Abstract:
Alpha–melanocyte-stimulating hormone (α-MSH) is a protein with known capacity for protection against cardiovascular ischemia–reperfusion (I/R) injury. This investigation evaluates the capacity of α-MSH to mitigate I/R effects in an isolated working rat heart model and determine the dependency of these alterations on the activity of heme oxygenase-1 (HO-1, hsp-32), a heat shock protein that functions as a major antioxidant defense molecule. Healthy male Sprague Dawley rats were used for all experiments. After treatment with selected doses of α-MSH, echocardiographic examinations were performed on live, anesthetized animals. Hearts were harvested from anesthetized rats pretreated with α-MSH and/or the HO-1 inhibitor SnPP, followed by cardiac function assessment on isolated working hearts, which were prepared using the Langendorff protocol. Induction of global ischemia was performed, followed by during reperfusion assessment of cardiac functions. Determination of incidence of cardiac arrhythmias was made by electrocardiogram. Major outcomes include echocardiographic data, suggesting that α-MSH has mild effects on systolic parameters, along with potent antiarrhythmic effects. Of particular significance was the specificity of dilatative effects on coronary vasculature, and similar outcomes of aortic ring experiments, which potentially allow different doses of the compound to be used to selectively target various portions of the vasculature for dilation.
Key Words: alpha–melanocyte-stimulating hormone, isolated working heart, echocardiography, vasodilatation
INTRODUCTION
Alpha–melanocyte-stimulating hormone (α-MSH) is a melanocortin family neuropeptide, expressed ubiquitously at various levels by a wide variety of cells.1 The biologically active protein is produced by proteolytic cleavage of adrenocorticotropic hormone, the cleavage product of proopiomelanocortin.2 The compound exerts its effect by interacting nonselectively as a full agonist with melanocortin receptors. Its major effect in normal homeostasis includes hair and skin pigmentation (through MC1 receptor activation), reproductive function, food intake regulation, and regulation of energy metabolism.3 The most significant of its functions in the context of this investigation is its role as an endogenous countermeasure to ischemia–reperfusion (I/R) injury.4,5 For example, rat studies of cardiac and hepatic responses in melanocortin-induced modulation of JAK/ERK/STAT signaling in animals subjected to extended myocardial ischemia/reperfusion revealed left ventricular cardioprotective abatement of inflammatory tissue damage by tumor necrosis factor–α and apoptotic cell depletion. There are protective effects mediated substantially through α-MSH–induced HO-1 protein expression and enzyme activity.6,7 Related studies have underscored the role of HO-1 in particular as an essential component of protection against I/R injury to cardiovascular tissue.8–11 Based on these findings, and in the context of ongoing efforts by authors of this report to characterize HO-1–dependent processes with applications in human medicine,12 this study was undertaken to evaluate the role of HO-1 in α-MSH–mediated cardiovascular effects. Here, isolated working hearts harvested from pretreated rats were subjected to I/R injury and evaluated for changes in cardiac physiological outcomes indicative of levels of adaptation to I/R-mediated disruption of normal cardiovascular function. The dependency of these changes on HO-1 activity was determined by selective use of the HO-1 inhibitor, SnPP.
METHODS
Animals
Male Sprague Dawley rats weighing 300–350 g (Charles River International, Inc, Wilmington, MA) were used for all experiments in this study. The animals were housed at room temperature (approximately 25°C), with lighting set to alternating dark and light periods of 12 hours. The rats were maintained on normal rodent chow and tap water ad libitum. All animals received humane care, in compliance with the “Principles of Laboratory Animal Care” according to the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals, formulated by the National Academy of Sciences, and published by the National Institute of Health (NIH Publication No. 86-23, revised 1985). All protocols involving animal use were approved by the Committee of Animal Research, University of Debrecen, Hungary (DE MÁB 45/2001 and DE MÁB 35/2007).
Chemical Reagents
α-MSH (CAS Number 581-05-5) was obtained from Sigma-Aldrich Ltd (St. Louis, MO), and Sn(IV) protoporphyrin IX dichloride (SnPP, MFCD#: MFCD00673909) was provided by Frontier Scientific Inc (Logan, UT). All other chemicals and buffer solutions were obtained from Sigma-Aldrich Ltd (Budapest, Hungary). Reagents used for isolated aortic ring experiments included the following: phenylephrine, a selective alpha adrenergic receptor agonist; α-MSH; Nω-nitro-l-arginine (NOARG), a nitric oxide (NO) synthase inhibitor; indomethacin (INDO), a cyclooxygenase inhibitor; and salts for Krebs–Henseleit buffer (Krebs solution). The Krebs solution used in these experiments was composed of NaCl: 118 mM, KCl: 4.7 mM, CaCl2: 2.5 mM, NaH2PO4: 1 mM, MgCl2: 1.2 mM, NaHCO3: 24.9 mM, glucose: 11.5 mM, and ascorbic acid: 0.1 mM, dissolved in redistilled water. Phenylephrine was dissolved in Krebs solution, whereas α-MSH was dissolved in Krebs solution after dispersed in 20 μL acetic acid:water (1:9) solution (vol/vol). NOARG and INDO were dissolved in 96% ethanol, and then NOARG solution was further diluted in physiological salt solution.
