Abstract
During the past decade, extracellular vesicles (EVs), which include apoptotic bodies, microvesicles, and exosomes, have emerged as important players in cell-to-cell communication in normal physiology and pathological conditions. EVs encapsulate and convey various bioactive molecules that are further transmitted to neighboring or more distant cells, where they induce various signaling cascades. The message delivered to the target cells is dependent on EV composition, which, in turn, is determined by the cell of origin and the surrounding microenvironment during EV biogenesis. Among their multifaceted role in the modulation of biological responses, the involvement of EVs in vascular development, growth, and maturation has been widely documented and their potential therapeutic application in regenerative medicine or angiogenesis-related diseases is drawing increasing interest. EVs derived from various cell types have the potential to deliver complex information to endothelial cells and to induce either pro- or antiangiogenic signaling. As dynamic systems, in response to changes in the microenvironment, EVs adapt their cargo composition to fine-tune the process of blood vessel formation. This article reviews the current knowledge on the role of microvesicles and exosomes from various cellular origins in angiogenesis, with a particular emphasis on the underlying mechanisms, and discusses the main challenges and prerequisites for their therapeutic applications.
Keywords: cell-derived microparticles, endothelial cells, exosomes, extracellular vesicles, regenerative medicine
Angiogenesis, defined as the formation of new blood vessels from a pre-existing vascular network, naturally occurs in an organism during growth and development and also in response to injury to restore a tissue’s blood supply and promote wound healing. The new vessels can be formed by either sprouting angiogenesis, where endothelial cells (ECs) form sprouts that grow toward an angiogenic stimulus, or intussusceptive angiogenesis, where interstitial tissues invade the existing vessels and form transvascular tissue pillars that expand and split the vessel.1 Sprouting angiogenesis comprises several steps: enzymatic degradation of the vessel’s basement membrane, EC proliferation, migration, sprouting, branching, and tube formation. The stabilization and maturation of the newly formed vascular structures require the recruitment of pericytes, the deposition of extracellular matrix, and mechanical stimulation by the shear stress. In healthy tissues, angiogenesis is tightly regulated by a precise balance between stimulatory and inhibitory signals.2 Abnormal blood vessel growth occurs when this balance is disturbed and is a major cause of numerous diseases, such as cancer, atherosclerosis, corneal neovascularization, rheumatoid arthritis, or ischemic diseases.
During the past decade, the vesicles released by different cell types have been shown to be important mediators during the process of blood vessel formation and, as such, they have attracted particular interest among researchers from various fields of biology and medicine, including angiogenesis.3,4 Longtime considered as inert debris or a hallmark of cell injury, extracellular vesicles (EVs), which include apoptotic bodies, microvesicles, and exosomes, have emerged as an important tool for intercellular communications in normal physiology and in pathophysiological conditions.5,6 Indeed, EVs function as the carriers of small bioactive molecules, such as peptides, proteins, lipids, and nucleic acids, that act as regulators in the recipient cells in a paracrine or endocrine manner.7–9
This article reviews the current knowledge on the role of microvesicles and exosomes from various cellular origins in angiogenesis, with a particular emphasis on the underlying mechanisms, and discusses the main challenges and prerequisites for their therapeutic applications.
Biogenesis of EVs
EVs are defined as heterogeneous plasma membrane vesicles released from various types of cells into biological fluids under both normal and stressed conditions.5 According to their size and biogenesis pathways, EVs can be divided into 3 main types: exosomes, microvesicles (also called microparticles), and apoptotic bodies. Table 1 shows the main characteristics of these different types of EVs.
Table 1.
Exosomes, Microvesicles, and Apoptotic Bodies: Main Characteristics
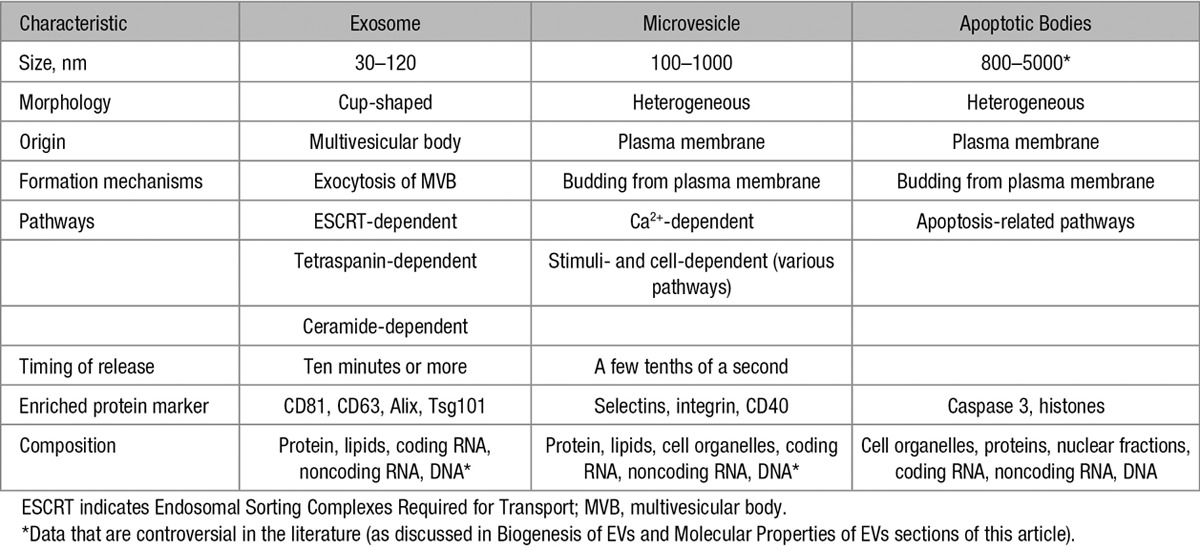
Exosomes are assumed to represent a homogeneous population with a size between 30 and 120 nm in diameter, and they typically display a cup-like shape.10 However, it was demonstrated that cells release distinct subpopulations of exosomes with heterogeneous sizes and compositions that elicit differential molecular and biological properties.11 Exosomes are derived from the endosomal system and are generated by the intraluminal budding of endosomal compartments, forming the intraluminal vesicles (ILVs) in intracellular multivesicular bodies. ILV formation constitutes the starting point of the exosome biogenesis process. The main mechanism of ILV formation is mediated by the Endosomal Sorting Complexes Required for Transport machinery.12 However, ILV invagination and exosome secretion are also regulated in an Endosomal Sorting Complexes Required for Transport–independent manner that includes tetraspanin microdomains and lipid rafts.8,13,14 After multivesicular bodies fuse with the plasma membrane of the parent cell, ILVs are released as exosomes into the extracellular environment. Similar to the various mechanisms proposed for the biogenesis of exosomes, a set of mechanisms has also been proposed for their release, involving Rab GTPases (Rab11/35, Rab27), the aforementioned tetraspanin and the SNARE (soluble N-ethylmaleimide-sensitive attachment protein receptor) complex.15–17
In contrast to exosomes, microvesicles, with a size ranging from 100 to 1000 nm in diameter, represent a more heterogeneous population and are formed by the outward blebbing of the plasma membrane. The release is visible within a few seconds after stimulation.16 In response to stimuli, outward blebbing may be dependent on various enzymes and mitochondrial or calcium signaling. During the blebbing process, a cytoskeleton reorganization and alterations of phospholipid symmetry occur. These processes may differ significantly between cell types.18–20 In this way, the modification of membrane asymmetry promotes the redistribution of aminophospholipids, principally phosphatidylserine, to the outer part of the plasma membrane. Microvesicle formation seems to occur selectively in the lipid-rich microdomains of the membrane, such as in lipid rafts or caveolae domains.21,22
Interestingly, several studies have reported that changes in the plasma membrane may be independent of asymmetry loss. These processes involve the Endosomal Sorting Complexes Required for Transport pathway or tetraspanin microdomains.14,17 The few studies that have analyzed the molecular mechanisms of microvesicle production clearly show that multiple complex pathways are involved. Depending on the starting stimulus, these include myosin light chain and Rho-associated kinase I and II, nuclear factor-κB, tumor necrosis factor–related apoptosis-inducing ligand, or p38 mitogen-activated protein kinase.6
Apoptotic bodies are another type of EVs that are exclusively released during the last steps of apoptosis by caspase-mediated cleavage and the consequent activation of Rho-associated kinase I.17,23 They are characterized by the presence of an externalized phosphatidylserine and a permeable membrane, and they are larger than exosomes and microvesicles. Several reports indicate that apoptotic bodies contain a variety of cellular materials, such as histones, DNA, cellular organelles, and membrane/cytosolic components.17,24 However, little is known about their molecular composition, and only a few recent studies have provided proteomic characterization.
Importantly, whether the passive transfer of cargo by apoptotic bodies has a functional involvement in intercellular communication is not well known, and it requires more studies.
