Abstract
BREVIPEDICELLUS (BP) encodes a class-I KNOTTED1-like homeobox (KNOX) transcription factor that plays a critical role in conditioning a replication competent state in the apical meristem, and it also governs growth and cellular differentiation in internodes and pedicels. To search for factors that modify BP signaling, we conducted a suppressor screen on bp er (erecta) plants and identified a mutant that ameliorates many of the pleiotropic defects of the parent line. Map based cloning and complementation studies revealed that the defect lies in the FILAMENTOUS FLOWER (FIL) gene, a member of the YABBY family of transcriptional regulators that contribute to meristem organization and function, phyllotaxy, leaf and floral organ growth and polarity, and are also known to repress KNOX gene expression. Genetic and cytological analyses of the fil-10 suppressor line indicate that the role of FIL in promoting growth is independent of its previously characterized influences on meristem identity and lateral organ polarity, and likely occurs non-cell-autonomously from superior floral organs. Transcription profiling of inflorescences revealed that FIL downregulates numerous transcription factors which in turn may subordinately regulate inflorescence architecture. In addition, FIL, directly or indirectly, activates over a dozen genes involved in glucosinolate production in part by activating MYB28, a known activator of many aliphatic glucosinolate biosynthesis genes. In the bp er fil-10 suppressor mutant background, enhanced expression of CYP71A13, AMIDASE1 (AMI) and NITRILASE genes suggest that auxin levels can be modulated by shunting glucosinolate metabolites into the IAA biosynthetic pathway, and increased IAA levels in the bp er fil-10 suppressor accompany enhanced internode and pedicel elongation. We propose that FIL acts to oppose KNOX1 gene function through a complex regulatory network that involves changes in secondary metabolites and auxin.
Introduction
Growth and development of terrestrial plants is guided by events occurring at meristems, zones where pluripotent stem cells perpetuate themselves and generate raw material for organ production. For aerial development, the shoot apical meristem (SAM) elaborates leaf, stem and flower anlagen at specific regions depending on complex temporal and spatial interactions between proteins, microRNAs and hormones [1,2]. The SAM shares common mechanisms of regulation with floral meristems, which form during the reproductive phase to generate sepals, petals, stamens and carpels, with an important difference being that floral meristems are determinate.
Genes affecting SAM and floral meristem patterning, maintenance, and function have been identified by both forward and reverse genetic screens. One family of genes that plays a prominent role in promoting meristem function throughout the plant life cycle is the class I KNOTTED-like homeobox (KNOX1) genes, which were named for the founding member, KNOTTED1 (KN1) from maize (reviewed in [3]). Leaf blades of the kn1 dominant mutant display knots of undifferentiated cells around lateral veins due to ectopic expression of the KN1 gene product [4,5]. In numerous monocot and dicot species, the expression of a variety of KNOX1 proteins in leaves conditions the production of ectopic meristems, implicating the factors as critical regulators of meristem function in a diverse array of plants [6–8].
In addition to their role in meristems, KNOX1 genes promote growth in aerial organs such as leaves, flowers and stems. For example, compound leaves of tomato are observed to branch and form supercompound leaves if either the LeT6 KNOX gene or the maize KN1 gene is ectopically expressed [9]. In tobacco, maize and Arabidopsis, ectopic expression of KNOX1 genes also results in alterations in leaf architecture [6, 8–13]. In rice and Arabidopsis, KNOX1 genes are known to promote both longitudinal and radial growth of stems [14–16].
A large number of factors interact with KNOX1 genes to influence meristem and organ growth and morphology (reviewed in [17]). KNOX1 proteins promote cytokinin biosynthesis to sponsor meristematic activity and cell division [18–20] and conversely, repress gibberellin function in meristems to support meristem maintenance [12, 21–22]. In many cases, KNOX1 genes are expressed in meristems but are downregulated as lateral organs are initiated, but they can be reactivated in compound leaf species [23]. Families of genes that encode the adaxializing factors ASYMMETRIC LEAVES1 (AS1) and ASYMMETRIC LEAVES2 (AS2) in Arabidopsis [24–26], PHANTASTICA in Antirhinnum and other species [27–28], and ROUGHSHEATH2 in maize [29–30] repress KNOX genes as well as genes encoding some of the abaxializing factors of the YABBY and KANADI families in leaf primordia (reviewed in [31]). In addition, some YABBY proteins play roles in negatively regulating KNOX genes in lateral organs [32]. Collectively, these antagonistic interactions assist in establishing distinct domains of gene expression that promote proper lateral organ polarity. In contrast to these well-established examples of hierarchical controls that pattern leaves, little is known of the factors that act coordinately with KNOX1 genes in stems to control morphogenesis.
We have previously characterized the expression and function of the Arabidopsis KNOX1 gene BREVIPEDICELLUS (BP), which is required to promote elongation and radial expansion of inflorescence stems and pedicels, short stems that orient flowers and siliques at an upright angle along inflorescences [15]. BP acts in a partially redundant manner with the ERECTA (ER) receptor protein kinase, as double mutant bp er pedicels develop downward bends that are due to growth suppression on the abaxial side. Pedicel abnormalities of the bp er mutant are spatially linked to the patterning of underlying vascular bundles that are continuous with associated floral organs, and nodal identity is translated downwards into subtending internodes [33]. This stimulated the hypothesis that BP and ER promote growth along pedicels and internodes at least in part by counteracting growth-repressive signals that originate from superior organs and are borne by the vasculature. To explore this further, we conducted a suppressor screen of bp er, and identified a point mutation in the FILAMENTOUS FLOWER (FIL) gene that suppresses many of the bp er pleiotropic phenotypes. FIL is a member of the YABBY family of transcriptional regulators, which play roles in leaf and floral organ polarity, organ growth, phyllotaxy and shoot apical meristem organization and function [34–43]. Our analyses indicate that the effect of the fil-10 suppressor mutation on pedicel development is also due to mobile signaling from the flower, and is not linked to the role of FIL in promoting abaxial organ fate. Subsequent microarray analyses revealed that numerous genes encoding glucosinolate (GSL) biosynthetic enzymes are repressed in the fil-10 suppressor, and the levels of many glucosinolate metabolites are significantly reduced. These changes in GSL levels are correlated with elevated auxin levels that likely influence inflorescence architecture.
Materials and methods
Biological materials
Unless otherwise stated the parent background was bp-2 er, for which extensive phenotypic and molecular analyses have been conducted [15, 33]. The fil-2, fil-3, fil-4 and fil-5 alleles were obtained from Dr. Gary Drews. The mutant alleles for ap1-1 (CS28), as2-101 (CS16274), and lug-1 (CS3081) were obtained from the Arabidopsis Biological Resource Center. Wildtype Landsberg Lan (La-1) was obtained from Dr. Detlef Weigel. Seeds of kan1-2, kan2-1, las-11 and yab3-2 mutants were obtained from Dr. John Bowman. Double and higher order mutants were constructed by crosses and validated by either visual phenotypes conferred by the mutant, and/or molecular genotyping (CAPS analysis where possible; direct sequencing for others as is described in S1 Table).
EMS mutagenesis and plant growth conditions
Approximately 10,000 bp-2 er seeds were placed in 50ml of 0.2% EMS (Aldrich) for 16h. Seeds were washed extensively with water and planted in 20cm plastic pots in Premier Promix PGX at a density of approximately 200 seeds per pot. M1 plants were grown under natural lighting conditions in a greenhouse. M2 seeds were regrown in Conviron growth chambers at 22°C under fluorescent lighting (125μE/m2) with a 16hr day:8hr night photoperiod.
Microscopy and morphometric analyses
Light, SEM and fluorescence microscopy were carried out as previously described [33]. Morphometric measurements were conducted with mature plants and as previously described [33]. For pedicel measurements, samples were taken from the lower nodes of the plant to ensure the acropetal gradient of development was not a complicating issue. In situ hybridizations were performed as described in Lincoln et al. [44]. For confocal microscopy of FIL::GFP plants, buds of 0.3 to 0.5mm were dissected and embedded in 4% agarose. The blocks were affixed to a sectioning plate with superglue and 80-120um sections cut using a Leica VT1000S vibratome. Sections were mounted in cold water and imaged using a Zeiss 510 Meta laser scanning confocal microscope with an excitation wavelength of 488nm and a pinhole adjustment of 1.74–1.81 Airy units. An emission bandpass filter of 510-530nm was used to collect GFP fluorescence. Images were edited using LSM image Browser software, version 3 (Zeiss).
Mapping of the fil-10 suppressor mutant
bp er fil-10, backcrossed twice to bp er, was crossed with Columbia harboring a mutation in the ER gene (Cer) to establish a mapping population. DNA from F2 plants displaying a bp er fil-10 or er fil-10 phenotype was used for simple sequence length polymorphisms (SSLPs) or dCAPS analyses [45]. For additional experiments involving microarray analysis and glucosinolate/auxin profiling, two additional backcrosses to the bp er parent line were performed.
Identification of the lesion in fil-10
The FIL gene was amplified by PCR from bp er fil-10 and bp er genomic DNA using FIL FOR: 5’ AAAAGATGTCTATGTCGTCTATGTCCTCC 3’ and FIL BACK: 5’ GAATCGGTTATATGCGGATGGGACTC 3’ primers. PCR products were gel purified (Qiagen) and both strands were sequenced using the FIL F/B primers (S1 Table). To validate sequencing results, the procedure was repeated on a second series of plants, and gave identical results.
Transgenic construction and analysis
To examine FIL protein localization, a GFP tagged version was generated by amplifying the FIL promoter (2.7kb) and coding region with the primers: FIL/GFP FOR 5’TCGGAGCTCGATTCTTCATATGTTAAGTTATGCTGA 3’ and FIL/GFP BACK 5’TAACCGGTGCAGGAGCGTAGAACCCTTCTTTCATCACC 3’ using Phusion (New England Biolabs) polymerase. These primers engineer 5’ Sac I and 3’ Age I sites to facilitate cloning into pEGAD. Sequencing confirmed an in-frame fusion of FIL with GFP, where the last eight amino acids of FIL are missing. The construct was mobilized into Agrobacterium strain GV3101, and used to transform bp fil-10 er plants via the floral dip procedure [46]. Transgenics were selected on 0.5X MS media containing 10μg/ml BASTA (Crescent Chemicals).
Microarray analyses and QRT-PCR
Inflorescences from five-week old plants were used as a source of total RNA for both microarray analyses and QRT-PCR. Older flowers were culled from the periphery of the inflorescence such that no buds of later than stage 13 (bud opening defined by Smyth et al. [47] were used. For microarray analysis, total RNA was prepared from inflorescences of bp er and bp er fil-10 plants in triplicate, using the Qiagen RNeasy system. RNA was reverse transcribed into cDNA pools using oligo dT, and the cDNA was amplified by in vitro transcription with biotinylated CTP to generate probes. Affymetrix ATH1 arrays were employed, and hybridization and washing conditions were carried out as described by the manufacturer. Detection/quantitation was facilitated by using an Affymetrix GeneChip scanner 3000. Raw data was subjected to GCOS/MAS normalization and a linear scaling factor was applied to set the TGT value to 500. The list was culled by discarding genes for which values were low and hence were called ‘absent’. Lists of UP/DOWN regulated genes were then obtained by sorting the Excel spreadsheet. Individual values from the triplicate samples were then examined and genes were removed from the list if the average value was skewed by an anomalous signal. Cutoff values were arbitrarily set at 2.5 fold and 1.9 fold to generate short and extended lists of genes influenced by FIL. Raw data and additional information can be accessed through the GEO accession number GSE86643. Analyses are presented in S2 and S3 Tables.
For QRT-PCR, total RNA was prepared as described above, and on-column DNAse digestion was undertaken, using RNAse free DNAse I (Invitrogen). cDNA pools were generated by reverse transcription of 1ug of total RNA, employing oligo dT as a primer and Superscript III reverse transcriptase (Invitrogen). An MJ Research instrument was used to amplify cDNAs to validate the microarray results and to test other putative target genes, using Sensifast SYBR mix (Bioline). Primers were designed by employing the open source Primer3 software. Primer efficiency tests were performed on dilutions of cDNA, and melting curves and gel analysis used to confirm primer specificity. Several potential reference genes were tested with both bp er and bp er fil cDNAs to determine the most reliable set. PP2a (At4g15415) and ACT7 (At5g09810) exhibited minimal variation and their primer efficiencies (E) and ΔCT values were averaged for normalization of target gene data. The relative expression ratio was calculated as described by Pfaffl [48], and pairwise type three Student’s t-tests conducted by transforming ΔCT values to linear terms by the equation (1+E)− ΔCT as described by Livak and Schmittgen [49]. Two independent biological experiments that employed three to four technical replicates were carried out for each primer set. The independent experiment is summarized in S1 Fig. A list of primers is provided in S1 Table.
Glucosinolate and auxin profiling
Inflorescences were dissected from five week old plants, their fresh weights recorded, and then placed in either 100% methanol (for glucosinolate profiling), or a solution of 80% methanol, 1% acetic acid (for IAA determination). Glucosinolate metabolites were identified and quantitated by HPLC as described by Kliebenstein et al. [50], and IAA levels were determined as described by Stokes et al. [51]. For IAA measurements, two independent experiments were carried out and revealed similar trends, and three experiments were conducted to profile glucosinolate metabolites, which also showed similar trends.
DR5::GUS analysis in bp er and bp er fil-10 genetic backgrounds
The DR5::GUS cassette was resected from a pBIN19 derivative with Sal I and EcoRI, and recloned into the Xho I/ EcoRI sites of pEGAD-link in order to use BASTA as a selectable marker. Following validation of primary transformants, T2 seeds were surface sterilized and germinated on media containing 0.5XMS salts, 5mM MES pH 5.7, 1% sucrose, and 10μg/ml BASTA. Ten day old seedlings were fixed in 90% acetone for 30 minutes on ice, followed by one wash each in cold water and x-gluc buffer (50mM phosphate buffer, pH7.2, 0.2% Triton X-100, 2mM potassium ferrocyanide, 2mM potassium ferricyanide). X-gluc buffer containing 1mM x-gluc (BioShop Canada) was added and seedlings were incubated in the dark at room temperature for 8 hours, then fixed/decolorized with an ethanol series. The alcohol was exchanged for 8:2:1 chloral hydrate:glycerol:water and following overnight incubation at 4°C, slides of individual seedlings were prepared, coverslipped, and photographed using a Nikon SMZ1500 stereomicroscope with a digital imaging system (Nikon Digital Sight D5 Fi1). To investigate DR5 copy number in the transgenic lines, multiplex PCR was employed using the primers EGADjunctionFOR/GUS genotype back to screen for DR5::GUS insertions, and AMIgenotypeFOR/AMIgenotypeBACK as a single copy gene control. Primer sequences and PCR conditions are given in S1 Table.
