Abstract
The role of glutamate in the regulation of neurogenesis is well-established, but the role of vesicular glutamate transporters (VGLUTs) and excitatory amino acid transporters (EAATs) in controlling adult neurogenesis is unknown. Here we investigated the implication of VGLUTs in the differentiation of subventricular zone (SVZ)-derived neural precursor cells (NPCs). Our results show that NPCs express VGLUT1-3 and EAAT1-3 both at the mRNA and protein level. Their expression increases during differentiation closely associated with the expression of marker genes. In expression analyses we show that VGLUT1 and VGLUT2 are preferentially expressed by cultured SVZ-derived doublecortin+ neuroblasts, while VGLUT3 is found on GFAP+ glial cells. In cultured NPCs, inhibition of VGLUT by Evans Blue increased the mRNA level of neuronal markers doublecortin, B3T and MAP2, elevated the number of NPCs expressing doublecortin protein and promoted the number of cells with morphological appearance of branched neurons, suggesting that VGLUT function prevents neuronal differentiation of NPCs. This survival- and differentiation-promoting effect of Evans blue was corroborated by increased AKT phosphorylation and reduced MAPK phosphorylation. Thus, under physiological conditions, VGLUT1-3 inhibition, and thus decreased glutamate exocytosis, may promote neuronal differentiation of NPCs.
Introduction
Glutamate plays key roles in the pathophysiology of cerebral ischemia and other neurodegenerative diseases [1–3]. Glutamate levels are regulated at the synaptic cleft by EAATs [4]. EAAT1 and EAAT2 are present in astrocytes, whereas EAAT3 and EAAT4 are located in neurons. Because of their biophysical properties, EAAT3 and EAAT4 could act as glutamate buffers by maintaining normal extracellular glutamate concentration, whereas excessive glutamate is withdrawn from the synapse by EAAT1 and EAAT2, thus preventing excitotoxicity [4].
In addition to the aforementioned mechanisms, glutamate concentrations are also regulated by modulating glutamate internalization into synaptic vesicles through VGLUTs 1, 2 and 3. VGLUT1 and VGLUT2 have a complementary distribution in the cortex and are also present in the caudate-putamen [5, 6]. VGLUT3 is found in the cortex and in the caudate-putamen among other structures, and it is less abundant than the other two isoforms [5]. Interestingly, VGLUTs are expressed by astrocytes in vitro [7]. Changes in VGLUT levels have been associated with several pathologies including schizophrenia, depression [8] or Parkinson’s disease [9].
We have previously proposed VGLUTs as possible pharmacological targets for stroke. We found that during early stages of reperfusion VGLUT1 is upregulated in the cortex (Cx) and striatum (St) whereas VGLUT2 and 3 are expressed by reactive glia in the ischemic corpus callosum (iCC) [10]. Interestingly, another work reported that stroke was associated with EAAT overexpression in glial cells within the iCC [11].
Adult neurogenesis has been clearly demonstrated in the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampus, with controversial findings regarding the cortex [12]. Under physiological conditions stem cells in the SVZ proliferate and migrate towards the olfactory bulb (OB) forming chains of neuroblasts that are surrounded by glial cells along the rostral migratory stream (RMS) [13–15]. It is known that the interaction between neuroblasts and glial cells during the migration and differentiation process is regulated by numerous growth factors [16]. Nonetheless, recent evidence also suggests an important role for neurotransmitters in the regulation of neurogenesis in both health and pathology [17]. Indeed, glutamate and GABA balance have been proven to influence cell differentiation and survival in the RMS [18–22].
During the past decade the key role of neurotransmitters such as glutamate, in the regulation of neurogenesis and glial glutamatergic signaling has been clearly stated [19, 23–28]. NPCs express different types of glutamate receptors depending on their developmental stage. In fact, the presence of transcripts coding for various subunits of NMDA, AMPA, kainate receptors and group I, II and III metabotropic receptors, have been detected in neurospheres derived from embryonic cortex, along with those coding for GABA receptors [12]. Interestingly, blockade of the metabotropic glutamate receptor 5 (mGluR5) by genetic deletion or pharmacological interventions reduced the number of BrdU + cells along the iCC [29] whilst deletion of mGluR7 increased NPC proliferation but reduced neuronal differentiation [30]. However, nothing is known about the possible expression and role of vesicular or membrane glutamate transporters in adult neurogenesis under both, physiological and pathological conditions.
The well documented role of glutamate on NPC proliferation, migration and survival along the RMS under physiological conditions [18–21] together with the observations that VGLUTs and EAATs are expressed in glial cells in the iCC [10, 11] led us to hypothesize that VGLUT expression could play a direct role on the neuronal differentiation of SVZ-derived NPCs both in health and disease.
In this work, we have characterized the expression of VGLUTs and EAATs on SVZ stem cell cultures during different stages of differentiation and studied the functional implication of VGLUTs in neurogenesis by means of pharmacological inhibition with Evans Blue. Our data suggest a role of glutamate and its transporters in adult neurogenesis.
Material and methods
All animal handling was performed in accordance with European Commission guidelines (2010/63/UE) and was approved by the Animal Research Committee at the Complutense University.
SVZ-derived stem cell culture
NPCs were isolated from P0-3 rat pups as described by [16]. Briefly, rats were decapitated and the brains were removed and placed into cold DMEM/F12/Glutamax (GIBCO, Thermo Fisher Scientific, Spain). Meninges were removed and 300 μm coronal slices were produced with a custom-made tissue chopper. The SVZ was then isolated and treated with trypsin/EDTA (0.125%) during 10 min at 37°C with gentle shaking every 3 min. To stop the reaction, cells were centrifuged (1 min at 1000 rpm) and rinsed three times with DMEM/F12/Glutamax. Cells were then resuspended at 20,000 cell/mL in DMEM/F12/Glutamax containing EGF (GIBCO; 10 ng/mL). Cells were allowed to grow as neurospheres for 4–5 days before starting the experiments. For induction of cell differentiation required for subsequent Western blotting or Immunocytochemistry, plates and coverslips were treated with 50 μg/mL Poly-D-lysine (Sigma-Aldrich, Spain) diluted in sterile water overnight. Neurospheres were then seeded and allowed to attach for 10 min. Media containing growth factors was gently removed and immediately replaced with media without growth factors. Differentiation was carried out for a maximal period of 20 days. When necessary, the activity of VGLUTs was inhibited by addition of increasing concentrations of the dye Evans blue [31], a competitor inhibitor of VGLUTs, at final concentrations of 0.1, 1 and 5 μM for times between 3 to 5 days of differentiation. Samples were then prepared for western blotting or immunocytochemistry as indicated.
Immunocytochemistry
Cells were fixed for 15 min with cold 4% paraformaldehyde in PBS. Washing steps were done 3 times with PBS 0.1% triton (PBS-T) before and after blocking and after incubation with primary and secondary antibodies. Blocking was carried out with 5% normal goat serum (NGS) or normal donkey serum (NDS) in PBS-T for 1 h depending on antibody combinations. Primary antibodies used were rabbit anti VGLUT1, rabbit anti VGLUT2 or rabbit anti VGLUT3 (Synaptic Systems, Germany, dilution 1:150), mouse anti GFAP (Sigma-Aldrich, dilution 1:250) or goat anti doublecortin (Santa Cruz Biotechnology, Spain, dilution 1:200). These antibodies were incubated overnight at 4°C in 1% serum in PBS-T. Secondary donkey anti rabbit alexa 488 or goat anti rabbit alexa 488 (Invitrogen, Thermo Fisher Scientific, Spain, dilution 1:250), biotinylated goat anti mouse (Invitrogen), or biotinylated donkey anti goat (Santa Cruz Biotechnology; dilution 1:500) were incubated for 1 h at room temperature (RT) in 1% serum PBS-T. For biotinylated antibody detection, an additional incubation step with streptavidin-texas red (Thermo Fisher 1:800) at RT in PBS-T was performed for 1 h. Cells where then washed 3 times with PBS and mounted on ProLong Gold Antifade with DAPI (Invitrogen). Preparations were allowed to dry for at least 24 h before performing detailed observation under the confocal microscope. Phenotype characterization of VGLUTs expression on DCX+ or GFAP+ cells at 3 and 7dd was performed by counting cells that expressed a VGLUT and either DCX or GFAP and normalized as percentage of the total cells present in a field as counted by total number of nuclei (DAPI+). At least 100 fields were counted in three different experiments at the mentioned time points.