Molecular Properties of EVs
In the past decades, numerous works have focused on providing an exhaustive and comprehensive characterization of EV content, leading to the creation of specialized online databases, such as EVpedia and vesiclepedia,25,26 which record the bioactive molecules and markers observed within these vesicles. Vesicle content specifically reflects the vesicle’s localization, cellular origin, and mechanism of secretion (Table 1).17,27,28 However, a set of proteins is commonly found in EVs. These proteins are associated with vesicle trafficking and biogenesis, such as tetraspanins (CD81, CD9, and CD63), specific stress proteins (HSP90 [heat shock proteins]), members of the Endosomal Sorting Complexes Required for Transport complexes (Tsg101, Alix), proteins involved in membrane fusion (Rabs, ARF6), and signaling proteins.8,15,29 It is now agreed on that these proteins, which were considered for years to be specific to exosomes, can also be detected in larger vesicles, such as microvesicles (Table 1). In addition, several recent reports point to a nonuniform distribution of these proteins in all EV subpopulations.12,30 Importantly, EVs also carry membrane receptors on their surface that are characteristic of the cells that they have been generated from. In addition to their surface molecules, EVs also carry important soluble mediators, such as cytokines, growth factors, and transcription factors.8,26 In addition, their lipid content displays a particular organization and composition that can be distinct from the parent cell, even though they share common features.8,31,32
The lipids that are generally enriched in EVs are sphingomyelin, cholesterol, phosphatidylserine, ceramide, and glycosphingolipids, which confer an EV structure that is close to the raft domains. Phosphatidylserine represents one of the characteristic EV markers that is expressed on their outer surface. Interestingly, microvesicles lacking phosphatidylserine have been recently identified.33–35 This finding suggests that phosphatidylserine externalization might not be the sole mechanism of EV biogenesis. A lack of phosphatidylserine-positive staining might also be because of very low expression of phosphatidylserine, which is undetectable by the current methods. However, a lack of phosphatidylserine-positive staining might also result from phosphatidylserine engagement with lactadherin, Del-1, or protein S, thereby preventing its recognition by labeled Annexin V. Furthermore, lipids are emerging as important factors in EV functions. Several lipids, such as eicosanoids, fatty acids, and cholesterol, have been described as key mediators that are able to activate signaling in the recipient cells. In addition to proteins and lipids, EVs can also incorporate genetic material, such as small and long, coding and noncoding RNA (mRNA, miRNA, and lncRNA), and other cytosolic components and molecules.36–39 Some studies have also reported the presence of genomic and mitochondrial DNA in the EVs.40,41 The mechanisms of DNA packaging in the EVs are still unclear, but it may involve cell apoptosis,42 the existence of plasma membrane associated DNA,43 or the release of genomic DNA in the cytosol.44–46
Interestingly, the lipid-bilayered EVs encapsulate their genetic cargo and protect it from enzymatic digestion.47 EVs may therefore serve as nucleic acid delivery vehicles, representing a new mechanism of genetic exchange between cells.36,48,49 For example, several studies have demonstrated that encapsulated mRNA can be exchanged between cells and can induce the reprogramming of hematopoietic progenitors and ECs.3,37 EVs can directly shuttle RNAs and transcription factors, which are capable of inducing phenotypic changes in the target cells.50,51 In addition, EVs carry a broad range of miRNAs, which are known to modulate gene expression by translational inhibition or by promoting the degradation of target mRNAs.52 Of note, EVs can be packaged with disease- and activation-specific bioactive molecules.53 Also, the microvesicles released from leukemia cells have been demonstrated to increase the global DNA methylation levels in recipient cells.54 Thus, the EV-mediated horizontal transfer of genetic material may directly modulate the phenotype and behavior of the recipient cells.3,55,56
EV-Mediated Intercellular Communication/Interaction With Target Cells
Many studies have documented the biological role of EVs as the mediators of cell communication. EVs convey information to the recipient cells that are present in the surrounding extracellular environment through several mechanisms. Some EVs can deliver their content through different types of endocytosis, such as clathrin-mediated endocytosis that is dependent or independent of receptors, macropinocytosis, and raft domain-mediated endocytosis.57,58 Once absorbed into the endosomal–lysosomal system, EVs can fuse with the organelle membrane and dump their contents into the cytoplasm. They can also fuse with the membrane of the recipient cell to release their cargo intracellularly, either directly or through specific receptors.59 They may also release their contents into the extracellular space and activate a fast response in the neighboring cells.16 Finally, the membrane surfaces of EVs can trigger signaling cascades through receptor/ligand interactions without internalization.5,16,60 Thus, EVs have the potential to deliver complex information to multiple cells in their tissue environment. They function as dynamic systems and are capable of adapting their content, and consequently their function, depending on both the cellular source and the stimulus that engendered their biogenesis.
EVs and Angiogenesis
EVs are generated from different cell types, and they can modulate angiogenesis by stimulating or inhibiting it. These effects are highly dependent on the EV content and surface molecule expression, which can be highly modulated by the stimulus used to induce EV production.
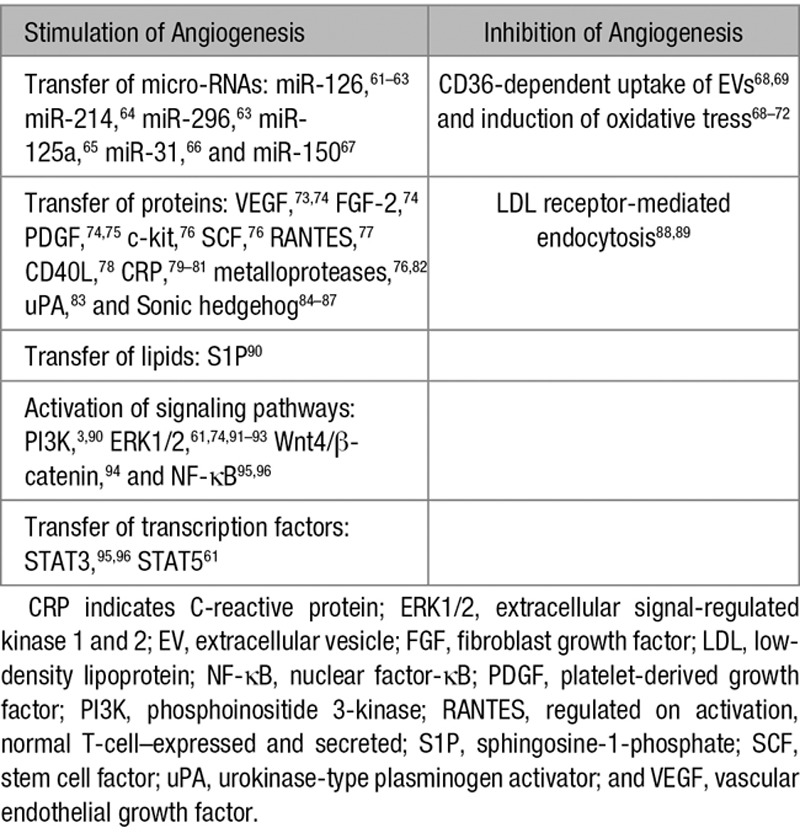
Table 2 summarizes the main mechanisms involved in the modulation of angiogenesis by EVs. During the past 10 years, a large amount of studies has documented the role of EVs in angiogenesis and highlighted their therapeutic potential. However, some of the earlier studies need to be revisited as they attributed the biological effect of the EVs without confirming the purity of the vesicle preparation. Here, we focus on the EVs derived from ECs, endothelial colony-forming cells, mesenchymal stem/stromal cells, platelets, leukocytes, and erythrocytes and discuss the underlying mechanisms.
Table 2.
Main Mechanisms Involved in the Modulation of Angiogenesis by EVs
EC-Derived EVs
Angiogenesis is a dynamic and tightly regulated process in which EC are in constant communication with their environment through multiple paracrine factors and cell-to-cell and cell-to-matrix interactions. Among the extracellular factors, the vesicles released by ECs have been described as important mediators in the formation of blood vessels. Figure 1 illustrates the main mechanisms involved in the modulation of angiogenesis by endothelial EVs. The angiogenic potential of the vesicles shed by EC was documented for the first time in 2002.82 This study evidenced that the EVs released by EC contain β1 integrin and the enzymatically active matrix metalloproteinases matrix metalloproteinase-2 and matrix metalloproteinase-9. The EVs were capable of promoting EC invasion and capillary-like structure formation in vitro.82 In addition, stimulation with VEGF (vascular endothelial growth factor) and FGF-2 (fibroblast growth factor-2) resulted in an increased amount of both the active and proenzyme forms of vesicle-associated metalloproteinases.82
Figure 1.
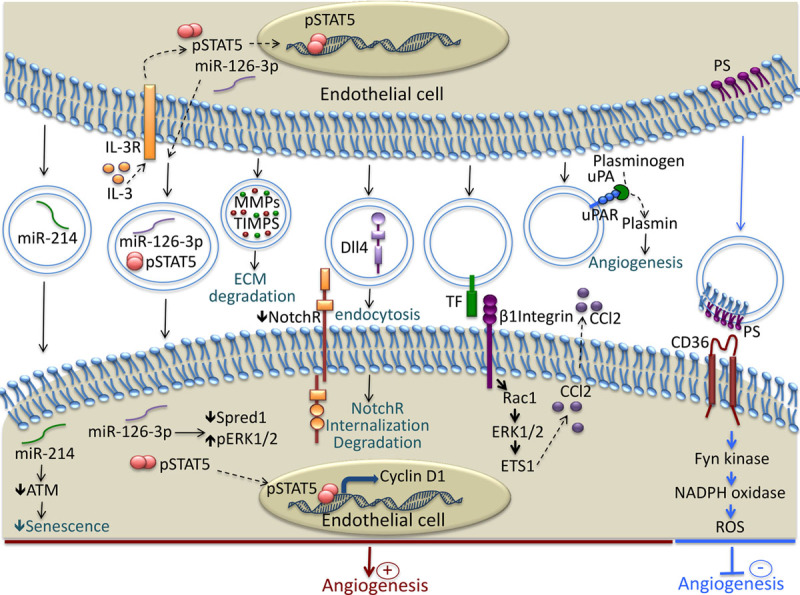
Mechanisms involved in the modulation of Angiogenesis by endothelial cell (EC)–derived extracellular vesicles (EVs). EC release EVs rich in micro-RNA such as miR-214 and miR-126, that are transferred to recipient EC and induce proangiogenic signaling. EVs contain functional matrix metalloproteinases that facilitate angiogenesis through the degradation of components of the extracellular matrix. Dll4 is transferred to EC by the EVs and induces Notch receptor internalization and tip cell formation. EVs bear, at their surface, a tissue factor that interacts with β1 integrin and induces Rac1-ERK1/2-ETS1 signaling, leading to the increased secretion of CCL2. EVs transport the complex uPA/uPAR, which stimulates angiogenesis through plasmin generation. The phosphatidylserine present on the surface of the EVs interacts with CD36 and induces Fyn kinase signaling, which leads to increased oxidative stress and the inhibition of angiogenesis. ATM indicates ataxia telangiectasia mutated; CCl2, chemokine c-c motif ligand 2; Dll4, Delta-like 4; ECM, extracellular matrix; ERK1/2, extracellular signal-related kinase 1 and 2; ETS1, avian erythroblastosis virus E26 homolog-1; IL-3R, interleukin-3 receptor; MMPs, matrix metalloproteinases; NotchR, Notch receptor; PS, phosphatidylserine; Rac1, Ras-related C3 botulinum toxin substrate 1; ROS, reactive oxygen species; TF, tissue factor; TIMPS, tissue inhibitor of metalloproteinases; uPA, urokinase plasminogen activator; and uPAR, urokinase plasminogen activator receptor.
