Abstract
Liver glycogen metabolism plays an important role in glucose homeostasis. Glycogen synthesis is mainly regulated by glycogen synthase that is dephosphorylated and activated by protein phosphatase 1 (PP1) in combination with glycogen-targeting subunits or G subunits. There are seven G subunits (PPP1R3A to G) that control glycogenesis in different organs. PPP1R3G is a recently discovered G subunit whose expression is changed along the fasting-feeding cycle and is proposed to play a role in postprandial glucose homeostasis. In this study, we analyzed the physiological function of PPP1R3G using a mouse model with liver-specific overexpression of PPP1R3G. PPP1R3G overexpression increases hepatic glycogen accumulation, stimulates glycogen synthase activity, elevates fasting blood glucose level, and accelerates postprandial blood glucose clearance. In addition, the transgenic mice have a reduced fat composition, together with decreased hepatic triglyceride level. Fasting-induced hepatic steatosis is relieved by PPP1R3G overexpression. In addition, PPP1R3G overexpression is able to elevate glycogenesis in primary hepatocytes. The glycogen-binding domain is indispensable for the physiological activities of PPP1R3G on glucose metabolism and triglyceride accumulation in the liver. Cumulatively, these data indicate that PPP1R3G plays a critical role in postprandial glucose homeostasis and liver triglyceride metabolism via its regulation on hepatic glycogenesis.
Circulating energy sources such as glucose are maintained in a homeostasis through coordinate regulation of energy uptake, consumption, storage, and release by liver, adipose tissue, skeletal muscles, and other tissues. Glycogen and triglyceride are two major forms of energy storage in the body and provide fuel needed during different phases of food deprivation, with glycogenolysis in the liver and skeletal muscles being primarily involved in maintaining blood glucose level during the early phase of fasting (1). After a meal, the postprandial blood glucose is mainly disposed by increased glucose uptake in peripheral tissues, such as liver and skeletal muscles. In particular, the liver takes up approximately one-third of the oral glucose load in the animal (2). As a glucose sensor, the liver actively contributes to the control of postprandial blood glucose homeostasis (3–5). Glucose is stored in the liver in the form of glycogen, and two key enzymes are primarily involved in hepatic glycogen metabolism: glycogen synthase (GS) and glycogen phosphorylase (GP). GS catalyzes the addition of glucose to the glycogen chain, and GP catalyzes the breakdown of glycogen to release glucose-1-phosphate. The phosphorylation of GS is associated with an inhibition of the enzyme activity, with its multiple residues phosphorylated by a plethora of protein kinases. On the other hand, the activity of GS is stimulated by dephosphorylation via glycogen synthase phosphatase (GSP). In contrast to GS, the activity of GP is activated by phosphorylation on one N-terminal serine by phosphorylase kinase and inhibited by dephosphorylation by protein phosphatase 1 (PP1) (6, 7). At the same time, elevated intracellular glucose binds activated GP and promotes the dephosphorylation and inhibition of its activity, and GSP is then released from the allosteric inhibitory effect of glycogen phosphorylase a (GPa) (8). In addition, glycose-6-phosphate (G6P) is an allosteric activator of GS, serving to make GS a better substrate for dephosphorylation and activation by PPs (9, 10), and the activity of GP correlates inversely with G6P concentration within the physiological range (11, 12). After a meal, the increase in intracellular G6P concentration consequently results in an increase in GS activity and inactivation of GP. In addition, the elevated insulin during a meal contributes to stimulation of GS activity by inhibition of glycogen synthase kinase-3 (GSK3) (13).Through these complex processes, feeding leads to activation of GS and inactivation of GP, resulting in glycogen accumulation in the liver.
PP1 forms a variety of distinct multimeric holoenzymes with the catalytic subunit of PP1 (PP1c) forming a complex with different regulatory and targeting subunits and plays a critical role in glucose metabolism (6, 7). Glycogen-targeting regulatory subunits (G subunits) serve to localize PP1c to the glycogen particles and modulate the activities of the glycogen-metabolizing enzymes through PP1-mediated dephosphorylation, functioning as a major GSP to dephosphorylate and activate GS and, in turn, stimulate glycogenesis. There are seven genes encoding G subunits (PPP1R3A to PPP1R3G) according to the GenBank database (6). PPP1R3A (GM) is mainly expressed in the skeletal muscle and heart and deletion of this gene leads to reduction of glycogen level in skeletal muscle, accompanied by glucose intolerance and insulin resistance (14). PPP1R3B (GL) is the primary G subunit expressed in the liver and mice with overexpression of a deregulated form of PPP1R3B displayed improved glucose tolerance (15). PPP1R3C (PPP1R5) is expressed in many tissues and mice with heterozygous deletion of PPP1R3C had reduced glycogen levels in many tissues, accompanied by progressive glucose intolerance, hyperinsulinemia, and insulin resistance with aging (16). PPP1R3D (PPP1R6) is mainly expressed in the brain and likely plays a function in glycogen accumulation in neurons (17). PPP1R3E is highly expressed in the liver and heart muscle in rodents (18). PPP1R3F is a membrane-associated G subunit and has been reported to regulate GS in astrocytoma cells (19). Collectively, these data indicate that G subunits play important roles in glycogen and glucose metabolism in various tissues.
Our recent study has demonstrated that PPP1R3G is another glycogen-targeting regulatory subunit of PP1 (20). Distinct from other G subunits, the expression of PPP1R3G protein changes concurrently with the fasting-feeding cycle in the mouse liver. PPP1R3G protein is up-regulated by fasting and rapidly down-regulated after refeeding. Overexpression of PPP1R3G markedly increases cellular glycogen levels via stimulation of GS activity. Such study also reveals that PPP1R3G is actively involved in the control of blood glucose homeostasis by regulating hepatic glycogenesis in a manner closely coordinated with the fasting-feeding cycle. To investigate the function of PPP1R3G on glycogen metabolism further, we generated a transgenic mouse model with liver-specific expression of PPP1R3G in this study. We found that elevated PPP1R3G expression markedly enhanced hepatic glycogen accumulation and increased the clearance rate of postprandial blood glucose. Interestingly, PPP1R3G overexpression in the liver could also lead to reduced hepatic triglyceride concentration in the fed state and improved hepatic steatosis on fasting. Therefore, our study has pinpointed the physiological roles of PPP1R3G in not only glucose homeostasis but also lipid metabolism.
Materials and Methods
Animal studies
All animal procedures and protocols were approved by the Institutional Animal Care and Use Committee of the Institute for Nutritional Sciences, Chinese Academy of Sciences. Blood samples were taken and other tissues of interest were snap-frozen in liquid nitrogen immediately after resection and stored at −80°C until further analysis. Food intake and body weight measurement were described previously (21).
Generation and characterization of PPP1R3G liver-specific transgenic mice
The transgenic mice with liver-specific expression of PPP1R3G were generated by Model Animal Research Center of Nanjing University (Nanjing, China). Tail biopsies of the mice were analyzed by genomic PCR. The presence of PPP1R3G transgene was detected by primers 5′-CCAATGAAATGCGAGGTAAG-3′ and 5′-AGAGCGGCGATATTCCTGTA-3′ and the PCR product was 411 bp in length.
RNA isolation and real-time quantitative PCR
RNA isolation, RT, and real-time PCR were carried out as described (22). The sequences of primers are as follows: 5′-TGCGAACAGGGAGAATACTG-3′ and 5′-CCTCTTTCCAAGTTCCGAAG-3′ for mouse PPP1R3G; 5′-GATCATTGCTCCTCCTGAGC-3′ and 5′-ACTCCTGCTTGCTGATCCAC-3′ for mouse β-actin.