Results
Identification of fil-10 as a suppressor of bp er phenotypes
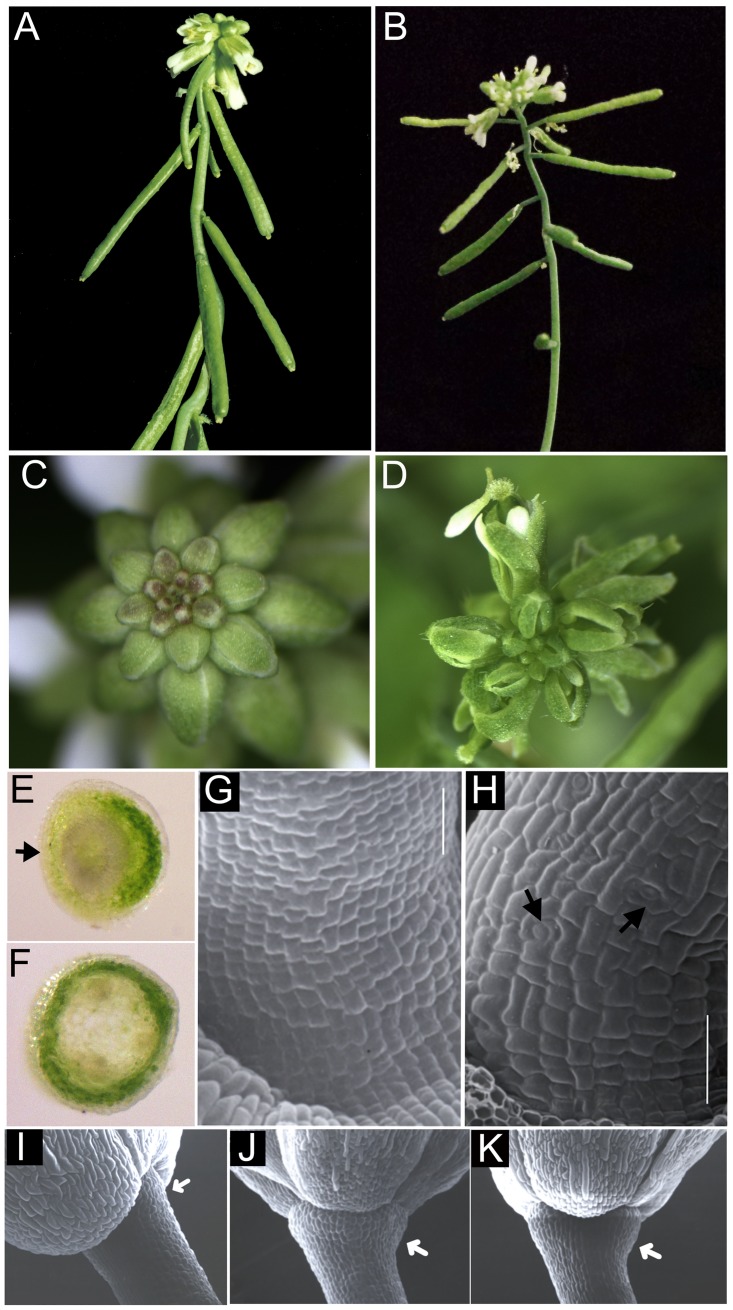
Wild-type Arabidopsis pedicels elongate as straight stems to support flowers and siliques at an upright angle along inflorescence axes. In bp er mutants, pedicel elongation is compromised and pedicels acquire bends that orient flowers at a downward angle (Fig 1A). To identify other genes controlling pedicel development, bp er seeds were mutagenized with EMS, and an M2 plant with elongated, perpendicular pedicels was identified (Fig 1B) and backcrossed to bp er. F2 plants segregated the suppressed phenotype in a 3:1 ratio, demonstrating that the novel phenotype is due to a recessive mutation at a single locus. Examination of unopened flowers with a dissecting microscope revealed narrow sepals that failed to fully conceal developing inner reproductive organs (Fig 1C and 1D). Further genetic and molecular characterization (see below) demonstrated allelism between the suppressor mutant and the FILAMENTOUS FLOWER (FIL) gene, and hereafter we refer to the mutant as fil-10.
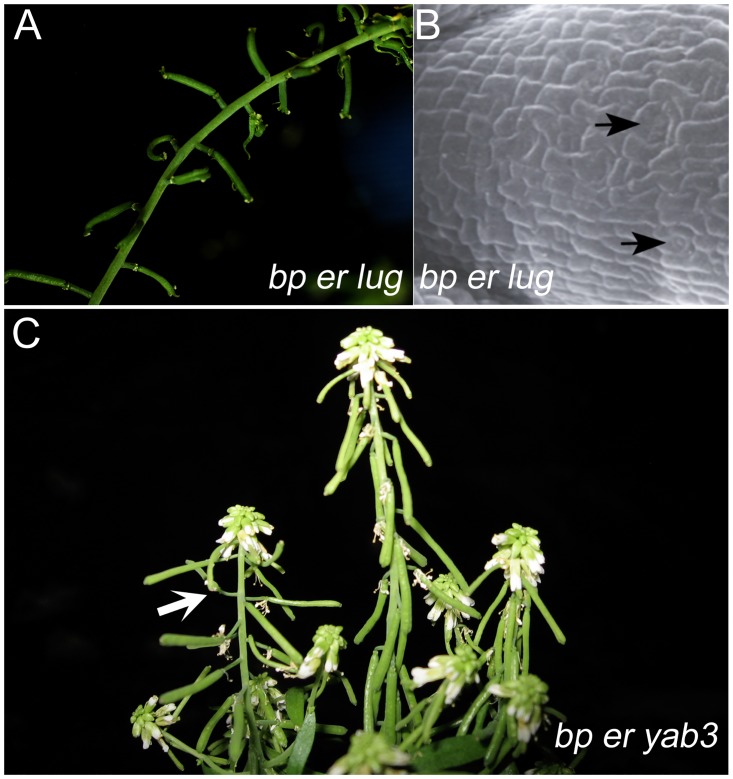
Fig 1. Suppression of bp er pedicel phenotypes by the fil-10 mutation.
A bp er plant showing short pedicels that bend downwards. (B) bp er fil-10 plant exhibiting enhanced internode growth and elongated pedicels perpendicular to the stem axis. The acute pedicel angle defect is partially ameliorated. (C) bp er inflorescence cluster with closed floral buds. (D) Young bp er fil-10 flowers with visible inner whorl organs due to aberrant sepal development. (E) Hand section of a bp er pedicel. Note the lack of chlorenchyma development on the abaxial side (arrow). (F) Hand section of a bp er fil-10 pedicel, revealing a continuous ring of chlorenchyma tissue. (G) The bp er pedicels display files of short cells on their abaxial sides and differentiation of guard cells is repressed. (H) In bp er fil-10, the pedicel stripe is confined to a narrow band of stomata free tissue on lateral sides, but abaxial cells are larger and assume the irregular shapes found in wild type. Differentiation of stomata is also observed (arrows) (I-K) Receptacles of bp er fil-10 (I), fil-10 er (J) and Ler (K). Note expansion in fil-10 er and Ler but lack of enlargement in bp er fil-10 (arrow). Bars in panels G and H are 50 μM.
Light microscopy of hand sections of pedicels showed that, in contrast to the disruptions of chlorenchyma tissue associated with the abaxial side of bp er pedicels (Fig 1E; [33]), bp er fil-10 pedicels displayed a continuous ring of chlorenchyma (Fig 1F). Similarly, while the epidermis of bp er pedicels exhibits files of short cells that lack stomata on abaxial and lateral sides (Fig 1G), this feature is strongly suppressed in bp er fil-10, which exhibits a relatively indistinct stripe of undifferentiated cells along the lateral sides, and a more wild-type array of irregularly shaped cells on other sides. In contrast to the bp er line, the pedicels of the suppressor line also differentiate guard cells on all sides (Fig 1H). Our previous work demonstrated that BP plays a role in receptacle enlargement as gauged by a constriction of tissue at the distal end of the pedicel in bp mutants [33]. However, unlike the suppression of other defects, the bp er fil-10 receptacles did not enlarge as they did the fil-10 er or Ler plants (Fig 1I–1K). Receptacle growth is enhanced by overexpression of BP [33] and our results indicate that the mechanism controlling pedicel morphogenesis is genetically separable from that regulating receptacle growth. While FIL contributes to growth and patterning of stems, pedicels and floral organs, it apparently does not play a role in receptacle enlargement.
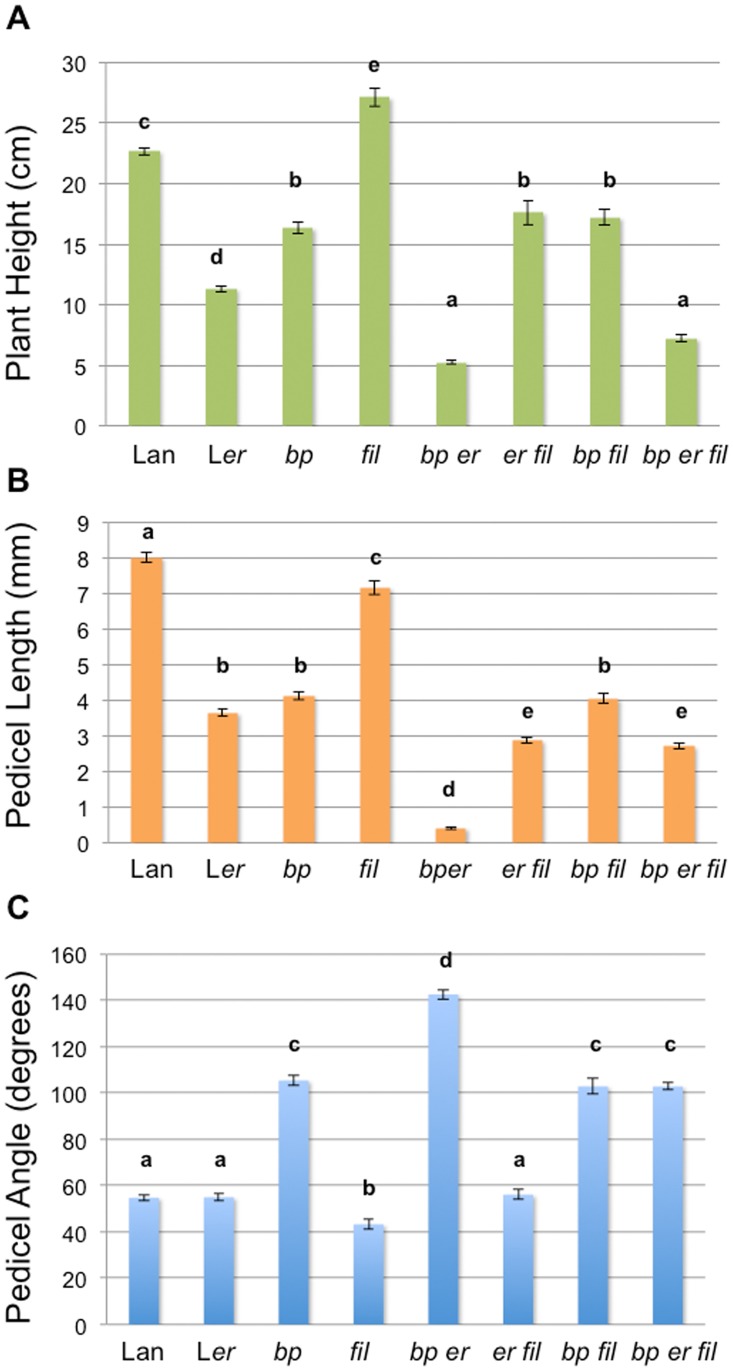
Developmental analyses of bp er fil-10 plants showed that bp er pedicel phenotypes are increasingly suppressed as development progresses (2.5mm ± 0.1mm pedicel length (pl); 108° ± 2° pedicel angle (pa) for flowers 1–5 and 2.9mm ± 0.1mm pl; 98° ± 2° pa for flowers 6–10). To examine interactions between fil-10, bp and er, height, pedicel length and pedicel projection angle comparisons were made between all possible genotypes. Relative to the baseline genotype Landsberg, mutations in both BP and ER result in compromised internode elongation, while fil-10 enhances growth (Fig 2A). These relationships are supported by the double mutant phenotypes in which either bp or er in combination with fil-10 conditions less robust growth than fil-10 alone. The effect on plant height is less pronounced when bp er is compared with the triple bp er fil mutant. Pedicel growth is also affected by the three genes in a manner similar to internode elongation (Fig 2B). The bp mutation significantly alters the pedicel angle and the angle becomes more pronounced by combining bp with er. The fil-10 mutation suppresses this effect, giving rise to perpendicular pedicels in the triple mutant (Fig 2C). In summary, the fil-10 suppressor partially ameliorates the bp er defects in internode and pedicel elongation, and conditions differential growth and development of pedicels to alter plant architecture.
Fig 2. Morphometric analyses and differential effects of mutations in bp, er, and/or fil.
Crosses were used to generate all combinations of single, double and triple mutants in a Landsberg (Lan) background. (A) Plant height was measured from the rosette to the inflorescence tip in six week old plants. (B-C) Mature, senescing plants were used to measure pedicel length (B) and angle (C). The error bars represent standard error of the mean. Data were compared by one way ANOVA using Tukey’s Honest Significant Differences method. Letters above the bars indicate significance categories where p< 0.01. For all measurements, n = 15–150. Similar trends were observed in two independent experiments.