Echocardiography
Echocardiographic examinations were performed on animals anesthetized with a mixture of ketamine and xylazine (50, 5 mg/kg), administered intramuscularly. Rats were positioned in a dorsal decubitus position, and the chest of each was shaved. Echocardiographic measurements were performed using Vivid E9 ultrasound system (GE Healthcare, Little Chalfont, United Kingdom), with an i13L type probe designed for rodent models at a working frequency of 14 MHz. Each examination was completed during a 20-minute time interval. Conventional measurements were made in 2 dimensions and M-mode, using standard views. Parasternal long axis views were recorded to ensure that the mitral and aortic valves, as well as the apexes were visualized. The parasternal short axis views were recorded at the midpapillary muscle level. M-mode acquisitions were performed at the midpapillary muscle level, either in parasternal long or short axis views. Measurements included assessment of interventricular and left ventricular free-wall thickness in diastole and systole (IVSs, IVSd), left ventricular internal diameter at end-diastole (LVIDd), and end-systole (LVIDs). End-systolic volume, end-diastolic volume, stroke volume (SV), and left ventricular mass were calculated. Fractional shortening was computed using the equation fractional shortening (FS) = [(LVIDd − LVIDs)/LVIDd] × 100, and ejection fraction was calculated using the formula (Teiholz): ejection fraction (EF) = (LVEDD2−LVESD2)/LVIDD2. All measurements were averaged over 3–5 consecutive cardiac cycles. Analyses of data were conducted by a trained cardiologist, who was blinded to experimental conditions to ensure objective interpretations.
Isolated Working Heart Preparation
Rats were anesthetized with a mixture of ketamine–xylazine (50/5 mg/kg) and administered heparin (1000 IU/kg) intravenously through the dorsal penile vein. After thoracotomy, hearts were excised, and placed into ice-cold perfusion buffer followed by aortic cannulation. Perfusion of hearts was conducted according to the Langendorff protocol for a 10-minute washout period at a constant perfusion pressure equivalent to 100 kPa. The perfusion medium was a modified Krebs–Henseleit bicarbonate buffer (118 mM NaCl, 4.7 mM KCl, 1.7 mM CaCl2, 25 mM NaHCO3, 0.36 mM KH2PO4, 1.2 mM MgSO4, and 10 mM glucose). The Langendorff apparatus was switched to “working” mode after the washout period as previously described.13 Baseline cardiac functions (as described below) were then assessed. In protocols I and II, these procedures were followed by 30 minutes of global ischemia and 120 minutes of reperfusion, during which cardiac parameters were measured at the 30-, 60-, 90-, and 120-minute timepoints. In protocol III, after recording baseline heart functions, instead of ischemia/reperfusion, 1 mL α-MSH solution (in concentration of 1 μmol/L) was injected directly into the system, through the aortic cannula of the apparatus, to monitor direct effect of α-MSH administration (see also Experimental Design).
Induction of Global Ischemia and Reperfusion in Isolated Hearts
Induction of global ischemia was performed during isolated working heart procedures of protocols I and II (see Isolated Working Heart Preparation and Experimental Design). After measurement of baseline cardiac functions, the atrial inflow and aortic outflow lines were clamped at a point close to the origin of the atrial and aortic cannulas, respectively, and the peristaltic pump (MASTERFLEX, Vernon Hills, IL) was halted. Reperfusion was initiated by unclamping the atrial inflow and aortic outflow lines, and starting the peristaltic pump. To prevent the myocardium from drying out during normothermic global ischemia, the thermostated glassware (in which hearts were suspended) was covered and the vapor content was kept at a constant level. Cardiac function was recorded and monitored throughout the experimental period using a computerized array consisting of silver electrodes and pressure transducers connected directly to isolated hearts (PowerLab; ADInstruments, Castle Hill, Australia). Before ischemia and during reperfusion, the values of heart rate (HR), coronary flow (CF), and aortic flow (AF) were registered. AF was measured by a timed collection of the buffer pumped out from the heart, and CF rate was measured by a timed collection of the coronary perfusate, dripped from the heart. Preselected exclusion criteria for these experiments were conducted to exclude isolated working hearts if ventricular arrhythmias occurred before the induction of ischemia.
Determination of Cardiac Arrhythmia Incidence
During evaluation of isolated working hearts, epicardial electrocardiogram (ECG) measurements were monitored using 2 silver electrodes attached directly to the heart. ECG readings were analyzed to determine the incidence of cardiac arrhythmias. Arrhythmias were analyzed based on their occurrence using a quantal approach: whether there was at least 1 arrhythmic event or not. Arrhythmias (ie, at least 1 arrhythmic event) were further classified into 2 groups defined as follows: (1) if the pump function of the heart recovered spontaneously from the arrhythmic event(s), it was considered to have “non-severe” arrhythmia and (2) if the heart failed to recover its pump function, it was considered to have “severe” arrhythmia, regardless of the type or mechanism of the arrhythmia or the number of arrhythmic events.
Isolated Aortic Ring Experiments
For each experiment, a rat was guillotined and then the abdominal aorta rapidly excised. Aortas from each animal were processed by sectioning 6, approximately 2-mm-wide ring segments from the vessels. One of the rings was deprived from its intimal layer using a small cotton swab. All rings were mounted horizontally at a resting tension of 10 mN, using a wire instrument, in a 10 mL vertical organ chamber (Experimetria TSZ-04) containing Krebs solution, oxygenated with 95% O2 and 5% CO2 (36°C; pH 7.4). The isometric contractile force of the circulatory muscle layers was measured by a transducer (Experimetria SD-01) and strain gauge (Experimetria SG-01D), and recorded by a polygraph (Medicor R-61 6CH Recorder).
