together with the distinct N-terminal amino acid sequence, makes CSP32 a novel antimicrobial peptide. In addition, CSP32 showed a strong structural similarity of its monomeric sequence to the 12-amino acid sequence of bacitracin A, a derivative of bacitracin, and the chemoinformatic analysis confirmed this result.
Bacitracin is an antibiotic produced by certain species, including B. licheniformis and B. subtilis. According to Bonsai: Bioinformatics Software Server (http://bioinfo.lifl.fr/), only 16 bacitracin derivatives have been identified to date. The FHDN motif is common in all bacitracin derivatives, including CSP32. According to an NCBI BLAST search, the sequence of CSP32 shows the highest homology to a bacteriocin-like compound (lichenin) from B. licheniformis [24]. Besides, the sequence also showed some degree of identity with AMPs from B. subtilis JM4 [10] with shared sequences EIxxIFHDN where x represents unidentified residues. CSP32 was found to be almost completely stable up to 90°C and at pH 5.0–12.0. The pH and temperature stability of CSP32 is comparable with that of lichenin and AMPs produced by the B. subtilis strain JM4 [10, 24]. CSP32 has molecular weight 1390.98 Da (theoretical) according to the first 12 amino acids, which have a high degree of similarity to bacitracin derivatives (monomeric peptides; Table 2), as determined by Edman degradation method. On the other hand, MALDI-TOF-MS showed molecular weight of 5697.90 Da for a complex of four peptides (consisting of 12 amino acid residues). We determined the structural similarity by comparing experimental ESI-MS/MS fragmentation of CSP32 and spectra of fragments derived from bacitracin A. MS-MS analysis of CSP32 and peptide sequence analysis in the fragmentation pattern revealed that the MS-MS molecular masses of 199.1, 356.2, 441.1, 852.4, 982.5, 1111.6, and 1337.7 are similar to the fragmentation pattern of bacitracin A. In addition, the standard bacitracin A fragmentation analysis (molecular weights 475.1595, 7112.1154, and 1422.8960) showed a pattern similar to that of CSP32. Furthermore, the findings from HR-QTOF-ESI/MS revealed that the quaternary structure consists of 11 peptide subunits (Table 3). On the basis of the results of three different experiments, clearly CSP32 is an oligomer of bacitracin, and Edman degradation method was able to read only the first 12 amino acids of the N terminus. The single band in tricine SDS-PAGE corresponded to molecular weight 5697.90 Da from MALDI-TOF-MS results. Just as other well-known AMPs, CSP32 is not sensitive to all the proteases tested, suggesting that it is nonproteinaceous as previously reported [24, 25]. Although high proteolytic-enzyme concentrations were necessary to inactivate the antimicrobial activity, some antimicrobials produced by Bacillus spp. are cyclic peptides containing unusual amino acids, which are more resistant to proteolytic enzymes. CSP32 may be a novel peptide because of its unique N-terminal amino acid sequence along with other biochemical properties. Effects of CSP32 were evaluated against various pathogenic Gram-positive and negative bacteria. CSP32 exerted antimicrobial action on Gram-positive microorganisms, in agreement with the effects of antimicrobial peptides from Bacillus sp. JM4 [10] and B. licheniformis A89 [26].
Besides, the antimicrobial effect of CSP32 against multidrug-resistant bacteria such as MRSA, VRSA, and VRE was comparable with the action of bacitracin or vancomycin. Nevertheless, CSP32 is slightly less potent than BCP61 [25]. Moreover, CSP32 showed strong antimicrobial activity against anaerobic pathogens P. acne and C. difficile. When we compared its activity with that of monensin, CSP32 was more effective than monensin against those pathogens (Table 5). Monensin is a polyether ionophore widely used in veterinary medicine for the prevention and treatment of coccidiosis in poultry and as a growth promoter in ruminants [27]. Although it is popular in many countries, its residues in food may pose a risk to health among sensitive individuals [27, 28]. Our results show that CSP32 may be useful for the reduction of methane gas production and for enhancement of feed efficacy via management of anaerobic intestinal microorganisms.
