Abstract
Kit ligand (KITL) plays indispensable roles both in primordial follicle activation and in the maintenance of meiotic arrest of the oocyte. The regulation of KITL expression in the ovary, however, remains largely unknown. In the zebrafish, there are 2 paralogues of KITL, kitlga and kitlgb, and 2 Kit receptors, kita and kitb. Consistent with the situation in mammals, kitlga is only expressed in the ovarian follicle cells, and its cognate receptor kita is expressed in the oocyte. In the present study, we demonstrated that the expression of kitlga was promoted by IGF-I through its receptor IGF-IR. The stimulation involved transcription but not translation, suggesting that the kitlga gene is likely a direct downstream target of IGF-I signaling. Further experiments showed that the stimulatory effect of IGF-I was mediated by phosphatidyl inositol 3-kinase (PI3K)-Akt pathway. IGF-I also activated MEK-ERK pathway; however, this pathway suppressed kitlga expression. The regulation of kitlga expression by IGF-I appeared to depend on the stage of follicle development with a greater induction at early stage than late stage. This may be related to changes in IGF-I signaling pathways and/or local paracrine environment. In support of this were the differential expression of IGF-I receptors (igf1ra and igf1rb) and responsiveness of IGF-I signaling pathways, especially the PI3K-Akt pathway. Furthermore, the IGF-I-induced kitlga expression was inhibited by epidermal growth factor, an oocyte-derived paracrine factor in the zebrafish follicle. This study provides evidence for a controlling mechanism underlying the regulation of KITL expression in the ovary.
It is well known that, as the center of the female reproductive system in vertebrates, the ovary is subject to multifactorial regulation. It is controlled not only by systemic endocrine hormones such as gonadotropins from the pituitary but also a variety of intraovarian paracrine factors including Kit ligand (KITL), IGF-I, epidermal growth factor (EGF), activin, and steroids (eg, estradiol), which form a complex communication network in the ovarian follicle (1, 2).
KITL/Kitl, also known as stem cell factor, mast cell growth factor, and steel factor is the product of Steel (Sl) locus in mice (3–5), whereas its receptor KIT (Kit) is coded by the White Spotting (W) locus (4–9). In the mouse ovary, Kitl is expressed in the granulosa cells whereas Kit is expressed in the oocyte and theca cells, suggesting a KITL/KIT-mediated granulosa-oocyte and granulosa-theca signaling (10, 11). Both in vivo and in vitro studies have demonstrated that the interaction of KITL with KIT is essential for oogenesis and folliculogenesis (12–16). In recent years, the Kit system has attracted increasing attention because a growing body of evidence indicates that KITL plays indispensable roles both in gonadotropin-independent activation of primordial follicles from the quiescent pool (17, 18) and in the maintenance of meiotic arrest before germinal vesicle breakdown (19–21). Of particular interest, KITL has also been demonstrated to be involved in the proliferation of granulosa cells in which the receptor KIT is absent (22, 23).
In contrast to the Kit system in mammals in which there is only one locus in the genome coding for KITL, which may exist as both soluble form (KITL1) and membrane-associated form (KITL2) due to alternative mRNA splicing and proteolytic processing (24), there are 2 paralogues of KITL, kitlga and kitlgb, and 2 Kit receptors, kita and kitb, in the zebrafish genome, probably as a result of whole-genome duplication specific to the fish lineage (25). We have recently demonstrated that the 2 forms of KITL and Kit receptor exhibit distinct spatial location within the follicle with kitlga being expressed in the somatic follicle cells and its preferred receptor kita located in the oocyte, similar to the situation in mammals. In contrast, kitlgb expression was exclusively restricted to the oocyte whereas its preferred receptor kitb was expressed in the surrounding follicle cells (26). Because the somatic follicle cells are the targets of various endocrine hormones and paracrine factors regulating folliculogenesis, the location of kitlga expression in these cells has led us to speculate that this ligand may be a key member of the Kit system in the zebrafish follicle that is subject to external regulation.
In mammals, there have been some studies on the regulation of KITL in the granulosa cells, but the information available remains limited. The expression of Kitl in bovine granulosa cells was stimulated by theca cell-derived keratinocyte growth factor and hepatocyte growth factor (27). Kitl expression is also regulated by pituitary-derived gonadotropins, but the regulation remains intricate because it varies depending on experimental conditions (19, 20, 27, 28). FSH and LH increased Kitl expression in cultured granulosa cells but LH reduced it in the antral follicles (19, 20, 27). It has been reported that the regulation of Kitl expression is subject to regulation by unidentified factors from the oocyte, and the effect of FSH appeared to depend on the stage of developing oocyte (28). A similar study was recently reported in the hen in which both oocyte conditioned medium and FSH reduced KITL expression in cultured granulosa cells (29).
In the zebrafish folliculogenesis, the expression of kitlga increases significantly when the follicles of primary growth (PG) stage are activated to enter the vitellogenic growth, or previtellogenic stage (PV), in contrast to kitlgb, which decreases its expression during the transition (25). A recent study from our laboratory showed that the first wave of PG-PV transition in the zebrafish life cycle, also defined as a marker for puberty onset, was closely associated with body growth, suggesting a role for factors from the somatotropic axis such as IGF-I in the activation of the reproductive axis (30). In support of this is the evidence that both IGF-I (igf1) and its receptors (igf1ra and igf1rb) significantly increase their expression during the PG-PV transition (our unpublished data). These observations have led us to hypothesize that the growth axis may influence the reproductive axis through IGF-I, and kitlga could be one of its potential target genes in the follicle.
The potential role of KITL as a mediating factor in the follicle downstream of IGF-I, a key endocrine hormone and paracrine growth factor of the growth axis, is supported by several lines of evidence in both mammalian models and the zebrafish. First, IGF-I receptor (Igf1r) is expressed in both granulosa cells and oocytes in mammals (31), and IGF-I itself can also be detected in the granulosa cells (31–33), suggesting that IGF-I (Igf1) may act in both endocrine and paracrine/autocrine manners to regulate granulosa cells and probably the oocyte as well in mammals. In the zebrafish, 3 IGF ligands (igf2a, igf2b, and igf3) and 2 isoforms of IGF receptor (igf1ra and igf1rb) could also be detected in both the follicle cells (granulosa and theca) and oocyte (34). As for the Kit system, the distribution pattern of kitlga and kita expression in the zebrafish follicle is the same as that in mammals; Kitlga produced in the peripheral follicle layer activates Kita in the oocyte (26), making kitlga a potential target for regulation by endocrine and paracrine factors including IGF-I. Second, both IGF-I and KITL enhance follicle growth and survival. The failure of IGF-I to interact with its receptor activates the Fas antigen-mediated cell death in the follicles through apoptosis (35). Similarly, inhibition of KITL-KIT interaction with antibody to KIT promotes the death of oocytes in vitro (22). Third, they both promote granulosa cell proliferation (22, 23, 36) and regulate steroidogenesis. For example, IGF-I increases estradiol and progesterone secretion from follicles cultured in vitro (37), and KITL-KIT interaction between granulosa and theca cells influences androgen output by theca cells (22, 27, 38). Finally, IGF-I exerts its effect by activating 2 major signal transduction pathways: the phosphatidylinositol 3-kinase (PI3K)-Akt and MAPK kinase (MEK)-ERK pathways in both mammals (39, 40) and zebrafish (41–43). Similarly, Kit also signals through both PI3K-Akt (44) and MEK-ERK pathways (45) in the ovary.
Despite the importance of IGF-I and its similar effects to those of KITL in the ovary, little is known about their regulatory relationship in follicle development. Limited studies have been reported on synergistic effects of IGF-I and KITL on germ cell survival in the ovary (46) and expression of steroidogenic enzymes in the theca cells (47), but not on their reciprocal regulation in the follicle.
To test the hypothesis that kitlga (Kitlga) could be a downstream target gene for IGF-I in the follicle and to demonstrate the regulatory relationship between IGF-I and the Kit system in the ovary, we undertook this study on regulation of kitlga expression by IGF-I using the primary culture of zebrafish follicle cells in which kitlga and IGF-I receptors (igf1ra and igf1rb) are both expressed (26, 34). Further experiments were carried out to investigate the intracellular mechanisms of IGF-I signaling that underlie the regulation of kitlga by IGF-I, focusing on PI3K-Akt and MEK-ERK pathways. We also examined the influence of other factors on IGF-I regulation of kitlga expression, including the developmental stage of follicles and local paracrine factors such as EGF.
Materials and Methods
Animals and chemicals
Zebrafish (Danio rerio) were obtained from a local tropical fish market and maintained in flow-through aquaria at 28 ± 1°C on a photoperiod of 14-hours light, 10 hours dark. The fish was fed twice a day with the commercial tropical fish feed Otohime S1 (Marubeni Nisshin Feed Co) and once with artemia. All experiments performed were licensed by the Government of the Hong Kong Special Administrative Region and endorsed by the Animal Experimentation Ethics Committee of The Chinese University of Hong Kong. Unless otherwise indicated, all common chemicals used were purchased from Sigma, USB Corporation, GE Healthcare, or Merck, enzymes from Promega Corp., and culture media from Gibco Invitrogen. Recombinant human IGF-I, actinomycin D, and cycloheximide were purchased from Sigma; and PPP, NVP-AEW541, LY294002, wortmannin, Akti, GW5074, FR180204, U0126 and PD98059 from Calbiochem. IGF-I was first dissolved in water, cycloheximide in ethanol and actinomycin D, PPP, NVP-AEW541, LY294002, wortmannin, Akti, GW5074, FR180204, U0126, and PD98059 in dimethylsulfoxide (DMSO). They were diluted to the desired concentrations with the medium before use. Antibodies for Akt (catalog no. 9272), phosphor-Akt (Ser473, catalog no. 9271L), ERK1/2 (MAPK3/1) (catalog no. 9102L), phospho-ERK1/2 (catalog no. 9101L) were from Cell Signaling Technology, and horseradish peroxidase-linked antirabbit IgG (catalog no. sc-2374) was from Santa Cruz Biotechnology.