Determination of Infarcted Areas of Isolated Hearts
Triphenyl-tetrazolium chloride (TTC) staining was used to determine infarcted areas of each heart, as a direct, postsacrifice approach. One hundred milliliters of 1% TTC solution in phosphate buffer (Na2HPO4 88 mM, NaH2PO4 1.8 mM) was administered directly through the side arm of the aortic cannula at the endpoint of isolated working heart procedure. TTC stained viable myocardium deep red, whereas potentially infarcted areas stayed pale as it was seen during the analysis. Hearts were stored at −80°C for a week and then sliced transversely to the apico-basal axis into 2–3-mm thick sections, weighed, blotted dry, and placed in between microscope slides, and scanned on a Hewlett-Packard Scanjet single pass flatbed scanner (Hewlett-Packard, Palo Alto, CA). Using NIH 1.61 image processing software, each image was subjected to equivalent degrees of background subtraction, brightness, and contrast enhancement for improved clarity and distinctness. Infarcted areas (pale areas, white pixels) of each slice were traced, and the respective areas were calculated by pixel density analysis. Infarcted areas and total area were measured by computerized planometry software: Scion for Windows Densitometry Image program (version Alpha 4.0.3.2, Scion Corporation, Meyer Instruments, Inc, Houston, TX). Infarct size was expressed as a percentage ratio of the infarcted zone to the total area in each heart (percentage of pixels).
Measurement of HO-1 Enzyme Activity in Heart Tissue
Activity of heme oxygenase-1 enzyme in myocardial tissue was measured according to the following general protocol: Tissue samples were homogenized in a solution containing HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) 10 mM, sucrose 32 mM, DTT (Dithiothreitol) 1 mM, EDTA 0.1 mM, soybean trypsin inhibitor 10 μg/mL, leupeptin 10 μg/mL, aprotinin 2 μg/mL, and pH 7.4 (homogentisate buffer). All reagents were purchased from Sigma-Aldrich, St. Louis, MO. The supernatant was collected by centrifugation of the homogenate for 30 minutes at 20,000g at 4°C. Assessment of heme oxygenase activity was done on each sample of supernatant, according to the procedures used by Tenhunen et al.14 Briefly, HO-1 enzyme activity was measured using a computer-based, spectrophotometric analysis of bilirubin formation from heme. This HO-1 enzymatic measurement protocol used a reaction mixture containing an aliquot of the supernatant, plus glucose-6-phosphate 2 mM, glucose-6-phosphate dehydrogenase 0.14 U/mL, heme 15 μM, NADPH 150 μM, rat liver cytosol as a source of biliverdin reductase 120 μg/mL, MgCl2 2 mM, and KH2PO4 100 mM. Each reaction mixture was incubated for 1 hour in the dark. The reactions were arrested by placing the samples on ice. Bilirubin formation was calculated on the basis of difference between optical densities obtained at 460 and 530 nm. The HO-1 activities were expressed in nanomols (nmol) of bilirubin formed per milligram protein per hour.
Western Blot Analysis for Expression of HO-1 Protein in Heart Tissue
Three hundred milligrams of frozen tissue from the left ventricular myocardium of each rat was homogenized in 800 μL homogenization buffer (25 mM Tris-HCl, pH 8, 25 mM sodium chloride, 4 mM sodium orthovanadate, 10 mM NaF (sodium fluoride), 10 mM sodium pyrophosphate, 10 nM Okadaic acid, 0.5 mM EDTA, 1 mM PMSF (phenylmethylsulfonyl fluoride), and 1x protease inhibitor cocktail), using a Polytron homogenizer. Homogenates were centrifuged at 2000 rpm at 4°C for 10 minutes. Supernatants were further centrifuged at 10,000 rpm at 4°C for 20 minutes. The resultant supernatants are cytosolic extracts that were used experimentally to evaluate this cell fraction. Samples (50 μg each) were next separated with sodium dodecyl sulfate polyacrylamide gel electrophoresis using 12% tris-glycine gel at 120 V for 90 minutes. Then, proteins were transferred onto nitrocellulose membranes at 100 V for 1 hour. Membranes were blocked as a countermeasure to nonspecific binding of probing antibody, using 5% dry milk powder, and then incubated overnight at 4°C with primary antibodies (1:1000 dilution; anti-GAPDH; anti–HO-1 rabbit monoclonal antibodies, Sigma-Aldrich). After overnight incubation, membranes were treated with horseradish peroxidase–conjugated goat anti–rabbit IgG (1:3000 dilution) secondary antibody. Chemiluminescent detection (ECL; Litmus Scientific, Advansta Inc, Menlo Park, CA) was used to identify bands. Detection was made using autoradiography for variable lengths of time with medical x-ray film (Agfa-Gevaert N.V., Mortsel, Belgium). Quantitative analysis of scanned blots was performed using the Scion for Windows Densitometry Image program (version Alpha 4.0.3.2, Scion Corporation).
Experimental Design
The experimental strategies used to analyze effects of α-MSH are summarized in Figure 1. For this work, 5 basic protocols were designed:
FIGURE 1.

Experimental design. Protocols shown here were used for different parts of the study. A, Protocol I: Investigation of effect of α-MSH pretreatment on cardiac HO-1 expression in ischemic–reperfusion injury. B, Protocol II: Evaluation of significance of the HO-1 pathway in cardioprotective effects of α-MSH. C, Protocol III: Estimation of direct cardiac effects of α-MSH on isolated hearts. D, Protocol IV: Analysis of direct effects of α-MSH on echocardiographic heart functions. E, Protocol V: Evaluation of vascular response of isolated aortic rings to α-MSH administration.