Recently, it was shown that the EVs generated in response to interleukin-3 stimulation promoted angiogenesis through the transfer of miR-126-3p and pSTAT5 into the recipient EC, leading to a lower Spred-1 level and increased ERK1/2 activation and cyclin D1 transcription (Figure 1).61 The involvement of miR-126 in angiogenesis was previously documented in another study, which showed that the presence of miR-126 in apoptotic bodies induced CXCL12 expression in the target cells and the recruitment of progenitor cells in mice with atherosclerosis and conferred an atheroprotective effect by increasing plaque stability.62 In addition, other micro-RNAs present in the EC-derived exosomes, such as miR-214, was shown to promote angiogenesis both in vitro and in vivo by repressing ATM expression and preventing senescence.64 During blood vessel formation, the selection of tip- and stalk cells depends on δ-like 4/Notch signaling.97 Interestingly, it was demonstrated that this signaling does not necessarily require direct cell contact, as δ-like 4 can be incorporated into endothelial exosomes and transferred to the target EC.98 These δ-like 4 containing exosomes stimulated the formation of capillary-like structures in vitro and in vivo through the internalization of the Notch receptor, followed by its degradation.98 Microparticles released from the microvascular EC were shown to contain a tissue factor and to trigger angiogenesis ex vivo and postischemic collateral vessel formation in vivo.91 The proangiogenic effect of the microparticles was mediated by β1 integrin interaction with the target EC, leading to Rac1-ERK1/2-ETS (Ras-related C3 botulinum toxin substrate 1-extracellular signal-related kinase 1 and 2-avian erythroblastosis virus E26 homolog-1) signaling and CCL2 production (Figure 1).91 EC-derived EVs also generate monomeric CRP (C-reactive protein), which was shown to be highly angiogenic.79–81 In addition, it was demonstrated that endothelial microparticles play an important role in plasmin generation, which can affect the in vitro tube formation of endothelial progenitor cells.83 This effect was dose dependent, as low amounts of endothelial microparticles increased tube formation, whereas a high concentration had an inhibitory effect.83 In line with this study, an increased concentration of EC-derived microparticles inhibited angiogenesis in the cultured sections of hearts by inducing eNOS (endothelial nitric oxide synthase) dysfunction.99 Recently, it was shown that EC-derived EVs inhibit tube-like structure formation by microvascular EC in vitro and in vivo. This antiangiogenic effect of EVs was triggered by reactive oxygen species in an NADPH (nicotinamide adenine dinucleotide phosphate) oxidase and Src family kinase-dependent manner and required the expression of CD36 on the target EC (Figure 1).68 The involvement of oxidative stress in the antiangiogenic effect of EV was also documented by other studies, showing that endothelial microparticles impaired acetylcholine-induced endothelial vasorelaxation and nitric oxide (NO) production in rat aortic rings and inhibited in vitro angiogenesis by increasing the oxidative stress.70,71
Endothelial Colony-Forming Cell–Derived EVs
Since the discovery of endothelial progenitor cells (EPC) by Asahara et al100 in 1997, there has been a considerable amount of debate surrounding the EPC identity and their functional role in postnatal neovascularization. Notably, the uptake of platelets microparticles and the subsequent platelet antigen acquisition by ex vivo cultured peripheral blood mononuclear cells has been recognized as a major confounding factor in the definition of the endothelial phenotype of circulating EPC.101 By contrast, endothelial colony-forming cells (ECFC) have been recognized as genuine EPCs, displaying an unambiguous endothelial differentiation potential, proliferative activity, and a specific capacity to self-assemble into functional blood vessels in vivo.102 Because these cells have higher vasculogenic potential compared with mature EC, EVs originating from ECFC have been proposed as potent mediators of proangiogenic signals. Several studies clearly demonstrated their capacity to stimulate blood vessel formation.63,103,104 For instance, ECFC-derived EVs were shown to incorporate into EC via interaction with α4 and β1 integrins, and they stimulated angiogenesis both in vitro and in vivo through the delivery of mRNA associated with eNOS and the PI3K/AKT signaling pathway.3 In addition, ECFC-derived EVs prevented capillary rarefaction and subsequent tissue damage in a rat model of kidney ischemia-reperfusion injury.63 The renoprotective action of EVs was abolished by the specific depletion of miR-126 and miR-296, indicating their critical role in the stimulation of angiogenesis. Additional in vitro studies demonstrated that the EV internalization by hypoxic EC was mainly mediated by L-selectin, and it restored the expression of proangiogeneic and antiapoptotic genes that were downregulated by hypoxia.63 Similar mechanisms of miRNA delivery have been involved in the capacity of ECFC-EV to promote neovascularization and limit muscle damage after hindlimb ischemia or to enhance the survival of human islet transplants.103,104 Recent studies reported that ECFC-derived exosomes promote cutaneous wound healing in diabetic rats by enhancing neovascularization in the wound sites. The exosomes mediated their proangiogenic potential by activating Erk1/2 signaling in EC and by stimulating the expression of several angiogenic molecules.92,93
Mesenchymal Stem/Stromal Cell–derived EVs
Mesenchymal stem/stromal cells (MSCs) have potent proangiogenic properties that have been attributed to their secretion of paracrine factors. However, it was recently suggested that among soluble factors, EVs play an important role in intercellular communication and are involved in angiogenesis. Figure 2 summarizes the main mechanisms involved in the modulation of angiogenesis by MSC-derived EVs. The functionality of the MSC secretome is strongly influenced by the microenvironment. For example, on hypoxia stimulation, bone-marrow–derived MSC released EVs that can be incorporated by EC, stimulated in vitro tube formation, and promoted angiogenesis in a rat myocardial infarction model.105 In addition, exosomes derived from MSC enhanced the tube formation of EC and stimulated neovascularization, leading to improved heart function after ischemic injury in a rat myocardial infarction model.105 Recently, it was demonstrated that EVs isolated from bone-marrow–derived MSC increased postischemic neuroangiogenesis in a way similar to that of MSC after focal cerebral ischemia in mice.106 In line with this study, after traumatic brain injury in rats, the administration of exosomes derived from MSC significantly improved functional recovery by promoting endogenous angiogenesis.107 The main mechanisms involved in the proangiogenic effect of MSC-derived exosomes in ischemic conditions involve the activation of the nuclear factor-κB pathway and the transfer of transcriptionally active STAT3 (Figure 2).95,96 In addition, exosomes that were isolated from bone marrow MSC were able to reduce infarct size and enhance blood flow recovery by increasing the density of functional capillaries after myocardial infarction in rats.105
Figure 2.
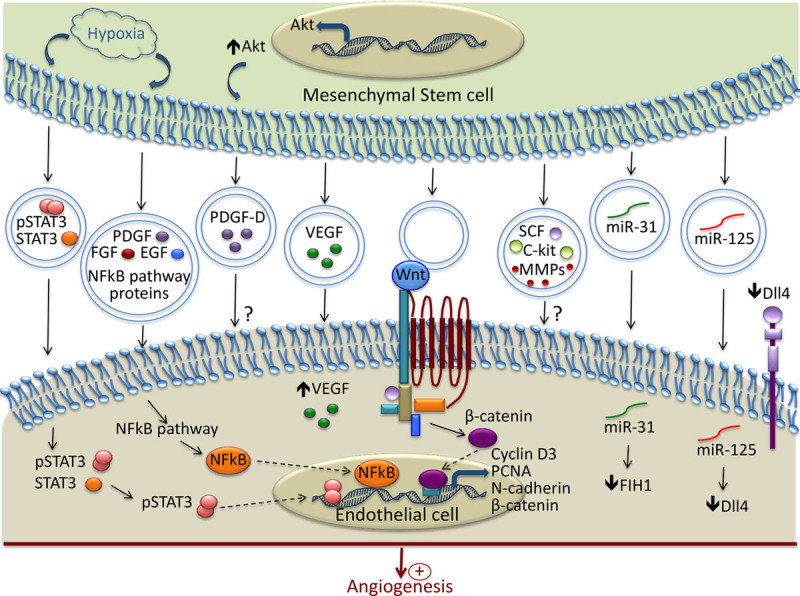
Mechanisms involved in the modulation of Angiogenesis by MSC-derived extracellular vesicles (EVs). In response to hypoxia, mesenchymal stem/stromal cells release EVs containing active pSTAT3 and nuclear factor (NF)-κB pathway–associated proteins, which are transferred to recipient endothelial cell (EC) and promote the transcription of proangiogenic proteins. EVs contain and transfer several growth factors to EC (PDGF, FGF, EGF, VEGF, SCF, and c-kit). Wnt is present in the EVs and through interaction with its receptor, promotes the transcription of several molecules involved in angiogenesis. EVs stimulate vessel formation through the transfer of various micro-RNA. Among them, miR-31 acts by suppressing the factor inhibiting HIF-1α, and miR-125 promotes tip cell specification by suppressing Delta-like 4 (Dll4). ? indicates that the exact mechanisms of their transfer and the underlying signaling pathways are not completely described; EGF, epidermal growth factor; FGF, fibroblast growth factor; FIH, factor inhibiting HIF-1α; HIF, hypoxia inducible factor; PCNA, proliferating cell nuclear antigen; PDGF, platelet-derived growth factor; SCF, stem cell factor; and VEGF, vascular endothelial growth factor.
Another important source of MSC is the umbilical cord. A hypoxia treatment of umbilical cord–derived MSC increased their production of EVs with proangiogenic potential. These EVs enhanced in vitro capillary-like structure formation and further improved blood flow recovery in a rat hindlimb ischemia model.108 After unilateral kidney ischemia in rats, the intravenous injection of EVs derived from umbilical cord MSC had protective effects by increasing the capillary vessel density through the delivery of VEGF.73 The exosomes released from Akt-overexpressing MSC derived from umbilical cord improved cardiac function after intravenous infusion in a rat model of acute myocardial infarction. In addition, the exosomes isolated from Akt-overexpressing MSC stimulated EC migration, proliferation, and tube-like formation in vitro, but they also increased the formation of blood vessels in vivo. This study identified PDGF-D (platelet-derived growth factor-D) as an important player in the exosome-mediated stimulation of angiogenesis.75 The activation of the Wnt4/β-catenin pathway was reported as another mechanism involved in the proangiogenic effect of human umbilical cord MSC-derived exosomes (Figure 2).94
Exosomes that were isolated from adipose tissue–derived MSC were also shown to promote angiogenesis in vitro and in vivo. This proangiogenic effect was because of the transfer of miR-125a that promoted tip cell specification through the direct suppression of δ-like 4 (Figure 2).65 The angiogenic potential of EVs can be greatly improved by modulating the culture conditions of MSC. Thus, PDGF treatment was shown to modulate EV protein composition, leading to an increased c-kit, SCF (stem cell factor), and metalloprotease content and, therefore, it enhanced their proangiogenic potential.76 Preconditioning of the adipose-derived stem cells with an endothelial differentiation medium upregulated the release of microvesicles and their proangiogenic properties through the delivery of miR-31 and the suppression of factor inhibiting HIF-1α (Figure 2).66
Induced pluripotent stem cells present an unlimited cellular source for MSC generation. Interestingly, it was reported that exosomes derived from induced pluripotent stem cells–differentiated MSC promoted angiogenesis in osteoporotic rats.109
Platelet-Derived EVs
In addition to their key role in hemostasis and thrombosis, platelets are involved in several biological processes such as inflammation, angiogenesis, and tissue regeneration. During the past 12 years, the role of platelet EVs in angiogenesis has been well documented. Figure 3 shows the main mechanisms involved in the modulation of angiogenesis by platelet-derived EVs. The amount of platelet microvesicles and their proteome are dependent on the stimulus used for their generation.110 Thus, different pathological contexts lead to the release of microparticles with a specific protein content that will further determine the message delivered to the neighboring cells.
Figure 3.