Immunoblotting analysis
The immunoblotting assay was described previously (23). The mouse antitubulin antibody was from Sigma-Aldrich. The anti-PPP1R3G was generated in our laboratory as previously reported (20).
Measurement of glycogen content and GS activity
The glycogen content of mouse tissues was measured as previously described (24). Measurement of the glycogen content in cells was performed as previously described (25). GS activity assay was modified from a previously described method (26, 27). Briefly, liver tissue was homogenized in 300 μL solution containing 50 mM Tris-HCl at pH 7.8, 10 mM EDTA, 2 mM EGTA, 100 mM NaF, protease inhibitor cocktail (Sigma-Aldrich), followed by centrifugation at 3600g for 5 minutes at 4°C. Four microliters of the supernatant was added to 36 μL of assay mixture 1 (25 mM Tris-HCl at pH 7.4, 100 mM KCl, 5 mM MgCl2, 10 mM G6P, and 0.5% glycogen) with or without 5 mM 5′-urdiphosphate-glucose (UDPG) (Sigma-Aldrich). After incubation at 37°C for 30 minutes, the reaction was stopped by heating for 3 minutes at 100°C and then centrifuged at 10 000 rpm for 5 minutes. Twenty microliters of the supernatant was added to 10 μL of assay mixture 2 (6 mM HEPES at pH 7.4, 100 mM KCl, 5 mM MgCl2, 2 mM phosphoenolpyruvate [PEP]; Sigma-Aldrich), 0.4 mM NADH (Roche Applied Science), 4 U pyruvate kinase per milliliter (Sigma-Aldrich), and 2 U lactate dehydroxylase per milliliter (Sigma-Aldrich). Absorbance at 340 nm was measured at 37°C for 20 minutes. GS activity was calculated as the ratio of activated GS activity (−G6P) vs the total GS activity (+G6P).
Glucose tolerance test (GTT), insulin tolerance test (ITT), and food tolerance test (FTT)
GTT and ITT were previously described (21). For GTT, the mice fasted overnight were injected ip with glucose at 1 g/kg. For ITT, the mice fasted for 4 to 6 hours were injected ip with insulin at 0.75 U/kg. For FTT, the mice fasted overnight were fed with normal chow. Blood was collected by venous bleeding from the tail vein at 0, 30, 60, and 120 minutes: after glucose injection (for GTT), after insulin injection (for ITT), or after feeding (for FTT). Blood glucose concentrations were measured by Glucometer Elite monitor (Abbott Diabetes Care).
Measurements of blood and liver samples
Hepatic lipids were extracted with chloroform-methanol (2:1) according to a published method (28). Serum and liver levels of triglycerides were determined by the Serum Triglyceride Determination Kit (Sigma-Aldrich). Serum levels of high-density lipoprotein (HDL), low-density lipoprotein, total cholesterol, alanine transaminase, and aspartate transaminase were determined by HDL, low-density lipoprotein, total cholesterol (TC), and Alanine Transaminase/Aspartate Transaminase Determination Kit (ShenSuoYouFu), respectively.
Isolation of primary hepatocytes
Mouse hepatocytes were isolated from livers of 8-week-old mice by a modified two-step collagenase perfusion protocol (29). In brief, the hepatocytes were plated on collagen I coated 12-well plates (2 × 105 cells/well) in 10% fetal bovine serum (Gibco). The medium was changed after 4 hours with DMEM supplemented with 10% fetal bovine serum, 20 U/mL penicillin, and 20 U/mL streptomycin.
PAS-staining and Oil Red staining
The PAS-staining assay was performed as previously described (20). Cells were fixed in 4% paraformaldehyde (PFA) and oxidized in 0.5% periodic acid. After rinsing three times with water, cells were stained with Schiff's reagent for 15 minutes and then washed for 5 minutes. After fixation of the livers with 10% formalin/PBS, hepatic lipid content was determined by 5-μm-thick frozen sections stained with Oil Red O (Sigma-Aldrich).
Statistical analysis
All results are expressed as means ± SEM unless indicated otherwise. Significant differences were assessed by two-tailed Student's t test for experiments with only two groups and ANOVA for experiments that contained more than two groups. P < .05 was considered statistically significant.
Results
Generation and characterization of transgenic mice with PPP1R3G specifically expressed in the liver
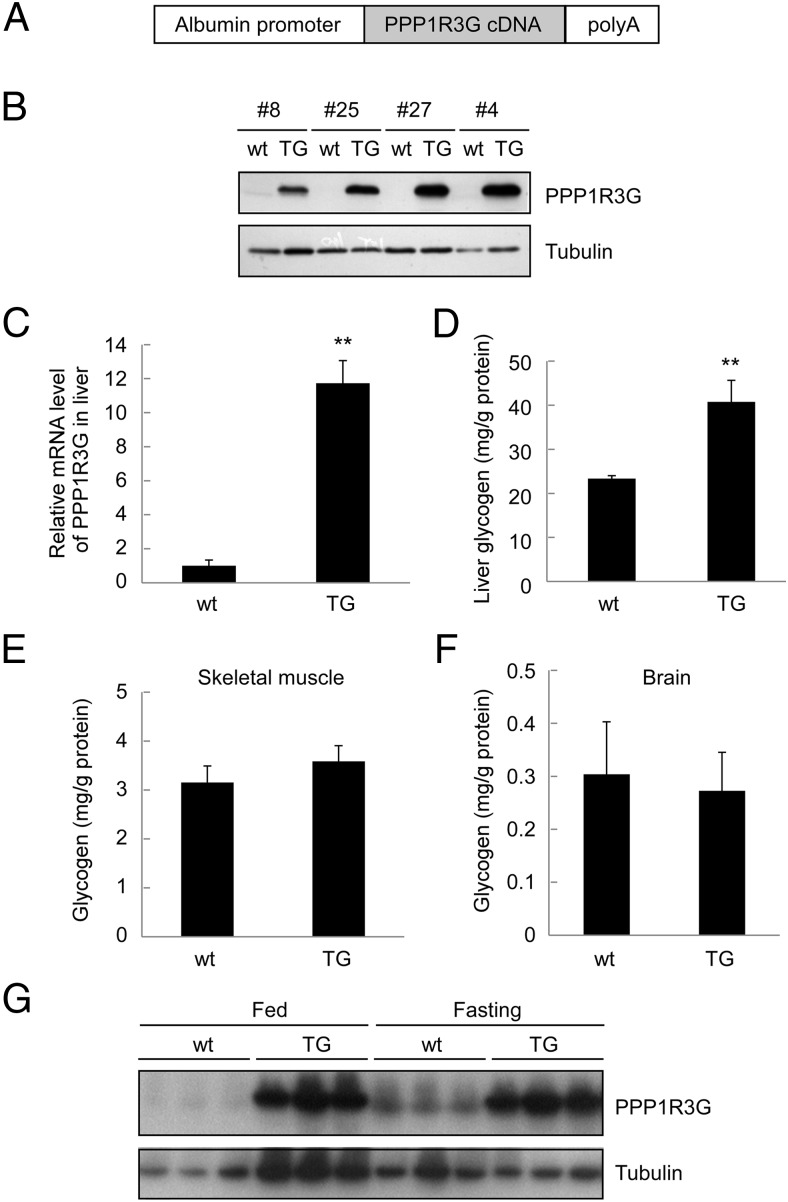
Our previous study had demonstrated that PPP1R3G, as a newly identified glycogen-targeting regulatory subunit of PP1, was able to regulate glycogen synthesis in the liver and modulate postprandial blood glucose homeostasis. To further investigate the role of PPP1R3G in glucose homeostasis and metabolism in vivo, we cloned the full-length mouse PPP1R3G cDNA downstream of albumin promoter to generate a transgenic mouse model with liver-specific expression of PPP1R3G (Figure 1A). Four founders were found to have overexpression of PPP1R3G protein in the liver (Figure 1B) and the offspring of founder number 27 was used in all subsequent studies. It is noteworthy that all the transgenic mice were apparently normal and the transgene was inherited in Mendelian ratio without adverse effects on reproduction and overall growth of the animals. We further analyzed the mRNA level of PPP1R3G by quantitative RT-PCR with RNA isolated from the livers in both wild-type and the transgenic mice. There was an approximate 12-fold increase in PPP1R3G mRNA level in the transgenic mice compared with the wild-type controls (Figure 1C). We also examined the effect of PPP1R3G overexpression on hepatic glycogen storage. In wild-type mice, the glycogen level in the liver was 23.3 mg/g protein (Figure 1D). In contrast, the liver glycogen level was increased to 40.7 mg/g protein in the transgenic mice, with approximately two-fold increase compared with the wild-type control (Figure 1D). On the other hand, the glycogen level was not altered in the skeletal muscle (Figure 1E) and brain (Figure 1F) in the transgenic mice. In addition, we analyzed hepatic PPP1R3G expression level under both fed and fasted conditions. As expected from our previous report (20), fasting was able to induce PPP1R3G expression (Figure 1G). However, PPP1R3G expression level in the liver was much higher in the transgenic mice than the wild-type animals under both feeding conditions (Figure 1G). Collectively, these results indicate that PPP1R3G was specifically overexpressed in the liver in the transgenic mice.