Characterization of fil-10 floral phenotypes
The fil-10 suppressor line exhibits reduced fecundity, producing short siliques with fewer viable seeds that may be due to reduced levels or viability of pollen. We assessed female viability by crossing Ler pollen into fil-10 er gynoecia. Siliques elongated and set seed, indicating that either an anther or male gametophyte defect underlies reduced fil-10 fertility. To distinguish between sporophytic and gametophytic possibilities, fil-10/+ er pollen was crossed into Ler gynoecia. The F1 plants appeared normal and were fully fertile. Genotyping revealed that 27/62 = 43.5% of plants were heterozygous for fil-10, consistent with the 50% value expected if fil-10 and wild-type pollen grains are equally viable. Thus, reduced seed set is due to a sporophytic defect probably related to low pollen yield.
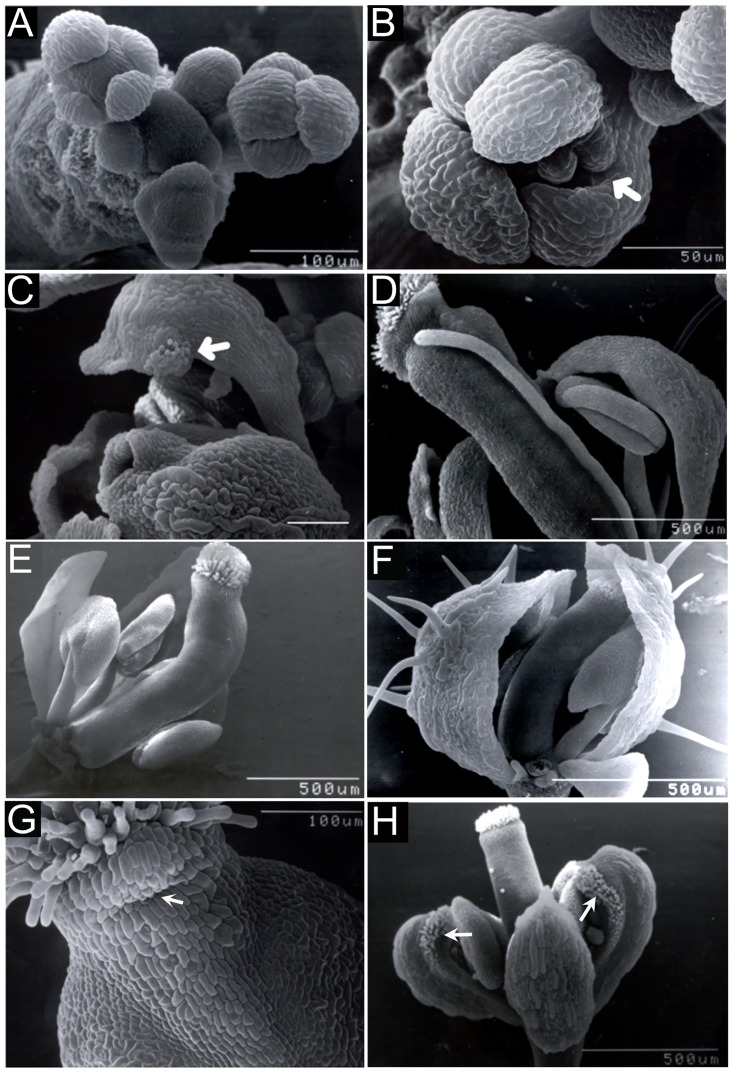
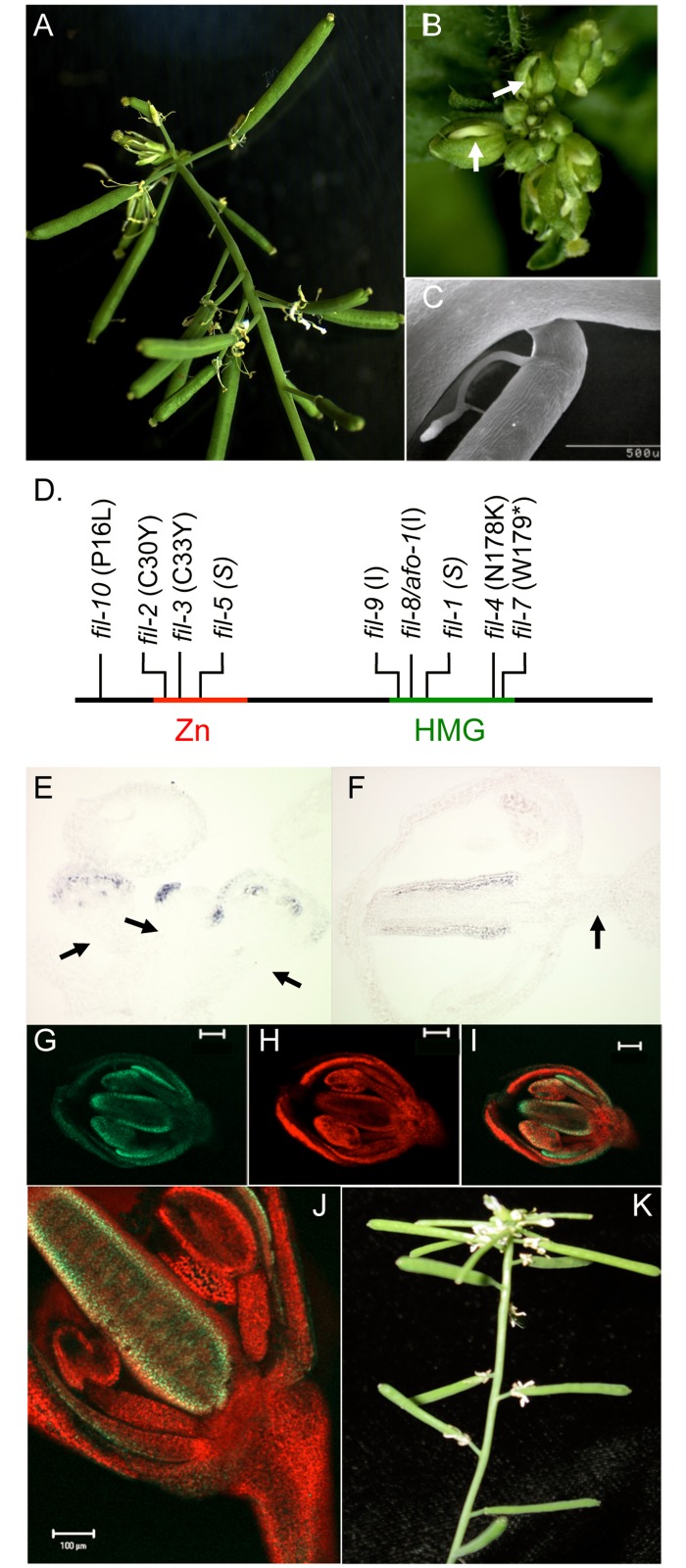
The fil-10 mutation also conditions floral morphological phenotypes when combined with er, but these are typically much less severe than those that have been reported for strong fil alleles [34–36,42]. fil-10 er floral meristems initiate normally and generally produce four symmetrically arranged sepals (Fig 3A) that do not elaborate bract-like organs. In accordance with the phenotype of older flowers, the margins of young sepals are often separated by gaps that expose inner whorl organs (Fig 3B). Partial sepal-to-carpel homeotic transformations occasionally manifest as stigmatic tissue formed at the tips of medial first whorl organs (Fig 3C). In other cases, first or third whorl organs develop as radial filaments (Fig 3D). In the fourth whorl, gynoecia are often crooked or bent (Fig 3E), likely due to contact of the gynoecium tip with the inner face of a sepal (Fig 3F) and protruding stylar tissue is observed on medial sides (Fig 3G). We also examined the effect of a stronger fil allele in the bp er background. While the fil-4 allele suppresses bp er in a similar fashion to fil-10 (Fig 4), bp er fil-4 plants display more severe stem and floral phenotypes that include phyllotaxy defects, the reduced floral cluster bearing type B flowers, and in many instances floral organ identity is severely compromised, manifested as filamentous organs (see S2 Fig). These defects mimic those of strong fil alleles. In summary, broad morphological defects in fil-10 er flowers support others’ findings that FIL plays an important role as a general regulator of floral organogenesis [34–36, 42], but define fil-10 as a weak allele that impinges upon both BP and ER signaling.
Fig 3. fil-10 conditions floral organ abnormalities.
(A-G) fil-10 er flowers. (A) Early inflorescences showing symmetrically located sepal primordia. (B) An early bud with a gap (arrow) between two sepals. (C) A flower formed late in development with stigmatic tissue (arrow) on the tip of a sepal. (D) A flower with a third whorl filament lacking an anther. (E) A gynoecium with a bend. (F) A gynoecium in the midst of bending due to sustained contact with the inner face of a lateral sepal. (G) Medial region of a gynoecium showing a bulge of style tissue (arrow) under the stigma. (H) fil-10 ap1-1 er flower showing transformation of medial sepals (arrows) into carpelloid organs.
Fig 4. Mutations in FIL and LAS affect inflorescence architecture in a similar fashion.
(A-C) bp er fil-4 plants showing elongated pedicels (A), upward-oriented floral buds with gaps between sepals (arrow; B) and bends in pedicels at filamentous organs (C). (D) Locations of characterized mutations in the FIL gene. The nature of each mutation is shown in parentheses: I = insertional mutant, S = splice junction mutant; the asterisk represents a stop codon. (E-F) In situ hybridization with a FIL probe showing expression in sepal primordia (central bud) and in floral organs of older, peripheral buds (E), and gynoecium valve expression in a stage 9 pedicel (F). Note the absence of FIL expression in pedicel tissue (arrows) at stages that precede the period of pedicel elongation [59]. (G-I) A collage of a stage 9 bud from a transgenic plant expressing a FILpro::FIL::GFP transgene. The left panel shows FIL::GFP expression on the abaxial side of floral organs; the middle panel is the chlorophyll autofluorescence (red channel) and the right panel is the merged image. (J) Mature flower illustrating FIL::GFP in floral organs only. (K) The bp er las-11 triple mutant exhibits a phenotype nearly identical to that of bp er fil-10.
fil-10 does not influence floral meristem identity
Previously we demonstrated that reduced floral meristem identity in leafy (lfy) mutants suppresses bp er pedicel phenotypes [33]. Reduced floral fate results in increased numbers of axillary stems and less prominent receptacles. Unlike lfy, our observations indicate that suppression of bp er pedicel phenotypes in fil-10 is not due to changes to floral identity. First, axillary branch number is similar between bp fil-10 er (1.9 ± 0.2) and bp er (2.1 ± 0.1). Second, fil-10 and fil-10 er receptacles enlarge (Fig 1J), but this feature is compromised when lfy is also mutant (in bp er lfy-5 [33]). Third, we crossed bp er fil-10 to ap1-1 er to examine the effect of fil-10 in another known floral identity mutant. Similar to the effect of lfy-5, ap1-1 suppressed the bp er pedicel phenotypes, but we also observed a novel floral phenotype that is not present in ap1-1 or fil-10 plants. In bp fil-10 ap1-1 er and fil-10 ap1-1 er flowers, medial first whorl organs of all flowers displayed carpel-like features that included stigmatic tissue at tips and along margins, style-like tissue adjacent to margins, ovules along margins and an overall hooded morphology (Fig 3H). Importantly, secondary flowers evident in axils of first-whorl organs in ap1-1 were never observed in fil-10 backgrounds, suggesting that fil-10 flowers are fully determinate. Collectively, these results indicate that fil-10 does not compromise floral identity as is the case for stronger fil alleles [34, 36], (and S2 Fig). Thus, FIL may interact with BP and ER to influence floral architecture and pedicel growth downstream of floral meristem fate specification.
fil-10 does not impact pedicel development through its effect on organ polarity
It is well established that FIL contributes to the emergence of organ polarity by specifying abaxial identity of lateral organs [35]. To determine whether a reduction in abaxial organ identity contributes to suppression in bp er fil-10, we crossed bp er with kanadi-1 and kanadi-2, which show abaxial-to-adaxial transformations in leaves and floral organs [38, 52–56]. We saw no evidence of suppression of bp er pedicel phenotypes in bp-4 kan1-2 er, bp-4 kan2-1 er or bp-4 kan1-2 kan2-1/+ er, suggesting that lateral organ polarity per se does not significantly influence pedicel morphology. Because the KAN genes are expressed in stem tissue where they play a role in vascular patterning [55] we also tested the relationship between organ polarity and pedicel development by removing the function of ASYMMETRIC LEAVES2 (AS2) from bp er fil-10 plants. KAN exerts its function in part by repressing AS2 [57], an adaxial regulator that is expressed in leaves and floral organs but not in internodes or pedicels [25, 58]. Because removal of AS2 from an er background increases abaxial fate in lateral organs [58], we reasoned that this could counteract the loss of abaxial identity due to the fil-10 mutation, phenocopying the bp er pedicel phenotypes. However, although quadruple bp er fil-10 as2-101 mutants gave rise to shorter pedicels, removal of AS2 did not affect pedicel angle (Table 1), consistent with the kan data suggesting that organ polarity does not significantly impact pedicel morphology.
Table 1. The influence AS2 on pedicel architecture.
| Genotypea | Pedicel Length (mm) | Pedicel Angle (degrees)b |
|---|---|---|
| bp er fil-10 | 2.75 ± 0.05 | 93.1 ± 0.9 |
| bp er fil-10 as2-101 | 1.75 ± 0.09 | 95.9 ± 1.3 |
aFor bp er fil-10, n = 189. For bp er fil-10 as2-101, n = 55.
bAngle between the inflorescence axis and the adaxial face of the pedicel.
Pairwise T-tests revealed that the change in pedicel length is statistically significant (p<0.005), while the change in pedicel angle is not (p = 0.34).
Identification and molecular characterization of fil-10
The original bp er suppressor mutation (termed sup2) was mapped to a 660kbp region on chromosome 2 between the T8M12 and GBF3 markers. Scanning annotation units in this chromosomal region showed that the YABBY gene FILAMENTOUS FLOWER (FIL) is located approximately halfway between the two markers. Similarities between fil and sup2 phenotypes, including compromised fecundity, filamentous organs, and style defects prompted us to test whether other fil alleles could suppress bp er. Crossing the intermediate fil-4 allele into bp er produced plants with elongated pedicels, although pedicels often bend down at filamentous structures formed on abaxial sides (Fig 4A–4C). We next crossed bp er fil-4 with bp er sup2 in a complementation test. Progeny plants exhibited a suppressed bp er phenotype, indicating that the lines contain mutations in the same gene. To confirm that FIL is mutated in sup2, FIL cDNA and genomic fragments isolated from bp er sup-2 plants were cloned and sequenced, revealing a P16L mutation located upstream from the Zn finger domain (Fig 4D). Taken together, these experiments indicate that the sup2 phenotype is due to a mutation in the FIL gene and we propose fil-10 as the allele designator.