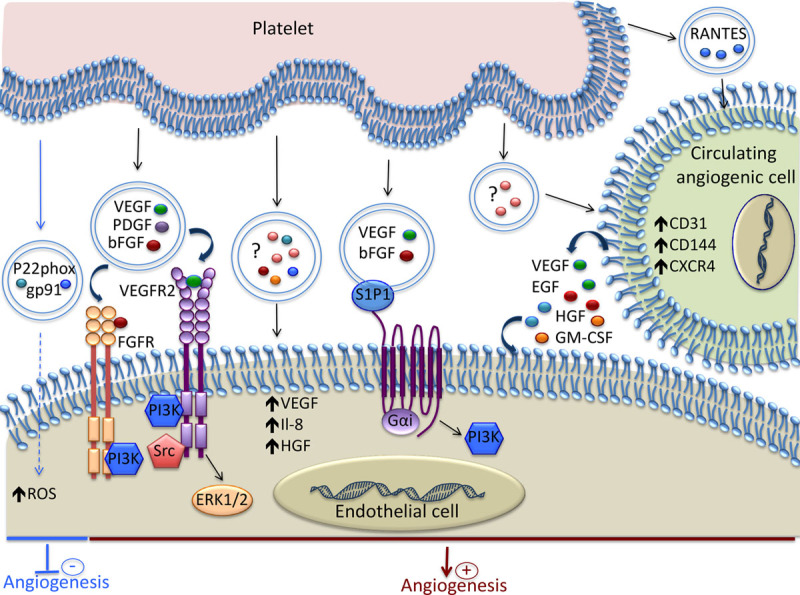
Mechanisms involved in the modulation of Angiogenesis by platelet-derived extracellular vesicles (EVs). Platelet-derived EVs contain various growth factors and chemokines that induce proangiogenic signaling in endothelial cell (EC). Spingosine-1-phosphate (S1P1), present on the EV surface, induces PI3K activation and, together with VEGF and bFGF, promotes angiogenesis. The EVs released by platelets stimulate the proangiogenic potential of circulating angiogenic cells by increasing their expression of both membrane molecules and soluble factors. Platelet-derived EVs can inhibit angiogenesis by transferring the p22phox and gp91 subunits of NADPH oxidase and increasing the oxidative stress in EC. ? indicates that the exact content of the EVs is not reported; bFGF, basic fibroblast growth factor; EGF, epidermal growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HGF, hepatocyte growth factor; NADPH, nicotinamide adenine dinucleotide phosphate; PI3K, phosphoinositide 3-kinase; RANTES, regulated on activation, normal T-cell–expressed and secreted; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor; and VEGFR, vascular endothelial growth factor receptor.
The role of platelet microparticles in angiogenesis was evidenced for the first time in 2004.90 This study showed that the platelet microparticles isolated from healthy donors were capable of promoting proliferation, migration, and tube formation of human umbilical vein EC through the cooperative effect of VEGF, FGF-2, and lipid components, such as spingosine-1-phosphate.90 These effects were essentially mediated via the pertussis toxin-sensitive G protein and the PI3 kinase pathway (Figure 3).
The proangiogenic activity of platelet microparticles was further supported by a study showing that EPC cultures contain platelet microparticles with the capacity to stimulate endothelial tube formation in vitro.101 This proangiogenic effect was attenuated after removing the microparticles by filtration or ultracentrifugation or by specifically inhibiting the formation of the platelet integrin complex αIIbβ3.
In a rat aortic ring model, platelet-derived microparticles exerted proangiogenic effects in a dose-dependent manner through the transfer of cytokines, such as VEGF, FGF-2, and PDGF, and the activation of PI3 kinase, src kinase, and ERK (Figure 3).74 These results were supported by increased in vitro tube-like formation and in vivo angiogenesis using agarose beads that contained platelet microparticles. In addition, in a model of chronic myocardial ischemia in rats, platelet microparticles were shown to increase the number of functional capillaries after injection into the myocardium.74 Platelet microvesicles were also documented as important mediators in tumor progression and angiogenesis in lung cancer through the upregulation of factors involved in tumor vascularization, such as interleukin-8, VEGF, and HGF (hepatocyte growth factor).111
It was also shown that platelet-derived EVs can have deleterious effects on EC. The exosomes isolated from septic patients contain the p22phox and gp91 subunits of NADPH oxidase and were able to produce reactive oxygen species (Figure 3).72 The incubation of these exosomes with EC enhanced their apoptosis. Further studies showed that platelets exposed to lipopolysaccharide or NO, but not thrombin or tumor necrosis factor-α, generated exosomes similar to those recovered from septic patients, and they induced apoptosis in EC through redox signaling.112
It was also shown that platelet-derived microparticles stimulated endogenous stem cell repair mechanisms after middle cerebral artery occlusion in rats. The delivery of platelet microparticles increased angiogenesis and neurogenesis at the infarct zone in a dose-dependent manner.113 These results were supported by another study that demonstrated the protective effect of platelet-derived microparticles against cerebral ischemic reperfusion injury.114
Platelet microparticles can also enhance the regenerative capacity of early outgrowth ECs by modulating their phenotype and secretome, as attested by the increased expression of CD31, VE-cadherin, and CXCR4, and the secretion of VEGF, EGF, HGF, and GM-CSF (granulocyte-macrophage colony-stimulating factor; Figure 3).115 In addition, platelet microparticles increased the ability of early outgrowth ECs to stimulate endothelial migration, proliferation, tube formation, and importantly, endothelial regeneration after injury in vivo. In line with this study, the pretreatment of circulating angiogenic cells with platelet microparticles isolated from peripheral blood of atherosclerotic patients led to increased neovascularization in rats with hindlimb ischemia.77 This proangiogenic effect was shown to be dependent on the release of RANTES by the platelet microparticles (Figure 3).
Leukocyte-Derived EVs
Leukocyte EVs may originate from lymphocytes, monocyte/macrophages, or neutrophils, and they have been shown to actively participate in vascular homeostasis. Several studies, mostly focusing on lymphocytes and monocytes, emphasized that leukocyte EVs may also play a role in angiogenesis. Depending on the stimuli used for their generation, the EVs derived from lymphocytes can exert either pro- or antiangiogenic effects. For instance, lymphocytes undergoing activation and apoptosis release microvesicles with proangiogenic properties, whereas microparticles shed from apoptotic lymphocytes inhibit angiogenesis.69,84,85
The microparticles generated from T lymphocytes after treatment with phytohemagglutinin, phorbol ester, and actinomycin D express Sonic hedgehog and induce the formation of capillary-like structures in vitro through FAK activation and the upregulation of ICAM-1 (intercellular adhesion molecule-1), RhoA, and VEGF.84 These microparticles expressing Sonic hedgehog favored neovascularization in a mouse model of hindlimb ischemia by activating the eNOS pathway and NO production in not only the ischemic area but also the aorta of mice.85 The stimulation of NO production by lymphocyte microparticles carrying Sonic hedgehog also had a beneficial effect in restoring endothelial function in mouse models of angiotensin II–induced hypertension and myocardial ischemia/reperfusion injury.86,87
In contrast, actinomycin D treatment of lymphocytes in the absence of activation signals resulted in a microparticle release that inhibited angiogenesis.69 Thus, in a model of in vivo corneal neovascularization and in vitro aortic rings, the microparticles generated after actinomycin D treatment suppressed angiogenesis through increased reactive oxygen species production, upregulation of CD36 on target EC and the consequent reduction of VEGFR2 and phospho-ERK levels.69 In addition, the antiangiogenic effect of microparticles was shown to present an interesting therapeutic application in pathological angiogenesis, such as retinopathies and tumor angiogenesis.88,89 The inhibition of angiogenesis by T-lymphocyte–derived microparticles was mediated by low-density lipoprotein receptor, as low-density lipoprotein receptor knockdown in target endothelial and tumor cells resulted in decreased microparticle uptake and abrogated the antiangiogenic effect.88,89 In the case of choroidal angiogenesis, it was demonstrated that the inhibitory effect of microparticles was dependent on the production of pigment epithelium-derived growth factor and nerve growth factor by retinal pigment epithelial cells and the activation of p75 neurotrophin receptor in choroidal microvascular cells.116 Recently, the same group evidenced that on CD36-dependent uptake, lymphocyte microparticles can exert their antiangiogenic effect by altering the expression of several angiogenic factors in macrophages.117
The microvesicle derived from monocytes were also shown to promote angiogenesis both in vitro and in vivo through the transfer of miR-150 to EC.67 THP-1 (human acute monocytic leukemia cell line)–derived EVs were also shown to generate monomeric CRP, which is highly angiogenic to vascular EC and therefore might impact on the process of vascularization.79–81 In addition, CD40 ligand–positive microparticles, which were isolated from human atherosclerotic plaques and of mostly macrophage origin, were able to stimulate endothelial proliferation and angiogenesis, suggesting that microparticles could participate in intraplaque neovascularization and plaque vulnerability.78
Erythrocyte-Derived EVs
In addition to their vital role in transporting oxygen to organs and tissues, erythrocytes were shown to participate in angiogenesis.118 A recent study demonstrated that the production of sphingosine-1-phosphate by erythrocytes is essential for vascular stabilization, maturation, and remodeling during embryonic development.118 The process of EV formation occurs during erythrocyte maturation and allows for the selective retention of certain proteins in the plasma membrane and the removal of noxious molecules, such as oxidized proteins.119 Vesicle release is accelerated during erythrocyte senescence and is considered a part of apoptosis-like form in these cells.120 Even though studies addressing the specific role of erythrocyte EVs in angiogenesis are still lacking, some studies suggest that erythrocyte-derived EVs may have a deleterious effect on EC, particularly in some pathological conditions, such as sickle cell disease and malaria. It was reported that EVs derived from malaria-infected erythrocytes altered EC gene expression and barrier properties through the transfer of miR-451a.121 In addition, erythrocyte microparticles were significantly elevated in patients with sickle cell disease, and they expressed more sphingosine-1-phosphate than erythrocyte microparticles from healthy donors.122 Sickle cell disease erythrocyte-derived MP are internalized by myeloid cells and promote proinflammatory cytokine production and EC adhesion in an ERK ½-ICAM-4–mediated fashion. These results suggest that circulating MP may contribute to the inflammation-rooted pathogenesis of the disease.122 In addition, it was evidenced that erythrocyte vesiculation produced microparticles loaded with heme that could be further transferred to EC, inducing oxidative stress and apoptosis.123 Heme-laden microparticles strongly reduced endothelium-dependent vasodilation and induced vasoocclusions in kidneys.123 These observations indicate that microparticles carrying heme may be a source of oxidant stress, contributing to endothelial dysfunction and vascular injury.
Toward EV-Based Therapeutic Angiogenesis
Based on the various studies discussed above, EVs have emerged as a major form of intercellular communication, playing important roles in angiogenesis and cardiovascular homeostasis. Their role in cellular signaling during vascular development, growth, and maturation has been extensively documented and has opened up new perspectives for their therapeutic application in regenerative medicine and tissue engineering as drug delivery vehicles modulating vascularization.
Since the early 2000s, EVs have been tested in clinical trials involving patients with melanoma,124 nonsmall cell lung cancers,125,126 colorectal cancer,126a and type I diabetes mellitus (NCT02138331). Although a small number of patients was included in the studies, these clinical trials demonstrated the feasibility and safety of EV administration in humans. Recently, a prospective clinical trial was initiated to evaluate the effect of autologous plasma-derived exosomes on wound healing in patients with intractable cutaneous ulcers (NCT02565264). This trial will provide important information about the feasibility and the efficiency of EV-based therapy in angiogenesis-related disorders.
Although the pro- and antiangiogenic effects of EVs from various sources have been reported, further in vivo studies are required to better understand the specific role of EVs in exchanging molecules between cell types. Several limitations need to be overcome to progress toward the use of EVs in the therapeutic modulation of angiogenesis. In particular, the standardization of the end product, quality control, and the associated costs remain major issues for EV-based therapies.