Protocol I: Investigation of Effect of α-MSH Pretreatment on Cardiac HO-1 Expression in Ischaemic–Reperfusion Injury
Sixteen healthy male Sprague Dawley rats weighing 300–330 g were divided into 2 groups (n = 8/group): I-a group received 0.5 mL saline subcutaneously 24 hours before the isolated working heart procedure (described in Isolated Working Heart Preparation); I-b group received 250 μg/body weight α-MSH solution (dissolved in saline) subcutaneously 24 hours before the isolated working heart operation. During isolated working heart method, hearts were exposed to ischemia/reperfusion (described in Induction of Global Ischemia and Reperfusion in Isolated Hearts). Samples from myocardial tissues were frozen on liquid nitrogen and stored at −80°C for further Western blot analysis (Fig. 1A).
Protocol II: Evaluation of Significance of the HO-1 Pathway in Cardioprotective Effects of α-MSH
In protocol II (Fig. 1B), another population of male Sprague Dawley rats (50 rats) was subdivided into 4 groups based on the pretreatments 24 hours before the isolated heart procedure (described in Isolated Working Heart Preparation): group II-a animals (n = 15) received subcutaneous saline injection of 0.5 mL; group II-b animals (n = 14) were injected with 250 μg/body weight α-MSH solution (diffused in saline) subcutaneously; group II-c animals (n = 11) received 50 μg/body weight SnPP diffused in 0.5 mL saline intraperitoneally together with 250 μg/body weight α-MSH solution subcutaneously; group II-d animals (n = 10) were injected only with 50 μg/body weight intraperitoneal SnPP solution. During isolated working heart method, hearts were exposed to ischemia/reperfusion (described in Induction of Global Ischemia and Reperfusion in Isolated Hearts). At the endpoint of the experiments, samples from myocardial tissues were either frozen in liquid nitrogen and stored at −80°C for further analysis of HO-1 activity or stained for determination of infarcted areas (TTC staining) and then frozen in liquid nitrogen and stored at −80°C for further analysis.
Protocol III: Estimation of Direct Cardiac Effects of α-MSH on Isolated Hearts
Hearts harvested from Sprague Dawley rats without any pretreatment (n = 8) were subjected to isolated working heart procedure (described in Isolated Working Heart Preparation) without ischemia/reperfusion. After 10 minutes of retrograde perfusion using modified Krebs–Henseleit buffer (washout period), the set-up was switched to anterograde-mode (working heart), and baseline parameters (AF, CF, AoP (aortic pressure), and HR) were recorded. After measuring baseline cardiac function, 1 mL alpha-MSH solution (containing 1 μmol/L alpha-MSH) solution was injected directly through the side arm of the aortic cannula into the system. After 5 minutes of distribution, cardiac parameters were recorded again to estimate direct effects of α-MSH (Fig. 1C).
Protocol IV: Analysis of Direct Effects of α-MSH on Echocardiograpic Heart Functions
As shown on Figure 1D, rats without any pretreatment (n = 8) were anesthetized using ketamine–xylazine combination, and the chest of each animal was shaved. The right jugular vein was exposed and cleaned from the adhering connective tissue, and a small incision was made on the wall of the vein, then a polyethylene catheter containing saline was introduced into it. The animal was placed into dorsal decubitus position and echocardiographic examination was performed (described in Echocardiography). After recording baseline parameters, 3 different doses of α-MSH (10, 100, 250 μg/kg, respectively) were injected intravenously through the catheter, and cardiac parameters were estimated and recorded again under the influence of the different (and cumulative) doses of α-MSH.
Protocol V: Evaluation of Vascular Response of Isolated Aortic Rings to α-MSH Administration
For each isolated aortic ring experiment (n = 8), a rat without any pretreatment was guillotined and then the abdominal aorta rapidly excised (described in Induction of Global Ischemia and Reperfusion in Isolated Hearts). After a 90-minute incubation in Krebs solution, 1 μM phenylephrine was administered to 5 of the 6 aortic rings, including the intima-deprived [ET- (endothelium-deprived)] specimen, for 2 minutes, followed by a 45-minute washout period. Next, these 5 rings were subjected to one of the following treatments: (1) Krebs solution alone for one of the intact rings (naive) and (2) for the intima-deprived ring (naive ET-), (3) 200 μM NOARG, (4) 3 μM INDO, and (5) 200 μM NOARG together with 3 μM INDO at the same time. Immediately after these treatments, these 5 preparations received 1 μM phenylephrine. After 30 minutes, during which the contractile force reached a plateau, an α-MSH concentration–response (E/c) curve was constructed (from 1 nM to 1 μM) for these aortic rings. In the case of the sixth aortic ring, (6) after the 90-minute incubation in Krebs solution, an α-MSH E/c curve was generated (from 1 nM to 1 μM). Protocol V (n = 8 animals) is illustrated in Figure 1E.
Data Analysis and Statistics
All data are presented as average magnitudes of each outcome in a group ± SEM. Normality of data sets was verified with the D'Agostino–Pearson omnibus test. A Student's t test was used to determine differences between 2 sets of data. More than 2 data sets were compared with 1-way analysis of variance followed by Tukey's posttesting. Difference of means was considered significant at P < 0.05. Statistical analysis was performed with GraphPad Prism 6.07 (GraphPad Software Inc, La Jolla, CA).