Total RNA isolation and reverse transcription (RT)
Total RNA was extracted from cultured follicle cells or follicles with Tri-Reagent (Molecular Research Center) according to the manufacture's protocol and our previous study (48). The RT was then performed at 37°C for 2 hours in a volume of 10 μL containing 0. 5 μg of oligo(dT), 1× RT buffer, 0.5 mM each deoxyribonucleotide triphosphate, 0.1 mM dithiothreitol, and 100 U of Moloney murine leukemia virus reverse transcriptase (Invitrogen).
Quantification of kitlga mRNA by real-time qPCR
Real-time quantitative PCR (qPCR) was performed to quantify the expression of kitlga and the housekeeping gene ef1a. The templates for standard curves were prepared by PCR amplification of cDNA fragments with specific primers (Table 1). After purification with a PCR Purification Kit (Qiagen), the amplified DNA amplicons were quantified with the software Quantity One (Bio-Rad Laboratories) using the Mass Ruler DNA marker (MBI Fermentas) as the standard, and the copy numbers of the DNA molecules were calculated before use as templates to construct standard curves in qPCR. All PCRs were performed in a total volume of 30 μL containing 10 μL template (RT reaction mix diluted at 1:15), 1× PCR buffer, 0.2 mM each deoxyribonucleotide triphosphate, 2.5 mM MgCl2, 0.75 U of Taq polymerase, 0.5× EvaGreen (Biotium), and 20 nM fluorescein (Bio-Rad) on the iCycler iQ Real-time PCR Detection System (Bio-Rad). The amplification protocol was 30 seconds at 94°C, 30 seconds at 60°C, and 30 seconds at 72°C, with a signal detection period of 7 seconds at 80°C. A melt curve analysis was performed at the end of the reaction to check the reaction specificity.
Table 1.
Primers Used in RT-PCR
| Gene | Strand | Sequence | Expected Size (bp) | Accession no. |
|---|---|---|---|---|
| ef1a | Sense | 5′-GGCTGACTGTGCTGTGCTGATTG-3′ | 409 | NM_131263 |
| Antisense | 5′-CTTGTCGGTGGGACGGCTAGG-3′ | |||
| Kitlga | Sense | 5′-AATTCATCAAGAGATGCTGAGGAC-3′ | 213 | AY929068 |
| Antisense | 5′-TGCAAACGGGATGGTGAGGAG-3′ | |||
| igf1ra | Sense | 5′-CCCGTGTTCACCTACCCA-3′ | 207 | NM 152968 |
| Antisense | 5′-TTGCCTTTGATGACTGTGC-3′ | |||
| igf1rb | Sense | 5′-GATGCGTCGCATGTGTGACAAGCCACT-3′ | 214 | BC163581 |
| Antisense | 5′-CAGTCAGTGATCCTGTCTGGCGGAAAT-3′ |
Primary follicle cell culture
The primary follicle cell culture of zebrafish ovary was performed according to our previous report (48). Briefly, the ovaries from about 20 female zebrafish were isolated and dispersed in a 100-mm petri dish containing 60% Leibovitz L-15 medium. After removing the full-grown follicles by sieving, the follicles of earlier stages were washed 5 times with medium M199. Afterward, the follicles were cultured in M199 with 10% fetal calf serum (Hyclone Laboratories) for 6 days at 28°C in 5% CO2 for the proliferation of follicle cells. The medium was changed once on day 3. The follicle cells were harvested by trypsinization and plated in 24-well plates at the density of about 2.5 × 105 cells/well for 24 hours. The cells were then starved with M199 without serum for 24 hours before treatment.
Isolation and incubation of ovarian follicles
After anesthetization on ice and decapitation, ovaries were removed from 10 to 20 female zebrafish and placed in a 100-mm Petri dish containing 60% Leibovitz L-15 medium. The follicles at early vitellogenic (EV; early stage III, ∼350 μm) and full-grown (FG; late stage III, ≥650 μm) stages were manually isolated. The staging of the follicles was based on our previous study (49).
The follicles were incubated as previously reported (50). In brief, the follicles were placed in a 24-well plate (∼40 follicles per well) with 500 μL medium in each well. After incubation with or without IGF-I for 3–6 hours at 28°C, the follicles were collected for extraction of RNA (6 hours) or protein (3 hours).
Western blotting
The cells or follicles in each well were lysed by adding 100 μL sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris-HCl [pH 6.8] at 25°C, 1% [wt/vol] SDS, 10% glycerol, 5% 2-mercaptoethanol). Then the plate was shaken immediately for a few times, and the extract from each well was transferred to a microcentrifuge tube. All samples were heated to 95–100°C for 5 minutes, cooled on ice, and microcentrifuged for 5 minutes. Western blotting was performed according to the manufacturer's protocol (Cell Signaling Technology). Briefly, samples (half from each well) were loaded and separated in the 12.5% SDS-PAGE gel in 1 × running buffer (25 mM Tris base, 0.2 M glycine, 0.1% [wt/vol] SDS), followed by blotting to a nitrocellulose membrane (Bio-Rad Laboratories) in the blotting buffer (25 mM Tris base, 0.2 M glycine, 20% methanol). The membrane was incubated in 25 mL blocking buffer (1× Tris-buffered saline-0.1% Tween 20 with 5% [wt/vol] nonfat dry milk) for 1 hour at room temperature and then incubated in 5 mL of diluted primary antibody (1:1000 in blocking buffer) at 4°C overnight. The membrane was washed 3 times for 5 minutes each with the washing buffer (1× Tris-buffered saline-0.1% Tween 20) and then incubated with horseradish peroxidaseconjugated secondary antibody (1:2000 in wash buffer) for 1 hour at room temperature. The membrane was then washed again and equilibrated with the developing solution (Western Blotting Luminol Reagent; Santa Cruz Biotechnology). The signals were detected on the Lumi-Imager F1 Workstation (Roche).
Data analysis
The ratio of expression level of kitlga to that of the internal control ef1a was calculated and then expressed as the fold change/percentage compared with the control or reference group. All values were expressed as the mean ± SEM, and the data were analyzed by one-way ANOVA, followed by Dunnett test using Prism 5 on Macintosh OS X (GraphPad Software).
Results
IGF-I stimulates expression of kitlga in cultured follicle cells via IGF-IR
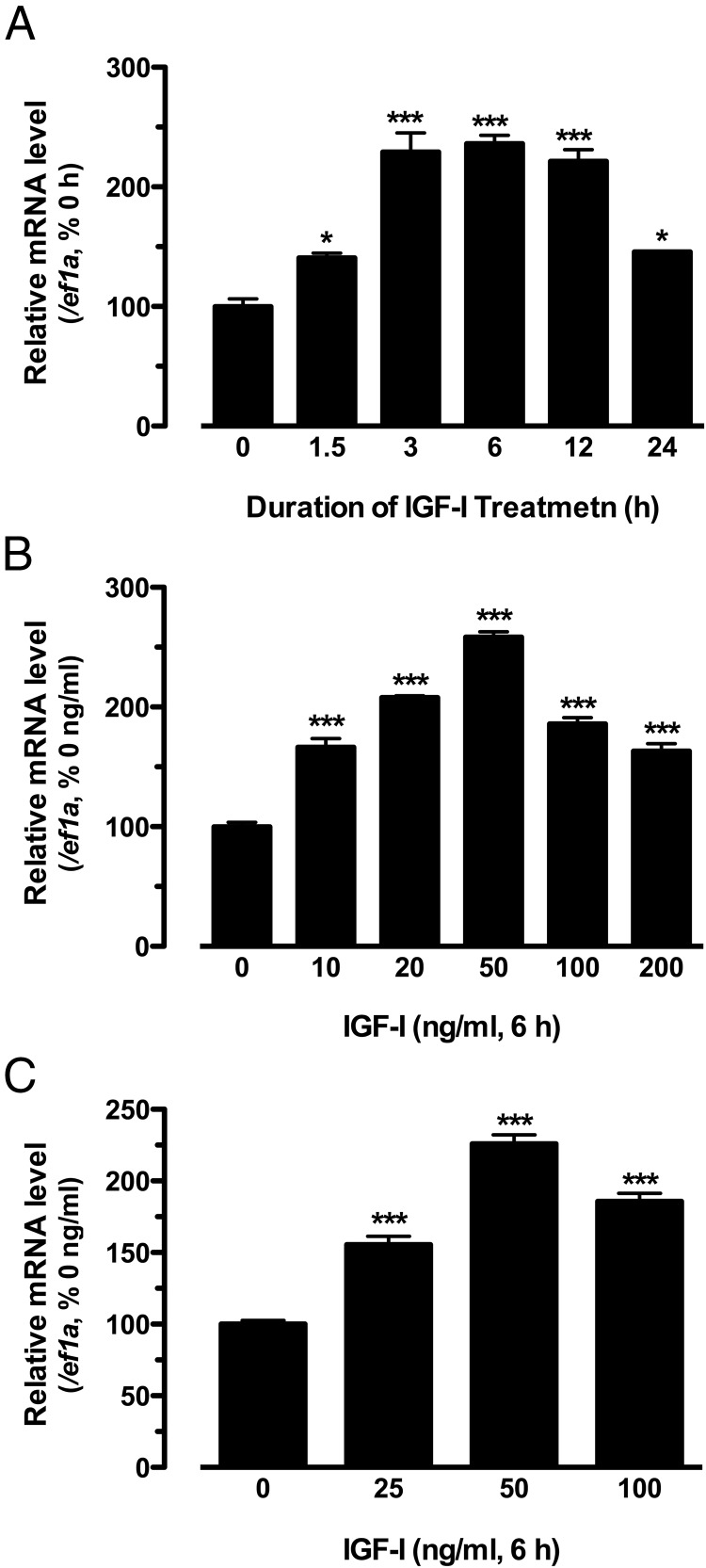
Treatment of the cultured zebrafish follicle cells with human IGF-I significantly increased the expression of kitlga in a time-dependent manner, and the maximal response was achieved at 3 hours and lasted until 12 hours of the treatment. Longer treatment for 24 hours caused a decline of the response (Figure 1A). The expression of kitlga also increased in response to IGF-I at 6 hours in a dose-dependent manner (0–200 ng/mL). The maximal response was observed at 50 ng/mL, and the response decreased at higher concentrations of 100 and 200 ng/mL (Figure 1B). The effect of IGF-I appeared to be specific because the housekeeping gene ef1a showed no response to IGF-I in either time course or dose response experiments (data not shown). To determine whether IGF-I can also promote the expression of kitlga in intact follicles, we treated the follicles at the EV stage with IGF-I. In agreement with its response in cultured follicle cells, kitlga also increased its expression in a dose-dependent manner in response to IGF-I with the maximal effect also achieved at 50 ng/mL (Figure 1C).