Cellular Source and Conditions for EV Production
One of the key points to consider is the choice of the cellular source and the conditions for a suitable quantity of clinical grade EV production. Recent studies suggest that several stimuli and culture conditions play an important role in the biogenesis and secretion of EVs with proangiogenic properties.61,82,96,127 In addition, it is important to evaluate how the patient’s pathological environment impacts the EV properties and therapeutic potential. Even though most of the preclinical works supporting the capacity of EVs for therapeutic angiogenesis have been done with EVs from cells that were isolated from healthy donors, there are emerging data showing that the proangiogenic capacity of MSC-derived EVs are impaired in patients with cardiovascular risk factors.128 Thus, donor-related variability may be responsible for the therapeutic differences observed among comparable EV fractions, requiring the definition of donor inclusion/exclusion criteria and potent markers to able to anticipate the therapeutic effects of EVs in vivo.
EV Concentration
One important question is what concentration of EVs should be used for the stimulation of angiogenesis without inducing any side effects? An overview of the literature shows that the effect of EVs on angiogenesis seems to be dose dependent. For instance, low amounts of endothelial microparticles (2×103 per well) were able to increase tube formation in vitro, whereas a high concentration had an inhibitory effect (2×105 per well).83 Another study reported that endothelial microparticles inhibited angiogenesis in the cultured sections of hearts when concentrations higher than 105 microparticles/mL were used.99 Platelet-derived microparticles exerted proangiogenic effects in vitro when doses between 30 and 100 μg of protein/mL were applied.74 However, when platelet microparticles were used to stimulate vascularization in vivo, a much higher concentration (250 μg/mL) was necessary to stimulate postischemic revascularization of the myocardium.74 In addition, it was reported that MSC-derived exosomes exerted the most potent angiogenic effect in vitro and in vivo at a concentration of 100 μg/mL.65 However, another study showed that a low concentration of MSC exosomes (1–10 μg/mL) was more effective for tube formation than a high concentration (100 μg/mL).95
All these observations demonstrate that the question on EV concentration remains controversial. It is difficult to address this question and compare the results from all these studies, as different culture conditions have been used for EV production and various methods have been used to quantify EVs.
Because of the small size of EVs and the technical limitations of nanosized particle analysis, EV quantification has been challenging to scientists. Several techniques have been used to quantify EVs, such as dynamic light scattering, nanoparticle tracking analysis, flow cytometry, and tunable resistive pulse sensing. However, a universal method for standardized EV quantification is still lacking. The studies mentioned above suggest that the optimal EV concentration for the stimulation of angiogenesis will depend on various factors, such as the cellular source of EVs, the stimulus used to induce EV release, EV content, the way of EV delivery in vivo, and also the pathological microenvironment. Determination of the EV concentration for the stimulation of angiogenesis is hampered not only by the various methods used for EV quantification but also by the different degrees of purity of the EV preparations.
Purity of EV Preparations
Another important aspect to consider is the purity of the different EV populations and the characterization of their content and biological properties. Several techniques have been developed to isolate EVs, such as differential ultracentrifugation, polymer-based precipitation, density gradients, microfiltration, and size-exclusion-based approaches.129 It is important to note that compared with ultracentrifugation and polymer-based precipitation, the development of flotation through density gradients, microfiltration, and size-exclusion chromatography for EV purification marked significant progress in the field.
A pure isolation of EVs from tissue culture supernatants and body fluids is hampered by the presence of macromolecular structures. For instance, the presence of bovine proteins and microvesicles in the culture media can impact the growth and phenotype of cultured cells, thereby influencing the quality and quantity of EVs produced in vitro.130 Furthermore, the RNAs, proteins, and lipids carried by EVs that are present in the bovine serum can also affect the results involving the proteomic, transcriptomic, and functional analysis of EVs. Thus, it is necessary to eliminate the EVs carried by the bovine serum before cell culture. Filtration of the culture media using 0.1-µm filters could help to eliminate microparticles, but not all the exosomes. Switching the cells to serum-free conditions during EV production could allow us to circumvent this problem; however, serum-free conditions may add additional stress to the cells and affect the nature and amount of secreted EVs.
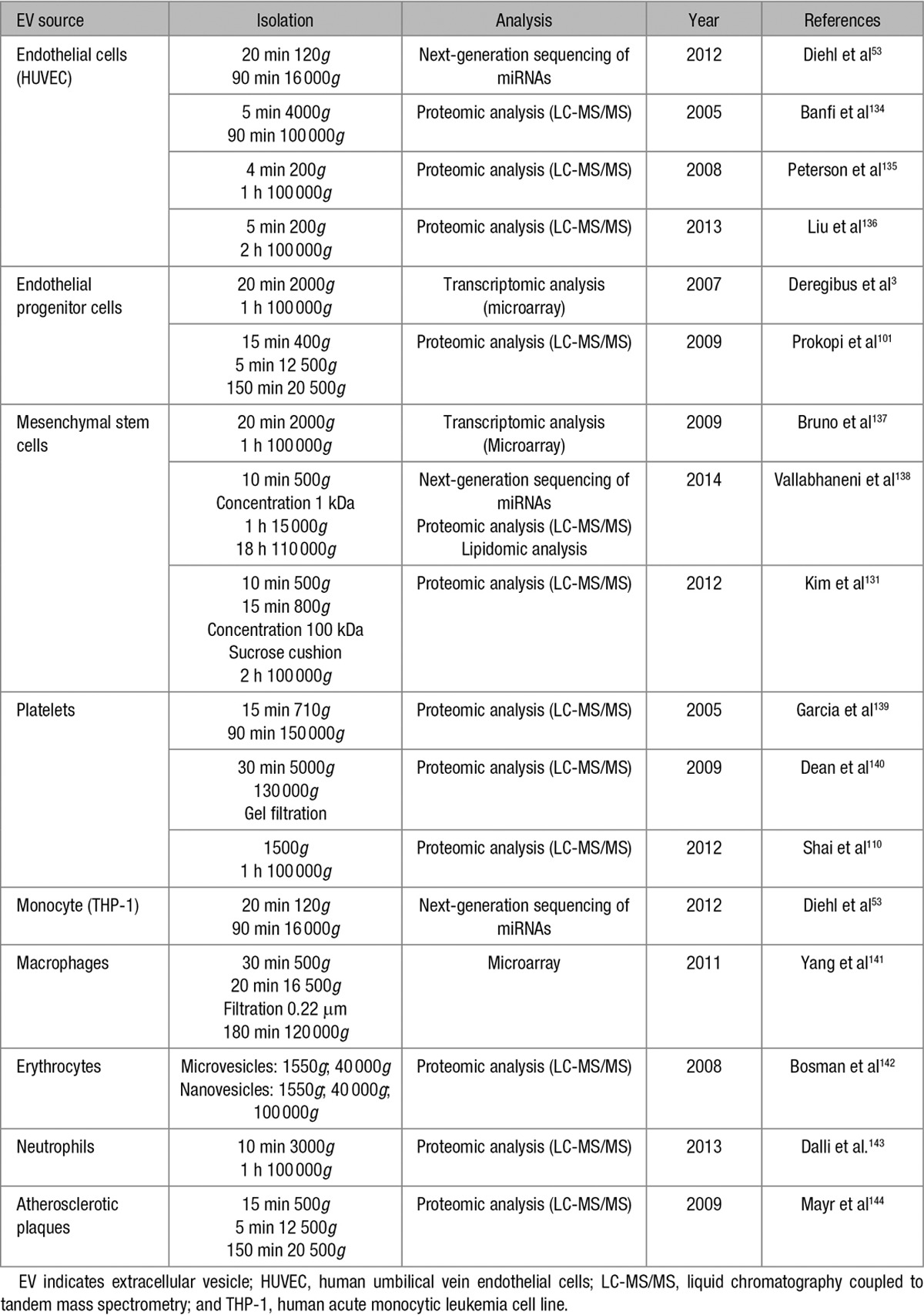
Within the EV population, many distinct subtypes of vesicles exist. However, the currently used methods do not allow for their efficient separation and can negatively impact their integrity, function, and therapeutic potential. Therefore, the development of new, more selective isolation techniques is necessary to achieve good purity in each vesicle population and to determine, in a more precise way, the properties and biological functions of each of them. Most of the molecules responsible for the therapeutic effects of EVs still remain to be identified and extensively characterized. To further explore their potential, proteomic and mRNA/miRNA microarray analysis of each vesicle population may provide useful information.131–133 Table 3 summarizes the main transcriptomic, proteomic, and lipidomic studies on the EVs that were derived from the cell types discussed in this review. In addition, the methods used for the isolation and purification of EVs varies considerably between different research groups and also varies depending on the tissue that they are extracted from. Indeed, considerable heterogeneity among EV preparations has been observed. Therefore, one of the most important challenges before applying EVs in therapeutics is the optimization and standardization of the methods of EV purification, enrichment, and characterization, which will allow increasing the homogeneity within EV preparations and will enable achieving good manufacturing practices.145
Table 3.
Transcriptomic, Proteomic, and Lipidomic Studies on EVs
EV Biodistribution After Administration
A better understanding of EV biodistribution after administration is an essential point for the safe successful clinical application of EVs in tissue regeneration. Although the substantial proangiogenic effects of EVs have been reported by several preclinical studies, our current knowledge of their biodistribution and clearance dynamics still remains limited. Several methods for EV labeling and tracking have been developed, such as reporter systems,146,147 fluorescent dye labeling, loading with superparamagnetic iron oxide nanoparticles148 or radioisotope labeling with clinically validated radio tracers.149 Several studies independently reported the short half-life of exogenous EVs that were artificially introduced in the circulation because of their retention and uptake in organs. In vivo tracking studies showed that intravenously administered EVs derived from kidney embryonic cells are taken up mainly by the kidney, followed by the liver and lung, whereas some EVs were retained by the heart, brain and muscle, albeit in low amounts.146 In addition, EVs derived from red blood cells showed increased uptake by the liver and bone, followed by the skin, muscle, spleen, kidney, and lung, whereas melanoma-derived EVs were mainly taken up by the lungs and spleen.8 Thus, the cellular source and the route of administration are the key determinants of EV tissue distribution.150 Therefore, establishing the best route of EV delivery in normal and pathological conditions may help improve the therapeutic efficiency of EVs. In addition, the efficiency of EV delivery could be improved by a better understanding of the mechanisms of EV uptake and interaction with the target cells, in particular, with the cells of the cardiovascular systems, as few studies are available.
Finally, further studies are necessary to evaluate the risk of adverse effects or side effects after EV administration. Although the EVs used in preclinical studies clearly lack the potential to directly induce tumor growth, the proproliferative and proangiogenic effects of EVs evoke the possibility that they could accelerate or participate in cancer progression. The procoagulant activity of EVs has been the subject of several studies, suggesting that another adverse effect associated with EV administration could be an increased risk of thrombosis. Moreover, because some EVs can both promote or inhibit angiogenesis, preclinical models that allow us to study the involvement of endogenous EVs, in addition to those that are exogenously administrated, are needed to better delineate the benefit/risk balance.