Figure 1.
Generation and characterization of transgenic mice with liver-specific expression of PPP1R3G. (A) A diagram depicting the transgenic construct. The full-length mouse PPP1R3G cDNA was cloned downstream of the albumin promoter. (B) Expression of PPP1R3G protein in the mouse liver from wild-type (wt) and transgenic mice (TG). Protein preparation from the mouse liver was used in Western blotting with an anti-PPP1R3G antibody. (C) Analysis of PPP1R3G mRNA level of mouse liver by real-time quantitative RT-PCR (qRT-PCR). The relative expression level of PPP1R3G compared with β-actin was shown as mean ± SEM (n = 8 for each group). **, P < .01 between the two groups. (D to F) Measurement of glycogen in liver (D), skeletal muscle (E), and brain (F). The tissues were isolated from nonfasted 4-month-old male animals (n = 7–8 for each group for D to F) and the results are reported as mean ± SEM; **, P < .01. (G) Hepatic PPP1G3G protein levels under both fed and fasting conditions as determined by Western blotting.
Regulation of liver-specific overexpression of PPP1R3G on glucose homeostasis
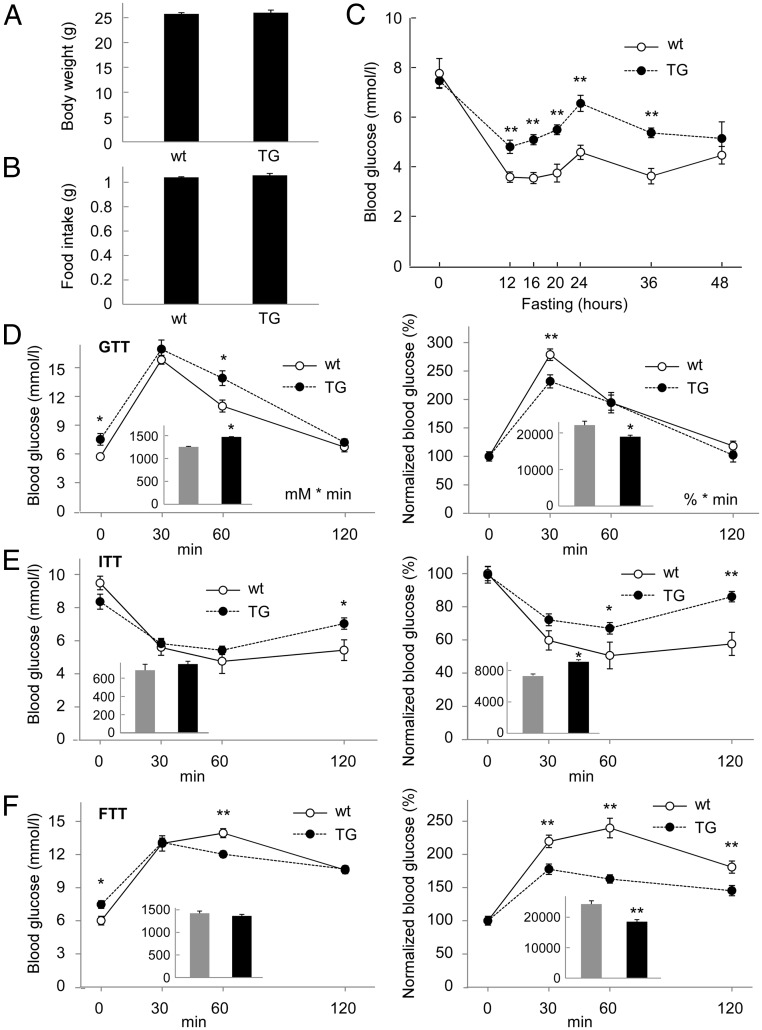
As the liver is one of the major organs involved in the regulation of glucose metabolism, we next analyzed the in vivo function of PPP1R3G overexpression on blood glucose homeostasis and insulin sensitivity. Under normal chow, there was no significant difference in body weight and food intake between the wild-type and transgenic mice (Figure 2, A and B). Although hepatic glycogen was increased in the transgenic mice (Figure 1D), the blood glucose level in the fed state was not altered by PPP1R3G overexpression (Figure 2C at time 0). However, the blood glucose level during fasting was changed by PPP1R3G overexpression. Compared with wild-type mice, the blood glucose level in the transgenic mice was consistently higher during fasting up to 36 hours since initiation of food abstinence (Figure 2C), indicating that the increased glycogen storage in the livers of these mice might contribute to elevated glucose release from the liver during starvation.
Figure 2.
Effect of PPP1R3G overexpression on blood glucose homeostasis. (A, B) Body weight and food intake of 3-month-old male wild-type and PPP1R3G transgenic mice (n = 8 for each group). (C) Blood glucose level was measured during fasting for different lengths of time (n = 6 for wild-type and n = 8 for transgenic mice, 7–8 weeks old). Glucose tolerance test (D), insulin tolerance test (E), and food tolerance test (F) were performed with the mice at 3 to 4 months of age (n = 8 for each group). Results shown in the right panels are expressed as a percentage of the initial blood glucose levels. The area under the curve (AUC) is shown in the inset for each data with the gray bar representing wild-type mice and the black bar representing transgenic mice. The units are “mM × minute” for unnormalized data and “% × minute” for normalized data. The data are shown as mean ± SEM.*, P < .05; **, P < .01.