Figure 1.
Effects of IGF-I on the expression of kitlga in cultured zebrafish follicle cells or intact follicles. A, Time course of IGF-I effect (50 ng/mL) on the expression of kitlga in cultured zebrafish follicle cells. B, Dose response of IGF-I effect on kitlga expression in cultured zebrafish follicle cells after 6 hours of treatment. C, Dose response of IGF-I effect on the expression of kitlga in intact EV follicles after 6 hours of treatment. The data were expressed as the percentage or fold change of the respective control group after normalization to the expression of the housekeeping gene, ef1a (mean ± SEM; n = 4). *, P < .05; ***, P < .001 vs control.
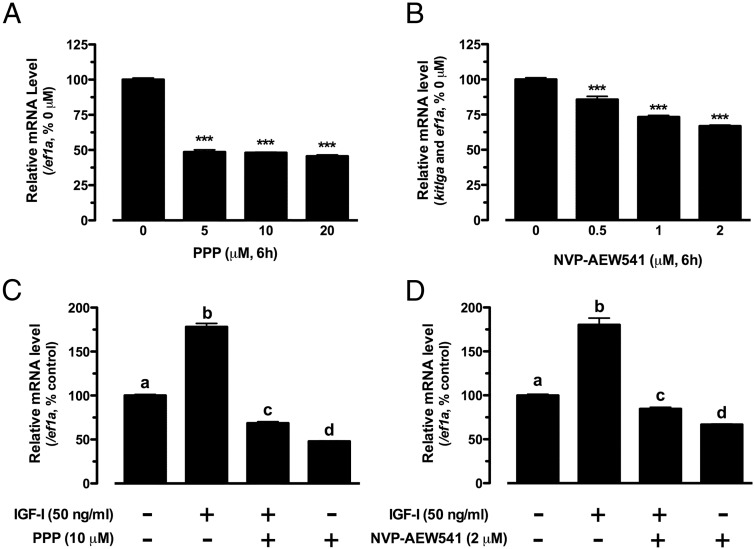
To demonstrate whether IGF-I acted via its receptor, IGF-IR (Igf1r), we tested 2 IGF-IR inhibitors, PPP and NVP-AEW541. Both PPP and NVP-AEW541 significantly reduced the basal expression of kitlga (Figure 2, A and B), and their presence also suppressed IGF-I-stimulated kitlga expression (Figure 2, C and D).
Figure 2.
Effects of PPP and NVP-AEW541 on the expression of kitlga in cultured zebrafish follicle cells. A and B, Dose response of PPP and NVP-AEW541 effects on the expression of kitlga in cultured zebrafish follicle cells after 6 hours of treatment. The data were normalized to the housekeeping gene ef1a and expressed as the percentage of the control group (mean ± SEM; n = 4). ***, P < .001 vs control. C and D, Effects of IGF-I on the expression of kitlga in the presence of PPP or NVP-AEW541 in cultured zebrafish follicle cells. Cultured zebrafish follicle cells were pretreated with PPP (10 μM) or NVP-AEW541 (2 μM) for 30 minutes before treatment with IGF-I (50 ng/mL) for 6 hours. The data are normalized to the housekeeping gene ef1a and expressed as the percentage of the control group (mean ± SEM; n = 4). Different letters indicate statistical significance (P < .05).
IGF-I stimulation of kitlga expression is transcription but not translation dependent
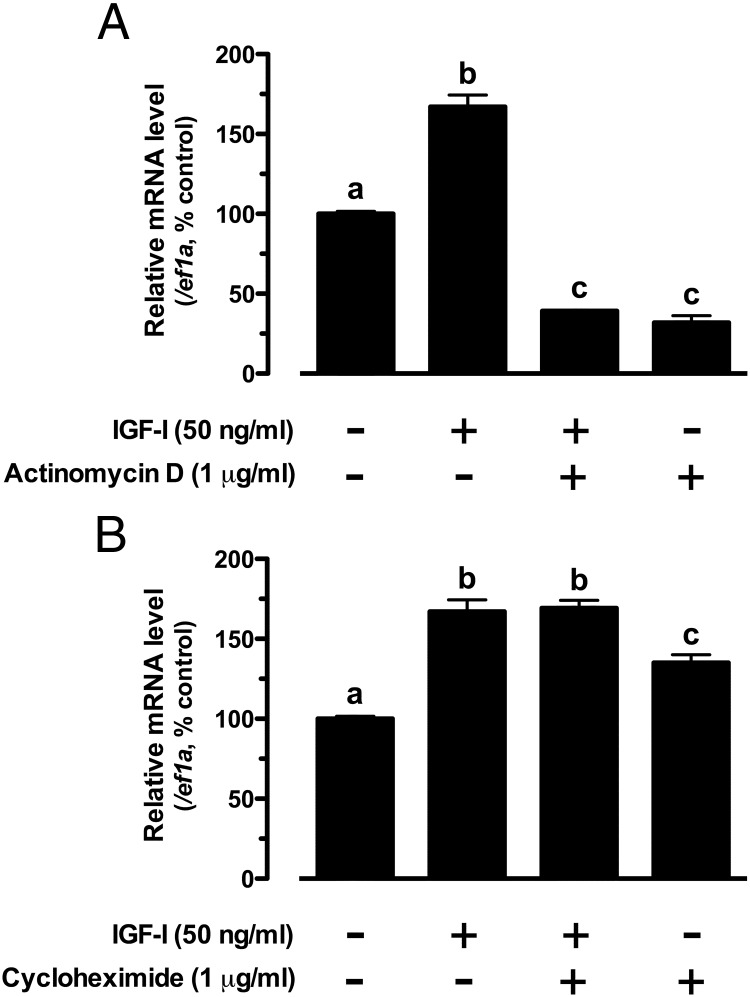
To further define the mechanism by which IGF-I regulates kitlga, we assessed the involvement of transcription and translation in IGF-I-induced kitlga expression by using specific transcription inhibitor actinomycin D and translation inhibitor cycloheximide. Treatment of cultured follicle cells with actinomycin D significantly reduced the basal expression level of kitlga and completely abolished the stimulatory effect of IGF-I, indicating a dependence of IGF-I action on transcription (Figure 3A). In contrast, cycloheximide had no effect on IGF-I-induced kitlga expression, suggesting no involvement of new protein synthesis in the regulation. Interestingly, cycloheximide alone seemed to further raise the basal level of kitlga expression (Figure 3B).
Figure 3.
Effects of actinomycin D (A) and cycloheximide (B) on IGF-I-induced kitlga expression in cultured zebrafish follicle cells. Cultured zebrafish follicle cells were pretreated with the drugs for 30 minutes before treatment with IGF-I (50 ng/mL) for 6 hours. The data are normalized to the housekeeping gene ef1a and expressed as the percentage of the control group (mean ± SEM; n = 4). Different letters indicate statistical significance (P < .05).