In accordance with the second objective, this study revealed that CSP32 significantly attenuates LPS-induced production of proinflammatory mediators NO, iNOS, and COX-2 in RAW 264.7 cells. Moreover, CSP32 suppressed the activation MAPKs (ERK, JUN, and p38) and of NF-κB by LPS (Fig 5A), and our data are consistent with the following mechanism: p65 binding activity and CSP32 prevent IκBα phosphorylation and degradation; the effects of CSP32 on both phenomena are dose-dependent (Fig 5B and 5C). Our results indicate that the inhibitory effects of CSP32 are mediated by iNOS and COX-2 suppression. Furthermore, the RT-PCR analysis suggests that mRNA levels of iNOS and COX-2 correlate with their protein levels. Therefore, the inhibitory effect of CSP32 on iNOS and COX-2 expression appears to be one of the mechanisms responsible for the anti-inflammatory action of AMPs. Hence, CSP32 suppresses the expression of genes implicated in inflammation. We evaluated production of proinflammatory cytokines in LPS-stimulated cells, specifically TNF-α, IL-1β, and IL-6. These cytokines are produced mainly by activated macrophages. Suppression of cytokine production is an important way to counter the inflammatory process [15–17]. Because AMPs have been reported to downregulate proinflammatory mediators [24], we next investigated the effects of CSP32 on the LPS-induced release of cytokines by ELISAs and RT-PCR. There was no change in basal cytokine expression after incubation with only CSP32 without LPS. Incubation with only LPS strongly upregulated the proinflammatory cytokines. After 24-h incubation with both LPS (1 μg/mL) and CSP32, there was a remarkable attenuation of this increased cytokine production and mRNA expression in RAW 264.7 cells. These results suggest that CSP32 is a potent inhibitor of LPS-induced iNOS and COX-2 mRNA and protein expression and cytokine production in RAW 264.7 cells (Fig 4), in line with other reports [15–17].
We are currently conducting such experiments as evaluation of peptide analogs, and luciferase assays. To the best of our knowledge, an oligomer consisting of peptide subunits likely to be a bacitracin derivative, i.e., purified CSP32—which has strong antimicrobial and anti-inflammatory properties, and was isolated from Bacillus sp. CS32 from fermented kimchi—has never been reported.
Conclusion
CSP32 has potent antimicrobial and anti-inflammatory activities and was purified from a Bacillus strain newly isolated from traditional Korean fermented kimchi. CSP32 is likely to be a novel oligomer of a bacitracin derivative because of its unique N-terminal amino acid sequence along with other biochemical properties. Our results show that CSP32 may be a suitable candidate for the development of a multifunctional peptide for the prevention or treatment of infections caused by multidrug-resistant and anaerobic pathogens as well as inflammation-related disorders.
Data Availability
All relevant data are included in the paper.
Funding Statement
Support was provided by: The National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (NRF-2015R1A2A1A15056120, NRF- 2015R1D1A1A 01059483); and Bio-industry Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (115073-2).