RESULTS
Echocardiographic Outcomes: Fractional Shortening and Ejection Fraction (Protocol IV)
Echocardiographic examinations shown in Figure 2 include (Fig. 2A) and (Fig. 2B). HRs and respiratory frequencies of each animal remained stable throughout the experiments. Moreover, no significant differences were noted between the independent measurements obtained by 2 blinded readers. FS and EF data correlated strongly with measurements of both the parasternal long and short axis views. FS after administration of 100 and 250 μg/kg α-MSH was significantly increased in comparison with this outcome evaluated before the injection (Control) of the material (FS100μg/kg: 53.79 ± 2.497 and FS250μg/kg: 55.00 ± 2.688 vs. FSControl: 45.69 ± 2.241). The same pattern was observed in EF results (EF100μg/kg: 88.31 ± 1.745, and EF250μg/kg: 89.21 ± 1.527 vs. EFControl: 81.52 ± 2.296). α-MSH at the dose of 10 μg/kg was observed to result in trends for increased EF and FS, but these increases did not reach statistical significance (FS10μg/kg: 52.50 ± 2.773 vs. FSControl: 45.69 ± 2.241; and EF10μg/kg: 87.04 ± 1.963 vs. EFControl: 81.52 ± 2.296). There were no significant changes observed in interventricular and left ventricular free-wall thicknesses and LV mass after the administration of a single dose of α-MSH.
FIGURE 2.
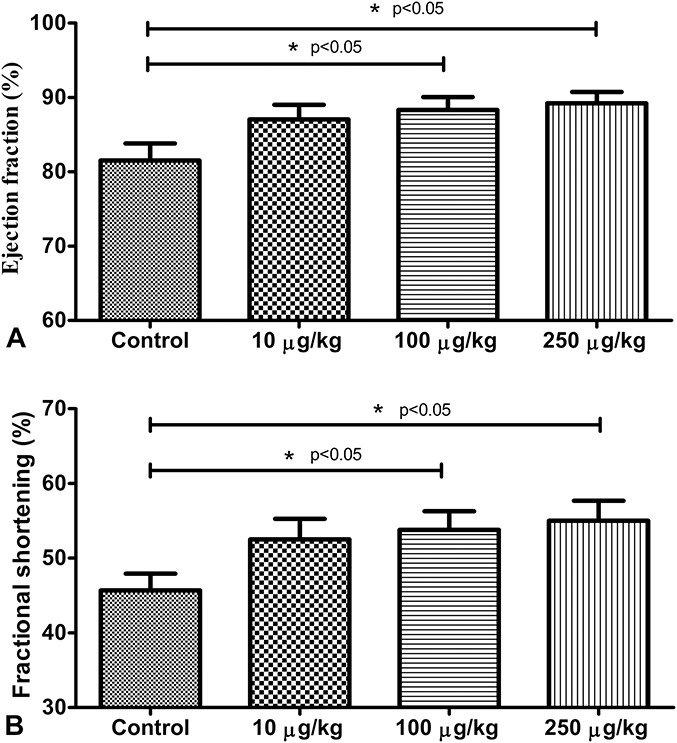
Echocardiographic outcomes. Effect of selected doses of α-MSH on ejection fraction (A) and fractional shortening (B). Echocardiographic examination was performed after ketamine–xylazine anesthesia. After recording baseline parameters (control), different doses of α-MSH (10, 100, 250 μg/kg, respectively) were injected intravenously through catheter, and cardiac parameters were estimated and recorded again. Results are shown as average values from each group of rats ± SEM of [%, (A)] and of [%, (B)]. *P < 0.05 compared with control.
Isolated Working Heart Results (Protocol II)
Data from the isolated working heart procedure of protocol I is not shown because similar outcomes observed using the same experimental circumstances have already been published by the authors of this report.15 Novel Western blot results of protocol I, which were produced by analyzation of frozen-stored myocardial tissue samples gained at the end of the isolated working heart procedure of protocol I are shown in Western Blot Analysis (Protocol I). The data presented in Figure 3 provide outcomes of cardiac function evaluations on isolated hearts using protocol II, to determine the effect of SnPP-mediated inhibition of HO-1 on HR, CF, AF, cardiac output (CO), and SV.
FIGURE 3.