We also analyzed the potential effect of PPP1R3G overexpression on glucose clearance and insulin sensitivity. The GTT revealed that fasting blood glucose level was higher in the transgenic mice than the wild-type control (Figure 2D, left panel). On normalization of the GTT results, it appeared that the relative clearance rate of blood glucose was slightly enhanced by PPP1R3G overexpression (Figure 2D, right panel, note that the normalized blood glucose level was reduced by PPP1R3G overexpression at 30-minute time point). The ITT demonstrated that the blood glucose level was higher in the transgenic mice at 60- and 120-minute points (Figure 2E), likely due to elevated hepatic glycogen degradation in response to insulin stimulation. We also performed a FTT to investigate the effect of PPP1R3G on postprandial blood glucose clearance. The mice were fasted overnight and then refed for different lengths of time. We observed that PPP1R3G transgenic mice had an obvious increase in postprandial glucose clearance in comparison with the wild-type controls as the blood glucose levels after feeding were markedly reduced by PPP1R3G overexpression (Figure 2F). Collectively, these data indicate that PPP1R3G overexpression in the liver is associated with an increase in fasting blood glucose level, together with acceleration of blood glucose clearance rates on either glucose injection (GTT) or feeding (FTT).
The effect of PPP1R3G overexpression on energy metabolism
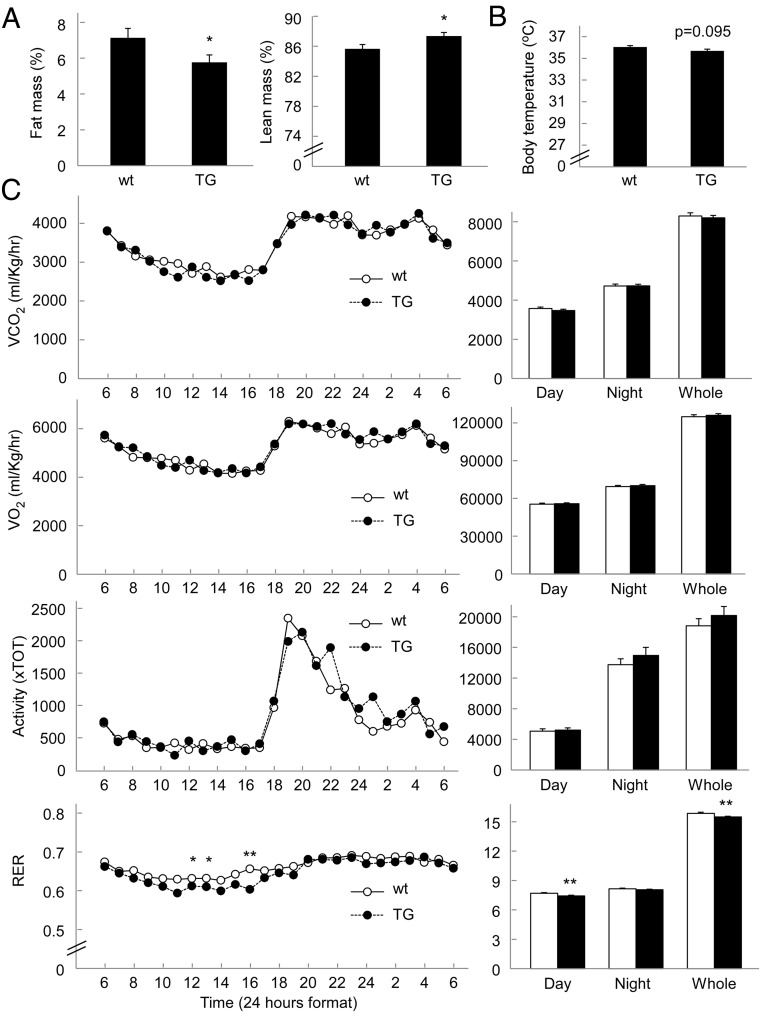
There are two major forms of energy storage in the body, glycogen and triglyceride, and both of them are closely implicated in energy metabolism. As our study revealed that PPP1R3G had a profound effect on liver glycogen storage and glucose homeostasis, we next investigated whether PPP1R3G imposed an effect on fat storage and energy metabolism. We first analyzed the proportions of fat mass and lean mass using nuclear magnetic resonance. Interestingly, the fat mass was significantly lower in the PPP1R3G transgenic mice (5.77% of fat/body) than the wild-type mice (7.13%) (Figure 3A). Consistently, the lean mass of the transgenic mice was higher than that of wild-type mice (Figure 3A). The weight of epididymal fat pad was also significantly reduced in the transgenic mice in comparison with wild-type mice (1.23 ± 0.05% fat pad/body weight in wild-type mice and 1.04 ± 0.05% fat pad/body weight in the transgenic mice, P < .05). These data, therefore, indicate that PPP1R3G overexpression is also associated with alteration of fat composition of the body.
Figure 3.
Effect of PPP1R3G overexpression on body composition, body temperature, and energy expenditure. (A) Body composition measured with nuclear magnetic resonance. (B) Rectal temperature. (C) Energy expenditure was measured by indirect calorimetry with the two groups of mice. Two- to 3-month-old male mice were measured for these parameters (n = 8 for each group). The cumulative data for the 24-hour period are shown in the right panel. Data are means ± SEM. *, P < .05; **, P < .01 between the two groups of mice.
The reduced fat mass in PPP1R3G transgenic mice could be explained by an increase in energy expenditure. To address this hypothesis, we measured energy expenditure by analyzing rectal temperature, indirect calorimetry, and physical activity. The rectal temperatures measured at 3:00 pm (at basal metabolic state) were slightly lower in the transgenic mice than the wild-type controls (Figure 3B). We did not observe significant differences in total energy expenditure (Figure 3C, shown as 24-h O2 consumption and 24-h CO2 output after being normalized to body mass). The physical activity was also not altered by PPP1R3G overexpression (Figure 3C). However, the respiratory exchange ratio (shown as VCO2/VO2) was slightly reduced in the transgenic mice during light phase (Figure 3C). Such data indicate that the transgenic mice may consume more fat as energy source than the control mice, likely contributing to reduction of fat mass in the body.
PPP1R3G overexpression alters hepatic triglyceride level
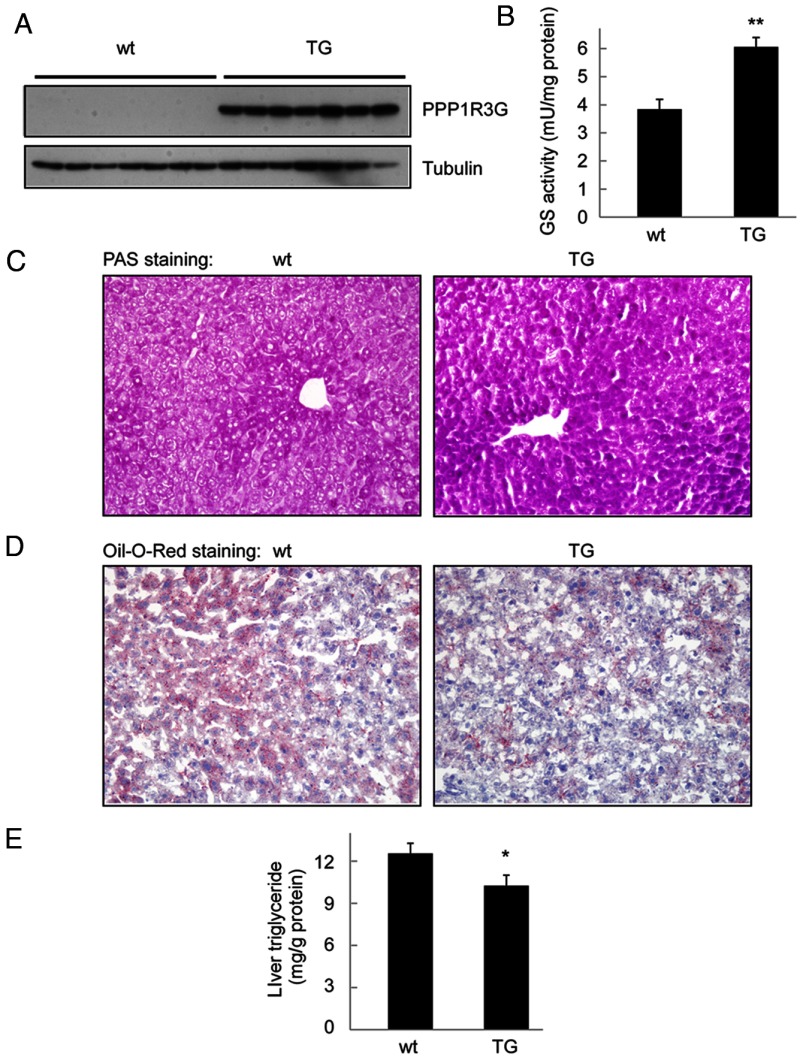
We carefully analyzed the effect of PPP1R3G overexpression on the storage of liver glycogen and triglyceride under both fed and fasted states. Under fed state, PPP1R3G protein was readily detectable in the liver of the transgenic mice, but not in the liver of wild-type controls (Figure 4A), consistent with our previous report revealing that PPP1R3G expression level is almost absent in the fed state (20). Also in agreement with our previous report, we found that GS activity was markedly elevated in the liver of the transgenic mice in the fed state (Figure 4B). PAS staining also confirmed that liver glycogen level was markedly elevated by PPP1R3G overexpression (Figure 4C).