References
- 1.Cook RJ, Thomashow LS, Weller DM, Fujimoto D, Mazzola M, Bangera G, et al. (1995) Molecular mechanisms of defense by rhizobacteria against root disease. Proc Natl Acad Sci U S A 92(10):4197–201. Epub 1995/05/09. PubMed Central PMCID: PMC41910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rana M, Chatterjee S, Kochhar S, Pereira B (2006) Antimicrobial peptides: a new dawn for regulating fertility and reproductive tract infections. J Endocrinol Reprod 2:88–95. [Google Scholar]
- 3.Bizani D, Brandelli A (2002) Characterization of a bacteriocin produced by a newly isolated Bacillus sp. Strain 8 A. J Appl Microbiol 93(3):512–9. Epub 2002/08/14. [DOI] [PubMed] [Google Scholar]
- 4.Sumi CD, Yang BW, Yeo I-C, Hahm YT (2014) Antimicrobial peptides of the genus Bacillus: a new era for antibiotics. Canadian journal of microbiology 61(2):93–103. doi: 10.1139/cjm-2014-0613 [DOI] [PubMed] [Google Scholar]
- 5.Tamang JP, Watanabe K, Holzapfel WH (2016) Review: diversity of microorganisms in global fermented foods and beverages. Frontiers in microbiology 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherif A, Ouzari H, Daffonchio D, Cherif H, Ben Slama K, Hassen A, et al. (2001) Thuricin 7: a novel bacteriocin produced by Bacillus thuringiensis BMG1.7, a new strain isolated from soil. Lett Appl Microbiol 32(4):243–7. Epub 2001/04/12. [DOI] [PubMed] [Google Scholar]
- 7.Ahern M, Verschueren S, van Sinderen D (2003) Isolation and characterisation of a novel bacteriocin produced by Bacillus thuringiensis strain B439. FEMS Microbiol Lett 220(1):127–31. Epub 2003/03/20. [DOI] [PubMed] [Google Scholar]
- 8.Dischinger J, Josten M, Szekat C, Sahl HG, Bierbaum G (2009) Production of the novel two-peptide lantibiotic lichenicidin by Bacillus licheniformis DSM 13. PLoS One 4(8):e6788 Epub 2009/08/27. Central PMCID: PMC2727956. doi: 10.1371/journal.pone.0006788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oscariz JC, Lasa I, Pisabarro AG (1999) Detection and characterization of cerein 7, a new bacteriocin produced by Bacillus cereus with a broad spectrum of activity. FEMS Microbiol Lett 178(2):337–41. Epub 1999/09/28. [DOI] [PubMed] [Google Scholar]
- 10.Wu S, Jia S, Sun D, Chen M, Chen X, Zhong J, et al. (2005). Purification and characterization of two novel antimicrobial peptides Subpeptin JM4-A and Subpeptin JM4-B produced by Bacillus subtilis JM4. Curr Microbiol 51(5):292–6. Epub 2005/10/08. doi: 10.1007/s00284-005-0004-3 [DOI] [PubMed] [Google Scholar]
- 11.Lee HJ, Joo YJ, Park CS, Kim SH, Hwang IK, Ahn JS, et al. (1999). Purification and characterization of a bacteriocin produced by Lactococcus lactis subsp. lactis H-559 isolated from kimchi. J Biosci Bioeng 88(2):153–9. Epub 2005/10/20. [DOI] [PubMed] [Google Scholar]
- 12.Kayalvizhi N, Gunasekaran P. Production and characterization of a low-molecular-weight bacteriocin from Bacillus licheniformis MKU3 (2008) Lett Appl Microbiol 47(6):600–7. Epub 2009/01/06. doi: 10.1111/j.1472-765X.2008.02473.x [DOI] [PubMed] [Google Scholar]
- 13.Khalil R, Elbahloul Y, Djadouni F, Omar S (2009) Isolation and partial characterization of a bacteriocin produced by a newly isolated Bacillus megaterium 19 strain. Pak J Nutr 8:242–50. [Google Scholar]