Morphological measurements
For morphological characterization of neuroblasts, goat anti doublecortin was incubated as detailed above and detected using biotinylated secondary donkey anti-goat (1:500) for 1 h at RT followed by 1 h incubation at RT with ABC complex (Vectastain, Vector labs, PALEX MEDICAL, S.A., Spain). After 3 washes in PBS, samples were incubated with 250 μl of PBS containing 500 μg/mL 3´3´diaminobenzidine and 0.01% H2O2 for 1 min. The reaction was stopped by repeated washes in PBS. Cells were then counterstained with hematoxylin for nuclear detection and dehydrated in 50% EtOH for 1 min and mounted on DePex mounting medium (Sigma). Images were acquired by bright field microscopy with a 40x objective for density and morphological characterization. Cells were classified as neuroblasts if they presented no clear axonal fiber (e.g. bipolar cells) when labelled with DCX, as compared to proto-neurons, which showed one specific axonal fiber with collateral protrusions characteristic of developing post-mitotic neurons on polarization stage 3–4[32] (S1 Fig). For axonal measurements, one fiber longer than 20 μM was selected. Length was quantified using the plug-in NeuronJ from ImageJ (NIH). A preliminary study on the suitability of DCX over MAP2 staining for morphological measurements was performed (S2 Fig).
Microscopy
Light microscopy was performed on a Leica DM LB2 microscope and a digital Leica DFC 320 camera. Confocal microscopy was performed on a multispectral Leica TCS-SP2-AOBS confocal microscope (Leica Microsystems, Barcelona, Spain).
Western blot analysis
Neurosphere cultures were homogenated in a protein extraction buffer containing 1% Triton, 10 mM Tris, 5 mM EDTA, 50 mM NaCl, 30 mM sodium pyrophosphate, 50 mM NaF, 100 μM orthovanadate, 500 μM PMSF, 5 μg/μL aprotinin and 0.025 μg/μL leupeptin, pH 7.4, and then centrifuged at 13,000 rpm at 4°C for 15 min. Supernatants were collected and stored at -80°C until used. The protein concentration was measured by the Bradford method (Quick Start Bradford Protein Assay; Bio-Rad, Spain) and 20 μg of protein were used for Western blot analysis. Western blots were carried out as previously described [10, 33]. Rabbit anti VGLUT1, rabbit anti VGLUT2 or rabbit anti VGLUT3 (Synaptic Systems, Göttingen, Germany); were used at a 1:2000 dilution. Guinea pig anti EAAT1, guinea pig anti EAAT2 or rabbit anti EAAT3 (Santa Cruz) were used at a 1:2000 dilution. Rabbit anti p-AKT (Ser473) or rabbit anti total AKT (Cell Signaling), rabbit anti MEK 1/2 and Rabbit anti p-MAPK(p44/42)(Thr202/Tyr204) (Cell Signaling), were used at a 1:2000 dilution. Mouse anti beta actin (Sigma) was used for loading control at a 1:2000 dilution. Band intensities were measured on a densitometric scanner, and normalized to β-actin expression. Quantification was performed using Image Gauge v. 4.0 (Science Lab. Fujifilm).
RT-PCR
The RNeasy Mini Kit was used for total RNA isolation. Reverse transcription (RT) was carried out for 1 h at 55°C with oligodeoxythymidylate primer using 1 μg of total RNA from each sample for complementary DNA synthesis.
Real time quantitative PCR was performed in order to determine the mRNA levels of VGLUT and EAAT isoforms and housekeeping β-actin by using the following specific primers synthesized at Sigma-Aldrich Co. (Table 1):
Table 1. Primers used for RTqPCR.
| Gen | Primer 5'->3' | Product lenght | pb | %GC | Tm | ||
|---|---|---|---|---|---|---|---|
| Glutamate Transporters | VGLUT1 | Fwd | GCTGTGTCATCTTCGTGAGG | 110 | 20 | 55 | 52,99 |
| Rev | CAGCCGACTCCGTTCTAAGG | 20 | 60 | 54,48 | |||
| VGLUT2 | Fwd | TGGTGCAGTACACTGGATGG | 123 | 20 | 55 | 53,82 | |
| Rev | CGTCTGTTATGGTTGGATGC | 20 | 50 | 51,23 | |||
| VGLUT3 | Fwd | GCAATGACAAAGCACAAGACC | 108 | 21 | 47,6 | 52,91 | |
| Rev | TTCCCCAGAAGCAAAGACC | 19 | 52,6 | 51,42 | |||
| EAAT1 | Fwd | TGTCTTCTCCATGTGCTTCG | 109 | 20 | 64,2 | 52,19 | |
| Rev | CGCTACCAATCTCATGATGG | 20 | 58,3 | 50,4 | |||
| EAAT2 | Fwd | ATTGGTGCAGCCAGTAGGCC | 100 | 20 | 50 | 63,8 | |
| Rev | TTCTATCCAGCAGCCAGTCC | 20 | 55 | 64,3 | |||
| EAAT3 | Fwd | CAAGCGTGAAGAAGTGAAGC | 137 | 20 | 50 | 52,1 | |
| Rev | TGATGCCGTCTGAGTACAGG | 20 | 55 | 53,39 | |||
|
Neurogenesis Markers |
DCX | Fwd | AGGTAACGACCAAGACGCAAATGG | 91 | 24 | 50 | 57,73 |
| Rev | AGGGCTTGTGGGTGTAGAGATAGG | 24 | 54,17 | 57,17 | |||
| NCAM | Fwd | GCCGGCAGTTTACAATGCTGCG | 112 | 22 | 59,09 | 59,87 | |
| Rev | ACGCTGATTTCTCCTTGGCTGGG | 23 | 56,62 | 59,19 | |||
| Beta 3 tubulin | Fwd | GTGAAGTCAGCATGAGGGAGATCG | 119 | 24 | 54,17 | 57,16 | |
| Rev | ATAGTTGCCGCTGGGGTCTATGCC | 24 | 58,33 | 60,65 | |||
| Nestin | Fwd | TACATACAGGACTCTGCTGGAGGC | 108 | 24 | 54,17 | 57,5 | |
| Rev | AGGAAATTCGGCTTCAGCTTGGGG | 24 | 54,17 | 59,46 | |||
| GFAP | Fwd | GCTCCAAGATGAAACCAACC | 119 | 20 | 50 | 51,24 | |
| Rev | CCAGCGACTCAACCTTCCT | 20 | 57,89 | 53,53 | |||
| SOX 2 | Fwd | ATCACAACAATCGCGGCGGC | 70 | 20 | 60 | 58,96 | |
| Rev | AGACGGGCGAAGTGCAATTGGG | 22 | 59,09 | 59,99 | |||
| TLX | Fwd | TTCTTCACAGCGGTCACGCAGC | 85 | 22 | 59,09 | 60,24 | |
| Rev | TCACGAGAGTCTGCCGTTCAGG | 22 | 59,09 | 58.35 | |||
| Control | rBACT | Fwd | GCCAACCGTGAAAAGATGA | 103 | 19 | 47,3 | 63,7 |
| Rev | TACGACCAGAGGCATACAGG | 20 | 55 | 67,4 | |||
Real-time PCR
The SYBR Green PCR Master Mix (Applied Biosystems, Thermo Fisher Scientific, Spain) and the 7900 HT Fast Real-Time PCR system (Applied Biosystems) were used to detect the real-time quantitative PCR products of reverse-transcribed cDNA samples, according to the manufacturer's instructions. q-PCR conditions were: 95°C (10 min) followed by 40 cycles of 15 seconds at 95°C and annealing for 1 minute at 60°C. Three independent quantitative PCR assays were performed for each gene and measured in triplicate. Three no-template controls (NTCs) were run for each quantitative PCR assay, and genomic DNA contamination of total RNA was controlled using RT minus controls (samples without the reverse transcriptase).
Statistics
Data were expressed as means±SEM values of three or four independent experiments with different cell batches, each one performed in triplicate. Statistical comparisons were performed by one-way analysis of variance (ANOVA) followed by Tukey post hoc test when analysis of variance was significant. Differences were regarded as significant for P <0.05. Statistical analyses were carried out using Sigmastat software (Systat Software, Inc., Germany).