Figure 4.
PPP1R3G overexpression increases hepatic glycogen content and GS activity while reduces hepatic triglyceride in the fed state. (A) Expression of PPP1R3G protein in the liver of 4-month-old male wild-type and transgenic mouse in the fed state. These animals were used in measurement of liver GS activity (B, n = 6 for each group), histological analysis using periodic acid Schiff (PAS) staining for glycogen (C), Oil-Red-O staining for triglyceride accumulation (D), and measurement of triglyceride level in the liver (E, n = 8 for each group). The data are shown as mean ± SEM. *, P < .05; **, P < .01.
As the fat mass was decreased in the transgenic mice (Figure 3A), we hypothesized that the triglyceride level in the liver might be affected by PPP1R3G overexpression. To address this hypothesis, we analyzed hepatic triglyceride in the fed state. Oil-Red-O staining revealed that hepatic triglyceride content was reduced in the transgenic mice in comparison with the wide-type animals (Figure 4D). Consistently, PPP1R3G overexpression led to a significant decrease in the triglyceride level in the liver in the fed state (Figure 4E). Together, these observations indicate that PPP1R3G overexpression could not only increase hepatic glycogen via elevation of GS activity, but also reduce the triglyceride content in the liver under fed state.
We next investigated the effect of PPP1R3G overexpression on liver glycogen accumulation and triglyceride accumulation under fasting state. Consistent with our previous observation (20), PPP1R3G protein could be detected in the liver in the wild-type mice on fasting overnight (Figure 5A, compared with Figure 4A). However, the expression level of PPP1R3G protein was much higher in the liver of the transgenic mice than the wild-type controls (Figure 5A). Consistently, the liver glycogen level and GS activity were both elevated by PPP1R3G overexpression under fasted state (Figure 5, B and C). Furthermore, the elevation of glycogen storage in the transgenic mice under fasted state was confirmed by PAS staining in the liver (Figure 5D).
Figure 5.
PPP1R3G overexpression improves hepatic steatosis under fasting conditions. (A) The expression of PPP1R3G protein in the liver of 4-month-old male wild-type and transgenic mouse in overnight-fasted state. These animals were used in measurement of hepatic glycogen content (B, n = 8 for each group), liver GS activity (C, n = 6 for each group), PAS staining (D), Oil-Red-O staining (E), measurement of hepatic triglyceride level (F, n = 7 for wild-type and n = 8 for transgenic mice). (F) Serum parameters in wild-type and the transgenic mice after overnight fasting (n = 8 for per group).The data are shown as mean ± SEM. *, P < .05; **, P < .01.
It was previously reported that fasting was able to induce hepatic steatosis in mice (30–32). We investigated the potential role of PPP1R3G overexpression on fasting-induced hepatic steatosis. Interestingly, Oil-Red-O staining revealed that hepatic steatosis was markedly improved by PPP1R3G overexpression (Figure 5E). Consistently, the transgenic mice also had a significant reduction in triglyceride content in the liver (Figure 5F). Furthermore, the serum triglyceride level was significantly reduced in the transgenic mice compared with wild-type animals, whereas other serum lipids were not altered (Figure 5G). In addition, there was no detectable difference in the levels of alanine transaminase and aspartate transaminase between the two types of animals, indicating that the elevated glycogen content in the transgenic mice was not associated with changes of liver function. These results collectively demonstrate that PPP1R3G overexpression is able to improve hepatic steatosis under fasting conditions.
PPP1R3G overexpression increases glycogen storage in primary hepatocytes
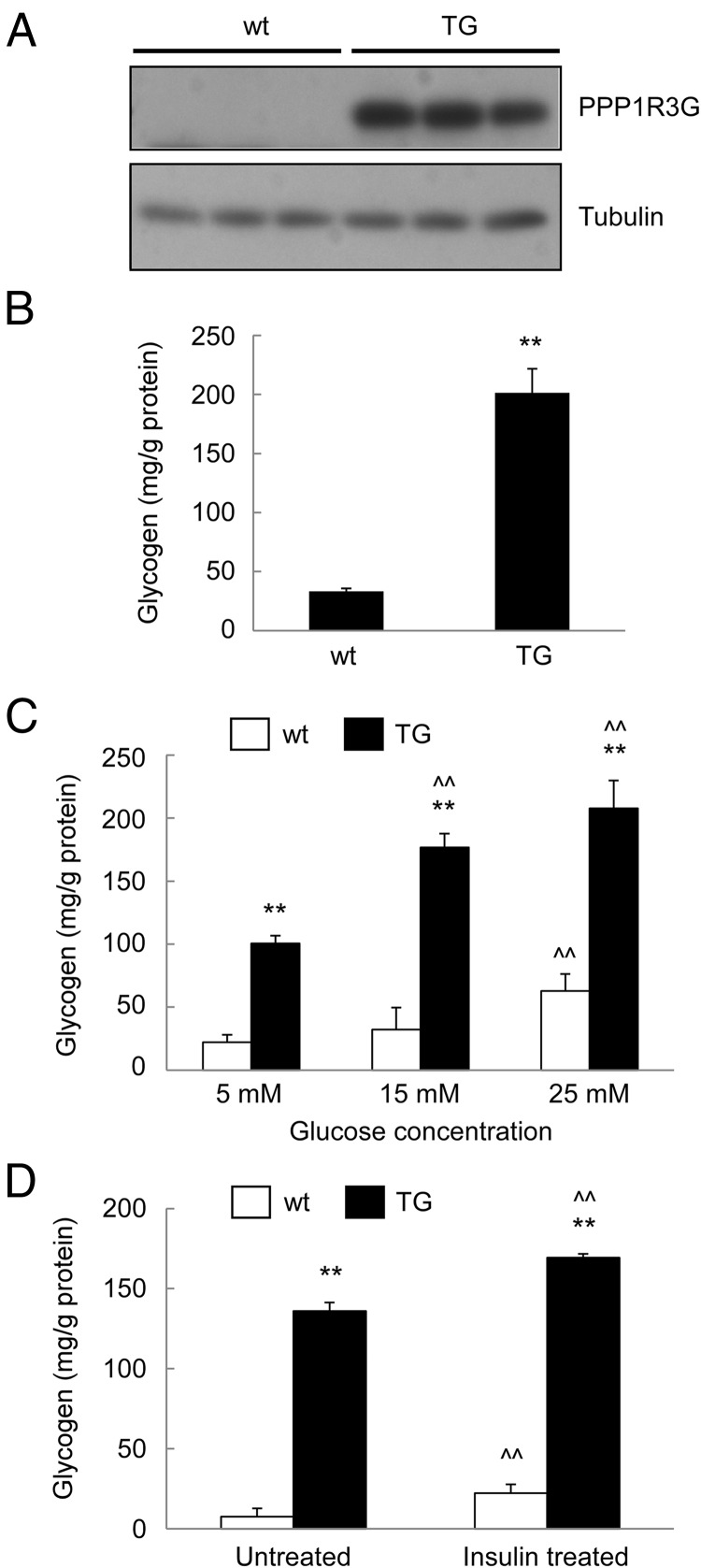
To further analyze the function of PPP1R3G on glycogen synthesis, we isolated primary hepatocytes from the livers of wild-type and transgenic mice. Consistent with the in vivo data, PPP1R3G protein was indeed highly expressed in primary hepatocytes isolated from the transgenic mice, but only at a low level in the hepatocytes derived from the wild-type animals (Figure 6A). The glycogen content in the hepatocytes was also markedly elevated by PPP1R3G overexpression (Figure 6B). We also analyzed the glucose-induced glycogen accumulation in the hepatocytes. As shown in Figure 6C, the glucose-induced glycogen synthesis in the hepatocytes was significantly enhanced by PPP1R3G overexpression in a dose-dependent manner. In addition, we found that insulin could stimulate glycogenesis in primary hepatocytes isolated from both types of animals (Figure 6D). These data collectively suggest that PPP1R3G could facilitate glycogenesis in primary hepatocytes, consistent with the observations made in vivo.