- 14.Rosenfeld Y, Shai Y (2006) Lipopolysaccharide (Endotoxin)-host defense antibacterial peptides interactions: Role in bacterial resistance and prevention of sepsis. Biochimica et Biophysica Acta (BBA)—Biomembranes. 1758(9):1513–22. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar D, Saha P, Gamre S, Bhattacharjee S, Hariharan C, Ganguly S, et al. (2008) Anti-inflammatory effect of allylpyrocatechol in LPS-induced macrophages is mediated by suppression of iNOS and COX-2 via the NF-kappaB pathway. Int Immunopharmacol 8(9):1264–71. Epub 2008/07/08. doi: 10.1016/j.intimp.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 16.Yoon WJ, Heo SJ, Han SC, Lee HJ, Kang GJ, Kang HK, et al. (2012) Anti-inflammatory effect of sargachromanol G isolated from Sargassum siliquastrum in RAW 264.7 cells. Arch Pharm Res 35(8):1421–30. Epub 2012/09/04. doi: 10.1007/s12272-012-0812-5 [DOI] [PubMed] [Google Scholar]
- 17.Yu HY, Kim KS, Lee YC, Moon HI, Lee JH (2012) Oleifolioside A, a New Active Compound, Attenuates LPS-Stimulated iNOS and COX-2 Expression through the Downregulation of NF-kappaB and MAPK Activities in RAW 264.7 Macrophages. Evid Based Complement Alternat Med 2012:637512 Epub 2012/08/23. PubMed Central PMCID: PMC3405816. doi: 10.1155/2012/637512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon WJ, Kim SS, Oh TH, Lee NH, Hyun CG. Cryptomeria japonica essential oil inhibits the growth of drug-resistant skin pathogens and LPS-induced nitric oxide and pro-inflammatory cytokine production (2009) Pol J Microbiol 58(1):61–8. Epub 2009/05/28. [PubMed] [Google Scholar]
- 19.Cho SS, Choi YH, Simkhada JR, Mander P, Park da J, Yoo JC. A newly isolated Streptomyces sp. CS392 producing three antimicrobial compounds (2012) Bioprocess Biosyst Eng 35(1–2):247–54. Epub 2011/09/13. doi: 10.1007/s00449-011-0599-7 [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen JH, Turnidge JD (2015) Susceptibility test methods: dilution and disk diffusion methods Manual of Clinical Microbiology, Eleventh Edition: American Society of Microbiology; p. 1253–73. [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards (2004) Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria—Sixth Edition: Approved Standard M11-A6. NCCLS, Wayne, PA, USA. [Google Scholar]
- 22.Choi YH, Na BH, Choi YS, Rahman MS, Kim MR, Jee J-P, et al. (2016) Anti-inflammatory function of 4-tert-butylphenyl salicylate through down-regulation of the NF-kappa B pathway. Archives of pharmacal research 39(3):429–36. doi: 10.1007/s12272-015-0679-3 [DOI] [PubMed] [Google Scholar]
- 23.Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure–activity relationships (1999) Biochem Pharmacol 58(5):759–65. [DOI] [PubMed] [Google Scholar]
- 24.Pattnaik P, Kaushik JK, Grover S, Batish VK (2001) Purification and characterization of a bacteriocin-like compound (Lichenin) produced anaerobically by Bacillus licheniformis isolated from water buffalo. J Appl Microbiol 91(4):636–45. Epub 2001/09/29. [DOI] [PubMed] [Google Scholar]
- 25.Choi YH, Cho SS, Simkhada JR, Yoo JC (2012) A novel thermotolerant and acidotolerant peptide produced by a Bacillus strain newly isolated from a fermented food (kimchi) shows activity against multidrug-resistant bacteria. Int J Antimicrob Agents 40(1):80–3. Epub 2012/05/15. doi: 10.1016/j.ijantimicag.2012.03.019 [DOI] [PubMed] [Google Scholar]
- 26.Mendo S, Faustino NA, Sarmento AC, Amado F, Moir AJ (2004) Purification and characterization of a new peptide antibiotic produced by a thermotolerant Bacillus licheniformis strain. Biotechnol Lett 26(2):115–9. Epub 2004/03/06. [DOI] [PubMed] [Google Scholar]
- 27.Chapman HD, Jeffers TK, Williams RB (2010) Forty years of monensin for the control of coccidiosis in poultry. Poult Sci 89(9):1788–801. Epub 2010/08/17. doi: 10.3382/ps.2010-00931 [DOI] [PubMed] [Google Scholar]
- 28.Lee HJ, Cho SS, Simkhada JR, Yoo JC (2009) Monoclonal antibody production and immunochemical detection of polyether antibiotics. Arch Pharm Res 32(3):437–41. Epub 2009/04/24. doi: 10.1007/s12272-009-1318-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included in the paper.