IGF-I stimulation of kitlga expression is mediated by the PI3K-Akt pathway
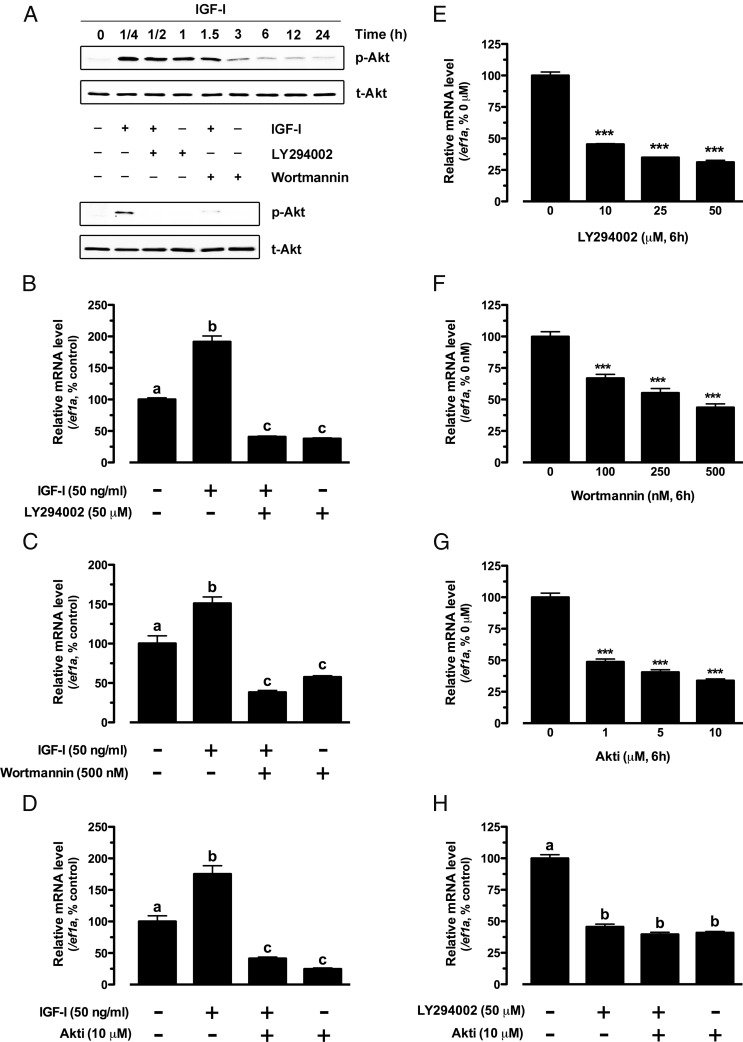
The PI3K-Akt pathway is well known to be the major intracellular pathway for IGF-I signaling in various cell types. As expected, IGF-I also significantly activated the PI3K-Akt pathway in cultured zebrafish follicle cells as shown by the increased phosphorylation of Akt in response to IGF-I. The phosphorylation reached the peak level at 15 minutes of treatment and lasted for more than 1.5 hours. The response of Akt to IGF-I could be abolished by PI3K inhibitors LY294002 and wortmannin (Figure 4A). To understand whether the PI3K-Akt pathway plays a role in the expression of kitlga, in particular the response to IGF-I, we tested kitlga expression in the presence of LY294002 and wortmannin as well as Akt inhibitor Akti in cultured follicle cells. Both PI3K and Akt inhibitors significantly decreased the basal level of kitlga expression in a dose-dependent manner (Figure 4, E–G). Cotreatment with LY294002 and Akti caused no further decrease in kitlga expression, suggesting that PI3K and Akt might work in the same signaling pathway in controlling kitlga expression (Figure 4H).
Figure 4.
Role of PI3K-Akt pathway in IGF-I-stimulated kitlga expression in cultured zebrafish follicle cells. A, Time course of IGF-I activation of Akt in the follicle cells (upper) and blockade of IGF-I-induced Akt phosphorylation by specific PI3K inhibitors, LY294002 and wortmannin (lower). Cultured zebrafish follicle cells were pretreated with LY294002 (50 μM) or wortmannin (500 nM) for 30 minutes before treatment with IGF-I (50 ng/mL) for 15 minutes. p-Akt, phosphorylated Akt; t-Akt, total Akt. B–D, Effect of IGF-I on the expression of kitlga in the presence of LY294002 (50 μM), wortmannin (500 nM) or Akti (10 μM) in cultured zebrafish follicle cells. The cells were pretreated with the drugs for 30 minutes before treatment with IGF-I (50 ng/mL) for 6 hours. E–H, Dose response of LY294002, wortmannin, and Akti effects on the basal expression of kitlga in cultured zebrafish follicle cells after 6 hours of treatment. The data were normalized to the housekeeping gene ef1a and expressed as the percentage of the control group (mean ± SEM; n = 4). Statistical significance was indicated by asterisk (***, P < .001) or different letters (P < .05).
To investigate whether the stimulatory effect of IGF-I on the expression of kitlga in the zebrafish follicle cells was mediated by the PI3K-Akt pathway, we treated the cells with IGF-I in the presence or absence of the above PI3K-Akt pathway inhibitors. All 3 inhibitors tested significantly suppressed or completely abolished the effect of IGF-I (Figure 4, B–D).
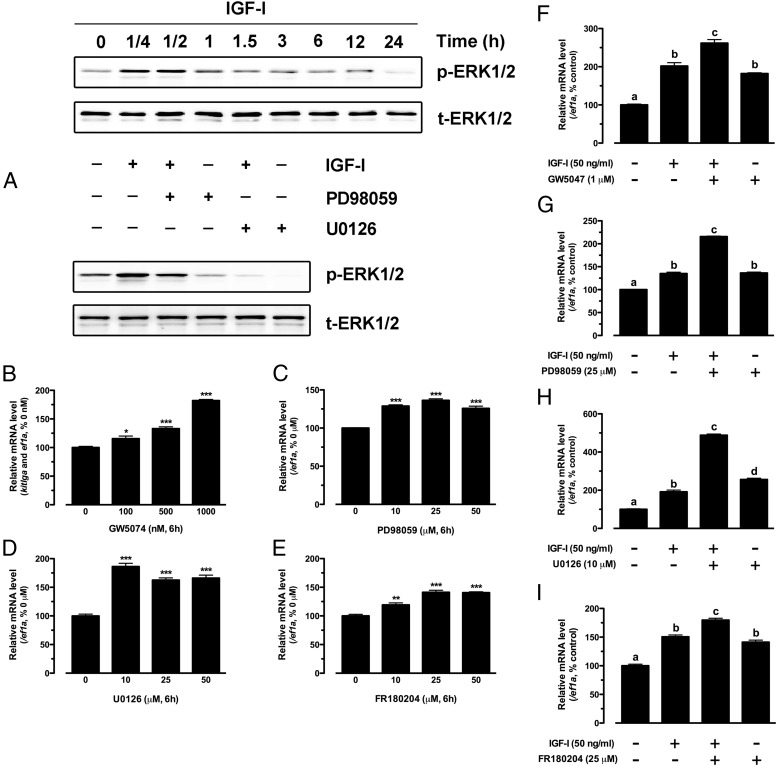
IGF-I stimulation of kitlga expression is attenuated by the MEK-ERK pathway
In addition to the PI3K-Akt pathway, IGF-I is also known to activate the MEK-ERK pathway in its target cells including the zebrafish (42). This is also true in cultured zebrafish follicle cells, as shown by a significant increase of ERK phosphorylation in response to IGF-I treatment. Although the activation also occurred quickly, with the peak level reached at 15 minutes, the magnitude of ERK response was much weaker compared with that of Akt, and it lasted for less than 1 hour, significantly shorter than that of Akt (1.5 hours). The phosphorylation could be significantly reduced or abolished by PD98059 and U0126, 2 potent inhibitors of MEK1/2 (Figure 5A). To examine whether the MEK-ERK pathway was involved in IGF-I-induced kitlga expression, we tested a series of drugs that target this pathway. Surprisingly, inhibition of the ERK pathway with GW5074, an inhibitor of c-Raf that is the immediate upstream kinase of MEK1/2, significantly increased rather than decreased the basal expression level of kitlga in a dose-dependent manner (Figure 5B). In agreement with GW5074, both MEK inhibitors U0126 and PD98059 also significantly increased basal kitlga expression (Figure 5, C and D). The same stimulatory effect was also obtained with FR180204, an inhibitor that directly suppresses ERK1/2 (Figure 5E).
Figure 5.
Effect of IGF-I on ERK1/2 phosphorylation in cultured zebrafish follicle cells. A, Time course of IGF-I activation of ERK1/2 in the follicle cells (upper) and blockade of IGF-I effect on ERK1/2 by specific MEK inhibitors, PD98059 and U0126 (lower). Cultured zebrafish follicle cells were pretreated with PD98059 (25 μM) or U0126 (10 μM) for 30 minutes before treatment with IGF-I (50 ng/mL) for 15 minutes. p-ERK1/2, phosphorylated ERK1/2; t-ERK1/2, total ERK1/2. B–E, Dose response of GW5074, PD98059, U0126, and FR180204 effects on the basal expression of kitlga in cultured zebrafish follicle cells after 6 hours of treatment. F–I, Effects of IGF-I on the expression of kitlga in the presence of GW5074 (1 μM), PD98059 (25 μM), U0126 (10 μM), and FR180204 (25 μM) in cultured zebrafish follicle cells. The cells were pretreated with the drugs for 30 minutes before treatment with IGF-I (50 ng/mL) for 6 hours. The data were normalized to the housekeeping gene ef1a and expressed as the percentage of the control group (mean ± SEM; n = 4). Statistical significance was indicated by asterisk (*, P < .05; **, P < .01; ***, P < .001) or different letters (P < .05).