Results
VGLUTs and EAATs are expressed at both the mRNA and protein level in SVZ-derived NPCs in primary culture in a time-dependent manner
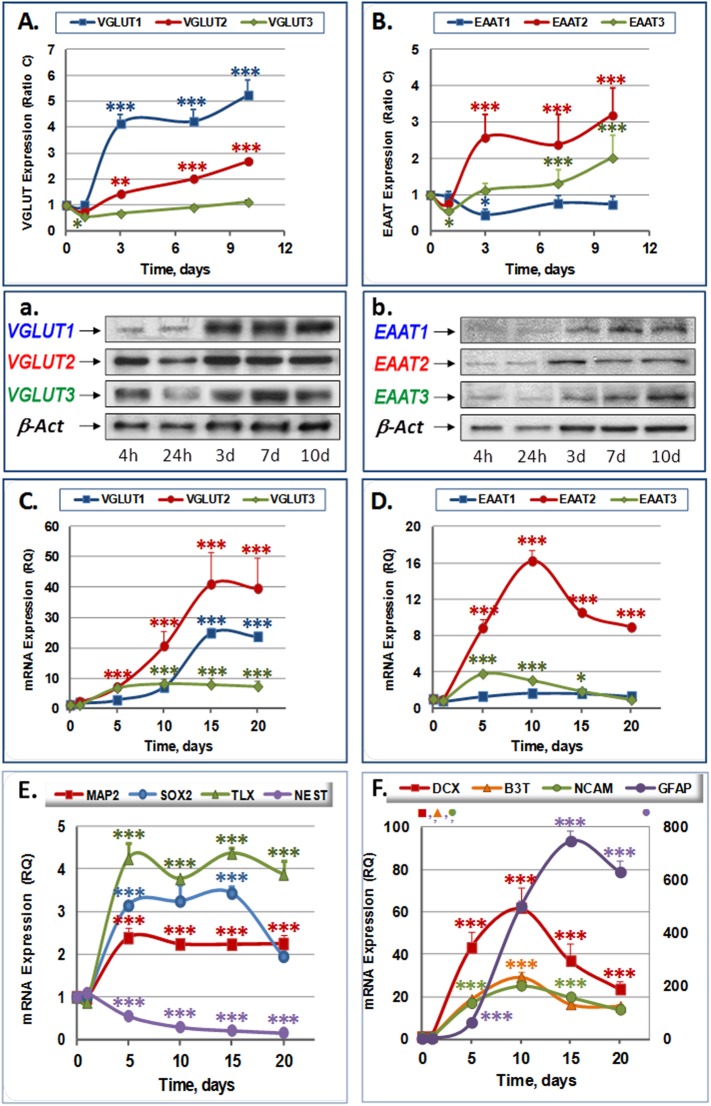
We first characterized the expression of VGLUTs and EAATs in SVZ cultures over 20 days by RT-qPCR. The analysis showed that VGLUT mRNAs are increasingly expressed during differentiation (Fig 1C). VGLUT1 mRNA increased by 6.88±0.88 fold at 10 days of differentiation (dd), reaching a maximum at 15 dd (24.78±1.73 fold). VGLUT2 mRNA was rapidly upregulated at 24 h after seeding (2.09±0.37 fold) and further increased until 15 dd (40.88±10.74 fold). VGLUT3 mRNA reached its maximum at 5–10 dd (8.01±1.75 fold) and remains stable thereafter (Fig 1C).
Fig 1. Time course evolution of glutamate transporters and differentiation markers in SVZ NPCs primary culture 1–20 days after differentiation.
A-B) Time course evolution of of VGLUTs (E) and EAAT (F) protein expression in NPCs: Quantitative analysis. a-b) Representative images of Western blot experiments of VGLUT1-3 (a) and EAAT1-3 (b). C-D) Time course evolution of of VGLUTs (C) and EAAT (D) mRNA expression in NPCs E-F). Time course evolution of mRNA expression of specific neurogenic markers: Nestin, MAP2, SOX2 and TLX (E) and DCX, B3T, NCAM and GFAP (F). A clear reduction in Nestin mRNA expression is evident along with increased MAP2, SOX2, TLX (E) and DCX, B3T, NCAM, GFAP (F) mRNA expression. Western results were normalized to β-actin and are mean ± SEM of values obtained in four experiments, each one performed by duplicate. Statistics compare the expression at indicated times against control 4 h after seeding.* p<0.05; ** p<0.01; ***p<0.001; ANOVA test followed by Tukey post hoc test.
By contrast, EAATs showed a very different expression pattern. EAAT1 mRNA remained low throughout the time interval examined. EAAT3 mRNA increased between 5 dd and 10 dd (3.77±0.08 fold), but then declined to control levels. Remarkably, only EAAT2 mRNA steadily increased in a way comparable to VGLUT, peaking at 10 dd (16.17±1.22 fold) and dropping afterwards. However, it remained significantly higher than control levels (Fig 1D).
Accordingly, western blot analysis showed a gradual increase of VGLUTs protein levels. Thus, VGLUT1-3 protein levels increased between 3–10 dd (Fig 1A). VGLUT1 was already elevated at 3 dd reaching a peak between 7–10 dd (5.24±0.56 fold compared to control). VGLUT2 steadily increased until the end of the experiment reaching a maximum of 2.69±0.08 fold with respect to control. VGLUT3 was transiently downregulated at one day (0.56±0.02 fold) and recovered thereafter. The abundance of mRNA and protein of these transporters obviously correlated well with each other. Notably, VGLUT expression was detected from the beginning of the neurosphere differentiation, which could suggest glutamate might be loaded into vesicles during neurosphere development.
On the contrary, EAAT1 protein was transiently reduced at 3 dd, but then recovered to control levels at 7 dd (Fig 1B). EAAT2 protein increased at 3 dd, reaching maximum levels of 2.57±0.64 fold relative to control (Fig 1B). EAAT3 mildly increased at 3 dd, reaching maximum levels of 2.01±0.62 fold at 10 dd (Fig 1B).
Changes in mRNA expression of VGLUTs and EAATs in SVZ NPCs are significantly correlated with the mRNA levels of neurogenic markers
To validate VGLUT and EAAT expression with regard to NPC differentiation we also studied the expression of various differentiation markers. mRNA expression of the intermediate filament Nestin and the transcription factors SOX2 (SRY (sex determining region Y)-box 2) and TLX (orphan nuclear receptor subfamily 2 group E member 1 (NR2E1)), were chosen as indicators of the presence of proliferative NPCs. Nestin is highly expressed in proliferating cells and is progressively reduced as differentiation advances [17]. SOX2 activates the expression of the transcription factor Hairy and enhancer of split 5 (Hes5) [34], which is involved in cell proliferation thus retaining NPCs in a proliferative state. TLX, which is also induced by SOX2 in neurogenic areas, represses the expression of the tumor suppressing gene phosphatase and tensin homologue (PTEN) and various micro RNAs involved in cell differentiation, while promoting the expression of Wnt7a and other factors involved in NPC proliferation [35]. As indicators of neurodifferentiation, we chose the cytoskeleton proteins doublecortin (DCX), Beta-3-tubulin (B3T), MAP2 and neural cell adhesion molecule (NCAM). B3T is a component of the tubulin cytoskeleton that constructs the axon and presents an almost exclusively neuronal expression [36]. MAP2 is a microtubule associated protein highly enriched in primary dendrites [32] while DCX is microtubule associated protein characteristic of migrating neurons [17]. NCAM is a glycoprotein expressed in the cell surface whose levels of glycosylation are directly related to neuroblast migration [17]. Thus a reduction of Nestin, TLX and SOX2, opposite to an increase in DCX, B3T, NCAM and GFAP were considered as hallmarks of cellular differentiation. DCX, B3T, NCAM and GFAP mRNAs were markedly increased (61.67±9.48, 29.13±2.53, 25.12±0.93 and 498.06±25.41 fold, respectively) at 10 dd (Fig 1F). Likewise, MAP2, SOX2 and TLX mRNAs increased at 5 dd of culture (2.38±0.23, 3.14±0.03 and 4.24±0.38 fold, respectively) and remained high during the remaining days of culture, with the exception of SOX2 mRNA, which significantly decreased at 15dd (3.42±0.16) (Fig 1E). Nestin mRNA remained constant during the first 24 h of differentiation and then decreased at 5 dd (0.56±0.05 fold), remaining low until the end of the experiment (Fig 1E). Furthermore, analysis of linear correlation (Spearman Rank Order Correlation) between VGLUT and EAAT expression in relation with specific markers of the different phases of the neurogenesis process showed that significant positive lineal correlations exist between VGLUT1 and VGLUT2 mRNAs (S3A Fig) and between VGLUT3 and EAAT2 or EAAT1 mRNAs (S3K Fig), as well as between EAAT2 and EAAT1 mRNAs (not shown). In agreement with the RTqPCR data, a significant lineal correlation was found between VGLUT1 and VGLUT2 mRNAs and the markers Nestin (negative) and GFAP (positive) (S3B–S3E Fig), while a significant positive lineal correlation was found between VGLUT3 mRNA with DCX, B3T, NCAM and GFAP mRNAs (S3G–S3J Fig) and between EAAT2 (or EAAT1 to a lesser extent) with DCX, B3T and NCAM mRNAs (S3L–S3O Fig), but not with GFAP mRNA (not shown). EAAT3 mRNA showed a significant positive lineal correlation with DCX mRNA (r = +0.89; P<0.05; not shown). Thus, the expression of glutamate transporters is characteristic of the differentiation process of SVZ-derived NSCs and is associated with the expression of neuroblast and glial markers and negatively associated with the immature precursor cell marker nestin.