Figure 6.
PPP1R3G overexpression increases glycogen level in primary hepatocytes. Primary hepatocytes isolated from wild-type and the transgenic mice were cultured overnight and used in the following experiments: analysis of PPP1R3G protein expression by immunoblotting (A), measurement of cellular glycogen content (B), cellular glycogen concentration after treatment for 24 hours with various glucose concentrations (C), and measurement of glycogen content after treatment with 100 nM insulin for 24 hours (D). The data are shown as mean ± SD (Student's t test for B and ANOVA for C and D). **, P < .01 as compared with wild-type and the transgenic mice with the same treatment. ^^, P < .01 as compared with the first group of mice with the same genotype.
Glycogen binding motif of PPP1R3G is crucial for the physiological functions of the protein
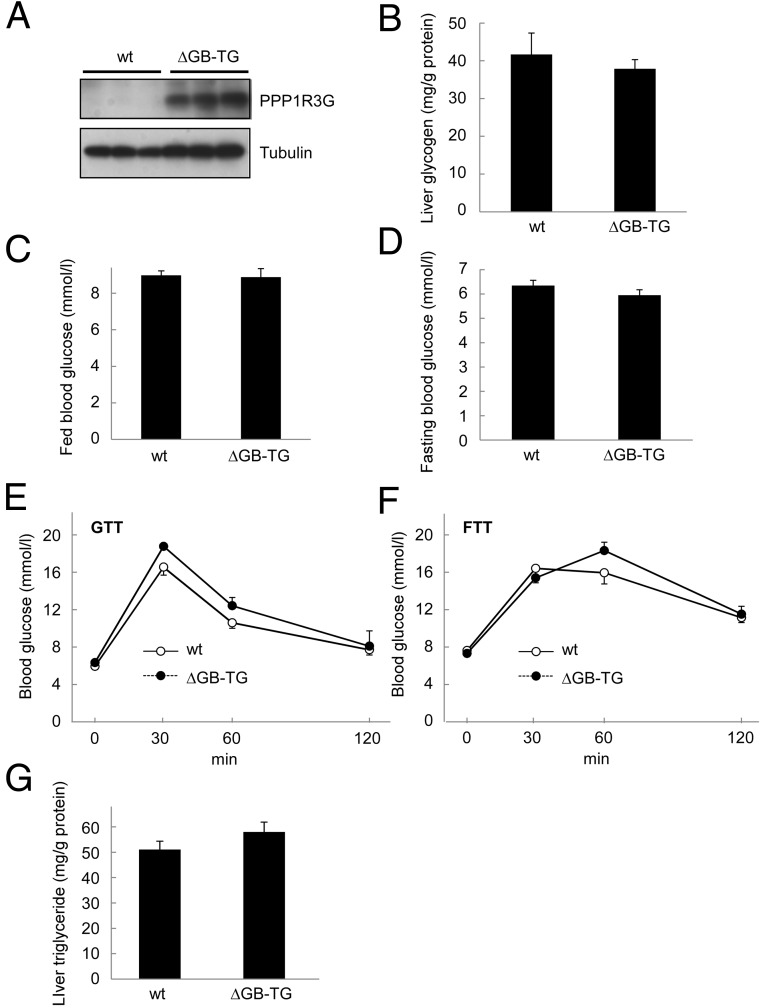
All G subunits possess a PP1-binding RVXF motif and a glycogen-binding site (6, 18, 33–36). Disrupting either binding site could abolish the effect of G subunits on glycogen synthesis (6, 36). To further address the importance of the glycogen-binding domain of PPP1R3G in glycogen metabolism in vivo, we generated another transgenic mouse with liver-specific expression of PPP1R3G that had a deletion of the glycogen binding domain (PPP1R3GΔGB) under the control of albumin promoter. The exogenous PPP1R3GΔGB protein was highly expressed in the liver by immunoblotting analysis (Figure 7A). Meanwhile, PPP1R3GΔGB was not detected in extrahepatic tissues (data not shown). As expected, overexpression of PPP1R3GΔGB was not associated with an increase in liver glycogen accumulation (Figure 7B). We also found that there was no significant difference in blood glucose levels between the two groups of mice in both fed state and fasted state (Figure 7, C and D). In addition, GTT and FTT revealed that the glucose clearance rate was not altered by PPP1R3GΔGB overexpression (Figure 7, E and F). The liver triglyceride level was also not changed by PPP1R3GΔGB overexpression (Figure 7G). Collectively, these data indicate that only the full-length PPP1R3G, but not the glycogen-binding domain-deleted mutant protein, was able to modulate liver glycogenesis, blood glucose clearance, and hepatic triglyceride accumulation in the mice.
Figure 7.
The glycogen binding domain is required for the physiological activities of PPP1R3G. (A) Analysis of PPP1R3G protein in the liver of 9-month-old male wild-type and the transgenic mice that expressed PPP1R3G containing deletion of glycogen binding domain (ΔGB) in the fed state. (B) Measurement of hepatic glycogen content (n = 5 for wild-type and n = 8 for the transgenic mice, respectively, the same for the following experiments). Blood glucose level was analyzed in the fed state (C) and in the overnight-fasted state (D). Glucose tolerance test (E) and food tolerance test (F) were performed in wild-type and the ΔGB transgenic mice. Hepatic triglyceride was measured in the fed state (G). Data are shown as mean ± SEM.