When tested in the presence of IGF-I, all the above MEK-ERK pathway inhibitors (GW5074 for c-Raf, U0126 and PD98059 for MEK1/2, and FR180204 for ERK1/2) increased both basal and IGF-I-stimulated kitlga expression (Figure 5, F–I)
IGF-I stimulation of kitlga expression is dependent on stage of follicle development
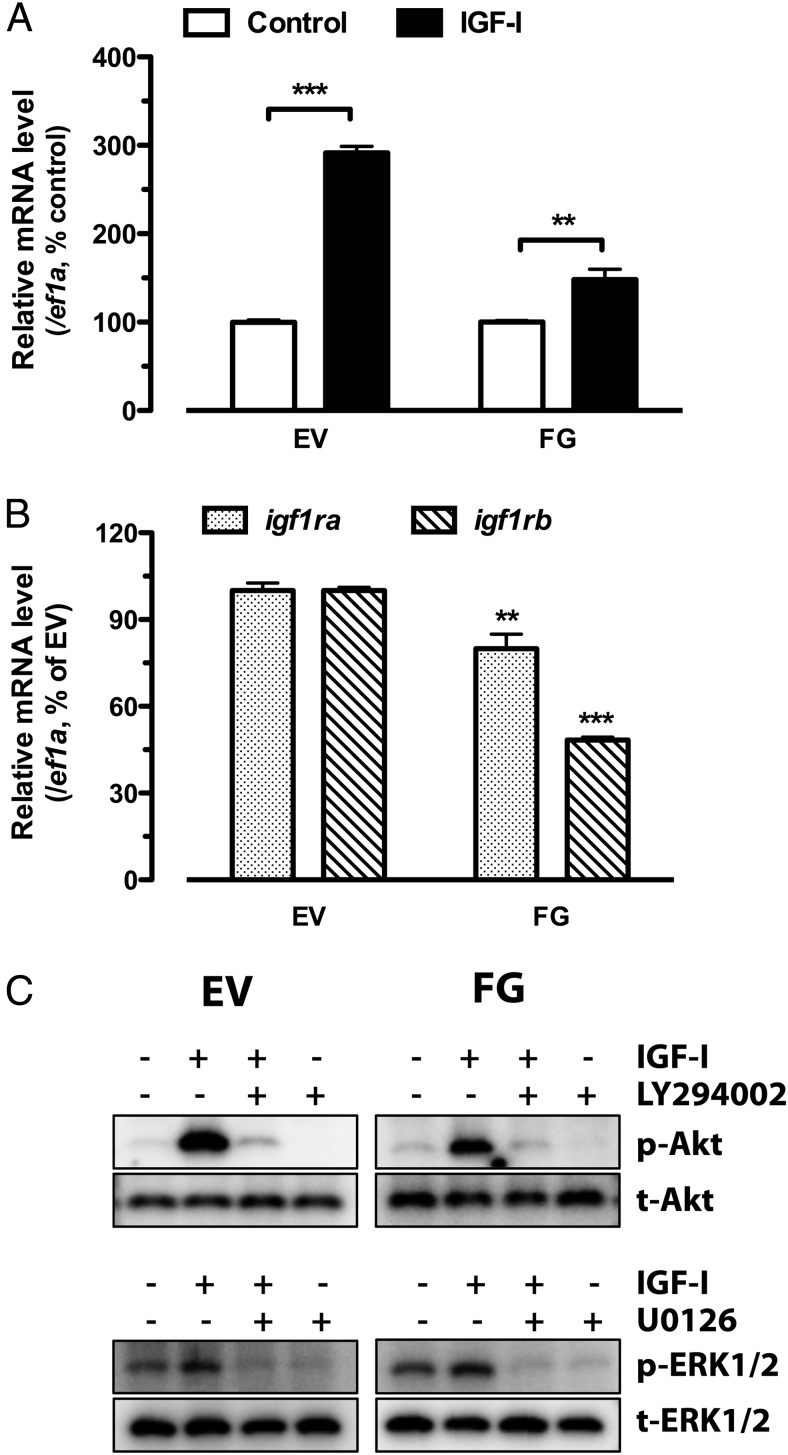
To understand whether the developmental stage of follicles influences IGF-I-stimulated kitlga expression in the follicle cells, we tested 2 stages of intact follicles, the EV and FG stages, which represent early and late vitellogenic growth, respectively. Interestingly, although kitlga expression increased at both stages in response to IGF-I after 6 hours of treatment, the responsiveness was much greater at the EV stage (∼300%) than that at the FG stage (∼150%) (Figure 6A).
Figure 6.
Temporal changes of IGF-I action and its signaling during follicle growth. A, Effect of IGF-I on kitlga expression in intact follicles of EV and FG stages. The follicles at EV and FG stages were treated with IGF-I (50 ng/mL) for 6 hours. B, Expression of IGF-I receptors (igf1ra and igf1rb) at EV and FG stages. C, Responsiveness of Akt and ERK1/2 to IGF-I at EV and FG stages. The data were normalized to the housekeeping gene ef1a and expressed as the percentage of the respective control group (mean ± SEM; n = 4). **, P < .01; ***, P < .001 vs control (A) or EV stage (B).
IGF-I signaling activity changes during folliculogenesis
To provide further evidence for the mechanism underlying the decreased kitlga responsiveness during follicle growth, we examined the expression of the 2 forms of IGF-I receptors (igf1ra and igf1rb) at EV and FG stages as well as changes of the responsiveness of PI3K-Akt and MEK-ERK pathways to IGF-I. Both forms of IGF-I receptors decreased their expression significantly from EV to FG stage, especially igf1rb (Figure 6B). Interestingly, whereas ERK phosphorylation did not show much difference in its response to IGF-I between these 2 stages, the IGF-I-induced phosphorylation of Akt showed a significant decline at FG stage compared with that at EV. We repeated the experiment 4 times, and the results were consistent (Figure 6C).
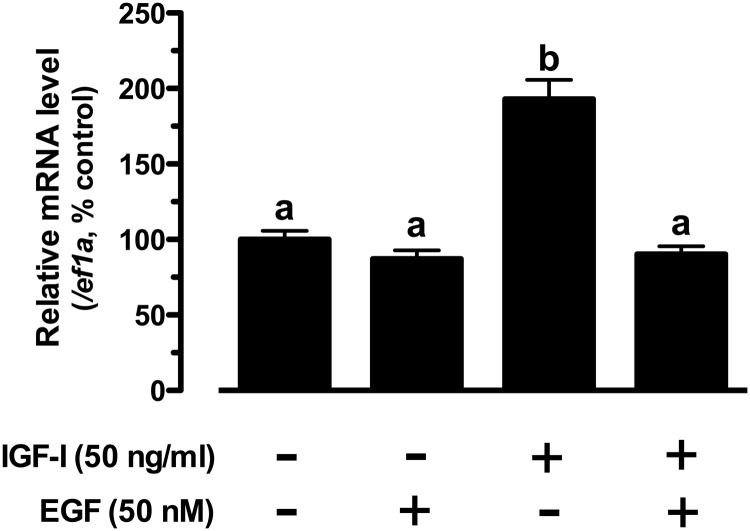
IGF-I stimulation of kitlga expression is influenced by EGF, a paracrine growth factor in the follicle that activates the ERK pathway
The inhibitory role of the ERK pathway in IGF-I-stimulated kitlga expression raised an interesting question on how the ERK pathway activation by a natural ligand would influence the IGF-I-induced kitlga expression. We chose to address this question by using EGF, a potent activator of ERK pathway in the zebrafish follicle cells (51, 52) and a paracrine growth factor released by the oocyte (53, 54). As shown in Figure 7, EGF had little effect on basal kitlga expression, but it completely abolished the IGF-I-stimulated kitlga expression (Figure 7).
Figure 7.
Interactive effects of IGF-I and EGF on kitlga expression. The follicle cells were treated with IGF-I (50 ng/ml) and EGF (50 nM) alone or in combination for 6 hours. The data were normalized to the housekeeping gene ef1a and expressed as the percentage of the control group (mean ± SEM, n = 4). Different letters indicate statistical significance (P < .05).
Discussion
It has been well demonstrated that both IGF-I and KITL play important roles in the ovary. For example, Igf1 knock-out mice are infertile, and the follicles fail to reach the antral stage, leading to anovulation (55, 56). Similarly, blockade of KIT function inhibits the activation and progression of primordial follicle development (17). These, together with our observations that kitlga (25) and IGF-I receptors (igf1ra and igf1rb) (our unpublished data) significantly increase their expression at the PG-PV transition and that the growth axis appears to play an important role in promoting the first wave of PG-PV transition in the zebrafish life cycle (30), prompted us to study their regulatory relationship in the zebrafish ovary.
The present study provides clear evidence that the expression of kitlga in the zebrafish follicle cells is regulated by IGF signaling, and the stimulatory effect of IGF-I involved transcription but not translation, suggesting that kitlga is likely an immediate downstream gene controlled by IGF signaling. The exact sources of IGF peptides that act on the follicle cells to up-regulate kitlga are not clear. Considering the widespread distribution of IGF peptides, it is conceivable that IGF-I and its related peptides may exert their effects in autocrine (from follicle cells), paracrine (from oocytes), and endocrine (from extraovarian sources) manners. Among the extraovarian tissues, the liver is the major origin of IGF-I, which is released from the hepatic cells to the circulation in response to pituitary GH (57). Interestingly, the inhibition of IGF signaling pathways at different points, including IGF-IR, PI3K-Akt, and c-Raf-MEK-ERK, not only affected the IGF-I-induced kitlga expression but also the basal levels. This suggests a possible involvement of autocrine regulation of kitlga by endogenous IGF peptides from the follicle cells because the experiment was performed on cultured follicle cells in serum-free medium, which is free of circulating IGF molecules from endocrine sources. In support of this is the evidence that IGF-II (igf2a and igf2b) and -III (igf3) were all expressed in the zebrafish ovary whereas IGF-I (igf1) expression was very low or undetectable (34). This suggests that IGF-I may act in the ovary mainly as an endocrine hormone from the growth axis, whereas IGF-II and -III are likely the ones that work locally as autocrine and paracrine factors. Evidence in mammals also supports the importance of intraovarian IGF ligands. Although there is a reduced growth rate and lower serum level of IGF-I in GH-deficient mice, the ovary appears normal in function, which is likely due to the GH-independent production of IGF-I in the ovary by the granulosa cells (56).