We observed that after 10 days of differentiation, the proliferation markers Nestin (which had been notably reduced), SOX2 and TLX remained largely stable. Meanwhile, the expression of the neuronal differentiation marker MAP2 did not increase to levels comparable to the neuroblast markers DCX and B3T, which abruptly decayed after this time thus indicating that neuroblasts in our conditions would remain in a state of proto-neurons or neuronal-like cells. Therefore time points below 10 days of differentiation were chosen for further western blot and immunocytochemistry analyses.
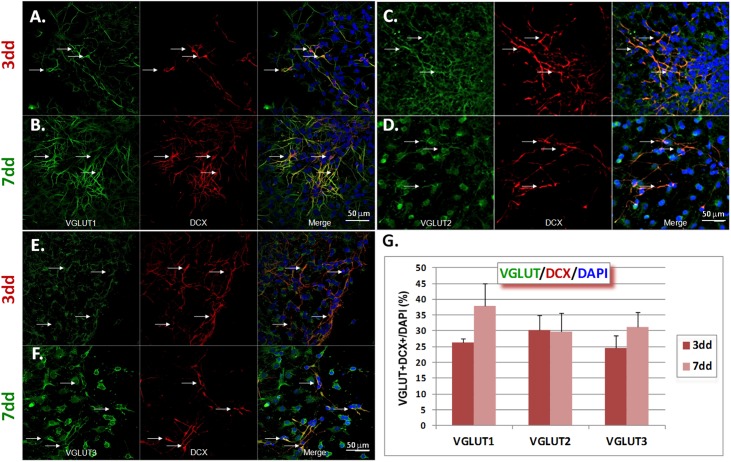
VGLUT1 and 2 are preferentially expressed by neuroblasts whereas VGLUT3 is preferentially expressed by glial cells
Using immunocytochemistry we next examined the characteristics of cells expressing VGLUT proteins at 3 and 7 dd. These time points allow sufficient NPC differentiation as judged by DCX and GFAP expression (Fig 1F). Given that cells were seeded as neurospheres, neuroblasts and glial cells spread away from them during differentiation (S1A Fig). Therefore, VGLUT+ cells were found in the periphery of the neurospheres where DCX+ and GFAP+ expressing cells could be distinguished from one another. DCX+ neuroblasts and neuronal-like cells or “proto-neurons” could be distinguished based on the presence of one characteristic axonal-like projection containing clear collateral branches and one minor primary dendrite compared to a bipolar neuroblast in which no axonal-like process can be defined (S1B Fig).
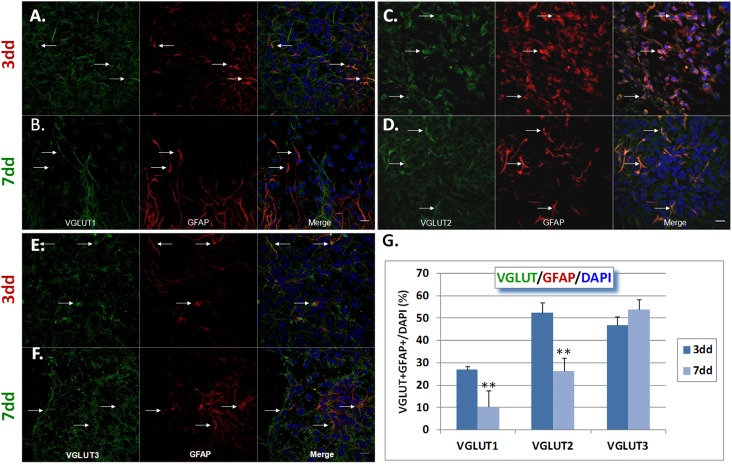
VGLUTs 1–3 were found on neuroblasts and glial cells (Figs 2 and 3 and S4 Fig). Therefore, we decided to characterize the number of VGLUT+DCX+ or VGLUT+GFAP+ cells in proportion to the total number of cells per field as detected by DAPI counterstaining. The results indicate that VGLUT1+DCX+ cells tend to increase between 3 and 7 dd, although this increase was not statistically significant (26.17±1.47% to 37.81±7.23%, p = 0.167) (Fig 2A, 2B and 2G). VGLUT2+DCX+ cells remained constant (30.35±4.58% vs. 29.81±5.79%, p = 0.945) (Fig 2C, 2D and 2G), as were VGLUT3+DCX+ cells (24.51±4.07% to 31.10±4.81% (p = 0.346) (Fig 2E, 2F and 2G). By contrast, VGLUT1+GFAP+ and VGLUT2+GFAP+ cells decreased significantly between 3 and 7 dd (27.08±3.01% to 10.45±2.47%, p<0.01, and 52.38±4.88% to 26.44±3.86% p<0.01, for VGLUT 1 and 2, respectively) (Fig 3A–3D and 3G), whereas VGLUT3+GFAP+ cells remained constant (46.76±9.16% to 53.66%±8.17, p>0.05) (Fig 3E, 3F and 3G). Hence, VGLUT1 was the predominant VGLUT on DCX+ cells whilst VGLUT3 was predominant on GFAP+ cells at 7 dd.
Fig 2. Co-expression of VGLUT1-3 and DCX in NPCs at 3 and 7 days of differentiation.
A-B) VGLUT1, DCX and merge images at 3 (A) and 7 (B) days of differentiation, respectively. (C-D) VGLUT2. (E-F) VGLUT3. Quantification was performed by counting of double labelled cells with respect to the total number of cells in the field as determined by DAPI staining. Data are expressed as mean ± SEM. Statistical analysis was done by Student t-test. 150–200 cells per image were analyzed; n = 10 images from 2 different cultures. Scale bar = 50 μm.
Fig 3. Co-expression of VGLUT1-3 and GFAP in NPCs at 3 and 7 days of differentiation.
A-B) VGLUT1, GFAP and merge images at 3 and 7 days of differentiation, respectively. (C-D) VGLUT2. (E-F) VGLUT3. A statistically significant reduction in the number of GFAP cells expressing either VGLUT1 or 2 was found between 3 and 7 dd (** P<0.01), but not for VGLUT3. Quantification was performed by counting of double labelled cells with respect to the total number of cells in the field as determined by DAPI staining. Data are expressed as mean ± SEM. Statistical analysis was done by Student t-test. 150–200 cells per image were analyzed; n = 10 images from 2 different cultures. Scale bar = 50 μm.
Intriguingly, VGLUT1 and 3 were abundant in B3T+ cells at 3dd (14.0±3.2% and 8.2±2.1% respectively) whereas VGLUT2 was present only in 4.3±1.4% of cells (S5 Fig). Since B3T is expressed in a later stage of neuronal development [17], this suggests that all VGLUTs might be important during early differentiation stages of SVZ-derived NPCs while DCX is still highly expressed. The fact that VGLUT3 maintains high levels of expression at 7 dd in glial cells, compared to VGLUT1 and 2, which undergo a significant reduction in the same period, indicates that this transporter may play the most important role in glutamate vesicle loading in NPC derived glial cells.