FIL is expressed in leaves and floral organs and acts to specify abaxial organ fates and promote blade outgrown, in part by repressing KNOX1 genes [32]. In addition, the finding that fil mutations suppress the bp er phenotype suggested that in this background, FIL might be ectopically expressed in pedicels to modulate their development. However, in situ hybridization with a FIL probe failed to detect FIL transcripts in bp er pedicel or internode tissue at all floral stages tested (Fig 4E and 4F), suggesting that FIL may function non-cell-autonomously from flowers to impact pedicel development. To more specifically test this hypothesis at the protein level, we constructed a FILpro::FIL::GFP transgene and generated transgenic lines in both wildtype and bp er plants. Examination of young buds revealed the characteristic abaxial domain expression of FIL, but in no case, at any stage of floral development, did we observe GFP fluorescence in developing pedicels (Fig 4G–4J). Moreover, pedicel angle defects begin to be manifest after about stage 11 of floral development [33], and the bulk of pedicel elongation also takes place after stage 11 [59], suggesting that pedicel development is spatially (and temporally) separated from FIL expression domains in floral organs. Finally, the introgression of the lateral suppressor (las-11) mutant into bp er confers a phenotype that is nearly identical to that of bp er fil-10 (Fig 4K). Recognizing that LAS regulates axillary meristem activity [60], and has been implicated in transducing the FIL non-cell-autonomous signal from peripheral domains of the meristem to the CZ [39], we reason that FIL’s effect on stem and pedicel development is likely mediated in a similar fashion. That the origin of the signal is superior to the pedicel is inferred by amelioration of the stripes of undifferentiated abaxial tissue that originate and are broadest at the receptacle in bp er, and trace the path of the vasculature down the inflorescence stem [15, 33], but which are suppressed in bp er fil mutants.
LEUNIG and YAB3 mutations differentially suppress the bp er phenotype
YABBY proteins are known to form complexes with Gro/Tup1 co-repressors such as LEUNIG (LUG) [40]. LUG is ubiquitously expressed and lug mutants show homeotic transformations in the flower [61]. In addition, LUG and its interacting partner protein SEUSS (SEU) act to control organ polarity and other aspects of plant development [62–64]. Upon crossing bp er and lug, we found that bp er lug-1 plants also exhibited suppressed pedicel phenotypes (Table 2) wherein pedicels are elaborated perpendicular to the stem axis and elongate to some extent (Fig 5A). The stomata-free stripe of cells on the abaxial side of bp er pedicels is also ameliorated, giving rise to normal epidermal patterning that includes stomatal development (Fig 5B).
Table 2. Effects of BP and LUG on pedicel morphology.
| Genotype | Pedicel Length (mm)a | Pedicel Angle (degrees)a,b |
|---|---|---|
| Ler | 3.7 ± 0.1 | 55 ± 2 |
| lug-1 er | 2.8 ± 0.2 | 48 ± 7 |
| bp er | 0.41 ± 0.03 | 143 ± 2 |
| bp er lug-1 | 0.99 ± 0.07* | 84 ± 3* |
a n>20. Each value represents the mean ± standard error.
bAngle between the inflorescence axis and the adaxial face of the pedicel.
* Pairwise T-tests reveal significant differences for both pedicel length and angle for bp er vs bp er lug-1 (p<0.05).
Fig 5. Suppressive effects of mutations in leunig and yabby3.
(A) bp er lug plant showing suppressed pedicel angles. (B) bp er lug abaxial pedicel showing enlarged cells and stomata (arrows). (C) bp er yab-3 plant. In rare cases, we observed pedicel suppression effects (arrow) of some axillary branches on plants which otherwise exhibited the bp er-like habit.
Given that some YABBY proteins are expressed in overlapping domains, interact physically with one another, and can rescue mutations in other YAB genes [40, 65, 66], we reasoned that mutations in YAB3, a close FIL relative, also might be able to suppress the bp er phenotype. We generated the bp er yab3 triple mutant but found that yab3 was ineffective in suppressing the bp er phenotype (Fig 5C). In very rare instances, secondary branches displayed some degree of suppression on plants that were otherwise bp er-like. Thus, the fil-10 suppression phenomenon generally cannot be phenocopied by yab3, although the two genes are functionally redundant in other contexts (e.g. vegetative development; [32, 35]).
Transcription profiling reveals possible mechanisms of FIL action
Our previous studies provided evidence for the existence of a vascular-borne signaling molecule whose synthesis, activation, or trafficking influences inflorescence architecture [33]. We therefore undertook transcription profiling experiments with bp er and bp er fil-10 inflorescences as a strategy to identify genes whose regulation is governed by FIL, anticipating that the identities of putative targets might suggest the nature of this signaling pathway. Triplicate samples of inflorescence RNA from the two genotypes were analyzed, and genes that exhibited more than a 2.5 fold change were functionally classified using both MapMan and Gene Ontogeny (GO) algorithms (S2/S3 Tables). The two lists are referred to hereafter as the UP list (genes upregulated in bp er fil-10, implying that FIL directly or indirectly represses these genes in bp er) and the DOWN list (genes downregulated in bp er fil-10, implying that FIL directly or indirectly activates these genes in bp er).
The UP list contains 71 genes. By normalizing these genes to their frequency in the functional classification groups, only the genes involved in RNA metabolism/transcription factor activity are over represented (p-value = 5.673−4). Twelve genes encode validated or putative transcription factors, including four Zn finger proteins, three AP2/EREBP domain factors, two homeobox domain proteins, one B3 domain protein, 1 JUMONJI family member, and one GeBP domain protein. A second category is a group of genes whose products are involved in regulated proteolysis. Lastly, there are 25 genes that encode products of unknown function, but in general there are no obvious patterns that implicate specific signaling pathways or other commonalities that inform how FIL executes its function. Rather, it appears likely that FIL may act in numerous processes by regulating a group of subordinate transcription factors.
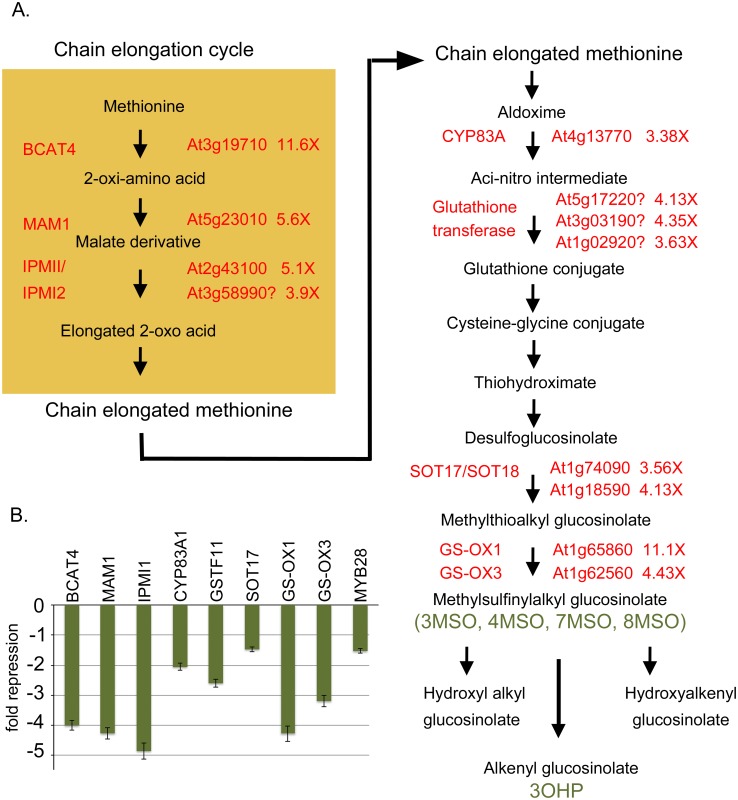
The DOWN list of 63 genes was parsed into several categories that are statistically overrepresented. Trends are observed for members of the miscellaneous and secondary metabolism category, and normalization to the reference set of all genes reveals these classes are overrepresented by 7 and 18 fold (p-values are 4.82 x 10−13 and 3.4 x 10−15, respectively). Ten of the secondary metabolism genes are known to be involved in the synthesis or modification of glucosinolates (GSLs) and an additional four are suspected to play roles in GSL metabolism based on the biochemical steps involved and the predicted enzymatic function (e.g. glutathione transferases). In a similar vein, several of the transcription factors on the UP list belong to families whose members are known to be physically associated with GSL gene promoters to modulate their expression [67]. Fig 6 shows a schematic of the aliphatic glucosinolate biosynthetic pathway, overlaid with genes whose expression is down regulated in bp er fil-10. Glucosinolate biosynthesis is initiated from tryptophan, phenylalanine, methionine or chain-elongated methionine derivatives [68]. The chain elongation cycle involves MAM1, IPMI isozymes, and BCAT4, whose collective function is to extend the amino acid derived substrates. These products feed into the central pathway that utilizes several cytochrome P450 monooxygenases, glutathione addition, and sulfotransferase and oxygenase activities to generate methylsulfinylalkyl glucosinolates. To validate the transcription profiling results we conducted QRT-PCR on several of the targets: MAM1, BCAT4, IPMI1, CYP83A1, SOT17, GS-OX1, GS-OX3 and GSTF11 (Fig 6B). QRT-PCR experiments revealed that all of the target genes tested are indeed downregulated in the fil-10 background, though generally not to the extent reported by the microarray analyses. In addition, the MYB28 gene, a known activator of aliphatic glucosinolate biosynthesis is also downregulated [69, 70]. Control of MYB28 by FIL may explain the wide-ranging changes in GSL gene expression.
Fig 6. Aliphatic glucosinolate biosynthesis genes are down-regulated by fil10.
(A) Schematic representation of the aliphatic glucosinolate biosynthetic pathway showing genes involved in various steps. The numbers beside the AGI identifiers indicate the change in expression of these genes in bp er fil-10 suppressor vs. the parent bp er line as gauged by microarray analysis. Question marks indicate uncertainly about the involvement of these genes in the indicated steps. The green text identifies specific glucosinolate metabolites that are products of the enzymatic steps and for which quantitative analysis was performed (see Table 3). (B) QRT-PCR analyses of selected GSL biosynthetic genes, confirming down regulation of these genes in bp er fil-10 verses the bp er parent. The GSTF11 gene (At3g03190) was selected for analysis as its expression pattern is very similar to that of FIL (eFP browser data) and the gene has been implicated in GSL biosynthesis. The relative expression ratio of the bp er fil-10 mutant is shown and error bars are the standard error of the mean. Pair-wise t-tests on linear transformed ΔCT values revealed that all differences are statistically significant (p<0.034).
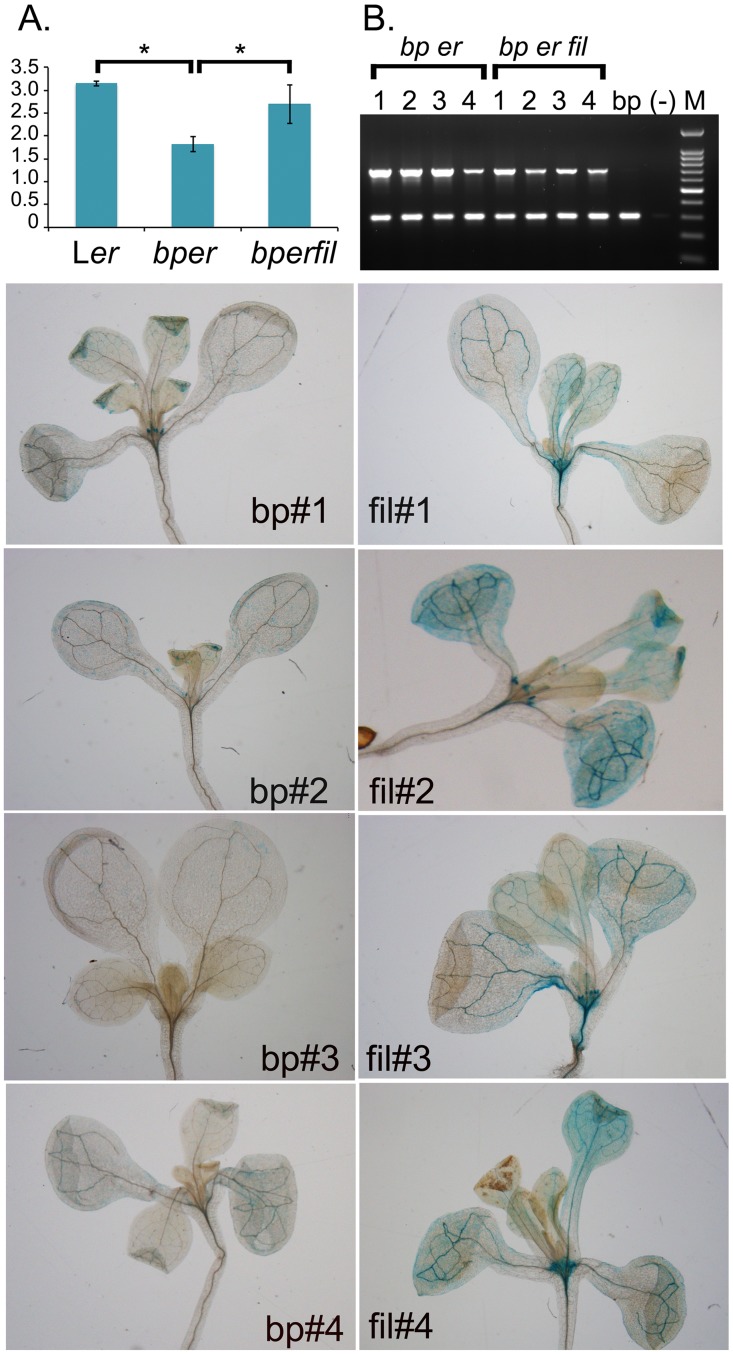
Downregulation of GSL biosynthetic genes led us to hypothesize that there is an altered glucosinolate metabolite pool in bp er fil-10 plants. To assess this, we conducted glucosinolate profiling on the single Ler mutant, the bp er double mutant and the bp er fil-10 suppressor. For many of the metabolites measured, mutations in either bp or fil led to significant changes in GSL metabolite levels (Table 3 and S2 Fig). The levels of several GSLs, including 3OHP, 4OHB, 4MSO, 7MSO, and 8MSO, were altered in the fil mutants in comparison to bp er but these were not consistent between fil-4 and fil-10 suggesting that, unlike the suppression phenotype, the GSL profiles are allele specific. Interestingly, the level of 3-indolyl methylglucosinolate (I3M) is elevated in both suppressor lines relative to the bp er parent, and this phenotype could be linked to the suppression ability of these alleles (S2 Fig). Indolic glucosinolates are derived from tryptophan, which also contributes the indole ring to auxins such as IAA. Given that mutations in several genes encoding enzymes involved in both aliphatic and aromatic GSL synthesis impact auxin metabolism [71–80], we reasoned that auxin levels might be altered in these plants. To investigate this hypothesis, we examined IAA levels in inflorescences from the three genotypes (Fig 7A). Ler inflorescences contain on average about 3ng/g FW of IAA. The dwarf-like double mutant bp er has lower levels of IAA (40% of Ler level), which may contribute to its diminutive stature. The bp er fil-10 suppressor line essentially restores IAA levels to that of Ler, and we postulate that elevated auxin levels are in part responsible for more robust growth of the suppressor line. The enhancement of auxin levels is corroborated by examining independent DR5::GUS transformants of bp er and bp er fil-10. In the bp er background, DR5::GUS signals mimic the wildtype pattern for auxin maxima [81], showing staining foci at leaf tips, hydathodes, young leaf primordial/stipules, root tips, and vascular tissues. In the bp er fil-10 suppressor background, the qualitative GUS staining pattern is mostly unchanged, but intensity is greater in all cases. This is particularly evident at the shoot apex and within the vascular tissues, and in most transformants, numerous cells within the leaf blade also display staining.