It is well known that IGF-I activates PI3K-Akt and c-Raf-MEK-ERK pathways in various systems including the zebrafish (39–43). Binding of IGF-I to its receptor initiates the recruitment of PI3K to the plasma membrane where it catalyzes the production of PIP3, which serves as the docking site for Akt binding and activation (13, 58, 59). In this study, we demonstrated that IGF-I increased zebrafish kitlga expression in the somatic follicle cells by activating PI3K-Akt pathway because its effect could be completely abolished by specific PI3K and Akt inhibitors. The exact physiologic significance of the increased Kitlga production in response to IGF-I is unknown at this moment. Because both IGF-I and KITL enhance follicle growth and survival in mammals (22, 35, 60) and both activate the PI3K-Akt signaling pathway (61), we hypothesize that, in addition to Akt activated by IGF-I itself, Kitlga from the follicle cells could be a downstream effector of IGF-I that further amplifies the growth and survival signal of the latter in the oocyte.
Recent studies in our laboratory have provided evidence for an important role of IGF-I in the reproduction of female zebrafish. We have observed that the onset of puberty in female zebrafish, which is characterized by the first wave of PG-PV transition in the ovary, largely depends on body growth rather than age (30). As a major component of the somatotropic axis, IGF-I may likely serve as an important endocrine factor for cross talks between the growth and reproductive axes. The present study provided evidence that IGF-I might advance the onset of puberty by enhancing the production of Kitlga in the somatic follicle layer via the PI3K-Akt pathway. The Kitlga in turn may act on the oocyte via its receptor Kita to promote the transition from PG to PV stage. This hypothesis is supported by the evidence in mammals that the Kit system and Akt pathway are both important for mammalian follicle activation or recruitment (13, 17, 62). The blockade or mutation of KIT and KITL, as well as inactivation of Akt, all lead to failure of follicle activation (13, 63). With the expression of IGF-IR in the oocytes in both mammals and fish (31, 34), IGF-I may also act on the oocytes directly.
In addition to the classical PI3K-Akt pathway, IGF-I can also activate the MEK-ERK pathway (39, 40). It was evidenced in the present study, however, that although it was activated by IGF-I in the zebrafish follicle cells, the MEK-ERK pathway was not responsible for the IGF-I-induced kitlga expression. In contrast to the PI3K-Akt pathway, the MEK-ERK pathway suppressed the expression of kitlga as the inhibition of 3 major components of the MEK-ERK pathway including c-Raf, MEK1/2, and ERK1/2 caused a significant increase in both basal and IGF-I-induced kitlga expression. Despite the inhibitory effect of the ERK pathway on kitlga expression, the net effect of IGF-I was stimulatory. This could be due to the fact that IGF-I had more robust effect in stimulating PI3K-Akt than MEK-ERK in both magnitude and duration. The physiological significance of the counterregulation of kitlga expression by the 2 signaling pathways of IGF-I is intricate and will be an interesting issue to investigate in future studies.
One potential role of the ERK-mediated inhibition of kitlga is likely related to final oocyte maturation. In mammals, KITL inhibits oocyte maturation (19, 20), and we have also observed an inhibitory effect of Kitlga on oocyte maturation in the zebrafish (26). In agreement with this, we have observed a decreased expression of kitlga before oocyte maturation in the zebrafish (25), which would favor the maturation by releasing the oocyte from a Kitlga-mediated inhibition. In contrast, although KITL and IGF-I share multiple activities in the ovary, IGF-I induces oocyte maturation, particularly in fish (64–67), including the zebrafish (34). The opposite effects of IGF-I and KITL on oocyte maturation have led us to hypothesize that IGF-I may promote the expression of kitlga through the PI3K-Akt pathway to activate and stimulate early follicle development including follicle activation and growth, while preventing precocious oocyte maturation. However, IGF-I may enhance final oocyte maturation partly by reducing its stimulation of kitlga expression in the follicle cells through either decreasing Akt or increasing ERK activity in later stages of follicle development. This idea was supported by our data that the responsiveness of kitlga to IGF-I was much higher in early vitellogenic follicles than that at full-grown follicles. To investigate whether this is due to a change of relative signaling intensity of the PI3K-Akt and MEK-ERK pathways during follicle development, we examined the responsiveness of Akt and ERK phosphorylation to IGF-I at early and late stages of follicle development. Interestingly, whereas Akt exhibited a significant decrease in its response to IGF-I at late stage, the phosphorylation of ERK showed little change (increased slightly in one experiment), supporting the idea that there is a decrease of the stimulatory pathway as compared with the inhibitory one. This change may be partly due to a decreased expression of IGF-I receptors from EV to FG as evidenced in the present study; however, this would not explain the relative stable response of ERK phosphorylation at the 2 stages. It is conceivable that the decreased kitlga expression and its response to IGF-I during follicle growth may likely be due to both reduced expression of IGF-I receptors and changes of relative signaling intensity of the 2 postreceptor pathways.
What causes the decrease of Akt signaling during zebrafish follicle development remains unknown. It has been reported in humans that the expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a lipid phosphatase that antagonizes the activity of PI3K by dephosphorylating PIP3 to PIP2, is induced by LH, leading to an inactivation of Akt and attenuation of IGF-I-induced granulosa cell proliferation (68). As the follicles grow, the expression of PTEN increases whereas the level of phosphorylated Akt attenuates in the granulosa cells (69, 70). It is therefore suggested that PTEN may be a trigger for proliferation/differentiation transition in the granulosa cells. Whether PTEN is the factor responsible for declined Akt phosphorylation during follicle development in the zebrafish will be interesting to explore in the future. In agreement with our observation, the IGF-I-induced ERK pathway is not attenuated by human chorionic gonadotropin in human granulosa cells (68). The ERK pathway is known to play an essential role in gonadotropin-induced oocyte maturation in mammals (71), as evidenced by the fact that ovulation does not occur in the ovary when ERK1/2 is depleted in the granulosa cells (72).
As reported earlier, recombinant zebrafish Kitlga inhibits oocyte maturation in vitro (26), and the expression of kitlga decreases significantly prior to oocyte maturation (25), which could be part of the mechanism that induces oocyte maturation. The attenuation of kitlga could be due to a reduced responsiveness to IGF-I in late stages of follicle growth, which might be caused by changed IGF-I signaling as discussed above. Because the developmental stage of follicles is determined by the stage of the enclosed oocyte, it is conceivable that the oocyte may release paracrine signals to influence Akt and ERK signaling in the follicle cells. One of the potential paracrine factors could be EGF and its related peptides. In mammals, it has been shown that LH induces oocyte maturation by stimulating transcription of EGF-like molecules, which in turn activates EGF receptor (EGFR) (73, 74). Further evidence showed that the transgenic mice expressing a mutated EGFR failed to ovulate in response to LH (73). Because ERK is the major intracellular pathway downstream of EGFR, these data suggest that an increased activity of ERK pathway in the follicle cells may be part of the signaling network responsible for oocyte maturation. According to our discovery in the present study, part of the function of the ERK pathway in the zebrafish follicle cells could be suppressing kitlga expression, which reduces an inhibitory tone on the oocyte. It follows that any signals increasing the intracellular level of ERK phosphorylation in the follicle cells will lead to a reduced kitlga expression and increased oocyte maturation. To test this hypothesis, we examined the effect of EGF on basal and IGF-I-induced kitlga expression. Our previous studies in the zebrafish have shown that EGF and its peptides are mostly expressed in the oocyte and their receptor EGFR is exclusively expressed in the somatic follicle cells, suggesting an oocyte-to-follicle cell signaling pathway (53, 54). Furthermore, the expression of EGFR increases to its peak level at FG follicles prior to oocyte maturation, suggesting an increased EGFR signaling at this stage of follicle development (53). Our recent study showed that EGF significantly increased ERK phosphorylation in the zebrafish follicle cells (51, 52). In agreement with the inhibitory effect of the ERK pathway on kitlga expression, EGF completely abolished IGF-I-induced kitlga expression in the present study. These experiments were performed in vitro on cultured follicle cells. How important the endogenous EGF and its peptides are in changing signaling pathways in the follicle cells remains unknown and will be an interesting question to address in the future.