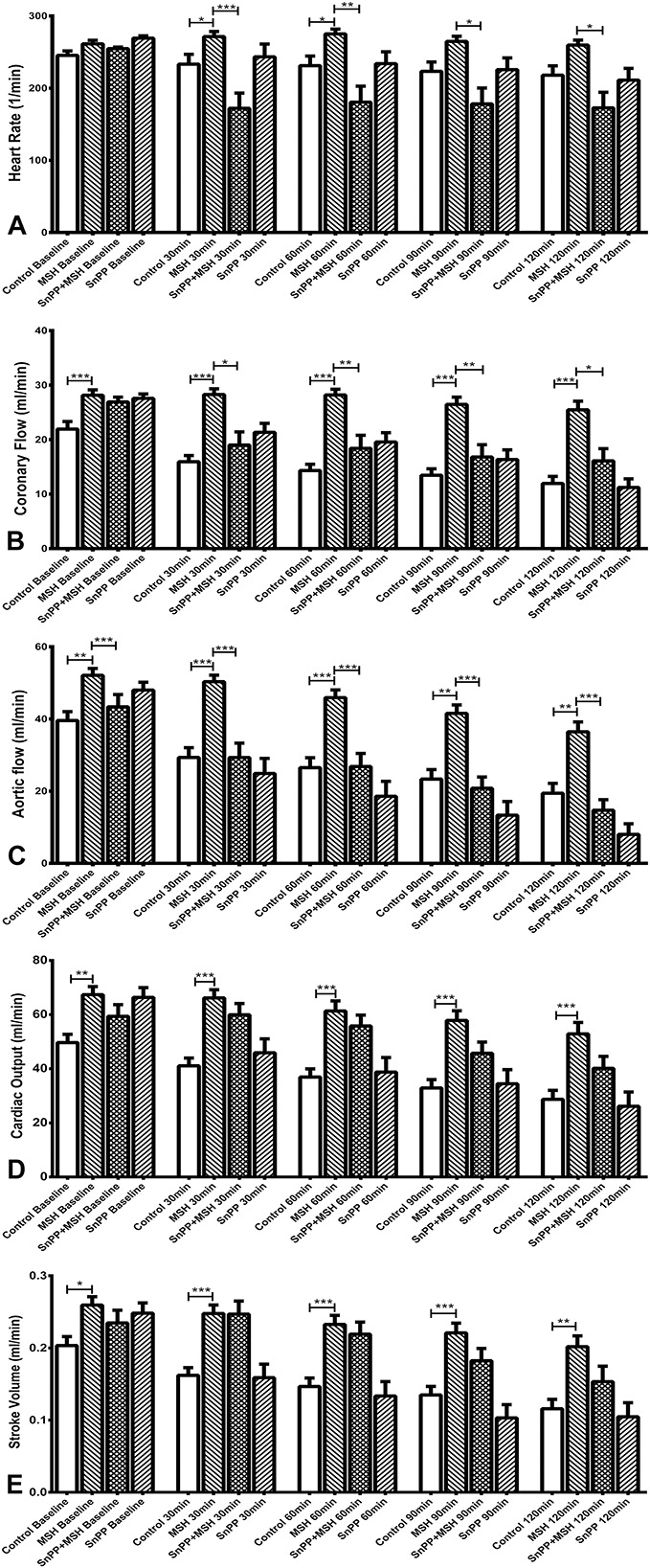
Effects of pretreatment of animals with α-MSH and/or the HO-1 inhibitor SnPP on cardiac functions in isolated working hearts (protocol II). Working hearts were isolated from 4 groups of rats which had been pretreated with injections of α-MSH and/or saline—and selected animals receiving injections of the HO-1 inhibitor SnPP, 24 hours before sacrifice and heart isolation. Treatments were as follows: 0.5 mL saline subcutaneously (group II-a, control); 250 μg/kg body weight α-MSH subcutaneously (group II-b, MSH); 50 μg/kg body weight SnPP intraperitonealy, plus 250 μg/kg body weight α-MSH subcutaneously (group II-c, SnPP + MSH); or 50 μg/kg body weight SnPP, intraperitonealy (group II-d, SnPP). Cardiac function parameters were recorded before ischemia (baseline), and at 30, 60, 90, and 120 minutes of reperfusion. Results are shown as average values from each group of rat hearts ± SEM of HR [bpm, (A)]; CF [mL/min, (B)]; AF [mL/min, (C)]; CO[ mL/min, (D)]; and SV [mL, (E)]. *P < 0.05, significant differences between groups. **P < 0.01, significant differences between groups. ***P < 0.001, significant differences between groups.
α-MSH and SnPP Effects on HR
Results of heart rate analyses, recorded by ECG during the isolated working heart procedure, are shown in Figure 3A. Here, it was observed that α-MSH treatment increased HR, especially at the first part of reperfusion period (P < 0.05), compared with the control group. SnPP treatment counteracted the HR-elevating effect of α-MSH (P < 0.05) throughout the whole reperfusion period (eg, at a 30-minute timepoint: 172.0 ± 21.22 bpm SnPP + MSH vs. 271.4 ± 7.117 bpm MSH; and at 60 minutes: SnPP + MSH 180.5 ± 22.28 bpm vs. 275.1 + 6.698 bpm MSH).
α-MSH and SnPP Effects on CF
CF values during the isolated working heart method, shown in Figure 3B, were also measured before ischemia (baseline), and at 30, 60, 90, and 120 minutes during reperfusion. As shown, α-MSH treatment improved preischemic CF values compared with controls (28.14 ± 0.99 vs. 21.93 ± 1.39 mL/min). CF values of α-MSH–treated animals, measured during reperfusion, were elevated at every timepoint, and differences were significant compared with any other groups (P < 0.05). CF values measured in MSH-treated animals after 120 minutes of reperfusion (25.45 ± 1.60 mL/min) were 16% higher than preischemic values of the control group (21.93 ± 1.39 mL/min), and decreased only by 10% versus preischemic levels observed in the alpha-MSH–treated group (28.14 ± 0.99 mL/min). The HO-1 inhibitor, SnPP, significantly counteracted the CF-elevating effect of MSH, as seen from the results of SnPP + MSH-treated group at every timepoint of the reperfusion period (P < 0.05).
α-MSH and SnPP Effects on AF
AF outcomes under the influence of α-MSH and modulation of HO-1 are shown in Figure 3C. Using protocol II for these experiments, AF was significantly increased in the α-MSH–treated group (52.07 ± 1.93 mL/min) compared with controls (39.57 ± 2.44 mL/min, P < 0.001) before the ischemic insult. Preischemic AF values in SnPP-treated and SnPP + MSH-treated groups were decreased compared with the MSH-group, but increased compared with the control group values (48.00 ± 2.16 and 43.36 ± 3.42 mL/min, respectively). AF values in the α-MSH–treated group were significantly elevated at any timepoints of reperfusion compared with the paired control values (P < 0.01). Even compared with preischemic controls, AF values in the MSH-treated group were elevated in all timepoints, except at 120 minutes of reperfusion. In addition, significant differences in AF between the α-MSH–treated group versus all other groups were measured during the reperfusion period (P < 0.01). Although baseline AF values of SnPP-treated animals were increased compared with control, they declined by the end of the reperfusion period (8.00 ± 2.94 mL/min) to 20% of control baseline values. Similar trends were observed in the SnPP + MSH-treated group, although these values remained higher at any timepoints than values of SnPP-only treated group, and over the whole reperfusion period.