Table 3. Glucosinolate metabolites in Ler, bp er and bp er fil-10.
| Genotype/ | 3OHP1 | 4OHB | 3MSO | 4MSO | 7MSO | 8MSO | I3M | 4OHI3M | NMO |
|---|---|---|---|---|---|---|---|---|---|
| Ler | 5.90±0.67 | 0.165 ± 0.02 | 0.069 ± 0.013 | 0.032 ± 0.003 | 0.113 ± 0.016 | 1.24 ± 0.18 | 0.16 ± 0.016 | 0.014 ± 0.004 | 0.022 ± 0.005 |
| bper | 6.0 ± 0.76 | 0.200 ± 0.04 | 0.123 ± 0.026 | 0.030 ± 0.003 | 0.135 ± 0.021 | 1.38 ± 0.29 | 0.08 ± 0.015 | 0.011 ± 0.004 | 0.013 ± 0.005 |
| bperfil | 4.59 ± 0.67 | 0.11 ± 0.03 | 0.179 ± 0.078 | 0.026 ± 0.01 | 0.106 ± 0.019 | 0.81 ± 0.10 | 0.09 ± 0.019 | 0.006 ± 0.004 | 0.012 ± 0.003 |
| T-tests2 | |||||||||
| Ler vs. bper | 0.7948348 | 0.05654988 | 0.000333266 | 0.241202909 | 0.058579013 | 0.2900458 | 4.31E-07 | 0.183791614 | 0.00650812 |
| bper vs bperfil | 0.0294717 | 0.00058607 | 0.100847632 | 0.406288507 | 0.029228242 | 0.0007679 | 0.0906205 | 0.097505342 | 0.787914392 |
1Abbreviations: 3OHP: 3-hydroxypropyl; 4OHB: 4-hydroxybutyl; 3MSO: 3-methysulfinyloctyl; 4MSO: 4-methysulfinyloctyl; 7MSO: 7- methysulfinyloctyl; 8MSO: 8-methysulfinyloctyl; I3M: indol-3-ylmethyl; 4OHI3M: 4-hydroxy-indol-3-ylmethyl; NMO: N-methoxy-indol-3-ylmethylglucosinolate.
2Student’s T-test was carried out for the pairwise comparisons of Ler vs bp er and bp er vs bp er fil. P-values are shown; confidence intervals of p< 0.05 are highlighted
Fig 7. Auxin levels are altered in bp and fil mutants.
(A) Auxin levels in Ler, bp er and bp er fil-10. Wildtype FIL is required for the bp er phenotype and is associated with lower auxin levels. Pairwise T-tests revealed significant differences between Ler and bp er (p < 0.001), and between bp er and bp er fil-10 (p = 0.01). (B) Multiplex PCR on four independent transformants of both bp er or bp er fil-10 harboring the auxin reporter DR5::GUS. The lower band represents a single copy control gene (AMI) while the upper band assesses the presence/level of the DR5::GUS reporter gene. The bp lane is a non-transformed control, (-) is no DNA template. Lower left panels: X-gluc stained seedlings of four independent bp er transformants. Lower right panels: X-gluc stained seedlings of four independent bp er fil-10 transformants. In all cases, the bp er fil-10 suppressor lines exhibited broader and more intense staining than the bp er lines, despite the fact that the copy number of the auxin reporter gene was similar or even lower in the bp er fil-10 lines (panel B).
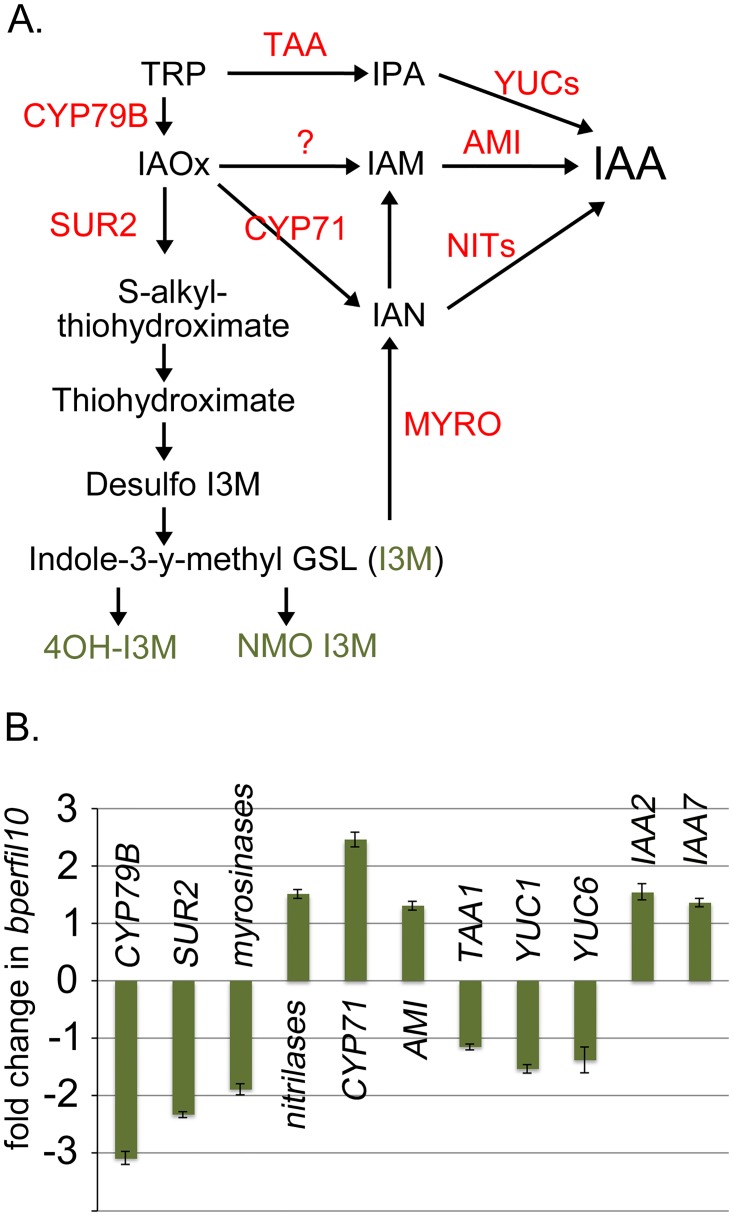
Despite a wealth of data on GSL biosynthetic mutants that influence auxin levels, the mechanistic connection between GSL biosynthesis and IAA production has not been elucidated. However, an aromatic pathway intermediate, IAOx, can be converted to IAA by reactions involving the intermediates IAN or IAM (reviewed in [82–83]), and in addition, IAA can be produced indirectly through GSL degradation by myrosinases (Fig 8A). To investigate these possibilities we conducted QRT-PCR on genes involved in indolic GSL biosynthesis and IAA biosynthesis. In general, the expression of most of these genes was either downregulated or unchanged, but changes in the expression of several genes are intriguing. First, direct IAA production through TAA and the YUCCA enzymes is likely reduced as TAA1, YUC1, and YUC6 were found to be downregulated in bp er fil-10 (Fig 8B). Importantly, the expression of CYP71A13 and an indole-3-actamide hydrolase (AMI1) are upregulated, which may provide a shunt to partition GSL metabolites into auxin biosynthesis. In addition, elevated expression of nitrilases may also convert IAN to IAA, though in an independent experiment, the nitrilases were found to be downregulated (see S1 Fig). As similar trends were observed for the other genes investigated, it is unclear why the nitrilases displayed this variation. QRT-PCR analysis of these genes in the bp er fil-4 background revealed higher levels of myrosinase mRNA, which may contribute to shunting indole-3-glucosinolate into the auxin biosynthesis pathway (S2 Fig). In addition, elevated levels of CYP71A13 mRNA may also contribute to conversion of GSL metabolites to IAA. We infer that in inflorescences, both the fil-4 and fil-10 suppressors orchestrate changes in the levels and shuttling of GLS metabolites that influence local auxin concentrations, conditioning changes in gene expression that affect shoot architecture. It is also likely that some of the uncharacterized genes, and/or those encoding enzymatic functions implicated in metabolite interconversions (e.g. cytochrome P450s) may provide a heretofore unrecognized means to alter auxin biology.
Fig 8. Changes in the expression of indolic glucosinolate and auxin biosynthesis genes in bp er fil-10.
(A) Inferred and speculative intersections of auxin and glucosinolate biosynthetic pathways. Some pathway steps are embellished with gene designations where empirical data implicate specific associations (red text). The green text identifies specific glucosinolate metabolites that are products of the enzymatic steps and for which quantitative analysis was performed (see Table 3). (B) QRT-PCR data on selected genes implicated in indolic glucosinolate and auxin metabolism. The relative level of transcripts in bp er fil-10 vs bp er is shown. Error bars represent standard error of the mean. Pairwise t-tests on linear transformed ΔCt revealed all differences to be statistically significant (p<0.02) except YUC1 (p = 0.069) and YUC6 (p = 0.55).
Discussion
Distinct plant species elaborate organs in genetically defined patterns, giving rise to the species’ characteristic inflorescence architecture and general plant habit. Mutant screens have identified a large number of genes that influence aspects of meristem specification and maintenance, boundary formation, phyllotaxy, organ identity, and hormone synthesis, transport and perception (reviewed in [84]). Class 1 KNOX genes play integral roles in many of these processes, and their expression is subject to activation or repression, spatially and/or temporally, by several well-characterized factors and auxin [3]. In general, the KNOX1 proteins condition a replication competent state and prevent differentiation in the meristem, and their expression is downregulated as cells are recruited into lateral organ primorida, yet other studies have revealed that reactivation of KNOX genes occurs in leaves of compound leaf species [23]. One class of negative regulators is the YABBY family of transcriptional repressors, which play roles in SAM activity, floral development, leaf lamina growth control, promotion of abaxial cell fate, and inflorescence phyllotaxy [34–36, 42].
Our studies reveal that mutant alleles of the YABBY family member FILAMENTOUS FLOWER suppress many of the developmental aberrations conditioned by the class 1 KNOX gene brevipedicellus. Previous studies have implicated FIL in downregulating KNOX genes in lateral organ primordia, and in higher order fil/yab mutants, ectopic meristem formation in leaves may be due to KNOX gene derepression [32]. It is thus possible that fil-10 mediated suppression of bp er phenotypes might be related to changes in the expression of other KNOX genes. In this regard, while our microarray data indicated that KNAT2 is upregulated by nearly 3 fold, QRT-PCR experiments revealed that the magnitude of this change is only 1.4 fold (S3 Fig). Moreover, the expression of KNAT6 and STM, known modulators of meristematic activity, are unchanged [85–88]. It therefore seems unlikely that KNOX gene reactivation plays a prominent role in rescuing the bp er phenotype. In all likelihood, the large number of genes that are affected by the fil-10 mutation, which includes more than twelve transcription factors, specify a complex network affecting numerous cellular processes that will be difficult to dissect. Two of these genes encode proteins with sequence similarity to the PLETHORA family that regulates inflorescence phyllotaxy by modulating local PIN1 activity [89], and our analyses of auxin in the bp er and the fil-10 suppressor lines, together with the phenotypic alterations they display, are consistent with localized changes to growth regulating molecules.
FIL acts non-cell autonomously to modulate development
FIL contributes to several aspects of inflorescence architecture. In vegetative development, FIL is expressed in young leaf primordia, along the abaxial sides of leaves, and in the peripheral zone of the SAM [34–36]. During early floral development, FIL expression is confined to cryptic bracts/sepals and later is found on abaxial sides of floral organs [35, 39]. Finally, during fruit development FIL is expressed in valve and presumptive valve margin cells where it contributes to the activation of genes required for valve margin development [35, 90]. In both developing leaves and fruit, FIL influences tissue identity in part by repressing KNOX genes, but apparently does so in a non-cell autonomous fashion. In leaf primordia, interruption of peripheral YAB1 (FIL or FIL/YAB3) expression alters meristem central zone activity to produce phyllotaxy defects, and in situ hybridization and reporter gene activities indicate that FIL is not expressed in the affected domains [39]. A suppressor screen identified LATERAL SUPPRESSOR (LAS) as a transducer of this mobile signal. Our introgression of the las-11 mutation into the bp er background resulted in architectural changes to plants that generally mimic the bp er fil phenotypes. Together with the in situ hybridization and FILpro::FIL::GFP reporter expression patterns (Fig 4), this observation indicates that the non-cell autonomous signalling that operates between PZ/CZ in leaf development is also employed to regulate pedicel and internode elongation and patterning. Finally, this regulatory module likely is key to repressing BP in the replum during fruit development. In fil and fil/yab3 mutant backgrounds, BP expression is enhanced in replum tissues, which are larger and differentiate stomata [91], a phenotype that is similar to stripe suppression and stomatal differentiation in bp er fil-10 pedicels (Fig 1). In fruits, the non overlapping expression patterns of medial (BP) and lateral (FIL) factors support the contention that FIL signals non autonomously from the adjacent lateral tissue to the medial (replum) tissue to influence replum morphogenesis [91]. Whether LAS is involved in this context is unknown, but it is clear that FIL employs one or more mobile signals to dictate multiple aspects of plant development in Arabidopsis.