Discussion
In this study, we investigated the in vivo function of PPP1R3G using a transgenic mouse model that has liver-specific overexpression of PPP1R3G. Under the control of an albumin promoter, PPP1R3G could be specifically expressed in the liver, but not in other tissues. Consistent with our previous report demonstrating that PPP1R3G is a G subunit that controls glycogen synthesis (20), PPP1R3G overexpression was able to significantly increase glycogen accumulation in the liver to about two-fold under constant feeding state (Figure 1D). Under fasting state, however, PPP1R3G overexpression could increase liver glycogen level by about four-fold (Figure 5B). Such increase in glycogenesis in the liver was associated with stimulation of GS activity by PPP1R3G overexpression (Figure 4B and 5C). One of the most obvious findings with the transgenic mice is that PPP1R3G is implicated in glucose homeostasis. Overexpression of PPP1R3G was able to markedly elevate fasting blood glucose level (Figure 2C), suggesting that the increased hepatic glycogen load is associated with increased release of glucose during starvation. The glucose clearance rate on either glucose load (Figure 2D) or feeding (Figure 2F) was significantly accelerated by PPP1R3G overexpression, indicating that PPP1R3G plays an important role in controlling postprandial blood glucose homeostasis. In addition to the regulation on glucose homeostasis, PPP1R3G also has an interesting effect on fat composition of the body. Overexpression of PPP1R3G was able to reduce fat mass while increasing lean mass in the mouse (Figure 3A). Studies with metabolic cage indicate that PPP1R3G overexpression was not able to change metabolic rate of the animals, whereas the transgenic mice might consume more fat as an energy source than the wild-type animals as reflected by a reduction in respiratory exchange ratio (Figure 3C).
One of the intriguing discoveries in this study is that PPP1R3G overexpression is associated with a reduction of triglyceride accumulation in the liver. Under fed state, PPP1R3G overexpression was able to significantly reduce liver triglyceride level (Figure 4, D and E). Under fasted state, PPP1R3G overexpression could markedly relieve fasting-induced hepatic steatosis (Figure 5, E and F). Interestingly, PPP1R3G overexpression is also associated with a reduced blood triglyceride level (Figure 5G). These findings suggest that PPP1R3G may change the balance of energy storage in the liver. As PPP1R3G plays a major role during postprandial phase, an increase in glycogen storage by elevated PPP1R3G expression would favor energy storage toward glycogen but not triglyceride after a meal. Meanwhile, as the liver is the major organ in controlling blood lipid levels, the reduction of triglyceride load in the liver would lead to reduction of blood triglyceride level. The observed association of liver glycogen metabolism with lipid metabolism in our study is supported by a recent finding that reveals the association of PPP1R3B polymorphism with blood lipid level (37). Overexpression of PPP1R3B in mouse liver by adenovirus results in significantly lower HDL-cholesterol level (37). Collectively, these observations indicate glycogen metabolism in the liver is closely associated with lipid metabolism in the body.
There are a total of seven G subunits (PPP1R3A to G) that are involved in glycogen metabolism (6). PPP1R3A (GM) is mainly expressed in the skeletal muscle and heart. Deletion of this gene leads to reduction of glycogen level in skeletal muscle, accompanied by increases in weight gain, fat deposition, and insulin resistance (14), suggesting that PPP1R3A plays a major function in glucose utilization in the skeletal muscle. PPP1R3B (GL) is the primary G subunit expressed in the liver in rodents. Overexpression of a deregulated form of PPP1R3B, which contains a Tyr284Phe substitution in GP, a GPa-binding region that prevents the protein from being inhibited by GPa, is associated with improvement of glucose tolerance (15). However, there was no significant accumulation of hepatic glycogen in the transgenic mice (15). Therefore, this study mainly indicates that during food deprivation, the GPa-mediated regulation of PPP1R3B may prevent futile glucose-glycogen cycling. However, how PPP1R3B is implicated in postprandial glucose homeostasis in vivo is currently unknown. PPP1R3C (PPP1R5) is expressed in many tissues and consistently the mice with heterozygous deletion of PPP1R3C had reduced glycogen levels in adipose tissue, liver, heart, and skeletal muscle, accompanied by progressive glucose intolerance, hyperinsulinemia, and insulin resistance with aging (16). At 18 months of age, the PPP1R3C heterozygous-deletion mice had an elevated fasting triglyceride level in serum, together with an elevation of triglyceride in the skeletal muscle (16). However, the mice had no change in hepatic triglyceride level. The increased lipid accumulation in the skeletal muscle was also associated with attenuation of insulin signaling (16). Therefore, such in vivo study with PPP1R3C gene disruption indicates this protein is mainly involved in regulating glycogen metabolism and insulin sensitivity in the skeletal muscle. Up until now, the in vivo functions of other three G subunits (PPP1R3D, PPP1R3E, andPPP1R3F) have not been characterized. Compared with the in vivo studies with PPP1R3A, PPP1R3B, and PPP1R3C as discussed before, PPP1R3G appears to have a more distinct function than other G subunits in the regulation of glycogen metabolism and glucose homeostasis. Our study indicates that the primary function of hepatic PPP1R3G is to regulate postprandial glucose homeostasis but not insulin sensitivity. Furthermore, PPP1R3G is actively involved in hepatic triglyceride accumulation as it changes the balance of energy preservation on a meal. However, it is noteworthy that the physiological functions of PPP1R3G in other tissues still await further characterization, such as using the strategy of gene deletion, in addition to the study using transgenic mouse model as reported here. Nevertheless, due to the paramount importance of PPP1R3G in postprandial glucose and lipid homeostasis in the liver, PPP1R3G may serve as an interesting target in the future for intervention of diseases with disruption of glucose and lipid metabolism.
Acknowledgments
This work was supported by research grants from National Natural Science Foundation of China (81021002, 81130077, and 81390353 to Y.C. and 30971660 to Y.P.), Ministry of Science and Technology of China (2012CB524900 to Y.C., 2010CB529506 to Y.P. and Z.W., and 2013BAI04B03 to Z.W.), and Chinese Academy of Sciences (KSCX2-EW-R-08 to Y.C.). Susie Chen is a summer student from University of Washington at Seattle, Washington, USA.
Y.C. is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis. Y.Z. and Y.C. conceptualized and designed the experiments. Y.Z., D.X., H.H., L.W., L.Z., X.J., X.R., X.L., P.C., and W.L. performed the experiments. S.C., Y.P., and Z.W. contributed the reagents and editorial assistance. Y.C. and Y.Z. analyzed the data and wrote the paper.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by research grants from National Natural Science Foundation of China (81021002, 81130077, and 81390353 to Y.C. and 30971660 to Y.P.), Ministry of Science and Technology of China (2012CB524900 to Y.C., 2010CB529506 to Y.P. and Z.W., and 2013BAI04B03 to Z.W.), and Chinese Academy of Sciences (KSCX2-EW-R-08 to Y.C.). Susie Chen is a summer student from University of Washington at Seattle, Washington, USA.
Footnotes
- FTT
- food tolerant test
- G6P
- glycose-6-phosphate
- GP
- glycogen phosphorylase
- GPa
- glycogen phosphorylase a
- GS
- glycogen synthase
- GSK
- glycogen synthase kinase-3
- GSP
- glycogen synthase phosphatase
- GTT
- glucose tolerance test
- HDL
- high-density lipoprotein
- ITT
- insulin tolerant test
- PP1
- protein phosphatase 1.