Conclusion
In addition to their role as promising biomarkers in various pathological conditions, EVs are important mediators in intercellular communication in normal physiology and disease. An increasing number of studies has documented the capacity of EVs to actively modulate the angiogenic programs in EC by acting on the key steps of vessel formation. Depending on the cellular source, the conditions in which they have been generated and their content, EVs may have either beneficial or detrimental effects on angiogenesis and tissue regeneration. EVs are released by various cell types, such as ECs, endothelial colony-forming cells, mesenchymal stem cells, platelets, or leucocytes, and are able to deliver powerful proangiogenic signals with subsequent beneficial effects in tissue repair. Specific conditions, such as hypoxia and angiogenic growth factor stimulation, favor the release of EVs with vasculogenic potential. However, some of the EVs produced by ECs, platelets, or lymphocytes can also exert an inhibitory activity on vessel growth, mainly by increasing the oxidative stress in target cells. Although the role of EVs in blood vessel formation is well documented and the main underlying mechanisms are identified, further studies are necessary to determine the precise role of each subpopulation of EVs. Indeed, one of the major challenges that the expanding field of EVs faces is the high diversity of vesicle subtypes and the complexity of specifically studying the characteristics, compositions and biological functions of each of them separately. Importantly, because some EVs can either promote or inhibit angiogenesis, animal models allowing us to study the specific involvement of endogenous EVs are needed to delineate when and how EVs can be protective.
A better understanding of EV biogenesis and the multifaced functions of EVs that defines the specific phenotypic characteristics and active components carried by each subpopulation of EVs will allow us to standardize the process of EV preparation for therapeutic applications. Together with the validation of accurate technologies for the clinical grade manufacturing and quality control of EVs, such advances may provide a better appraisal of the benefit-risk ratio, sustaining the currently emerging therapeutic applications of EVs in regenerative medicine or in angiogenesis-related diseases.
Sources of Funding
This work was supported by the A*MIDEX project (no ANR-11-IDEX-0001-02), funded by the Investissements d’Avenir French Government program, and managed by the French National Research Agency and INSERM.
Disclosures
None.
Footnotes
Extracellular Vesicles
Chantal Boulanger, Guest Editor
Nonstandard Abbreviations and Acronyms
- EC
- endothelial cells
- ECFC
- endothelial colony-forming cells
- EPC
- endothelial progenitor cells
- EVs
- extracellular vesicles
- MSC
- mesenchymal stem/stromal cells
References
- 1.Patel-Hett S, D’Amore PA. Signal transduction in vasculogenesis and developmental angiogenesis. Int J Dev Biol. 2011;55:353–363. doi: 10.1387/ijdb.103213sp. doi: 10.1387/ijdb.103213sp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 4.Gai C, Carpanetto A, Deregibus MC, Camussi G. Extracellular vesicle-mediated modulation of angiogenesis. Histol Histopathol. 2016;31:379–391. doi: 10.14670/HH-11-708. doi: 10.14670/HH-11-708. [DOI] [PubMed] [Google Scholar]
- 5.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 7.Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol Cell Physiol. 2014;306:C621–C633. doi: 10.1152/ajpcell.00228.2013. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- 8.Yáñez-Mó M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3 doi: 10.1002/0471143030.cb0322s30. Unit 3.22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 11.Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KE, Sadik M, Alaarg A, Smith CI, Lehtiö J, El Andaloussi S, Wood MJ, Vader P. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016;6:22519. doi: 10.1038/srep22519. doi: 10.1038/srep22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Théry C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 13.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 14.Andreu Z, Yáñez-Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 16.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–372. doi: 10.1016/j.tcb.2015.01.004. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17:170. doi: 10.3390/ijms17020170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson MC, Hillery CA, Hogg N. Circulating membrane-derived microvesicles in redox biology. Free Radic Biol Med. 2014;73:214–228. doi: 10.1016/j.freeradbiomed.2014.04.017. doi: 10.1016/j.freeradbiomed.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leroyer AS, Anfosso F, Lacroix R, Sabatier F, Simoncini S, Njock SM, Jourde N, Brunet P, Camoin-Jau L, Sampol J, Dignat-George F. Endothelial-derived microparticles: Biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb Haemost. 2010;104:456–463. doi: 10.1160/TH10-02-0111. doi: 10.1160/TH10-02-0111. [DOI] [PubMed] [Google Scholar]
- 20.Curtis AM, Edelberg J, Jonas R, Rogers WT, Moore JS, Syed W, Mohler ER., 3rd. Endothelial microparticles: sophisticated vesicles modulating vascular function. Vasc Med. 2013;18:204–214. doi: 10.1177/1358863X13499773. doi: 10.1177/1358863X13499773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morel O, Toti F, Morel N, Freyssinet JM. Microparticles in endothelial cell and vascular homeostasis: are they really noxious? Haematologica. 2009;94:313–317. doi: 10.3324/haematol.2008.003657. doi: 10.3324/haematol.2009.003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 23.EL Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 24.van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 25.Kim D-K, Kang B, Kim OY, et al. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. doi: 10.1371/journal.pbio.1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Østergaard O, Nielsen CT, Iversen LV, Jacobsen S, Tanassi JT, Heegaard NH. Quantitative proteome profiling of normal human circulating microparticles. J Proteome Res. 2012;11:2154–2163. doi: 10.1021/pr200901p. doi: 10.1021/pr200901p. [DOI] [PubMed] [Google Scholar]
- 28.Jeppesen DK, Nawrocki A, Jensen SG, Thorsen K, Whitehead B, Howard KA, Dyrskjøt L, Ørntoft TF, Larsen MR, Ostenfeld MS. Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors. Proteomics. 2014;14:699–712. doi: 10.1002/pmic.201300452. doi: 10.1002/pmic.201300452. [DOI] [PubMed] [Google Scholar]
- 29.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 30.Urbanelli L, Buratta S, Sagini K, Ferrara G, Lanni M, Emiliani C. Exosome-based strategies for Diagnosis and Therapy. Recent Pat CNS Drug Discov. 2015;10:10–27. doi: 10.2174/1574889810666150702124059. [DOI] [PubMed] [Google Scholar]
- 31.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–4099. doi: 10.1002/pmic.200800109. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 32.Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–1571. doi: 10.1002/pmic.201200329. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- 33.Larson MC, Woodliff JE, Hillery CA, Kearl TJ, Zhao M. Phosphatidylethanolamine is externalized at the surface of microparticles. Biochim Biophys Acta. 2012;1821:1501–1507. doi: 10.1016/j.bbalip.2012.08.017. doi: 10.1016/j.bbalip.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou S, Grillo D, Williams CL, Wasserstrom JA, Szleifer I, Zhao M. Membrane phospholipid redistribution in cancer micro-particles and implications in the recruitment of cationic protein factors. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connor DE, Exner T, Ma DD, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost. 2010;103:1044–1052. doi: 10.1160/TH09-09-0644. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]
- 36.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 37.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 38.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Würdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koppers-Lalic D, Hogenboom MM, Middeldorp JM, Pegtel DM. Virus-modified exosomes for targeted RNA delivery; a new approach in nanomedicine. Adv Drug Deliv Rev. 2013;65:348–356. doi: 10.1016/j.addr.2012.07.006. doi: 10.1016/j.addr.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J Neural Transm (Vienna) 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 41.Nazarenko I, Rupp AK, Altevogt P. Exosomes as a potential tool for a specific delivery of functional molecules. Methods Mol Biol. 2013;1049:495–511. doi: 10.1007/978-1-62703-547-7_37. doi: 10.1007/978-1-62703-547-7_37. [DOI] [PubMed] [Google Scholar]
- 42.Atkin-Smith GK, Tixeira R, Paone S, Mathivanan S, Collins C, Liem M, Goodall KJ, Ravichandran KS, Hulett MD, Poon IK. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun. 2015;6:7439. doi: 10.1038/ncomms8439. doi: 10.1038/ncomms8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng J, Torkamani A, Peng Y, Jones TM, Lerner RA. Plasma membrane associated transcription of cytoplasmic DNA. Proc Natl Acad Sci U S A. 2012;109:10827–10831. doi: 10.1073/pnas.1208716109. doi: 10.1073/pnas.1208716109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iraci N, Leonardi T, Gessler F, Vega B, Pluchino S. Focus on extracellular vesicles: physiological role and signalling properties of extracellular membrane vesicles. Int J Mol Sci. 2016;17:171. doi: 10.3390/ijms17020171. doi: 10.3390/ijms17020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen YJ, Le Bert N, Chitre AA, Koo CX, Nga XH, Ho SS, Khatoo M, Tan NY, Ishii KJ, Gasser S. Genome-derived cytosolic DNA mediates type I interferon-dependent rejection of B cell lymphoma cells. Cell Rep. 2015;11:460–473. doi: 10.1016/j.celrep.2015.03.041. doi: 10.1016/j.celrep.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 46.Byrd AK, Zybailov BL, Maddukuri L, Gao J, Marecki JC, Jaiswal M, Bell MR, Griffin WC, Reed MR, Chib S, Mackintosh SG, MacNicol AM, Baldini G, Eoff RL, Raney KD. Evidence that G-quadruplex DNA accumulates in the cytoplasm and participates in stress granule assembly in response to oxidative stress. J Biol Chem. 2016;291:18041–18057. doi: 10.1074/jbc.M116.718478. doi: 10.1074/jbc.M116.718478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orozco AF, Jorgez CJ, Horne C, Marquez-Do DA, Chapman MR, Rodgers JR, Bischoff FZ, Lewis DE. Membrane protected apoptotic trophoblast microparticles contain nucleic acids: relevance to preeclampsia. Am J Pathol. 2008;173:1595–1608. doi: 10.2353/ajpath.2008.080414. doi: 10.2353/ajpath.2008.080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waldenström A, Gennebäck N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One. 2012;7:e34653. doi: 10.1371/journal.pone.0034653. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernås M, Lötvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5:e15353. doi: 10.1371/journal.pone.0015353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aliotta JM, Pereira M, Li M, Amaral A, Sorokina A, Dooner MS, Sears EH, Brilliant K, Ramratnam B, Hixson DC, Quesenberry PJ. Stable cell fate changes in marrow cells induced by lung-derived microvesicles. J Extracell Vesicles. 2012;1 doi: 10.3402/jev.v1i0.18163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aliotta JM, Pereira M, Johnson KW, de Paz N, Dooner MS, Puente N, Ayala C, Brilliant K, Berz D, Lee D, Ramratnam B, McMillan PN, Hixson DC, Josic D, Quesenberry PJ. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp Hematol. 2010;38:233–245. doi: 10.1016/j.exphem.2010.01.002. doi: 10.1016/j.exphem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 53.Diehl P, Fricke A, Sander L, Stamm J, Bassler N, Htun N, Ziemann M, Helbing T, El-Osta A, Jowett JB, Peter K. Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res. 2012;93:633–644. doi: 10.1093/cvr/cvs007. doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu X, You Y, Li Q, Zeng C, Fu F, Guo A, Zhang H, Zou P, Zhong Z, Wang H, Wu Y, Li Q, Kong F, Chen Z. BCR-ABL1-positive microvesicles transform normal hematopoietic transplants through genomic instability: implications for donor cell leukemia. Leukemia. 2014;28:1666–1675. doi: 10.1038/leu.2014.51. doi: 10.1038/leu.2014.51. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai J, Wu G, Jose PA, Zeng C. Functional transferred DNA within extracellular vesicles. Exp Cell Res. 2016;349:179–183. doi: 10.1016/j.yexcr.2016.10.012. doi: 10.1016/j.yexcr.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, Xiao ZD. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. 2014;289:22258–22267. doi: 10.1074/jbc.M114.588046. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prada I, Meldolesi J. Binding and fusion of extracellular vesicles to the plasma membrane of their cell targets. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17081296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loyer X, Vion AC, Tedgui A, Boulanger CM. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res. 2014;114:345–353. doi: 10.1161/CIRCRESAHA.113.300858. doi: 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- 61.Lombardo G, Dentelli P, Togliatto G, Rosso A, Gili M, Gallo S, Deregibus MC, Camussi G, Brizzi MF. Activated Stat5 trafficking via endothelial cell-derived extracellular vesicles controls IL-3 pro-angiogenic paracrine action. Sci Rep. 2016;6:25689. doi: 10.1038/srep25689. doi: 10.1038/srep25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 63.Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, Tetta C, Camussi G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412–427. doi: 10.1038/ki.2012.105. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 64.van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MA, Pegtel DM, Stoorvogel W, Würdinger T, Verhaar MC. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006, S1. doi: 10.1182/blood-2013-02-478925. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- 65.Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129:2182–2189. doi: 10.1242/jcs.170373. doi: 10.1242/jcs.170373. [DOI] [PubMed] [Google Scholar]
- 66.Kang T, Jones TM, Naddell C, Bacanamwo M, Calvert JW, Thompson WE, Bond VC, Chen YE, Liu D. Adipose-derived stem cells induce angiogenesis via microvesicle transport of miRNA-31. Stem Cells Transl Med. 2016;5:440–450. doi: 10.5966/sctm.2015-0177. doi: 10.5966/sctm.2015-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J, Zhang Y, Liu Y, et al. Microvesicle-mediated transfer of microRNA-150 from monocytes to endothelial cells promotes angiogenesis. J Biol Chem. 2013;288:23586–23596. doi: 10.1074/jbc.M113.489302. doi: 10.1074/jbc.M113.489302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramakrishnan DP, Hajj-Ali RA, Chen Y, Silverstein RL. Extracellular vesicles activate a CD36-dependent signaling pathway to inhibit microvascular endothelial cell migration and tube formation. Arterioscler Thromb Vasc Biol. 2016;36:534–544. doi: 10.1161/ATVBAHA.115.307085. doi: 10.1161/ATVBAHA.115.307085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang C, Mwaikambo BR, Zhu T, Gagnon C, Lafleur J, Seshadri S, Lachapelle P, Lavoie JC, Chemtob S, Hardy P. Lymphocytic microparticles inhibit angiogenesis by stimulating oxidative stress and negatively regulating VEGF-induced pathways. Am J Physiol Regul Integr Comp Physiol. 2008;294:R467–R476. doi: 10.1152/ajpregu.00432.2007. doi: 10.1152/ajpregu.00432.2007. [DOI] [PubMed] [Google Scholar]
- 70.Brodsky SV, Zhang F, Nasjletti A, Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol. 2004;286:H1910–H1915. doi: 10.1152/ajpheart.01172.2003. doi: 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- 71.Mezentsev A, Merks RM, O’Riordan E, Chen J, Mendelev N, Goligorsky MS, Brodsky SV. Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H1106–H1114. doi: 10.1152/ajpheart.00265.2005. doi: 10.1152/ajpheart.00265.2005. [DOI] [PubMed] [Google Scholar]
- 72.Janiszewski M, Do Carmo AO, Pedro MA, Silva E, Knobel E, Laurindo FR. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: a novel vascular redox pathway. Crit Care Med. 2004;32:818–825. doi: 10.1097/01.ccm.0000114829.17746.19. [DOI] [PubMed] [Google Scholar]
- 73.Zou X, Gu D, Xing X, Cheng Z, Gong D, Zhang G, Zhu Y. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am J Transl Res. 2016;8:4289–4299. [PMC free article] [PubMed] [Google Scholar]
- 74.Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67:30–38. doi: 10.1016/j.cardiores.2005.04.007. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 75.Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, Qian H, Xu W, Zhu W. Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl Med. 2017;6:51–59. doi: 10.5966/sctm.2016-0038. doi: 10.5966/sctm.2016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014;12:26. doi: 10.1186/1478-811X-12-26. doi: 10.1186/1478-811X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohtsuka M, Sasaki K, Ueno T, Seki R, Nakayoshi T, Koiwaya H, Toyama Y, Yokoyama S, Mitsutake Y, Chibana H, Itaya N, Okamura T, Imaizumi T. Platelet-derived microparticles augment the adhesion and neovascularization capacities of circulating angiogenic cells obtained from atherosclerotic patients. Atherosclerosis. 2013;227:275–282. doi: 10.1016/j.atherosclerosis.2013.01.040. doi: 10.1016/j.atherosclerosis.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 78.Leroyer AS, Rautou PE, Silvestre JS, Castier Y, Lesèche G, Devue C, Duriez M, Brandes RP, Lutgens E, Tedgui A, Boulanger CM. CD40 ligand+ microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J Am Coll Cardiol. 2008;52:1302–1311. doi: 10.1016/j.jacc.2008.07.032. doi: 10.1016/j.jacc.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 79.Braig D, Nero TL, Koch HG, et al. Transitional changes in the CRP structure lead to the exposure of proinflammatory binding sites. Nat Commun. 2017;8:14188. doi: 10.1038/ncomms14188. doi: 10.1038/ncomms14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turu MM, Slevin M, Matou S, West D, Rodríguez C, Luque A, Grau-Olivares M, Badimon L, Martinez-Gonzalez J, Krupinski J. C-reactive protein exerts angiogenic effects on vascular endothelial cells and modulates associated signalling pathways and gene expression. BMC Cell Biol. 2008;9:47. doi: 10.1186/1471-2121-9-47. doi: 10.1186/1471-2121-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Habersberger J, Strang F, Scheichl A, Htun N, Bassler N, Merivirta RM, Diehl P, Krippner G, Meikle P, Eisenhardt SU, Meredith I, Peter K. Circulating microparticles generate and transport monomeric C-reactive protein in patients with myocardial infarction. Cardiovasc Res. 2012;96:64–72. doi: 10.1093/cvr/cvs237. doi: 10.1093/cvr/cvs237. [DOI] [PubMed] [Google Scholar]
- 82.Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–680. doi: 10.1016/S0002-9440(10)64887-0. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lacroix R, Sabatier F, Mialhe A, Basire A, Pannell R, Borghi H, Robert S, Lamy E, Plawinski L, Camoin-Jau L, Gurewich V, Angles-Cano E, Dignat-George F. Activation of plasminogen into plasmin at the surface of endothelial microparticles: a mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood. 2007;110:2432–2439. doi: 10.1182/blood-2007-02-069997. doi: 10.1182/blood-2007-02-069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soleti R, Benameur T, Porro C, Panaro MA, Andriantsitohaina R, Martínez MC. Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis. 2009;30:580–588. doi: 10.1093/carcin/bgp030. doi: 10.1093/carcin/bgp030. [DOI] [PubMed] [Google Scholar]
- 85.Benameur T, Soleti R, Porro C, Andriantsitohaina R, Martínez MC. Microparticles carrying Sonic hedgehog favor neovascularization through the activation of nitric oxide pathway in mice. PLoS One. 2010;5:e12688. doi: 10.1371/journal.pone.0012688. doi: 10.1371/journal.pone.0012688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marrachelli VG, Mastronardi ML, Sarr M, Soleti R, Leonetti D, Martínez MC, Andriantsitohaina R. Sonic hedgehog carried by microparticles corrects angiotensin II-induced hypertension and endothelial dysfunction in mice. PLoS One. 2013;8:e72861. doi: 10.1371/journal.pone.0072861. doi: 10.1371/journal.pone.0072861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agouni A, Mostefai HA, Porro C, Carusio N, Favre J, Richard V, Henrion D, Martínez MC, Andriantsitohaina R. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J. 2007;21:2735–2741. doi: 10.1096/fj.07-8079com. doi: 10.1096/fj.07-8079com. [DOI] [PubMed] [Google Scholar]
- 88.Yang C, Xiong W, Qiu Q, Shao Z, Shao Z, Hamel D, Tahiri H, Leclair G, Lachapelle P, Chemtob S, Hardy P. Role of receptor-mediated endocytosis in the antiangiogenic effects of human T lymphoblastic cell-derived microparticles. Am J Physiol Regul Integr Comp Physiol. 2012;302:R941–R949. doi: 10.1152/ajpregu.00527.2011. doi: 10.1152/ajpregu.00527.2011. [DOI] [PubMed] [Google Scholar]
- 89.Yang C, Gagnon C, Hou X, Hardy P. Low density lipoprotein receptor mediates anti-VEGF effect of lymphocyte T-derived microparticles in Lewis lung carcinoma cells. Cancer Biol Ther. 2010;10:448–456. doi: 10.4161/cbt.10.5.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim HK, Song KS, Chung JH, Lee KR, Lee SN. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124:376–384. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 91.Arderiu G, Peña E, Badimon L. Angiogenic microvascular endothelial cells release microparticles rich in tissue factor that promotes postischemic collateral vessel formation. Arterioscler Thromb Vasc Biol. 2015;35:348–357. doi: 10.1161/ATVBAHA.114.303927. doi: 10.1161/ATVBAHA.114.303927. [DOI] [PubMed] [Google Scholar]
- 92.Zhang J, Chen C, Hu B, Niu X, Liu X, Zhang G, Zhang C, Li Q, Wang Y. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci. 2016;12:1472–1487. doi: 10.7150/ijbs.15514. doi: 10.7150/ijbs.15514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li X, Jiang C, Zhao J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J Diabetes Complications. 2016;30:986–992. doi: 10.1016/j.jdiacomp.2016.05.009. doi: 10.1016/j.jdiacomp.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 94.Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, Zhu Y, Wu L, Pan Z, Zhu W, Qian H, Xu W. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015;4:513–522. doi: 10.5966/sctm.2014-0267. doi: 10.5966/sctm.2014-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anderson JD, Johansson HJ, Graham CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappab signaling. Stem Cells. 2016;34:601–613. doi: 10.1002/stem.2298. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24:1635–1647. doi: 10.1089/scd.2014.0316. doi: 10.1089/scd.2014.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3:a006569. doi: 10.1101/cshperspect.a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RC, Sargent I, Li JL, Harris AL. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 99.Ou ZJ, Chang FJ, Luo D, Liao XL, Wang ZP, Zhang X, Xu YQ, Ou JS. Endothelium-derived microparticles inhibit angiogenesis in the heart and enhance the inhibitory effects of hypercholesterolemia on angiogenesis. Am J Physiol Endocrinol Metab. 2011;300:E661–E668. doi: 10.1152/ajpendo.00611.2010. doi: 10.1152/ajpendo.00611.2010. [DOI] [PubMed] [Google Scholar]