Changes in auxin and glucosinolate profiles modulates meristem activity
BP expression is linked to auxin metabolism, as exemplified by its ectopic expression in leaves of axr1 and pin1 mutants, and in leaves of plants treated with auxin transport inhibitors [92]. Such studies implicate auxin as a negative regulator of some KNOX1 genes, possibly acting through ARF6/ARF8 [93]. Conversely, chromatin immunoprecipitation of maize KNOTTED1 target loci, coupled with RNAseq, revealed that genes involved in auxin biosynthesis, transport and signaling are upregulated in dominant Kn1-N mutants [94]. Although we have not performed similar studies on bp mutant plants, we found a reciprocal relationship in which loss of KNOX1 (bp) function is correlated with reduced IAA levels in inflorescences (Figs 7 and 8). This in turn is associated with reductions in internode and pedicel elongation, and other developmental/tissue identity phenotypes. These data are consistent with the existence of a negative regulatory loop by which KNOX1 genes could attenuate their own expression by enhancing auxin biosynthesis, transport and/or signaling.
Auxin is implicated in many facets of plant development and in responses to external stimuli. We propose that changes in auxin levels underpin the growth habit differences between bp er and the bp er fil suppressor lines. There are numerous literature reports that support this contention. For example, in arf6/arf8 auxin response mutants of both Arabidopsis and tomato, internode and/or floral organ elongation is compromised [93,95]. Second, in crm/big/tir3 mutants that exhibit shortened internodes and pedicels, the basis of this defect is linked to aberrant polar auxin transport [96–99]. Indeed DR5 reporter signals in crm1-1 and big-j588 mutants is very much attenuated relative to wildtype [98, 100], suggesting lower auxin levels in this background, and pCYCB1;1::CYCB1;1-GUS signals were also reduced [99], implying that one role of CRM/BIG/TIR3 is to promote cell division. These authors also conducted morphometric analyses of well characterized auxin signaling mutants, axr1-12, arf1-3 arf2-6, and nph4-1 arf19-1, and showed that in all cases, shorter pedicels and internodes are due to defects in both cell size and cell number [99]. We previously reported that bp conditions similar cellular and tissue defects versus the Ler parent line [15], and herein we demonstrate that auxin levels in seedlings and/or inflorescences are significantly lower in bp er than in either Ler or bp er fil-10. Taken together, the data support the hypothesis that lower auxin levels are related to the stunted growth of bp er plants and that the molecular mechanisms that restore auxin levels serve to promote more robust growth in bp er fil-10 plants.
A remaining question is how might fil-10 influence auxin levels? The microarray data revealed no substantial changes in known auxin biosynthetic genes and QRT-PCR experiments indicate that the auxin-related genes tested (TAA, YUC1, YUC6, which in wildtype are most highly expressed at the shoot apex and/or in young floral buds [101]) are significantly downregulated. Although other pathways exist to synthesize IAA [82,83] the microarray data implicated downregulation of MYB28 and altered regulation of a number of glucosinolate metabolism genes as potentially creating a metabolic shunt from GSL pathways into those that produce IAA. MYB28 is part of a group of R2R3 MYB genes that activates aliphatic GSL biosynthetic genes [68–70, 102]. Loss and gain-of-function studies of MYB28 reveal that perturbing GLS can give rise to developmental defects and uncovers a reciprocal relationship between aliphatic glucosinolates and indolic glucosinolates (particularly I3M; [68–70]. Interestingly, mutant analysis of other GSL biosynthetic genes also reveals crosstalk between the aliphatic and indolic pathways [66, 75, 103, 104], but the intersections of these two pathways are not entirely clear. It is possible that the enzymes involved could utilize both aliphatic and indolic substrates, but the enzymology data is sparse (e.g. there is a 50 fold higher affinity for IAoX by CYP83B, vs CYP83A, [72]). Thus, while pathway intermediates have been determined by feeding experiments, the flux of metabolites is not linear and in all likelihood there are multiple points where shunts and feedback steps exist.
Although MYB28 is downregulated in fil-10, the GSL biosynthetic genes that are affected are only a subset (three of ten affected genes) of those known to be altered by 35S::MYB28 overexpression [70]. It is therefore likely that the influence of FIL on other genes is a major contributing factor to the elevated IAA levels we observe. In the fil-10 suppressor, up-regulation of both CYP71A13 [105] and AMI [106] could provide a means to channel IAoX to IAA, and in the fil-4 suppressor, CYP71A13, AMI, and myrosinase activity could contribute to this shunt. Feeding experiments with radiolabelled TRP showed that labeled IAN enrichment is lower than that of labeled IAA, implying that IAN is not a direct product of IAoX [107, 108], and therefore there are likely to be other yet-to-be-defined reactions by which IAA is synthesized. In this regard, our microarray data reveal altered regulation of numerous genes encoding proteins of unknown function, including at least six cytochrome P450s. Functional analysis of these genes may further refine our knowledge of auxin metabolism.
Lastly, FIL physically interacts with the LEUNIG/SEUSS co-repressor complex [40, 109], which also has been shown to interact with other regulators of floral development and inflorescence architecture (e.g. AP1 and SEP3; [110]). Mutations in SEUSS (seu) and the SEUSS-LIKE (slk) genes condition auxin resistant growth phenotypes and exhibit reduced sensitivity to auxin [63, 111, 112]. Conversely, in gynoecia, lug and seu mutants exhibit increased sensitivity to inhibitors of polar auxin transport [113], and in Antirrhinum, mutations in the LUG homolog STYLOSA are associated with altered vascular development in leaves, hypersensitivity towards auxin and polar auxin transport inhibitors, and reduced polar auxin transport [109]. The lug-1 mutant conditions a suppressed bp er phenotype that is similar to that of the fil-10 suppressor (Figs 1B and 6A), suggesting that the two proteins may act cooperatively to coordinate inflorescence architecture through their influences on auxin biosynthesis, transport and perception.
Glucosinolate metabolites can influence development and physiological processes
Our previous studies led us to postulate that BP acts to countermand the action of a vascular-borne growth repressor, but the nature of this signalling molecule has been elusive. Our observations that both bp and fil mutants alter glucosinolate profiles led us to consider the possibility that this repressor is linked to GSL metabolism. Evidence for this hypothesis is circumstantial but multifaceted. First, many genes involved in glucosinolate metabolism are predominantly expressed in vascular tissues and glucosinolates are known to be transported via the vasculature [114–116]. Second, indole-3-carbinol (I3C), a GSL breakdown product, has been shown to be an auxin antagonist, inhibiting auxin signalling and inducing growth arrest by interacting with the TIR1 auxin receptor [117, 118]. Third, although some molecules such as I3C are induced by herbivory, other GSL by-products are produced in unchallenged plants [119], and some are known to have growth inhibitory effects. Raphanusanin, generated from some GSL molecules by myrosinase action, is known to underpin blue light induced phototropism by inhibiting growth on the illuminated side of radish seedlings [120, 121], and exogenous application of raphanusanin in pea seedlings inhibits hypocotyl elongation and releases lateral buds from apical dominance [120, 122]. Our array analyses show that some hypothetical myrosinases are differentially expressed and could contribute to the generation of such inhibitory molecules. These genes represent intriguing targets for future functional genomics studies. Fourth, it is clear that glucosinolate metabolite levels can influence gene expression [123], as well as physiological processes such as flowering time [124–126]. Lastly, in seedlings treated with individually purified GLS molecules, changes in the transcriptome and developmental aberrations were observed (Kliebenstein lab, unpublished results). Collectively, these observations point to glucosinolate metabolites as contributors involved in fine tuning growth and development in addition to their well-established roles in orchestrating responses to biotic and abiotic stimuli.
Supporting information
RNA from inflorescences of bp er and bp er fil-10 was isolated and subjected to QRT-PCR. The fold change in bp er fil-10 is shown. This is an independent experiment relative to the data presented in Figs 6 and 8.
(TIF)
(A.) Inflorescence stem exhibiting a reduced floral cluster, consisting of type B flowerless pedicels (arrows). (B.) bp er fil-4 inflorescence revealing the conversion of floral organs to filamentous structures. (C.) PCR analysis of RNA splicing. gDNA represents genomic Ler DNA, (-) is no DNA template reaction, and bp er, bp er fil-4, and bp er fil-10 are cDNAs amplified from the relevant genotypes. DNA sequencing revealed that the fil-4 mutation is due to a G to A base change at the exon 6 splice donor sequence. Note the congruence of the bper and bperfil10 bands (337bp amplicon indicative of proper splicing of exon 5), and the larger 756bp amplicon in bp er fil-4, due to missplicing and the inclusion of intron 5 in the final mRNA. (D.) QRT-PCR analysis of glucosinolate metabolism genes. The expression pattern of these genes in the fil-4 suppressor is different from that of the fil-10 suppressor (see Figs 6 and 8), and the magnitude of the differences vs. the bp er parent line is much reduced. Elevated expression of myrosinases and CYP71A13 (CYP71) may provide avenues to shunt glucosinolate intermediates to IAA biosynthesis. (E-G.) Glucosinolate profiling of Ler, bp er, bp er fil-4 and bp er fil-10. Graphs showing comparisons where Student’s T-tests reveal statistical significance are shown. (H.) T-test values for all pair-wise comparisons. Those with p-values of less than 0.05 are highlighted in grey. Note that I3M, which can be converted to IAA via myrosinase/nitrilase activities, is elevated (see Fig 8 for pathway).
(TIF)
QRT-PCR of bp er and bp er fil-10 inflorescence RNA reveals no significant changes in the expression of these KNOX genes in the two genotypes.
(TIF)
(PDF)
(XLSX)
(XLSX)
Acknowledgments
We thank ABRC and Drs. John Bowman, Gary Drews and Hai Huang for providing seeds, and Drs. John Bowman and Marty Yanofsky for providing clones of FIL and AG for in situ hybridization probes. We are also indebted to Patricia Lam and Salma Rawof for help with mapping, Ayako Nambara for assistance with IAA measurements, Raymond Orr for microscopy assistance, Thanh Nguyen for microarray analyses, Rashida Patel for imaging the FIL::GFP plants, Dr. Sohee Kang for statistical advice, and Drs. Ron Dengler and Clare Hasenkampf for sharing equipment and advice on the project.
Data Availability
All relevant data underlying the findings of this study are publicly available at GEO (accession number GSE86643).