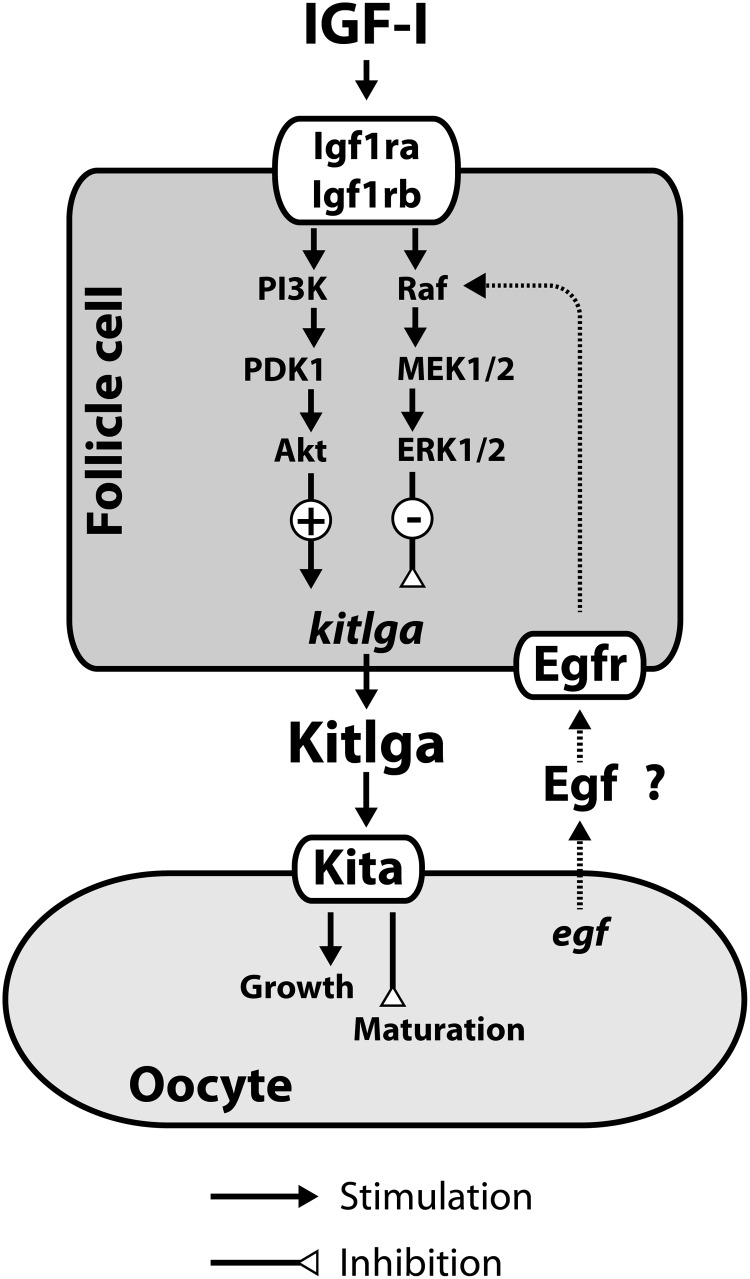
In summary, the present study provided evidence that IGF-I was a potent regulatory factor that up-regulated the expression of kitlga in the zebrafish follicle cells. The stimulation involved transcription but not translation, indicating that the kitlga gene is a direct downstream target of IGF-I. The effect of IGF-I on kitlga was mediated via PI3K-Akt but not the MEK-ERK pathway although both were activated by IGF-I. In contrast, the ERK pathway seemed to play a negative role in controlling kitlga expression. The stage-dependent response of kitlga expression to IGF-I at EV and FG stages might be due to changes in relative signaling intensity of the PI3K-Akt and ERK pathways, and paracrine factors from the oocyte such as EGF could be one of the factors that induce such changes in the signaling network (Figure 8).
Figure 8.
Hypothetical model for IGF-I regulation of kitlga expression and its signaling mechanisms in the zebrafish follicle cells. Kitlga from the follicle cells may promote oocyte growth while suppressing maturation during vitellogenic stage, and its high expression level may likely be maintained by growth factors such as IGF-I via the PI3K-Akt pathway. When the follicle approaches the preovulatory stage, the expression of kitlga reduces and its response to IGF-I diminishes, which is probably due to a decreased signaling of the stimulatory PI3K-Akt pathway and a relatively constant or slightly increased MEK-ERK pathway. Paracrine signals from the oocyte such as EGF family may contribute to the shifting of the relative signaling intensity of the 2 pathways.
Acknowledgments
This work was supported by grants (CUHK457805, 458706, 464707, 464308, and 464409) from the Research Grants Council of the Hong Kong Special Administrative Region (to W.G).
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by grants (CUHK457805, 458706, 464707, 464308, and 464409) from the Research Grants Council of the Hong Kong Special Administrative Region (to W.G).
Footnotes
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- EV
- early vitellogenic
- FG
- full grown
- IGF-IR
- IGF-I receptor
- KITL
- Kit ligand
- MEK
- MAPK kinase
- PG
- primary growth
- PI3K
- phosphatidylinositol 3-kinase
- PTEN
- phosphatase and tensin homolog
- PV
- previtellogenic
- qPCR
- quantitative PCR
- RT
- reverse transcription
- SDS
- sodium dodecyl sulfate.
References
- 1. Ge W. Intrafollicular paracrine communication in the zebrafish ovary: the state of the art of an emerging model for the study of vertebrate folliculogenesis. Mol Cell Endocrinol. 2005;237:1–10. [DOI] [PubMed] [Google Scholar]
- 2. Richards JS. Perspective: the ovarian follicle–a perspective in 2001. Endocrinology. 2001;142:2184–2193. [DOI] [PubMed] [Google Scholar]
- 3. Copeland NG, Gilbert DJ, Cho BC, et al. . Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell. 1990;63:175–183. [DOI] [PubMed] [Google Scholar]
- 4. Huang E, Nocka K, Beier DR, et al. . The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. [DOI] [PubMed] [Google Scholar]
- 5. Matsui Y, Zsebo KM, Hogan BL. Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature. 1990;347:667–669. [DOI] [PubMed] [Google Scholar]
- 6. Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. [DOI] [PubMed] [Google Scholar]
- 7. Yarden Y, Kuang WJ, Yang-Feng T, et al. . Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fantl WJ, Johnson DE, Williams LT. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–481. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Z, Zhang R, Joachimiak A, Schlessinger J, Kong XP. Crystal structure of human stem cell factor: implication for stem cell factor receptor dimerization and activation. Proc Natl Acad Sci USA. 2000;97:7732–7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Besmer P, Manova K, Duttlinger R, et al. . The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev Suppl. 1993;125–137. [PubMed] [Google Scholar]
- 11. Horie K, Takakura K, Taii S, et al. . The expression of c-kit protein during oogenesis and early embryonic development. Biol Reprod. 1991;45:547–552. [DOI] [PubMed] [Google Scholar]
- 12. Doneda L, Klinger FG, Larizza L, De Felici M. KL/KIT co-expression in mouse fetal oocytes. Int J Dev Biol. 2002;46:1015–1021. [PubMed] [Google Scholar]
- 13. Jacinto E, Facchinetti V, Liu D, et al. . SIN1/MIP1 maintains rictor-mtor complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. [DOI] [PubMed] [Google Scholar]
- 14. Thomas FH, Vanderhyden BC. Oocyte-granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reprod Biol Endocrinol. 2006;4:19.16611364 [Google Scholar]
- 15. Moniruzzaman M, Miyano T. KIT-KIT ligand in the growth of porcine oocytes in primordial follicles. J Reprod Dev. 2007;53:1273–1281. [DOI] [PubMed] [Google Scholar]
- 16. Hutt KJ, McLaughlin EA, Holland MK. KIT/KIT ligand in mammalian oogenesis and folliculogenesis: roles in rabbit and murine ovarian follicle activation and oocyte growth. Biol Reprod. 2006;75:421–433. [DOI] [PubMed] [Google Scholar]
- 17. Yoshida H, Takakura N, Kataoka H, Kunisada T, Okamura H, Nishikawa SI. Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev Biol. 1997;184:122–137. [DOI] [PubMed] [Google Scholar]
- 18. Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–4271. [DOI] [PubMed] [Google Scholar]
- 19. Ismail RS, Okawara Y, Fryer JN, Vanderhyden BC. Hormonal regulation of the ligand for c-kit in the rat ovary and its effects on spontaneous oocyte meiotic maturation. Mol Reprod Dev. 1996;43:458–469. [DOI] [PubMed] [Google Scholar]
- 20. Ismail RS, Dubé M, Vanderhyden BC. Hormonally regulated expression and alternative splicing of kit ligand may regulate kit-induced inhibition of meiosis in rat oocytes. Dev Biol. 1997;184:333–342. [DOI] [PubMed] [Google Scholar]
- 21. Thomas FH, Ismail RS, Jiang JY, Vanderhyden BC. Kit ligand 2 promotes murine oocyte growth in vitro. Biol Reprod. 2008;78:167–175. [DOI] [PubMed] [Google Scholar]
- 22. Reynaud K, Cortvrindt R, Smitz J, Driancourt MA. Effects of Kit Ligand and anti-Kit antibody on growth of cultured mouse preantral follicles. Mol Reprod Dev. 2000;56:483–494. [DOI] [PubMed] [Google Scholar]
- 23. Otsuka F, Shimasaki S. A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: its role in regulating granulosa cell mitosis. Proc Natl Acad Sci USA. 2002;99:8060–8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang EJ, Nocka KH, Buck J, Besmer P. Differential expression and processing of two cell associated forms of the kit-ligand: KL-1 and KL-2. Mol Biol Cell 1992;3:349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yao K, Ge W. Kit system in the zebrafish ovary: evidence for functional divergence of two isoforms of kit (Kita and Kitb) and kit ligand (Kitlga and Kitlgb) during folliculogenesis. Biol Reprod. 2010;82:1216–1226. [DOI] [PubMed] [Google Scholar]
- 26. Yao K, Ge W. Spatial distribution and receptor specificity of zebrafish kit system–evidence for a Kit-mediated bi-directional communication system in the preovulatory ovarian follicle. PLoS ONE. 2013;8:e56192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parrott JA, Skinner MK. Thecal cell-granulosa cell interactions involve a positive feedback loop among keratinocyte growth factor, hepatocyte growth factor, and Kit ligand during ovarian follicular development. Endocrinology. 1998;139:2240–2245. [DOI] [PubMed] [Google Scholar]
- 28. Joyce IM, Pendola FL, Wigglesworth K, Eppig JJ. Oocyte regulation of kit ligand expression in mouse ovarian follicles. Dev Biol. 1999;214:342–353. [DOI] [PubMed] [Google Scholar]
- 29. Kundu MC, Wojtusik J, Johnson PA. Expression and regulation of kit ligand in the ovary of the hen. Gen Comp Endocrinol. 2012;179:47–52. [DOI] [PubMed] [Google Scholar]
- 30. Chen W, Ge W. Gonad differentiation and puberty onset in the zebrafish: evidence for the dependence of puberty onset on body growth but not age in females. Mol Reprod Dev. 2013;80:384–392. [DOI] [PubMed] [Google Scholar]
- 31. Zhou J, Chin E, Bondy C. Cellular pattern of insulin-like growth factor-I (IGF-I) and IGF-I receptor gene expression in the developing and mature ovarian follicle. Endocrinology. 1991;129:3281–3288. [DOI] [PubMed] [Google Scholar]
- 32. Adashi EY, Resnick CE, Payne DW, et al. . The mouse intraovarian insulin-like growth factor I system: departures from the rat paradigm. Endocrinology. 1997;138:3881–3890. [DOI] [PubMed] [Google Scholar]