α-MSH and SnPP Effects on CO and SV
CO (Fig. 3D) and SV values (Fig. 3E) in α-MSH–pretreated animals were significantly elevated at all timepoints compared with their matched controls. However, SnPP failed to significantly counteract this effect.
Effect of α-MSH Administration Directly Onto Isolated Working Hearts (Protocol III)
Figure 4 shows the effects on cardiac function resulting from addition of 1 μmol/L α-MSH directly to isolated working hearts (MSH+) through the aortic cannula of the Langendorff apparatus on which the hearts are mounted, compared with baseline values (MSH−). As shown, α-MSH treatment resulted in significantly elevated CF (Fig. 4B) and CF/CO% (Fig. 4D) in comparison with baseline values (P < 0.05). No significant effects of α-MSH treatment were observed in AF, CO, HR, or SV in comparison with baseline values.
FIGURE 4.
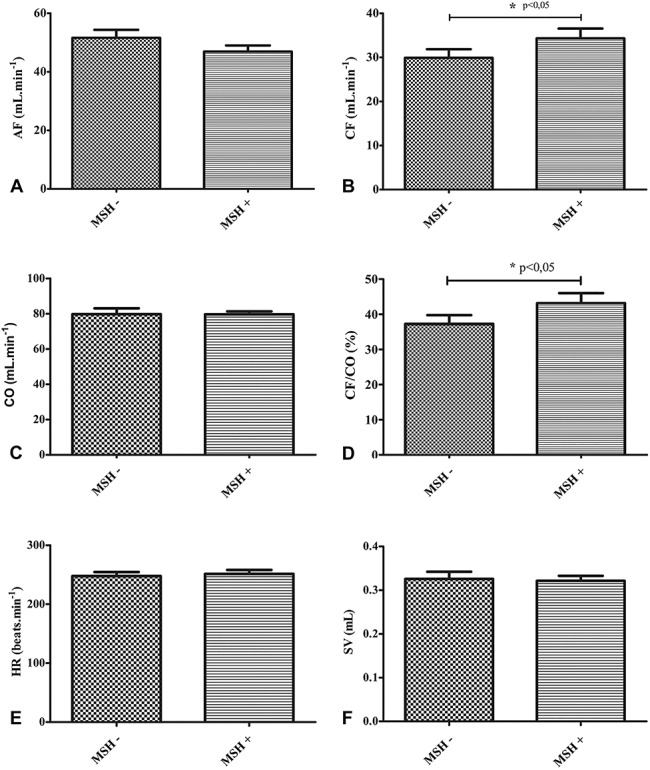
Influence of direct administration of α-MSH addition to isolated working heart functions (protocol III). Isolated working hearts excised from groups of ketamine-xylazine–anesthetized rats were treated with 1.0 mL 1 μmol/L α-MSH (MSH+) directly into the Langendorff system in which the hearts were mounted. Baseline values are designated as MSH−. Administration of α-MSH solutions to hearts was accomplished through the aortic cannula of the apparatus. Results are shown as average values from each group of rats ± SEM of AF [mL/min, (A)]; CF [mL/min, (B)]; , mL/min, (C)]; the ratio of CF to CO in % [CF/CO, %, (D)]; HR [bpm, (E)]; and SV [mL, (F)]. *P < 0.05, significant differences between groups as shown.
Determination of Incidence and Severity of Ventricular Arrhythmias (Protocol II)
Outcomes of ECG analyses, shown in Figure 5, demonstrate that no arrhythmic events were detected in 80% of the control rats. However in 6.7% and in 13.3%, nonsevere and severe arrhythmias were found in this group, respectively. No arrhythmic events were determined in the MSH-treated group. In the SnPP + MSH-treated animals, 36.4% of isolated hearts showed no signs of arrhythmia, whereas in 27.2% of cases nonsevere and in further 36.4% of cases severe arrhythmic events were observed. In the SnPP-only treated group, 30.0% of isolated hearts showed no arrhythmia, whereas in 60.0% nonsevere and in 10.0% severe arrhythmic cases were detected.
FIGURE 5.
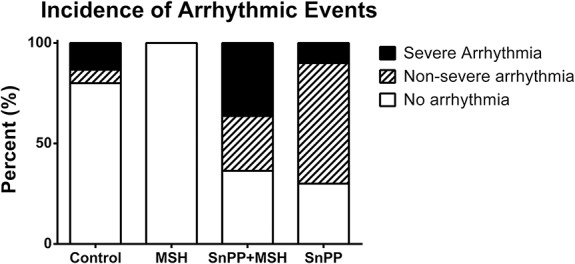
Effects of pretreatment of animals with α-MSH and the HO-1 inhibitor SnPP on cardiac arrhythmias in isolated working hearts (protocol II). Working hearts were isolated from 4 groups of rats which had been pretreated with 0.5 mL saline subcutaneously (control); 250 μg/kg body weight α-MSH subcutaneously (MSH); 50 μg/kg body weight SnPP intraperitonealy, plus 250 μg/kg body weight α-MSH subcutaneously (SnPP + MSH); or 50 μg/kg body weight SnPP, intraperitonealy (SnPP). Arrhythmic events in which hearts failed to recover their pump function are classed as severe, whereas those hearts in which pump function was spontaneously restored after arrhythmia were classed as nonsevere.