VGLUT1 and VGLUT2 inhibition increases neuronal differentiation
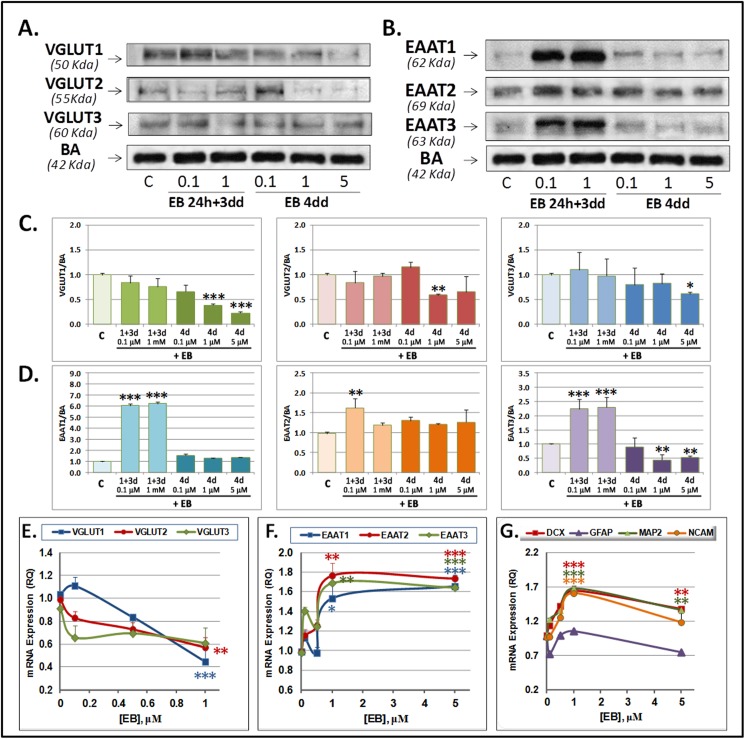
To elucidate the involvement of VGLUTs in neurogenesis, we administered the competitive inhibitor of vesicular glutamate uptake Evans Blue (EB) [37], which blocks VGLUTs by interfering with their cation-binding sites [38]. Cells were acutely treated either for 24 h and then allowed to recover for 3 days, or continuously treated with increasing EB concentrations during 4 days. While acute EB treatment did not significantly alter VGLUT protein levels, chronic treatment significantly reduced all VGLUTs by more than 50% (Fig 4A and 4C). Continuous EB also reduced VGLUT mRNA expression (Fig 4E). Surprisingly, protein and mRNA levels of all EAATs were increased following acute but not chronic EB treatment, with the exception of EAAT3, which was mildly reduced at the protein level (Fig 4B, 4D and 4F). However, concentrations higher than 1 μM EB increased the mRNA levels of all EAATs (Fig 4F). Interestingly, mRNA levels of DCX, NCAM and MAP2 were significantly increased by 70% by 1 μM EB (Fig 4G). Of note, only 5 μM EB was able to reduce the number of cells on zone B of the cell culture (S1A and S6A Figs). Continuous EB exposure increased levels of Nestin, SOX2 and TLX, arguing against a possible reduction of glutamate transporter levels as a consequence of increased cell mortality (S6B Fig).
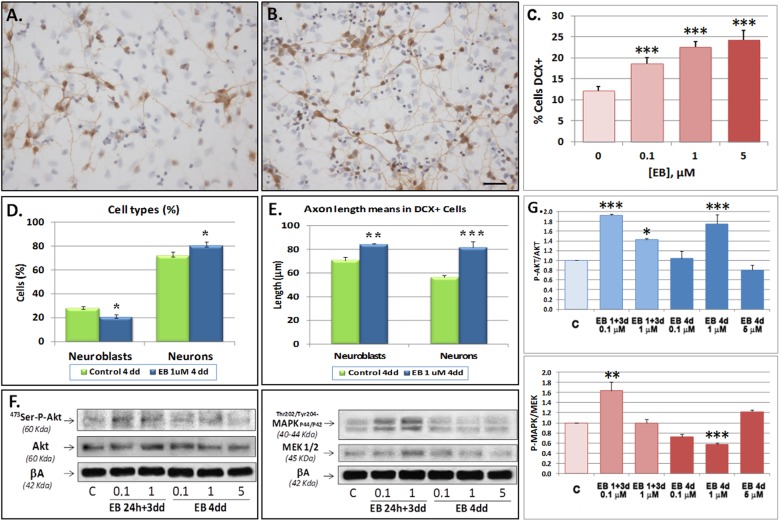
Fig 4.
Evans Blue (EB) effect on VGLUT and EAAT expression at the protein (A-D) and the mRNA level (E-F) and on mRNA expression of neurogenic markers (G) in SVZ NPCs in primary culture. Cells were acutely (24 h EB + 3 dd) or chronically (4 dd) treated with EB at indicated concentrations and then analyzed by RTqPCR for mRNA expression. A-B) Representative Westerns blots of VGLUTs (A) and EAATs (B) and quantification normalized to β-actin (C-D). (E-G). Graphs show data of VGLUTs (E), EAATs (F) or DCX, GFAP, MAP2 and NCAM (G) mRNA expression. Data are means ± SEM of three experiments each one performed by duplicate in different cultures. Statistical significances against controls were performed by One Way ANOVA followed by Tukey post hoc test, when analysis of variance was significant. (*) P<0.05, (**) P<0.01 and (***) P<0,001.
More detailed analyses using concentrations from 0.1 to 5 μM EB revealed a concentration dependent increase in the number of DCX+ cells (Fig 5A–5C), indicating that VGLUT1 and/or VGLUT2 inhibition promoted neuronal differentiation. In our cell cultures, some DCX+ cells indeed acquired a neuronal-like shape based on the presence of one characteristically longer neurite and several branches emerging from it, which was reminiscent of an axonal process, as opposed to the more usual bipolar shape of neuroblasts (S1B Fig). Quantitative analysis at 4dd revealed that EB at 1μM significantly decreased the number of neuroblasts from 28.09±4.26% to 20.92±5.49% but increased the number of neuronal like cells from 70.57±6.99% to 83.85±2.62% (Fig 5D). The length of neurites in neuroblasts and neuronal-like cells significantly increased from 70.57±2.85 μm to 83.85±1.07 μm and from 56.21±1.76 μm to 81.4±5.26 μm, respectively (Fig 5E).
Fig 5. Evans Blue increases neuronal differentiation of SVZ-derived NPCs.
A-D: NPCs immunocytochemical analysis of DCX+ immunolabelled cells. DCX+ cells are observed at 3 days of differentiation without EB (A) or with 1 μM chronic EB treatment (B). Note that DCX+ cells show more evident neurites than the control cells. C) Percentage of DCX+ cells increased in a concentration dependent manner under chronic treatment with EB at 3 days of differentiation. Chronic EB treatment significantly reduced the proportion of neuroblasts and increased the proportion of “neuronal-like” cells. D) Neuroblast and neuron percentages. E) Neurite length in neuroblasts and neurons (μm). Cells were characterized on area “B” (See S1 Fig). n = 900 cells/treatment. (F-G) Acute or chronic treatment of cells promoted 473Ser-Akt and phosphorylation and 202Thr/204-Tyr-MAPK P44/42 phosphorylation. Cells were acutely or chronically (4 dd) exposed to EB at the indicated concentrations and analyzed for P-AKT/AKT and P-MAPK/MEK. F) Representative Western Blot of P-AKT/AKT (left) or P-MAPK/MEK. G) Quantification of results for three different experiments. Statistical analysis was performed by One Way ANOVA, followed by Tukey post hoc test when analysis of variance was significant. Comparisons of percentages of neuroblasts or “neuronal-like” cells were done by Student-T test. (*) p<0.05, (**) p<0.01 and (***) p<0,001.
Neuronal polarization is controlled by 473Ser-AKT phosphorylation, which in turn inhibits GSK3β thus activating microtubule polymerization and allowing axonal specification [39, 40]. Notably, EB treatment at 1μM for 4 days increased AKT phosphorylation by 1.75±0.19-fold whereas it decreased MAPK p44/42 phosphorylation to 0.57±0.03-fold (Fig 5F–5G). These results corroborated the notion that cell differentiation was induced by VGLUT downregulation.
Discussion
During the past decade, glutamate and its receptors have been proven to play a critical role in the regulation of in neuroblast migration and survival along the glial tube and on NPC survival [17, 19, 29, 30], while it is also known that glutamate-mediated calcium currents are necessary for NPC neuronal differentiation of human NPCs [41]. However, a specific role of the glutamate transporters VGLUT and EAAT in the regulation of adult neurogenesis has so far not been documented. Previous work of our group showed that VGLUT1 and EAAT2 expression increases after focal cerebral ischemia, correlating positively with neurological damage, which suggests that VGLUT1 could influence brain remodeling and recovery by controlling the amount of glutamate that is loaded in presynaptic vesicles in the recovering brain. Additionally, VGLUT2 and 3 were also found in the iCC after stroke [10]. In agreement with our previous work, EAATs were found to be expressed by glial cells in the iCC [11]. Therefore, we wondered whether VGLUTs could be expressed in SVZ-derived NPCs and whether they play a role in neuronal differentiation. In order to test the role of glutamate transporters in neurogenesis, we characterized the expression of VGLUTs and EAATs in SVZ-derived NPC cultures at different stages of differentiation in vitro.