- 33. Wandji SA, Wood TL, Crawford J, Levison SW, Hammond JM. Expression of mouse ovarian insulin growth factor system components during follicular development and atresia. Endocrinology. 1998;139:5205–5214. [DOI] [PubMed] [Google Scholar]
- 34. Nelson SN, Van Der Kraak G. Characterization and regulation of the insulin-like growth factor (IGF) system in the zebrafish (Danio rerio) ovary. Gen Comp Endocrinol. 2010;168:111–120. [DOI] [PubMed] [Google Scholar]
- 35. Quirk SM, Harman RM, Cowan RG. Regulation of Fas antigen (Fas, CD95)-mediated apoptosis of bovine granulosa cells by serum and growth factors. Biol Reprod. 2000;63:1278–1284. [DOI] [PubMed] [Google Scholar]
- 36. Zhou J, Refuerzo J, Bondy C. Granulosa cell dna synthesis is strictly correlated with the presence of insulin-like growth factor I and absence of c-fos/c-jun expression. Mol Endocrinol. 1995;9:924–931. [DOI] [PubMed] [Google Scholar]
- 37. Demeestere I, Gervy C, Centner J, Devreker F, Englert Y, Delbaere A. Effect of insulin-like growth factor-I during preantral follicular culture on steroidogenesis, in vitro oocyte maturation, and embryo development in mice. Biol Reprod. 2004;70:1664–1669. [DOI] [PubMed] [Google Scholar]
- 38. Parrott JA, Skinner MK. Direct actions of kit-ligand on theca cell growth and differentiation during follicle development. Endocrinology. 1997;138:3819–3827. [DOI] [PubMed] [Google Scholar]
- 39. Adams TE, McKern NM, Ward CW. Signalling by the type 1 insulin-like growth factor receptor: interplay with the epidermal growth factor receptor. Growth Factors. 2004;22:89–95. [DOI] [PubMed] [Google Scholar]
- 40. Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr Pharm Des. 2007;13:663–669. [DOI] [PubMed] [Google Scholar]
- 41. Onuma TA, Ding Y, Abraham E, Zohar Y, Ando H, Duan C. Regulation of temporal and spatial organization of newborn GnRH neurons by IGF signaling in zebrafish. J Neurosci. 2011;31:11814–11824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pozios KC, Ding J, Degger B, Upton Z, Duan C. IGFs stimulate zebrafish cell proliferation by activating MAP kinase and PI3-kinase-signaling pathways. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1230–1239. [DOI] [PubMed] [Google Scholar]
- 43. Kamei H, Ding Y, Kajimura S, Wells M, Chiang P, Duan C. Role of IGF signaling in catch-up growth and accelerated temporal development in zebrafish embryos in response to oxygen availability. Development. 2011;138:777–786. [DOI] [PubMed] [Google Scholar]
- 44. John GB, Shidler MJ, Besmer P, Castrillon DH. Kit signaling via PI3K promotes ovarian follicle maturation but is dispensable for primordial follicle activation. Dev Biol. 2009;331:292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jin X, Han CS, Yu FQ, Wei P, Hu ZY, Liu YX. 2005 Anti-apoptotic action of stem cell factor on oocytes in primordial follicles and its signal transduction. Mol Reprod Dev. 70:82–90. [DOI] [PubMed] [Google Scholar]
- 46. Morita Y, Manganaro TF, Tao XJ, Martimbeau S, Donahoe PK, Tilly JL. Requirement for phosphatidylinositol-3′-kinase in cytokine-mediated germ cell survival during fetal oogenesis in the mouse. Endocrinology. 1999;140:941–949. [DOI] [PubMed] [Google Scholar]
- 47. Huang CT, Weitsman SR, Dykes BN, Magoffin DA. Stem cell factor and insulin-like growth factor-I stimulate luteinizing hormone-independent differentiation of rat ovarian theca cells. Biol Reprod. 2001;64:451–456. [DOI] [PubMed] [Google Scholar]
- 48. Pang Y, Ge W. Gonadotropin regulation of activin βA and activin type IIA receptor expression in the ovarian follicle cells of the zebrafish, Danio rerio. Mol Cell Endocrinol. 2002;188:195–205. [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Ge W. Developmental profiles of activin βA, βB, and follistatin expression in the zebrafish ovary: evidence for their differential roles during sexual maturation and ovulatory cycle. Biol Reprod. 2004;71:2056–2064. [DOI] [PubMed] [Google Scholar]
- 50. Pang Y, Ge W. Activin stimulation of zebrafish oocyte maturation in vitro and its potential role in mediating gonadotropin-induced oocyte maturation. Biol Reprod. 1999;61:987–992. [DOI] [PubMed] [Google Scholar]
- 51. Chung CK, Ge W. Epidermal growth factor differentially regulates activin subunits in the zebrafish ovarian follicle cells via diverse signaling pathways. Mol Cell Endocrinol. 2012;361:133–142. [DOI] [PubMed] [Google Scholar]
- 52. Chung CK, Ge W. Human chorionic gonadotropin (hCG) induces MAPK3/1 phosphorylation in the zebrafish ovarian follicle cells independent of EGF/EGFR pathway. Gen Comp Endocrinol. 2013;188:251–257. [DOI] [PubMed] [Google Scholar]
- 53. Tse AC, Ge W. Spatial localization of EGF family ligands and receptors in the zebrafish ovarian follicle and their expression profiles during folliculogenesis. Gen Comp Endocrinol. 2010;167:397–407. [DOI] [PubMed] [Google Scholar]
- 54. Wang Y, Ge W. Cloning of epidermal growth factor (EGF) and EGF receptor from the zebrafish ovary: evidence for EGF as a potential paracrine factor from the oocyte to regulate activin/follistatin system in the follicle cells. Biol Reprod. 2004;71:749–760. [DOI] [PubMed] [Google Scholar]
- 55. Baker J, Hardy MP, Zhou J, et al. . Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918. [DOI] [PubMed] [Google Scholar]
- 56. Zhou J, Kumar TR, Matzuk MM, Bondy C. Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol Endocrinol. 1997;11:1924–1933. [DOI] [PubMed] [Google Scholar]
- 57. Hull KL, Harvey S. Growth hormone: roles in male reproduction. Endocrine. 2000;13:243–250. [DOI] [PubMed] [Google Scholar]
- 58. Alessi DR, Andjelkovic M, Caudwell B, et al. . Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 59. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex. Science. 2005;307:1098–1101. [DOI] [PubMed] [Google Scholar]
- 60. Porter DA, Vickers SL, Cowan RG, Huber SC, Quirk SM. Expression and function of Fas antigen vary in bovine granulosa and theca cells during ovarian follicular development and atresia. Biol Reprod. 2000;62:62–66. [DOI] [PubMed] [Google Scholar]
- 61. Roskoski R., Jr Signaling by Kit protein-tyrosine kinase–the stem cell factor receptor. Biochem Biophys Res Commun. 2005;337:1–13. [DOI] [PubMed] [Google Scholar]
- 62. Reddy P, Liu L, Adhikari D, et al. . Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. [DOI] [PubMed] [Google Scholar]
- 63. Kissel H, Timokhina I, Hardy MP, et al. . Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 2000;19:1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mukherjee D, Mukherjee D, Sen U, Paul S, Bhattacharyya SP. In vitro effects of insulin-like growth factors and insulin on oocyte maturation and maturation-inducing steroid production in ovarian follicles of common carp, Cyprinus carpio. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:63–77. [DOI] [PubMed] [Google Scholar]
- 65. Paul S, Pramanick K, Kundu S, Bandyopadhyay A, Mukherjee D. Involvement of PI3 kinase and MAP kinase in IGF-I- and insulin-induced oocyte maturation in Cyprinus carpio. Mol Cell Endocrinol. 2009;309:93–100. [DOI] [PubMed] [Google Scholar]
- 66. Kagawa H, Kobayashi M, Hasegawa Y, Aida K. Insulin and insulin-like growth factors I and II induce final maturation of oocytes of red seabream, Pagrus major, in vitro. Gen Comp Endocrinol. 1994;95:293–300. [DOI] [PubMed] [Google Scholar]
- 67. Weber GM, Sullivan CV. In vitro hormone induction of final oocyte maturation in striped bass (Morone saxatilis) follicles is inhibited by blockers of phosphatidylinositol 3-kinase activity. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:467–473. [DOI] [PubMed] [Google Scholar]
- 68. Goto M, Iwase A, Harata T, et al. . IGF1-induced AKT phosphorylation and cell proliferation are suppressed with the increase in PTEN during luteinization in human granulosa cells. Reproduction. 2009;137:835–842. [DOI] [PubMed] [Google Scholar]
- 69. Goto M, Iwase A, Ando H, Kurotsuchi S, Harata T, Kikkawa F. PTEN and Akt expression during growth of human ovarian follicles. J Assist Reprod Genet. 2007;24:541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Froment P, Bontoux M, Pisselet C, Monget P, Dupont J. PTEN expression in ovine granulosa cells increases during terminal follicular growth. FEBS Lett. 2005;579:2376–2382. [DOI] [PubMed] [Google Scholar]
- 71. Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology. 2002;143:2221–2232. [DOI] [PubMed] [Google Scholar]
- 72. Fan HY, Liu Z, Shimada M, et al. . MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hsieh M, Lee D, Panigone S, et al. . Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. [DOI] [PubMed] [Google Scholar]