Funding Statement
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada (RGPIN 90057-06 to CDR and RGPIN-2014-03621 to EN), by grants from the National Science Foundation (IOS 1339125 and MCB 1330337 to DJK) and by a grant from the United States Department of Agriculture (Hatch project CA-D-PLS-7033-H to DJK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Barton MK. Twenty years on: the inner workings of the shoot apical meristem, a developmental dynamo. Dev Biol. 2010; 341:95–113. 10.1016/j.ydbio.2009.11.029 [DOI] [PubMed] [Google Scholar]
- 2.Stahl Y, Simon R. Plant primary meristems: shared functions and regulatory mechanisms. Curr Opin Plant Biol. 2010;13:53–58. 10.1016/j.pbi.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 3.Hay A, Tsiantis M. KNOX genes: versatile regulators of plant development and diversity. Development. 2010;137:3153–3165. 10.1242/dev.030049 [DOI] [PubMed] [Google Scholar]
- 4.Vollbrecht E, Reiser L, Hake S. Shoot meristem size is dependent on inbred background and presence of the maize homeobox gene, knotted1. Development. 2000;127:3161–3172. [DOI] [PubMed] [Google Scholar]
- 5.Smith LG, Greene B, Veit B, Hake S. A dominant mutation in the maize homeobox gene, KNOTTED-1, causes its ectopic expression in leaf cells with altered fates. Development. 1992;116:21–30. [DOI] [PubMed] [Google Scholar]
- 6.Chuck G, Lincoln C, Hake S. KNAT1 induces lobed leaves with ectopic meristems when overexpressed in Arabidopsis. Plant Cell. 1996;8:1277–1289. 10.1105/tpc.8.8.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams-Carrier RE, Lie YS, Hake S, Lemaux PG. Ectopic expression of the maize kn1 gene phenocopies the Hooded mutant of barley. Development 1997; 124: 3737–3745. [DOI] [PubMed] [Google Scholar]
- 8.Sentoku N, Sato Y, Matsuoka M. Overexpression of rice OSH genes induces ectopic shoots on leaf sheaths of transgenic rice plants. Dev Biol. 2000;220:358–364. 10.1006/dbio.2000.9624 [DOI] [PubMed] [Google Scholar]
- 9.Hareven D, Gutfinger T, Parnis A, Eshed Y, Lifschitz E. The making of a compound leaf: genetic manipulation of leaf architecture in tomato. Cell. 1996; 84:735–744. [DOI] [PubMed] [Google Scholar]
- 10.Schneeberger RG, Becraft PW, Hake S, Freeling M. Ectopic expression of the KNOX homeobox gene rough sheath1 alters cell fate in the maize leaf. Genes Dev. 1995;9:2292–2304. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto T, Kamiya N, Ueguchi-Tanaka M, Iwahori S, Matsuoka M. KNOX homeodomain protein directly suppresses the expression of a gibberellin biosynthetic gene in the tobacco shoot apical meristem. Genes Dev. 2001; 15:581–590. 10.1101/gad.867901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay A, Kaur H, Phillips A, Hedden P, Hake S, Tsiantis M. The gibberellin pathway mediates KNOTTED1-type homeobox function in plants with different body plans. Curr Biol. 2002;12:1557–1565. [DOI] [PubMed] [Google Scholar]
- 13.Shani E, Burko Y, Ben-Yaakov L, Berger Y, Amsellem Z, Goldshmidt A, et al. Stage-specific regulation of Solanum lycopersicum leaf maturation by class 1 KNOTTED1-LIKE homeobox proteins. Plant Cell. 2009;21:3078–3092. 10.1105/tpc.109.068148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato Y, Sentoku N, Miura Y, Hirochika H, Kitano H, Matsuoka M. Loss-of-function mutations in the rice homeobox gene OSH15 affect the architecture of internodes resulting in dwarf plants. EMBO J. 1999; 18:992–1002. 10.1093/emboj/18.4.992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douglas SJ, Chuck G, Dengler RE, Pelecanda L, Riggs CD. KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell. 2002; 14:547–558. 10.1105/tpc.010391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venglat SP, Dumonceaux T, Rozwadowski K, Parnell L, Babic V, Keller W, et al. The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc Natl Acad Sci U S A. 2002;99:4730–4735. 10.1073/pnas.072626099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamant O, Pautot V. Plant development: A TALE story. Comptes Rendus Biologies. 2010;333:371–381. 10.1016/j.crvi.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 18.Jasinski S, Piazza P, Craft J, Hay A, Woolley L, Rieu I, et al. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Current Biology. 2005;15:1560–1565. 10.1016/j.cub.2005.07.023 [DOI] [PubMed] [Google Scholar]
- 19.Yanai O, Shani E, Dolezal K, Tarkowski P, Sablowski R, Sandberg G, et al. Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Current Biology. 2005;15:1566–1571. 10.1016/j.cub.2005.07.060 [DOI] [PubMed] [Google Scholar]
- 20.Scofield S, Murray JA. KNOX gene function in plant stem cell niches. Plant Mol Biol. 2006;60:929–946. 10.1007/s11103-005-4478-y [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto T, Kamiya N, Ueguchi-Tanaka M, Iwahori S, Matsuoka M. KNOX homeodomain protein directly suppresses the expression of a gibberellin biosynthetic gene in the tobacco shoot apical meristem. Genes Dev. 2001;15:581–590. 10.1101/gad.867901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolduc N, Hake S. The maize transcription factor KNOTTED1 directly regulates the gibberellin catabolism gene ga2ox1. Plant Cell. 2009;21:1647–1658. 10.1105/tpc.109.068221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar M, Ori N. Compound leaf development in model plant species. Current Opin Plant Biol. 2014; 23:61–69. [DOI] [PubMed] [Google Scholar]
- 24.Byrne ME, Simorowski J, Martienssen RA. ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis. Development 2002; 129,1957–1965. [DOI] [PubMed] [Google Scholar]
- 25.Lin WC, Shuai B, Springer PS. The Arabidopsis LATERAL ORGAN BOUNDARIES-domain gene ASYMMETRIC LEAVES2 functions in the repression of KNOX gene expression and in adaxial-abaxial patterning. Plant Cell. 2003;15:2241–2252. 10.1105/tpc.014969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikezaki M, Kojima M, Sakakibara H, Kojima S, Ueno Y, Machida C, Machida Y. Genetic networks regulated by ASYMMETRIC LEAVES1 (AS1) and AS2 in leaf development in Arabidopsis thaliana: KNOX genes control five morphological events. Plant J. 2010; 61:70–82. 10.1111/j.1365-313X.2009.04033.x [DOI] [PubMed] [Google Scholar]
- 27.Tattersall AD, Turner L, Knox MR, Ambrose MJ, Ellis THN, Hofer JMI. The mutant crispa reveals multiple roles for PHANTASTICA in pea compound leaf development. Plant Cell 2005; 17, 1046–1060. 10.1105/tpc.104.029447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHale NA, Koning RE. PHANTASTICA regulates development of the adaxial mesophyll in Nicotiana leaves. Plant Cell 2004; 16: 1251–1262. 10.1105/tpc.019307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneeberger R, Tsiantis M, Freeling M, Langdale JA. The rough sheath2 gene negatively regulates homeobox gene expression during maize leaf development. Development 1998; 125: 2857–2865. [DOI] [PubMed] [Google Scholar]
- 30.Timmermans MC, Hudson A, Becraft PW, Nelson T. ROUGH SHEATH2: a Myb protein that represses knox homeobox genes in maize lateral organ primordia. Science 1999; 284: 151–153. [DOI] [PubMed] [Google Scholar]
- 31.Machida C, Nakagawa A, Kojima S, Takahashi H, Machida Y. The complex of ASYMMETRIC LEAVES (AS) proteins plays a central role in antagonistic interactions of genes for leaf polarity specification in Arabidopsis. Wiley Interdisciplinary Reviews-Developmental Biology. 2015; 4:655–671. 10.1002/wdev.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumaran MK, Bowman JL, Sundaresan V. YABBY polarity genes mediate the repression of KNOX homeobox genes in Arabidopsis. Plant Cell. 2002; 14:2761–2770. 10.1105/tpc.004911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douglas SJ, Riggs CD. Pedicel development in Arabidopsis thaliana: Contribution of vascular positioning and the role of the BREVIPEDICELLUS and ERECTA genes. Dev Biol. 2005; 284:451–463. 10.1016/j.ydbio.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 34.Sawa S, Ito T, Shimura Y, Okada K. FILAMENTOUS FLOWER controls the formation and development of Arabidopsis inflorescences and floral meristems. Plant Cell. 1999; 11:69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development. 1999; 126:4117–4128. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Atkinson A, Otsuga D, Christensen T, Reynolds L, Drews GN. The Arabidopsis FILAMENTOUS FLOWER gene is required for flower formation. Development. 1999; 126:2715–2726. [DOI] [PubMed] [Google Scholar]
- 37.Kumaran MK, Ye D, Yang WC, Griffith ME, Chaudhury AM, Sundaresan V. Molecular cloning of ABNORMAL FLORAL ORGANS: a gene required for flower development in Arabidopsis. Sex Plant Repro. 1999; 12:118–122. [Google Scholar]
- 38.Eshed Y, Izhaki A, Baum SF, Floyd SK, Bowman JL. Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development. 2004; 131:2997–3006. 10.1242/dev.01186 [DOI] [PubMed] [Google Scholar]
- 39.Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y. Signals derived from YABBY gene activities in organ primordia regulate growth and partitioning of Arabidopsis shoot apical meristems. Plant Cell. 2008; 20(5):1217–1230. 10.1105/tpc.107.057877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stahle MI, Kuehlich J, Staron L, von Arnim AG, Golz JF. YABBYs and the transcriptional corepressors LEUNIG and LEUNIG HOMOLOG maintain leaf polarity and meristem activity in Arabidopsis. Plant Cell. 2009; 21:3105–3118. 10.1105/tpc.109.070458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarojam R, Sappl PG, Goldshmidt A, Efroni I, Floyd SK, Eshed Y, et al. Differentiating Arabidopsis shoots from leaves by combined YABBY activities. Plant Cell. 2010;22:2113–2130. 10.1105/tpc.110.075853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lugassi N, Nakayama N, Bochnik R, Zik M. A novel allele of FILAMENTOUS FLOWER reveals new insights on the link between inflorescence and floral meristem organization and flower morphogenesis. BMC Plant Biol. 2010;10:131 10.1186/1471-2229-10-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonaccorso O, Lee JE, Puah L, Scutt CP, Golz JF. FILAMENTOUS FLOWER controls lateral organ development by acting as both an activator and a repressor. BMC Plant Biol. 2012;12:176 10.1186/1471-2229-12-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lincoln C, Long J, Yamaguchi J, Serikawa K, Hake S. A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell. 1994; 6:1859–1876. 10.1105/tpc.6.12.1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konieczny A, Ausubel FM. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 1993;4:403–410. [DOI] [PubMed] [Google Scholar]
- 46.Clough SJ, Bent AF. Foral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998; 16:735–743. [DOI] [PubMed] [Google Scholar]
- 47.Smyth DR, Bowman JL, Meyerowitz EM. Early flower development in Arabidopsis. Plant Cell 1990; 2: 755–767. 10.1105/tpc.2.8.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfaffl MW. A new mathematical model for relative quantification in real time RT-PCR. Nuc Acids Res. 2001; 29: E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livak KJ, Schmittgen TD. Analysis of relative gen expression data using real-time quantitative PCR and the 2-ΔΔΔΔCT method. Methods 2001; 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 50.Kliebenstein DJ, Kroymann J, Brown P, Figuth A, Pedersen D, Gershenzon J, et al. Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiology. 2001;126:811–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stokes ME, Chattopadhyay A, Wilkins O, Nambara E, Campbell MM. interplay between sucrose and folate modulates auxin signaling in Arabidopsis. Plant Physiol. 2013; 162:1552–1565. 10.1104/pp.113.215095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eshed Y, Baum SF, Bowman JL. Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell. 1999;99(2):199–209. [DOI] [PubMed] [Google Scholar]
- 53.Eshed Y, Baum SF, Perea JV, Bowman JL. Establishment of polarity in lateral organs of plants. Curr Biol. 2001; 11:1251–1260. [DOI] [PubMed] [Google Scholar]
- 54.Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. KANADI regulates organ polarity in Arabidopsis. Nature. 2001;411:706–9. 10.1038/35079629 [DOI] [PubMed] [Google Scholar]
- 55.Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A, et al. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr Biol. 2003;13(20):1768–74. [DOI] [PubMed] [Google Scholar]
- 56.Pekker I, Alvarez JP, Eshed Y. Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell. 2005;17:2899–2910. 10.1105/tpc.105.034876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu G, Lin WC, Huang T, Poethig RS, Springer PS, Kerstetter RA. KANADI1 regulates adaxial-abaxial polarity in Arabidopsis by directly repressing the transcription of ASYMMETRIC LEAVES2. Proc Natl Acad Sci USA 2008; 105: 16392–16397. 10.1073/pnas.0803997105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu L, Xu Y, Dong A, Sun Y, Pi L, Xu Y, et al. Novel as1 and as2 defects in leaf adaxial-abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development. 2003;130:4097–4107. [DOI] [PubMed] [Google Scholar]
- 59.Bundy MGR, Thompson OA, Sieger MT, Shpak ED (2012) Patterns of Cell Division, Cell Differentiation and Cell Elongation in Epidermis and Cortex of Arabidopsis pedicels in the Wild Type and in erecta. PLoS ONE 7(9): e46262 10.1371/journal.pone.0046262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greb T, Clarenz O, Schafer E, Muller D, Herrero R, Schmitz G, et al. Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes & Development. 2003;17:1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu ZC, Meyerowitz EM. LEUNIG regulates AGAMOUS expression in Arabidopsis flower development. 1995;121:975–991. [DOI] [PubMed] [Google Scholar]
- 62.Franks RG, Wang C, Levin JZ, Liu Z. SEUSS, a member of a novel family of plant regulatory proteins, represses floral homeotic gene expression with LEUNIG. Development. 2002;129:253–263. [DOI] [PubMed] [Google Scholar]
- 63.Bao F, Azhakanandam S, Franks RG. SEUSS and SEUSS-LIKE transcriptional adaptors regulate floral and embryonic development in Arabidopsis. Plant Physiol. 2010;152(2):821–836. 10.1104/pp.109.146183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grigorova B, Mara C, Hollender C, Sijacic P, Chen X, Liu Z. LEUNIG and SEUSS co-repressors regulate miR172 expression in Arabidopsis flowers. Development. 2011;138(12):2451–2456. 10.1242/dev.058362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meister RJ, Oldenhof H, Bowman JL, Gasser CS. Multiple protein regions contribute to differential activities of YABBY proteins in reproductive development. Plant Physiology. 2005;137:651–662. 10.1104/pp.104.055368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallagher TL, Gasser CS. Independence and interaction of regions of the INNER NO OUTER protein in growth control during ovule development. Plant Physiol. 2008; 147:306–315. 10.1104/pp.107.114603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li B, Gaudinier A, Tang M, Taylor-Teeples M, Nham NT, Gaffari C, et al. Promoter-based integration in plant defense regulation. Plant Physiol. 2014; 166:1803–1820. 10.1104/pp.114.248716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sønderby I E, Geu-Flores F, Halkier BA Biosynthesis of glucosinolates—gene discovery and beyond. Trends Plant Sci. 2010;15: 283–290. 10.1016/j.tplants.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 69.Gigolashvili T, Yatusevich R, Berger B, Muller C, Flugge U. The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 2007; 51:247–261. 10.1111/j.1365-313X.2007.03133.x [DOI] [PubMed] [Google Scholar]