We found that the expression of VGLUT and EAAT in SVZ-derived NPCs increase throughout the differentiation process at both the mRNA and the protein level. VGLUT and EAAT expression correlated well with the expression of neuronal and glial differentiation markers, whilst there was an overall negative correlation with Nestin, showing that vesicular and membrane glutamate transporter expression is a hallmark of NPC differentiation. Furthermore, VGLUT1 and 2 are preferentially expressed by neuroblasts, whereas VGLUT3 is preferentially expressed by glial cells. Thus, VGLUT1 and 2 could be contributing mainly to neuronal differentiation whilst VGLUT3, in concert with EAAT2 (and to a lesser extent with EAAT1), could be contributing mainly to glial differentiation of NPCs in vitro. Importantly the stimulation of the P2Y4 receptor on embryonic stem cells was found to induce neuronal differentiation along with VGLUT expression, however VGLUT function was not evaluated in this study [42].
Pharmacological VGLUT inhibition with EB increased mRNA expression of the neurogenic markers DCX, B3T and MAP2, the number of NPCs expressing DCX and the differentiation from neuroblasts to neurons as judged by increased axonal length, together with activation of pAKT, which through inhibition of GSK3β can regulate axonal and dendritic specification [43], thereby suggesting that VGLUTs activity averted the entry of NPCs into neuronal differentiation. Importantly, increased signalling through the PI3K/Akt/NF-κB pathway and GSK3β inhibition has been shown to increase EAAT expression [44–46]. Thus, it could be speculated that EB promoted the EAAT expression under acute stimulation by a mechanism that is then silenced during continuous stimulation. On the other hand a VGLUT repressor might have been expressed under continuous EB stimulation through this pathway. Indeed when exposed to EB 1μM, VGLUTs and EAATs showed a differential regulation (Fig 4). Of note, the activation of AKT could drive mTOR-Akt-NF-κB cascade activation which has been shown to play critical roles to up-regulate GLT-1 after oxygen glucose deprivation [44].
EB is a very potent inhibitor of VGLUT activity [37], however a pharmacological approach often has the problem of lack of specificity. EB is also impermeable to the blood-brain-barrier, thus limiting the continuation of these studies in vivo. Further studies using siRNA to specifically delete the different isoforms of VGLUTs, or other VGLUT inhibitors such as Chicago Sky Blue 6, which has been used in vivo to study the contribution of VLGUT2 to neuropathic pain [46], will further enhance our understanding of the role of each of these transporters on neuronal differentiation both in vivo and in vitro. However, a study of how adult neurogenesis could be altered in adult animals lacking VGLUTs seems unlikely since VGLUT1 -/- animals show lethality after the third week of age [47] whilst VGLUT2 -/- die right after birth [48]. Thus, strategies must be developed to selectively delete each VGLUT on SVZ-NPCs so that their contribution to neurogenesis in health and disease can be further confirmed.
Although VGLUTs have been clearly shown to influence plasticity in a wide variety of contexts of health and disease [48, 49], to the best of our knowledge, this is the first work showing an active role of presynaptic proteins in the process of NPC differentiation in vitro. Nevertheless the involvement of other proteins of the SNARE complex, necessary for proper vesicle docking [50], remain to be elucidated in this context. Our data indicate that VGLUT-mediated glutamate release from SVZ-derived neuroblasts or astrocytes provides an autocrine signal that controls neuroblast migration and differentiation. This is of the utmost importance in the context of a brain trauma in which glutamate is highly dysregulated, such as stroke, a condition in which VGLUT regulation and function has been barely characterized [10]. The inhibitory control exerted by VGLUT activity on neural differentiation could thus regulate the timing at which the cell becomes a neuron, allowing for correct migration and synaptic integration within the surviving neuronal network by preventing the cell differentiating at the wrong location.
Conclusions
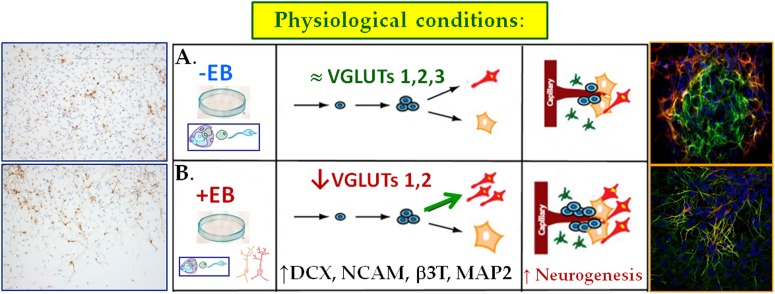
In short, in the light to our results we propose that (Fig 6):
Fig 6. Proposed model for the role of VGLUTs in neuronal differentiation of cultured SVZ-derived neural precursor cells.
Under physiological conditions, VGLUT1-2 inhibition, and thus decreased glutamate exocytosis, may promote neuronal differentiation of NPCs.
NPCs express VGLUT1-3 and EAAT1-3 both at the mRNA and protein level.
Their expression augments throughout NSC differentiation.
The inhibition of VGLUT expression increases adult NSC neuronal differentiation.
Under physiological conditions, VGLUT1-3 inhibition promotes neuronal differentiation and migration of NPCs.
Supporting information
A) Organization of NPCs in culture: Cells migrate from zone A (center) to zone B (periphery) where they acquire their final phenotype. Double immunocytochemistry of nestin (red) and VGLUT2 (green) at three days of differentiation. Dapi (blue) was used for nuclear staining. B) Comparison between a neuroblast (★) and a neuronal-like cell or “proto-neuron” (▲), both expressing DCX. Asterisk: a typical bipolar neuroblast cell. Arrow head: A cell that we have considered as a proto-neuron. Of note the neuroblast has two large projections from which an axon is indistinguishable, and almost no collateral branching. The proto-neuron has a characteristic primary axonal like extension (bracket) with several primary and secondary collateral branches and primary dendrites emerging from the cell body (arrow). Scale bar = 7 μm.
(TIF)
A) MAP2 shows an expression pattern with highest expression on the axonal shaft (*) while DCX is ditributed all allong the cell. B-D) MAP2 allows the detection of the axonal fiber (B) while DCX allows the detection of the axon, axonal tip (C; arrow head) and collateral fibers (C; arrows), as evidenced in the merge image (D). This allows a better estimation of axonal length on DCX+ cells. E) Protoneurons also present higher expression of MAP2 on the basis of the axonal shaft (*). F-H) MAP2 shows faint expression on collaterals (F; arrows) while DCX labelling clearly stains not only the axonal shaft but also axonal collaterals (G; arrows), as shown in the merged image (H). Scale bar: 20 μM (A, E); 10 μM (B-D; F-H).
(TIF)
Linear regression analysis of correlations between: A) VGLUT1 and VGLUT2 vs. each other and vs. NEST or GFAP (B-E). F-J. Correlations between VGLUT3 and different neurogenic markers. K) Correlations between EAAT2 and VGLUT3 each other and between EAAT2 and indicated neurogenic markers (L-O). Straight line equations, correlation coefficients (r) and statistical significances of regression analyses are indicated in each plot. Regression analysis and statistics were performed by the Spearman Rank Order Correlation Test.* p<0.05; ** p<0.01 and *** p<0.001.
(TIF)
A representative image of the punctate pattern found for all VGLUTs proteins in our culture conditions is shown. VGLUTs can be found in different cell populations (compare asterisk vs. arrow). In this case the colocalization of a VGLUT with DCX, is shown, thus demonstrating that VGLUTs are expressed in neuroblasts
(TIF)
A-B) VGLUT1, B3T, DAPI and merge images at 3 days of cell differentiation. C-D) VGLUT2. E-F) VGLUT3. Quantification was performed by counting of double labelled cells with respect to the total cells in the field as determined by DAPI staining. Data are expressed as mean ± SEM. 150–200 cells per image were analyzed; n = 10 images from 2 different cultures. Scale bar = 20 μm.
(TIF)
A) Total cell number was estimated by counting hematoxylin dyed nuclei on zone B of the cell culture. Only 5 μM EB was able to significantly reduce the number of nuclei (259±21 cells/field versus 377±20 cells/field for the control; n = 20 fields) in zone B of the cell culture. B) Nestin, SOX2 and TLX were increased after prolonged incubation with different concentrations of EB suggesting that EB may increase proliferation even in the absence of growth factors. Data are means ± SEM of three experiments each one performed by duplicate in different cultures. Statistical significances against controls were performed by One Way ANOVA followed by Tukey post hoc test, when analysis of variance was significant. (*) P<0.05, (**) P<0.01 and (***) P<0,001.
(TIF)
Acknowledgments
This study was supported by the grants SAF2010-20337 from Ministry of Economy and Competitiveness (MINECO, Spain), GR35/10-B and GR3/14 (Complutense University of Madrid-Santander Bank) and RD06/0026/0012 (Institute of Health Carlos III, ISCIII) to MJ. Oset-Gasque. E. Sánchez-Mendoza has a contract from RD06/0026/0012.