- 100.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 101.Prokopi M, Pula G, Mayr U, Devue C, Gallagher J, Xiao Q, Boulanger CM, Westwood N, Urbich C, Willeit J, Steiner M, Breuss J, Xu Q, Kiechl S, Mayr M. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732. doi: 10.1182/blood-2009-02-205930. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- 102.Critser PJ, Yoder MC. Endothelial colony-forming cell role in neoangiogenesis and tissue repair. Curr Opin Organ Transplant. 2010;15:68–72. doi: 10.1097/MOT.0b013e32833454b5. doi: 10.1097/MOT.0b013e32833454b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ranghino A, Cantaluppi V, Grange C, Vitillo L, Fop F, Biancone L, Deregibus MC, Tetta C, Segoloni GP, Camussi G. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol. 2012;25:75–85. doi: 10.1177/039463201202500110. doi: 10.1177/039463201202500110. [DOI] [PubMed] [Google Scholar]
- 104.Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MC, Galimi F, Romagnoli R, Salizzoni M, Tetta C, Segoloni GP, Camussi G. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant. 2012;21:1305–1320. doi: 10.3727/096368911X627534. doi: 10.3727/096368911X627534. [DOI] [PubMed] [Google Scholar]
- 105.Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem. 2015;37:2415–2424. doi: 10.1159/000438594. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 106.Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–867. doi: 10.3171/2014.11.JNS14770. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang HC, Liu XB, Huang S, Bi XY, Wang HX, Xie LX, Wang YQ, Cao XF, Lv J, Xiao FJ, Yang Y, Guo ZK. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21:3289–3297. doi: 10.1089/scd.2012.0095. doi: 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, Hu B, Wang Y, Li X. Exosomes Secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12:836–849. doi: 10.7150/ijbs.14809. doi: 10.7150/ijbs.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shai E, Rosa I, Parguiña AF, Motahedeh S, Varon D, García Á. Comparative analysis of platelet-derived microparticles reveals differences in their amount and proteome depending on the platelet stimulus. J Proteomics. 2012;76 Spec No:287–296. doi: 10.1016/j.jprot.2012.02.030. doi: 10.1016/j.jprot.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 111.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 112.Gambim MH, do Carmo Ade O, Marti L, Veríssimo-Filho S, Lopes LR, Janiszewski M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care. 2007;11:R107. doi: 10.1186/cc6133. doi: 10.1186/cc6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hayon Y, Dashevsky O, Shai E, Brill A, Varon D, Leker RR. Platelet microparticles induce angiogenesis and neurogenesis after cerebral ischemia. Curr Neurovasc Res. 2012;9:185–192. doi: 10.2174/156720212801619018. [DOI] [PubMed] [Google Scholar]
- 114.Shan LY, Li JZ, Zu LY, Niu CG, Ferro A, Zhang YD, Zheng LM, Ji Y. Platelet-derived microparticles are implicated in remote ischemia conditioning in a rat model of cerebral infarction. CNS Neurosci Ther. 2013;19:917–925. doi: 10.1111/cns.12199. doi: 10.1111/cns.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mause SF, Ritzel E, Liehn EA, Hristov M, Bidzhekov K, Müller-Newen G, Soehnlein O, Weber C. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122:495–506. doi: 10.1161/CIRCULATIONAHA.109.909473. doi: 10.1161/CIRCULATIONAHA.109.909473. [DOI] [PubMed] [Google Scholar]
- 116.Tahiri H, Yang C, Duhamel F, Omri S, Picard E, Chemtob S, Hardy P. p75 neurotrophin receptor participates in the choroidal antiangiogenic and apoptotic effects of T-lymphocyte-derived microparticles. Invest Ophthalmol Vis Sci. 2013;54:6084–6092. doi: 10.1167/iovs.13-11896. doi: 10.1167/iovs.13-11896. [DOI] [PubMed] [Google Scholar]
- 117.Tahiri H, Omri S, Yang C, et al. Lymphocytic microparticles modulate angiogenic properties of macrophages in laser-induced choroidal neovascularization. Sci Rep. 2016;6:37391. doi: 10.1038/srep37391. doi: 10.1038/srep37391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xiong Y, Yang P, Proia RL, Hla T. Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J Clin Invest. 2014;124:4823–4828. doi: 10.1172/JCI77685. doi: 10.1172/JCI77685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Delobel J, Prudent M, Rubin O, Crettaz D, Tissot JD, Lion N. Subcellular fractionation of stored red blood cells reveals a compartment-based protein carbonylation evolution. J Proteomics. 2012;76 Spec No:181–193. doi: 10.1016/j.jprot.2012.05.004. doi: 10.1016/j.jprot.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 120.Föller M, Huber SM, Lang F. Erythrocyte programmed cell death. IUBMB Life. 2008;60:661–668. doi: 10.1002/iub.106. doi: 10.1002/iub.106. [DOI] [PubMed] [Google Scholar]
- 121.Mantel PY, Hjelmqvist D, Walch M, et al. Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat Commun. 2016;7:12727. doi: 10.1038/ncomms12727. doi: 10.1038/ncomms12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Awojoodu AO, Keegan PM, Lane AR, Zhang Y, Lynch KR, Platt MO, Botchwey EA. Acid sphingomyelinase is activated in sickle cell erythrocytes and contributes to inflammatory microparticle generation in SCD. Blood. 2014;124:1941–1950. doi: 10.1182/blood-2014-01-543652. doi: 10.1182/blood-2014-01-543652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Camus SM, De Moraes JA, Bonnin P, et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood. 2015;125:3805–3814. doi: 10.1182/blood-2014-07-589283. doi: 10.1182/blood-2014-07-589283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Escudier B, Dorval T, Chaput N, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Besse B, Charrier M, Lapierre V, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5:e1071008. doi: 10.1080/2162402X.2015.1071008. doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126a.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther J Am Soc Gene Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Anderson JD, Johansson HJ, Graham CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappab signaling. Stem Cells. 2016;34:601–613. doi: 10.1002/stem.2298. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Togliatto G, Dentelli P, Gili M, Gallo S, Deregibus C, Biglieri E, Iavello A, Santini E, Rossi C, Solini A, Camussi G, Brizzi MF. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: impact on clinical applications. Int J Obes (Lond) 2016;40:102–111. doi: 10.1038/ijo.2015.123. doi: 10.1038/ijo.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gardiner C, Di Vizio D, Sahoo S, Théry C, Witwer KW, Wauben M, Hill AF. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5:32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shelke GV, Lässer C, Gho YS, Lötvall J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, Hwang D, Kim KP, Kim DW. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839–849. doi: 10.1021/pr200682z. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 132.Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O’Brien D, van Wijnen AJ, Lerman LO. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551:55–64. doi: 10.1016/j.gene.2014.08.041. doi: 10.1016/j.gene.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Banfi C, Brioschi M, Wait R, Begum S, Gianazza E, Pirillo A, Mussoni L, Tremoli E. Proteome of endothelial cell-derived procoagulant microparticles. Proteomics. 2005;5:4443–4455. doi: 10.1002/pmic.200402017. doi: 10.1002/pmic.200402017. [DOI] [PubMed] [Google Scholar]
- 135.Peterson DB, Sander T, Kaul S, Wakim BT, Halligan B, Twigger S, Pritchard KA, Jr, Oldham KT, Ou JS. Comparative proteomic analysis of PAI-1 and TNF-alpha-derived endothelial microparticles. Proteomics. 2008;8:2430–2446. doi: 10.1002/pmic.200701029. doi: 10.1002/pmic.200701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu Y, Huang W, Zhang R, Wu J, Li L, Tang Y. Proteomic analysis of TNF-α-activated endothelial cells and endothelial microparticles. Mol Med Rep. 2013;7:318–326. doi: 10.3892/mmr.2012.1139. doi: 10.3892/mmr.2012.1139. [DOI] [PubMed] [Google Scholar]
- 137.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vallabhaneni KC, Penfornis P, Dhule S, Guillonneau F, Adams KV, Mo YY, Xu R, Liu Y, Watabe K, Vemuri MC, Pochampally R. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6:4953–4967. doi: 10.18632/oncotarget.3211. doi: 10.18632/oncotarget.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Garcia BA, Smalley DM, Cho H, Shabanowitz J, Ley K, Hunt DF. The platelet microparticle proteome. J Proteome Res. 2005;4:1516–1521. doi: 10.1021/pr0500760. doi: 10.1021/pr0500760. [DOI] [PubMed] [Google Scholar]
- 140.Dean WL, Lee MJ, Cummins TD, Schultz DJ, Powell DW. Proteomic and functional characterisation of platelet microparticle size classes. Thromb Haemost. 2009;102:711–718. doi: 10.1160/TH09-04-243. doi: 10.1160/TH09-04-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang JD, Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bosman GJ, Lasonder E, Luten M, Roerdinkholder-Stoelwinder B, Novotný VM, Bos H, De Grip WJ. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–835. doi: 10.1111/j.1537-2995.2007.01630.x. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 143.Dalli J, Montero-Melendez T, Norling LV, Yin X, Hinds C, Haskard D, Mayr M, Perretti M. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol Cell Proteomics. 2013;12:2205–2219. doi: 10.1074/mcp.M113.028589. doi: 10.1074/mcp.M113.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mayr M, Grainger D, Mayr U, Leroyer AS, Leseche G, Sidibe A, Herbin O, Yin X, Gomes A, Madhu B, Griffiths JR, Xu Q, Tedgui A, Boulanger CM. Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ Cardiovasc Genet. 2009;2:379–388. doi: 10.1161/CIRCGENETICS.108.842849. doi: 10.1161/CIRCGENETICS.108.842849. [DOI] [PubMed] [Google Scholar]
- 145.Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol. 2016;228:R57–R71. doi: 10.1530/JOE-15-0201. doi: 10.1530/JOE-15-0201. [DOI] [PubMed] [Google Scholar]
- 146.Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW, Tannous BA, Breakefield XO. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483–494. doi: 10.1021/nn404945r. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lai CP, Kim EY, Badr CE, Weissleder R, Mempel TR, Tannous BA, Breakefield XO. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun. 2015;6:7029. doi: 10.1038/ncomms8029. doi: 10.1038/ncomms8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hu L, Wickline SA, Hood JL. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn Reson Med. 2014;74:266–271 doi: 10.1002/mrm.25376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hwang do W, Choi H, Jang SC, Yoo MY, Park JY, Choi NE, Oh HJ, Ha S, Lee YS, Jeong JM, Gho YS, Lee DS. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using (99m)Tc-HMPAO. Sci Rep. 2015;5:15636. doi: 10.1038/srep15636. doi: 10.1038/srep15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wiklander OP, Nordin JZ, O’Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]