References
- 1. Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. [DOI] [PubMed] [Google Scholar]
- 2. Moore MC, Cherrington AD, Wasserman DH. Regulation of hepatic and peripheral glucose disposal. Best Prac Res Clin Endo Metab. 2003;17(3):343–364. [DOI] [PubMed] [Google Scholar]
- 3. Shoemaker WC, Elwyn DH. Liver: functional interactions within the intact animal. Annu Rev Physiol. 1969;31:227–268. [DOI] [PubMed] [Google Scholar]
- 4. Postic C, Dentin R, Girard J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 2004;30(5):398–408. [DOI] [PubMed] [Google Scholar]
- 5. Hers HG. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. [DOI] [PubMed] [Google Scholar]
- 6. Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev. 2004;84(1):1–39. [DOI] [PubMed] [Google Scholar]
- 7. Brady MJ, Saltiel AR. The role of protein phosphatase-1 in insulin action. Recent Prog Hormone Res. 2001;56:157–173. [DOI] [PubMed] [Google Scholar]
- 8. Nuttall FQ, Gilboe DP, Gannon MC, Niewoehner CB, Tan AW. Regulation of glycogen synthesis in the liver. Am J Med. 1988;85(5A):77–85. [DOI] [PubMed] [Google Scholar]
- 9. Villar-Palasi C. Substrate specific activation by glucose 6-phosphate of the dephosphorylation of muscle glycogen synthase. Biochim Biophys Acta. 1991;1095(3):261–267. [DOI] [PubMed] [Google Scholar]
- 10. Shulman RG, Rothman DL. Enzymatic phosphorylation of muscle glycogen synthase: a mechanism for maintenance of metabolic homeostasis. Proc Natl Acad Sci USA. 1996;93(15):7491–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aiston S, Andersen B, Agius L. Glucose 6-phosphate regulates hepatic glycogenolysis through inactivation of phosphorylase. Diabetes. 2003;52(6):1333–1339. [DOI] [PubMed] [Google Scholar]
- 12. Larner J, Roach PJ, Huang LC, Brooker G, Murad F, Hazen R. Hormonal control of glycogen metabolism. Adv Exp Med Biol. 1979;111:103–123. [DOI] [PubMed] [Google Scholar]
- 13. Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993;12(2):803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delibegovic M, Armstrong CG, Dobbie L, Watt PW, Smith AJ, Cohen PT. Disruption of the striated muscle glycogen targeting subunit PPP1R3A of protein phosphatase 1 leads to increased weight gain, fat deposition, and development of insulin resistance. Diabetes. 2003;52(3):596–604. [DOI] [PubMed] [Google Scholar]
- 15. Kelsall IR, Rosenzweig D, Cohen PT. Disruption of the allosteric phosphorylase a regulation of the hepatic glycogen-targeted protein phosphatase 1 improves glucose tolerance in vivo. Cell Signalling. 2009;21(7):1123–1134. [DOI] [PubMed] [Google Scholar]
- 16. Crosson SM, Khan A, Printen J, Pessin JE, Saltiel AR. PTG gene deletion causes impaired glycogen synthesis and developmental insulin resistance. J Clin Invest. 2003;111(9):1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rubio-Villena C, Garcia-Gimeno MA, Sanz P. Glycogenic activity of R6, a protein phosphatase 1 regulatory subunit, is modulated by the laforin-malin complex. Int J Biochem Cell Biol. 2013;45(7):1479–1488. [DOI] [PubMed] [Google Scholar]
- 18. Munro S, Ceulemans H, Bollen M, Diplexcito J, Cohen PT. A novel glycogen-targeting subunit of protein phosphatase 1 that is regulated by insulin and shows differential tissue distribution in humans and rodents. FEBS J. 2005;272(6):1478–1489. [DOI] [PubMed] [Google Scholar]
- 19. Kelsall IR, Voss M, Munro S, Cuthbertson DJ, Cohen PT. R3F, a novel membrane-associated glycogen targeting subunit of protein phosphatase 1 regulates glycogen synthase in astrocytoma cells in response to glucose and extracellular signals. J Neurochem. 2011;118(4):596–610. [DOI] [PubMed] [Google Scholar]
- 20. Luo X, Zhang Y, Ruan X, et al. . Fasting-induced protein phosphatase 1 regulatory subunit contributes to postprandial blood glucose homeostasis via regulation of hepatic glycogenesis. Diabetes. 2011;60(5):1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han R, Lai R, Ding Q, et al. . Apolipoprotein A-I stimulates AMP-activated protein kinase and improves glucose metabolism. Diabetologia. 2007;50(9):1960–1968. [DOI] [PubMed] [Google Scholar]
- 22. Feng L, Xie X, Ding Q, et al. . Spatial regulation of Raf kinase signaling by RKTG. Proc Natl Acad Sci USA. 2007;104(36):14348–14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu L, Wang L, Wang X, et al. . Hepatic deletion of Smad7 in mouse leads to spontaneous liver dysfunction and aggravates alcoholic liver injury. PloS One. 2011;6(2):e17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lo S, Russell JC, Taylor AW. Determination of glycogen in small tissue samples. J Appl Physiol. 1970;28(2):234–236. [DOI] [PubMed] [Google Scholar]
- 25. Worby CA, Gentry MS, Dixon JE. Malin decreases glycogen accumulation by promoting the degradation of protein targeting to glycogen (PTG). J Biol Chem. 2008;283(7):4069–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Danforth WH. Glycogen synthetase activity in skeletal muscle. Interconversion of two forms and control of glycogen synthesis. J Biol Chem. 1965;240:588–593. [PubMed] [Google Scholar]
- 27. Morifuji M, Sakai K, Sugiura K. Dietary whey protein modulates liver glycogen level and glycoregulatory enzyme activities in exercise-trained rats. Exp Biol Med. 2005;230(1):23–30. [DOI] [PubMed] [Google Scholar]
- 28. Wang Q, Li S, Jiang L, et al. . Deficiency in hepatic ATP-citrate lyase affects VLDL-triglyceride mobilization and liver fatty acid composition in mice. J Lipid Res. 2010;51(9):2516–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neufeld DS. Isolation of rat liver hepatocytes. Methods Mol Biol. 1997;75:145–151. [DOI] [PubMed] [Google Scholar]
- 30. Heijboer AC, Donga E, Voshol PJ, et al. . Sixteen hours of fasting differentially affects hepatic and muscle insulin sensitivity in mice. J Lipid Res. 2005;46(3):582–588. [DOI] [PubMed] [Google Scholar]
- 31. Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J Clin Invest. 1999;103(11):1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hashimoto T, Cook WS, Qi C, Yeldandi AV, Reddy JK, Rao MS. Defect in peroxisome proliferator-activated receptor α-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem. 2000;275(37):28918–28928. [DOI] [PubMed] [Google Scholar]
- 33. Armstrong CG, Doherty MJ, Cohen PT. Identification of the separate domains in the hepatic glycogen-targeting subunit of protein phosphatase 1 that interact with phosphorylase a, glycogen and protein phosphatase 1. Biochem J. 1998;336(Pt 3):699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fong NM, Jensen TC, Shah AS, Parekh NN, Saltiel AR, Brady MJ. Identification of binding sites on protein targeting to glycogen for enzymes of glycogen metabolism. J Biol Chem. 2000;275(45):35034–35039. [DOI] [PubMed] [Google Scholar]
- 35. Wu J, Liu J, Thompson I, Oliver CJ, Shenolikar S, Brautigan DL. A conserved domain for glycogen binding in protein phosphatase-1 targeting subunits. FEBS Lett. 1998;439(1–2):185–191. [DOI] [PubMed] [Google Scholar]
- 36. Liu J, Brautigan DL. Glycogen synthase association with the striated muscle glycogen-targeting subunit of protein phosphatase-1. Synthase activation involves scaffolding regulated by β-adrenergic signaling. J Biol Chem. 2000;275(34):26074–26081. [DOI] [PubMed] [Google Scholar]
- 37. Teslovich TM, Musunuru K, Smith AV, et al. . Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]