We would like to thank Dr. J. Sánchez-Prieto for his helpful comments, to Nuria García-Font, Mina Borbor and Santiago Camblor-Perujo for technical assistance in the preparation of the manuscript and Javier Morón-Oset for proofreading the English of this manuscript.
Authors declare no conflict of interest.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the grants SAF2010-20337 and BFU-2013-43163-R from Ministry of Economy and Competitiveness (MINECO, Spain), GR35/10-B and GR3/14 (Complutense University of Madrid-Santander Bank) and RD06/0026/0012 (Institute of Health Carlos III, ISCIII). E. Sánchez-Mendoza has a contract from RD06/0026/0012. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11(4):369–80. Epub 2012/03/24. PubMed Central PMCID: PMC3964179. doi: 10.1016/S1474-4422(12)70039-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castillo J, Alvarez-Sabin J, Davalos A, Diez-Tejedor E, Lizasoain I, Martinez-Vila E, et al. [Consensus review. Pharmacological neuroprotection in cerebral ischemia: is it still a therapeutic option?]. Neurologia. 2003;18(7):368–84. Epub 2003/09/25. [PubMed] [Google Scholar]
- 3.Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol. 2006;26(7–8):1057–83. Epub 2006/05/20. doi: 10.1007/s10571-006-9008-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8(12):935–47. Epub 2007/11/08. doi: 10.1038/nrn2274 [DOI] [PubMed] [Google Scholar]
- 5.Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, et al. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123(4):983–1002. Epub 2004/01/31. [DOI] [PubMed] [Google Scholar]
- 6.Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435(3):379–87. Epub 2001/06/15. [DOI] [PubMed] [Google Scholar]
- 7.Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24(11):2633–42. Epub 2004/03/19. doi: 10.1523/JNEUROSCI.3770-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takamori S. VGLUTs: 'exciting' times for glutamatergic research? Neurosci Res. 2006;55(4):343–51. Epub 2006/06/13. doi: 10.1016/j.neures.2006.04.016 [DOI] [PubMed] [Google Scholar]
- 9.Kashani A, Betancur C, Giros B, Hirsch E, El Mestikawy S. Altered expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in Parkinson disease. Neurobiol Aging. 2007;28(4):568–78. Epub 2006/03/28. PubMed Central PMCID: PMC1976623. doi: 10.1016/j.neurobiolaging.2006.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-Mendoza E, Burguete MC, Castello-Ruiz M, Gonzalez MP, Roncero C, Salom JB, et al. Transient focal cerebral ischemia significantly alters not only EAATs but also VGLUTs expression in rats: relevance of changes in reactive astroglia. J Neurochem. 2010;113(5):1343–55. Epub 2010/04/07. doi: 10.1111/j.1471-4159.2010.06707.x [DOI] [PubMed] [Google Scholar]
- 11.Arranz AM, Gottlieb M, Perez-Cerda F, Matute C. Increased expression of glutamate transporters in subcortical white matter after transient focal cerebral ischemia. Neurobiol Dis. 2009;37(1):156–65. Epub 2009/10/07. doi: 10.1016/j.nbd.2009.09.019 [DOI] [PubMed] [Google Scholar]
- 12.Nakamichi N, Takarada T, Yoneda Y. Neurogenesis mediated by gamma-aminobutyric acid and glutamate signaling. J Pharmacol Sci. 2009;110(2):133–49. Epub 2009/06/02. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol. 1998;36(2):234–48. Epub 1998/08/26. [DOI] [PubMed] [Google Scholar]
- 14.Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494(3):415–34. Epub 2005/12/02. doi: 10.1002/cne.20798 [DOI] [PubMed] [Google Scholar]
- 15.Bath KG, Lee FS. Neurotrophic factor control of adult SVZ neurogenesis. Dev Neurobiol. 2010;70(5):339–49. Epub 2010/02/27. PubMed Central PMCID: PMC2917621. doi: 10.1002/dneu.20781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agasse F, Bernardino L, Kristiansen H, Christiansen SH, Ferreira R, Silva B, et al. Neuropeptide Y promotes neurogenesis in murine subventricular zone. Stem Cells. 2008;26(6):1636–45. Epub 2008/04/05. doi: 10.1634/stemcells.2008-0056 [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Mendoza E, Bellver-Landete V, Merino JJ, Gonzalez MP, Martinez-Murillo R, Oset-Gasque MJ. Review: Could neurotransmitters influence neurogenesis and neurorepair after stroke? Neuropathol Appl Neurobiol. 2013;39(7):722–35. Epub 2013/08/15. doi: 10.1111/nan.12082 [DOI] [PubMed] [Google Scholar]
- 18.Platel JC, Dave KA, Bordey A. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J Physiol. 2008;586(16):3739–43. Epub 2008/05/10. PubMed Central PMCID: PMC2538924. doi: 10.1113/jphysiol.2008.155325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platel JC, Dave KA, Gordon V, Lacar B, Rubio ME, Bordey A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 2010;65(6):859–72. Epub 2010/03/30. PubMed Central PMCID: PMC2861893. doi: 10.1016/j.neuron.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platel JC, Heintz T, Young S, Gordon V, Bordey A. Tonic activation of GLUK5 kainate receptors decreases neuroblast migration in whole-mounts of the subventricular zone. J Physiol. 2008;586(16):3783–93. Epub 2008/06/21. PubMed Central PMCID: PMC2538932. doi: 10.1113/jphysiol.2008.155879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platel JC, Lacar B, Bordey A. GABA and glutamate signaling: homeostatic control of adult forebrain neurogenesis. J Mol Histol. 2007;38(6):602–10. Epub 2008/03/01. [DOI] [PubMed] [Google Scholar]
- 22.Platel JC, Stamboulian S, Nguyen I, Bordey A. Neurotransmitter signaling in postnatal neurogenesis: The first leg. Brain Res Rev. 2010;63(1–2):60–71. Epub 2010/03/02. PubMed Central PMCID: PMC2862802. doi: 10.1016/j.brainresrev.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, Wang WZ, Comte I, Pastrana E, Tran PB, Brown J, et al. Dopamine stimulation of postnatal murine subventricular zone neurogenesis via the D3 receptor. J Neurochem. 2010;114(3):750–60. Epub 2010/05/19. PubMed Central PMCID: PMC2913229. doi: 10.1111/j.1471-4159.2010.06799.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu W, Gu C, Jiang W, Wester P. Neurotransmitter synthesis in poststroke cortical neurogenesis in adult rats. Stem Cell Res. 2009;4(2):148–54. Epub 2010/01/22. doi: 10.1016/j.scr.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 25.Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29(3):450–60. Epub 2004/02/12. doi: 10.1038/sj.npp.1300320 [DOI] [PubMed] [Google Scholar]
- 26.Coronas V, Bantubungi K, Fombonne J, Krantic S, Schiffmann SN, Roger M. Dopamine D3 receptor stimulation promotes the proliferation of cells derived from the post-natal subventricular zone. J Neurochem. 2004;91(6):1292–301. Epub 2004/12/09. doi: 10.1111/j.1471-4159.2004.02823.x [DOI] [PubMed] [Google Scholar]
- 27.Gundersen V, Storm-Mathisen J, Bergersen L. Neuroglial Transmission. Physiol Rev. 2015;95(3):695–726. doi: 10.1152/physrev.00024.2014 [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Lozada Z, Ortega A. Glutamatergic Transmission: A Matter of Three. Neural Plasticity. 2015;2015:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Giorgi-Gerevini V, Melchiorri D, Battaglia G, Ricci-Vitiani L, Ciceroni C, Busceti CL, et al. Endogenous activation of metabotropic glutamate receptors supports the proliferation and survival of neural progenitor cells. Cell Death Differ. 2005;12(8):1124–33. Epub 2005/06/11. doi: 10.1038/sj.cdd.4401639 [DOI] [PubMed] [Google Scholar]
- 30.Xia W, Liu Y, Jiao J. GRM7 regulates embryonic neurogenesis via CREB and YAP. Stem Cell Reports. 2015;4(5):795–810. Epub 2015/04/30. PubMed Central PMCID: PMC4437472. doi: 10.1016/j.stemcr.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juge N, Yoshida Y, Yatsushiro S, Omote H, Moriyama Y. Vesicular glutamate transporter contains two independent transport machineries. J Biol Chem. 2006;281(51):39499–506. Epub 2006/10/19. doi: 10.1074/jbc.M607670200 [DOI] [PubMed] [Google Scholar]
- 32.Ransome MI, Turnley AM. Erythropoietin promotes axonal growth in a model of neuronal polarization. Mol Cell Neurosci. 2008;38(4):537–47. Epub 2008/07/01. doi: 10.1016/j.mcn.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 33.Perez-Rodriguez R, Roncero C, Olivan AM, Gonzalez MP, Oset-Gasque MJ. Signaling mechanisms of interferon gamma induced apoptosis in chromaffin cells: involvement of nNOS, iNOS, and NFkappaB. J Neurochem. 2009;108(4):1083–96. Epub 2009/01/15. doi: 10.1111/j.1471-4159.2008.05862.x [DOI] [PubMed] [Google Scholar]