- 70.Sønderby IE, Hansen BG, Bjarnholt N, Ticconi C, Halkier BA, Kliebenstein DJ. A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLOS One 2007; 12:e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delarue M, Prinsen E, Onckelen HV, Caboche M, Bellini C. sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. Plant J. 1998;14:603–611. [DOI] [PubMed] [Google Scholar]
- 72.Bak S, Feyereisen R. The involvement of two p450 enzymes, CYP83B1 and CYP83A1, in auxin homeostasis and glucosinolate biosynthesis. Plant Physiol. 2001;127:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bak S, Tax FE, Feldmann KA, Galbraith DW, Feyereisen R. CYP83B1, a cytochrome P450 at the metabolic branch point in auxin and indole glucosinolate biosynthesis in Arabidopsis. Plant Cell. 2001;13:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reintanz B, Lehnen M, Reichelt M, Gershenzon J, Kowalczyk M, Sandberg G, et al. bus, a bushy Arabidopsis CYP79F1 knockout mutant with abolished synthesis of short-chain aliphatic glucosinolates. Plant Cell. 2001;13:351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tantikanjana T, Yong JW, Letham DS, Griffith M, Hussain M, Ljung K, et al. Control of axillary bud initiation and shoot architecture in Arabidopsis through the SUPERSHOOT gene. Genes Dev. 2001;15:1577–1588. 10.1101/gad.887301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, et al. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 2002; 16:3100–3112. 10.1101/gad.1035402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grubb CD, Zipp BJ, Ludwig-Muller J, Masuno MN, Molinski TF, Abel S. Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J. 2004;40:893–908. 10.1111/j.1365-313X.2004.02261.x [DOI] [PubMed] [Google Scholar]
- 78.Mikkelsen MD, Naur P, Halkier BA. Arabidopsis mutants in the C-S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant J. 2004;37:770–777. [DOI] [PubMed] [Google Scholar]
- 79.Maharjan PM, Dilkes BP, Fujioka S, Pencik A, Ljung K, Burow M, et al. Arabidopsis gulliver1/superroot2-7 identifies a metabolic basis for auxin and brassinosteroid synergy. Plant Journal. 2014;80:797–808. 10.1111/tpj.12678 [DOI] [PubMed] [Google Scholar]
- 80.Kong W, Li Y, Zhang M, Jin F, Li J. A Novel Arabidopsis microRNA promotes IAA biosynthesis via the indole-3-acetaldoxime pathway by suppressing superroot1. Plant Cell Physiol. 2015;56:715–726. 10.1093/pcp/pcu216 [DOI] [PubMed] [Google Scholar]
- 81.Aloni R, Schwalm K, Langhans M, Ullrich CI. Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 2003; 216, 841–853. [DOI] [PubMed] [Google Scholar]
- 82.Ljung K. Auxin metabolism and homeostasis during plant development. Development 2013; 140:943–950. 10.1242/dev.086363 [DOI] [PubMed] [Google Scholar]
- 83.Tivendale ND, Ross JJ, Cohen JD. The shifting paradigms of auxin biosynthesis. Trends Plant Sci. 2014; 19:44–51. [DOI] [PubMed] [Google Scholar]
- 84.Hepworth SR, Pautot VA. Beyond the Divide: Boundaries for patterning and stem cell regulation in plants. Front Plant Sci 2015; 6:1052 10.3389/fpls.2015.01052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Long JA, Barton MK. The development of the apical embryonic pattern in Arabidopsis. Development 1998; 125: 3027–3035. [DOI] [PubMed] [Google Scholar]
- 86.Belles-Boix E, Hamant O, Witiak SM, Morin H, Traas J, Pautot V. KNAT6:an Arabidopsis homeobox gene involved in meristem activity and organ separation. Plant Cell 2006; 18: 1900–1907. 10.1105/tpc.106.041988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ragni L, Belles-Boix E, Gunl M, Pautot V. Interaction of KNAT6 and KNAT2 with BREVIPEDICELLUS and PENNYWISE in Arabidopsis inflorescences. Plant Cell 2008; 20: 888–900. 10.1105/tpc.108.058230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao M, Yang S, Chen C-Y, Li C, Shan W, Lu W, et al. Arabidopsis BREVIPEDICELLUS Interacts with the SWI2/SNF2 chromatin remodeling ATPase BRAHMA to regulate KNAT2 and KNAT6 expression in control of inflorescence architecture. PLoS Genet 2015; 11(3): e1005125 10.1371/journal.pgen.1005125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prasad K, Grigg SP, Barkoulas M, Yadav RM, Sanchez-Perez GF, Pinon V, Blilou I, Hofhuis H, Dhonukshe P, Galinha C, Mahonen AP, Muller WH, Raman S, Verkleij AJ, Snel B, Reddy GV, Tsiantis M, Scheres B. Arabidopsis PLETHORA transcription factors control phyllotaxis. Current Biol 2011; 21:1123–1128. [DOI] [PubMed] [Google Scholar]
- 90.Dinneny JR, Weigel D, Yanofsky MF. A genetic framework for fruit patterning in Arabidopsis thaliana. Development 2005; 132: 4687–4696. 10.1242/dev.02062 [DOI] [PubMed] [Google Scholar]
- 91.Gonázlez-Reig S, Ripoll JJ, Vera A, Yanofsky MF, Martınez-Laborda A. Antagonistic Gene activities determine the formation of pattern elements along the mediolateral axis of the Arabidopsis fruit. PLoS Genet 2012; 8(11): e1003020 10.1371/journal.pgen.1003020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hay A, Barkoulas M, Tsiantis M. ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development. 2006;133:3955–61. 10.1242/dev.02545 [DOI] [PubMed] [Google Scholar]
- 93.Tabata R, Ikezaki M, Fujibe T, Aida M, Tian C, Ueno Y, et al. Arabidopsis AUXIN RESPONSE FACTOR6 and 8 regulate jasmonic acid biosynthesis and floral organ development via repression of class 1 KNOX Genes. Plant Cell Physiology. 2010;51:164–175. 10.1093/pcp/pcp176 [DOI] [PubMed] [Google Scholar]
- 94.Bolduc N, Yilmaz A, Mejia-Guerra MK, Morohashi K, O'Connor D, Grotewold E, et al. Unraveling the KNOTTED1 regulatory network in maize meristems. Genes Dev. 2012;26:1685–1690. 10.1101/gad.193433.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu N, Wu S, Van Houten J, Wang Y, Ding B, Fei Z, Clarke TH, Reed JW, van der Knaap E. Down-regulation of AUXIN RESPONSE FACTORS 6 and 8 by microRNA 167 leads to floral development defects and female sterility in tomato. J Exp Botany 2014; 65(9): 2507–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid biding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 1997; 9: 745–757. 10.1105/tpc.9.5.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J. BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev 2001; 15:1985–1997. 10.1101/gad.905201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamaguchi N, Suzuki M, Fukaki H, Morita-Terao M, Komeda Y. CRM1/BIG-mediated auxin action regulates Arabidopsis inflorescence development. Plant Cell Physiol 2007; 48(9): 1275–1290. 10.1093/pcp/pcm094 [DOI] [PubMed] [Google Scholar]
- 99.Yamaguchi N, Komeda Y. The role of CORYMBOSA1/BIG and auxin in the growth of Arabidopsis pedicel and internode. Plant Sci 2013; 209:64–74. 10.1016/j.plantsci.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 100.Guo X, Lu W, Ma Y, Qin Q, Hou S. The BIG gene is required for auxin-mediated growth in Arabidopsis. Planta 2012; 237:1135–1147. [DOI] [PubMed] [Google Scholar]
- 101.Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, et al. A gene expression map of Arabidopsis thaliana development. Nat Gen 2005; 37: 501–506. [DOI] [PubMed] [Google Scholar]
- 102.Malitsky S, Blum E, Less H, Venger I, Elbaz M, Morin S, et al. The transcript and metabolite networks affected by the two clades of Arabidopsis glucosinolate biosynthesis regulators. Plant Physiol. 2008;148:2021–2049. 10.1104/pp.108.124784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inze D. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 1995; 7:1405–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sugawara S, Hishiyama S, Jikumarua Y, Hanada A, Nishimura T, Koshiba T, Zhao Y, Kamiaya Y, Kasahara H. Biochemical analyses of indole-3-acetaldoxime- dependent auxin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA 2009; 106: 5430–5434. 10.1073/pnas.0811226106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nafisi M, Goregaoker S, Botanga CJ, Glawischnig E, Olsen CE, Halkier BA, Glazebrook J. Arabidopsis cytochrome P450 monooxygenase 71A13 catalyzes the conversion of indole-3-acetaldoxime in camalexin synthesis. Plant Cell 2007; 19:2039–2052. 10.1105/tpc.107.051383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pollman S, Neu D, Weiler EW. Molecular cloning and characterization of an amidase from Arabidopsis thaliana capable of converting indole-3-acetamide into the plant growth hormone, indole-3-acetic acid. Phytochemistry 2002; 62: 293–300. [DOI] [PubMed] [Google Scholar]
- 107.Normanly J, Cohen JD, Fink GR Arabidopsis thaliana auxotrophs reveal a tryptophan-independent biosynthetic pathway for indole-3-acetic acid. Proc Natl Acad Sci USA 1993; 90:10355–10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Muller A, Hillebrand H, Weiler EW. Indole-3-acetic acid is synthesized from L-tryptophan in Arabidopsis thaliana. Planta 1998; 206:362–369. 10.1007/s004250050411 [DOI] [PubMed] [Google Scholar]
- 109.Navarro C, Efremova N, Golz JF, Rubiera R, Kuckenberg M, Castillo R, et al. Molecular and genetic interactions between STYLOSA and GRAMINIFOLIA in the control of Antirrhinum vegetative and reproductive development. Development. 2004;131:3649–3659. 10.1242/dev.01205 [DOI] [PubMed] [Google Scholar]
- 110.Srihar VV, Surendrarao A, Liu Z. APETALA1 and SEPALLATA3 interact with SEUSS to mediate transcription repression during flower development. Development 2006; 133:3159–3166. 10.1242/dev.02498 [DOI] [PubMed] [Google Scholar]
- 111.Pfluger J, Zambryski P. The role of SEUSS in auxin response and floral organ patterning. Development. 2004;131:4697–4707. 10.1242/dev.01306 [DOI] [PubMed] [Google Scholar]
- 112.Lee JE, Lampugnani ER, Bacic A, Golz JF. SEUSS and SEUSS-LIKE 2 coordinate auxin distribution and KNOXI activity during embryogenesis. Plant J. 2014;80:122–135. 10.1111/tpj.12625 [DOI] [PubMed] [Google Scholar]
- 113.Staldal V, Sohlberg JJ, Eklund DM, Ljung K, Sundberg E. Auxin can act independently of CRC, LUG, SEU, SPT and STY1 in style development but not apical-basal patterning of the Arabidopsis gynoecium. New Phytol. 2008; 180:798–808. 10.1111/j.1469-8137.2008.02625.x [DOI] [PubMed] [Google Scholar]
- 114.Chen S, Petersen BL, Olsen CE, Schulz A, Halkier BA. Long-distance phloem transport of glucosinolates in Arabidopsis. Plant Physiol. 2001; 127:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nour-Eldin HH, Andersen TG, Burow M, Madsen SR, Jorgensen ME, Olsen CE, et al. NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature. 2012;488:531–534. 10.1038/nature11285 [DOI] [PubMed] [Google Scholar]
- 116.Jørgensen ME, Nour-Eldin HH, Halkier BA. Transport of defense compounds from source to sink: lessons learned from glucosinolates. Trends Plant Sci. 2015; 20:508–514. 10.1016/j.tplants.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 117.Katz E, Nisani S, Sela M, Behar H, Chamovitz DA. The effect of indole-3-carbinol on PIN1 and PIN2 in Arabidopsis roots. Plant Signal Behav. 2015;10:e1062200 10.1080/15592324.2015.1062200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Katz E, Nisani S, Yadav BS, Woldemariam MG, Shai B, Obolski U, et al. The glucosinolate breakdown product indole-3-carbinol acts as an auxin antagonist in roots of Arabidopsis thaliana. Plant J. 2015;82:547–555. 10.1111/tpj.12824 [DOI] [PubMed] [Google Scholar]
- 119.Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science. 2009;323:101–106. 10.1126/science.1163732 [DOI] [PubMed] [Google Scholar]
- 120.Hasegawa T, Yamada K, Kosemura S, Yamamura S, Hasegawa K. Phototropic stimulation induces the conversion of glucosinolate to phototropism-regulating substances of radish hypocotyls. Phytochemistry. 2000;54(3):275–9. [DOI] [PubMed] [Google Scholar]
- 121.Yamada K, Hasegawa T, Minami E, Shibuya N, Kosemura S, Yamamura S, Hasegawa KI Induction of myrosinase gene expression and myrosinase activity in radish hypocotyls by phototropic stimulation. J Plant Physiol. 2003; 160: 255–259. 10.1078/0176-1617-00950 [DOI] [PubMed] [Google Scholar]
- 122.Nakajima E, Yamada K, Kosemura S, Yamamura S, Hasegawa K. Effects of the auxin-inhibiting substances raphanusanin and benzoxazolinone on apical dominance of pea seedlings. Plant Growth Regulation. 2001;35:11–15. [Google Scholar]
- 123.Wentzell AM, Rowe HC, Hansen BG, Ticconi C, Halkier BA, Kliebenstein DJ. Linking metabolic QTLs with network and cis-eQTLs controlling biosynthetic pathways. PLOS Genetics. 2007: 3:1687–1701. 10.1371/journal.pgen.0030162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kerwin R, Feusier J, Corwin J, Rubin M, Lin C, Muok A, et al. Natural genetic variation in Arabidopsis thaliana defense metabolism genes modulates field fitness. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kerwin RE, Jimenez-Gomez JM, Fulop D, Harmer SL, Maloof JN, Kliebenstein DJ. Network quantitative trait loci mapping of circadian clock outputs identifies metabolic pathway-to-clock linkages in Arabidopsis. Plant Cell. 2011;23:471–485. 10.1105/tpc.110.082065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jensen LM, Jepsen HS, Halkier BA, Kliebenstein DJ, Burow M. Natural variation in cross-talk between glucosinolates and onset of flowering in Arabidopsis. Front Plant Sci. 2015;6:697 10.3389/fpls.2015.00697 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNA from inflorescences of bp er and bp er fil-10 was isolated and subjected to QRT-PCR. The fold change in bp er fil-10 is shown. This is an independent experiment relative to the data presented in Figs 6 and 8.
(TIF)
(A.) Inflorescence stem exhibiting a reduced floral cluster, consisting of type B flowerless pedicels (arrows). (B.) bp er fil-4 inflorescence revealing the conversion of floral organs to filamentous structures. (C.) PCR analysis of RNA splicing. gDNA represents genomic Ler DNA, (-) is no DNA template reaction, and bp er, bp er fil-4, and bp er fil-10 are cDNAs amplified from the relevant genotypes. DNA sequencing revealed that the fil-4 mutation is due to a G to A base change at the exon 6 splice donor sequence. Note the congruence of the bper and bperfil10 bands (337bp amplicon indicative of proper splicing of exon 5), and the larger 756bp amplicon in bp er fil-4, due to missplicing and the inclusion of intron 5 in the final mRNA. (D.) QRT-PCR analysis of glucosinolate metabolism genes. The expression pattern of these genes in the fil-4 suppressor is different from that of the fil-10 suppressor (see Figs 6 and 8), and the magnitude of the differences vs. the bp er parent line is much reduced. Elevated expression of myrosinases and CYP71A13 (CYP71) may provide avenues to shunt glucosinolate intermediates to IAA biosynthesis. (E-G.) Glucosinolate profiling of Ler, bp er, bp er fil-4 and bp er fil-10. Graphs showing comparisons where Student’s T-tests reveal statistical significance are shown. (H.) T-test values for all pair-wise comparisons. Those with p-values of less than 0.05 are highlighted in grey. Note that I3M, which can be converted to IAA via myrosinase/nitrilase activities, is elevated (see Fig 8 for pathway).
(TIF)
QRT-PCR of bp er and bp er fil-10 inflorescence RNA reveals no significant changes in the expression of these KNOX genes in the two genotypes.
(TIF)
(PDF)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data underlying the findings of this study are publicly available at GEO (accession number GSE86643).