- 34.Zhu G, Shi W, Fan H, Zhang X, Xu J, Chen Y, et al. HES5 promotes cell proliferation and invasion through activation of STAT3 and predicts poor survival in hepatocellular carcinoma. Exp Mol Pathol. 2015;99(3):474–84. Epub 2015/09/08. doi: 10.1016/j.yexmp.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 35.Islam MM, Zhang CL. TLX: A master regulator for neural stem cell maintenance and neurogenesis. Biochim Biophys Acta. 2015;1849(2):210–6. Epub 2014/06/17. PubMed Central PMCID: PMC4265312. doi: 10.1016/j.bbagrm.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katsetos CD, Legido A, Perentes E, Mork SJ. Class III beta-tubulin isotype: a key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. J Child Neurol. 2003;18(12):851–66; discussion 67. Epub 2004/01/23. doi: 10.1177/088307380301801205 [DOI] [PubMed] [Google Scholar]
- 37.Roseth S, Fykse EM, F. F. Uptake of L-glutamate into rat brain synaptic vesicles: effect of inhibitors that bind specifically to the glutamate transporter. Journal of Neurochemistry. 1995;65(1):96–103. [DOI] [PubMed] [Google Scholar]
- 38.Preobraschenski J, Zander J, Suzuki T, Ahnert-Hilger G, Jahn R. Vesicular glutamate transporters use flexible anion and cation binding sites for efficient accumulation of neurotransmitter. Neuron 2014;84(6):1287–301. doi: 10.1016/j.neuron.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 39.Yoshimura T, Arimura N, Kaibuchi K. Signaling networks in neuronal polarization. J Neurosci. 2006;26(42):10626–30. Epub 2006/10/20. doi: 10.1523/JNEUROSCI.3824-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schramm J, Schulte D. A fast and simple differentiation protocol to study the pro-neurogenic activity of soluble factors in neurospheres. Neurosci Lett. 2014;562:69–74. Epub 2014/01/15. doi: 10.1016/j.neulet.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 41.Whitney NP, Peng H, Erdmann NB, Tian C, Monaghan DT, Zheng JC. Calcium-permeable AMPA receptors containing Q/R-unedited GluR2 direct human neural progenitor cell differentiation to neurons. FASEB J. 2008;22(8):2888–900. Epub 2008/04/12. PubMed Central PMCID: PMC2493446. doi: 10.1096/fj.07-104661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uda Y, Xu S, Matsumura T, Takei Y. P2Y4 Nucleotide Receptor in Neuronal Precursors Induces Glutamatergic Subtype Markers in Their Descendant Neurons. Stem Cell Reports. 2016;6(4):474–82. Epub 2016/03/15. PubMed Central PMCID: PMC4834041. doi: 10.1016/j.stemcr.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10(5):319–32. Epub 2009/04/21. doi: 10.1038/nrn2631 [DOI] [PubMed] [Google Scholar]
- 44.Weng HR, Gao M, Maixner DW. Glycogen synthase kinase 3 beta regulates glial glutamate transporter protein expression in the spinal dorsal horn in rats with neuropathic pain. Exp Neurol. 2014;252:18–27. Epub 2013/11/28. PubMed Central PMCID: PMC3946993. doi: 10.1016/j.expneurol.2013.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li LB, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, et al. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem. 2006;97(3):759–71. Epub 2006/04/01. doi: 10.1111/j.1471-4159.2006.03743.x [DOI] [PubMed] [Google Scholar]
- 46.Wang ZT, Yu G, Wang HS, Yi SP, Su RB, Gong ZH. Changes in VGLUT2 expression and function in pain-related supraspinal regions correlate with the pathogenesis of neuropathic pain in a mouse spared nerve injury model. Brain Res. 2015;1624:515–24. Epub 2015/08/25. doi: 10.1016/j.brainres.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 47.Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, et al. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101(18):7158–63. Epub 2004/04/23. PubMed Central PMCID: PMC406482. doi: 10.1073/pnas.0401764101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallen-Mackenzie A, Wootz H, Englund H. Genetic inactivation of the vesicular glutamate transporter 2 (VGLUT2) in the mouse: what have we learnt about functional glutamatergic neurotransmission? Ups J Med Sci. 2010;115(1):11–20. Epub 2010/03/02. PubMed Central PMCID: PMC2853350. doi: 10.3109/03009730903572073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santos MS, Foss SM, Park CK, Voglmaier SM. Protein interactions of the vesicular glutamate transporter VGLUT1. PLoS One. 2014;9(10):e109824 Epub 2014/10/22. PubMed Central PMCID: PMC4198130. doi: 10.1371/journal.pone.0109824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sudhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80(3):675–90. Epub 2013/11/05. PubMed Central PMCID: PMC3866025. doi: 10.1016/j.neuron.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Organization of NPCs in culture: Cells migrate from zone A (center) to zone B (periphery) where they acquire their final phenotype. Double immunocytochemistry of nestin (red) and VGLUT2 (green) at three days of differentiation. Dapi (blue) was used for nuclear staining. B) Comparison between a neuroblast (★) and a neuronal-like cell or “proto-neuron” (▲), both expressing DCX. Asterisk: a typical bipolar neuroblast cell. Arrow head: A cell that we have considered as a proto-neuron. Of note the neuroblast has two large projections from which an axon is indistinguishable, and almost no collateral branching. The proto-neuron has a characteristic primary axonal like extension (bracket) with several primary and secondary collateral branches and primary dendrites emerging from the cell body (arrow). Scale bar = 7 μm.
(TIF)
A) MAP2 shows an expression pattern with highest expression on the axonal shaft (*) while DCX is ditributed all allong the cell. B-D) MAP2 allows the detection of the axonal fiber (B) while DCX allows the detection of the axon, axonal tip (C; arrow head) and collateral fibers (C; arrows), as evidenced in the merge image (D). This allows a better estimation of axonal length on DCX+ cells. E) Protoneurons also present higher expression of MAP2 on the basis of the axonal shaft (*). F-H) MAP2 shows faint expression on collaterals (F; arrows) while DCX labelling clearly stains not only the axonal shaft but also axonal collaterals (G; arrows), as shown in the merged image (H). Scale bar: 20 μM (A, E); 10 μM (B-D; F-H).
(TIF)
Linear regression analysis of correlations between: A) VGLUT1 and VGLUT2 vs. each other and vs. NEST or GFAP (B-E). F-J. Correlations between VGLUT3 and different neurogenic markers. K) Correlations between EAAT2 and VGLUT3 each other and between EAAT2 and indicated neurogenic markers (L-O). Straight line equations, correlation coefficients (r) and statistical significances of regression analyses are indicated in each plot. Regression analysis and statistics were performed by the Spearman Rank Order Correlation Test.* p<0.05; ** p<0.01 and *** p<0.001.
(TIF)
A representative image of the punctate pattern found for all VGLUTs proteins in our culture conditions is shown. VGLUTs can be found in different cell populations (compare asterisk vs. arrow). In this case the colocalization of a VGLUT with DCX, is shown, thus demonstrating that VGLUTs are expressed in neuroblasts
(TIF)
A-B) VGLUT1, B3T, DAPI and merge images at 3 days of cell differentiation. C-D) VGLUT2. E-F) VGLUT3. Quantification was performed by counting of double labelled cells with respect to the total cells in the field as determined by DAPI staining. Data are expressed as mean ± SEM. 150–200 cells per image were analyzed; n = 10 images from 2 different cultures. Scale bar = 20 μm.
(TIF)
A) Total cell number was estimated by counting hematoxylin dyed nuclei on zone B of the cell culture. Only 5 μM EB was able to significantly reduce the number of nuclei (259±21 cells/field versus 377±20 cells/field for the control; n = 20 fields) in zone B of the cell culture. B) Nestin, SOX2 and TLX were increased after prolonged incubation with different concentrations of EB suggesting that EB may increase proliferation even in the absence of growth factors. Data are means ± SEM of three experiments each one performed by duplicate in different cultures. Statistical significances against controls were performed by One Way ANOVA followed by Tukey post hoc test, when analysis of variance was significant. (*) P<0.05, (**) P<0.01 and (***) P<0,001.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.