NOARG and INDO Effect on α-MSH–Mediated Vascular Tone Response (Protocol V)
Vascular Response to Phenylephrine (Precontraction)
In the naive, naive ET-, 200 μM NOARG, 3 μM INDO, and 3 μM INDO + 200 μM NOARG animals, the contractile force of aortic rings before dosing with α-MSH E/c curve (in mN, mean ± SEM) were 3.47 ± 0.37, 4.21 ± 0.41, 4.46 ± 0.39, 1.98 ± 0.26, and 3.68 ± 0.4, respectively, with a significant difference between the naive and 3 μM INDO groups (P < 0.01).
Vascular Response to α-MSH
Treatment with α-MSH did not directly result in significant alteration of the resting vascular tone (data not shown). Conversely, the precontracted aortic rings exhibited significant relaxation in response to α-MSH (Fig. 6). The maximal relaxing effect induced by 1 μM α-MSH concentration (in percentage of the initial contractile force, mean ± SEM) did not differ significantly among the naive, naive ET-, 200 μM NOARG, 3 μM INDO, and 3 μM INDO + 200 μM NOARG groups (20.01 ± 4.64, 21.65 ± 4.53, 16.75 ± 5.41, 21.24 ± 6.73, and 23.71 ± 5.7, respectively). Thus, the effect of α-MSH on the increased vascular tone (due to α1 adrenoceptor stimulation) proved to be independent from the presence or absence of endothelium as well as from the ability of endothelium to produce prostacyclin (PGI2) or NO.
FIGURE 6.
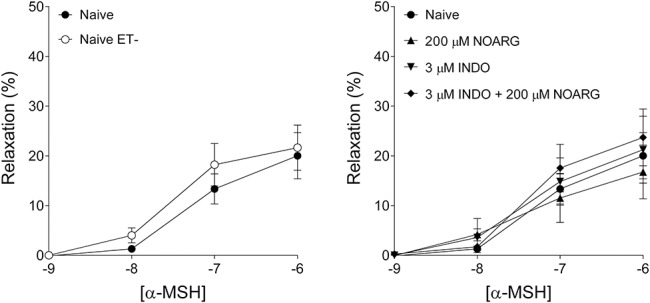
α-MSH effects on vascular tone. Effects of α-MSH on tone of rat abdominal aortic rings precontracted with 1 μM phenylephrine, in rings from which intimal endothelium had been debrided (naive ET-); and rings from which endothelium remained intact (naive). Effects of α-MSH on contractile force of naive, versus naive ET-rings are shown in the left panel, with influence of NOARG, INDO, and NOARG + INDO on α-MSH–treated naive rings shown in the right panel. For both diagrams, the x axis denotes the common logarithm of the molar concentration of α-MSH, and the y axis indicates the effect as a percentage decrease of the initial contractile force. The symbols show the responses to α-MSH averaged within the groups (±SEM).
Effects of α-MSH and HO-1 Inhibition on Ischemia-Reperfusion–Induced Cardiac Infarct Zone Extent (Protocol II)
As shown in Figure 7, significant changes were observed in the extent of infarcted areas (expressed in % of total area) of hearts from protocol II-treated animals, after ischemia/reperfusion injury. TTC staining of hearts harvested from α-MSH–pretreated groups revealed significantly lower infarct zone extent, in comparison with tissue sections from hearts from vehicle-treated control animals (19.74 ± 1.044% vs. 41.91 ± 3.922%) (P < 0.05). This cardioprotective effect was completely counteracted by SnPP pretreatment of α-MSH–treated animals (42.15 ± 3.235% vs. 19.74 ± 1.044%) (P < 0.05). Moreover, SnPP treatment alone was found to increase infarcted areas (53.17 ± 2.347%).
FIGURE 7.
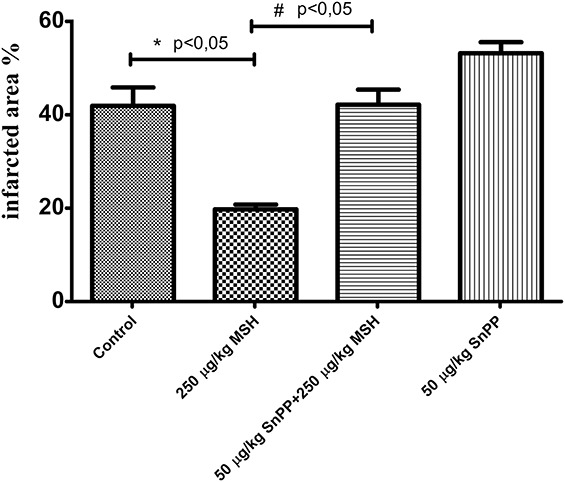
Effect of α-MSH and HO-1 inhibition on infarcted zone magnitude (protocol II). Rats administered subcutaneous saline (group II-a), or subcutaneous 250 μg/kg body weight α-MSH (group II-b), or intraperitoneal injections of 50 μg/kg body weight SnPP plus 250 μg/kg body weight subcutaneous α-MSH (group II-c), or 50 μg/body weight SnPP (group II-d), followed by sacrifice and ischemia/reperfusion injury during isolated working hearts according to protocol II. Staining of infarcted tissue was accomplished by administration to working hearts of 1% TTC solution in phosphate buffer, through the side arm of an aortic cannula. Hearts were sliced transversely, blotted dry, placed in between microscope slides, and scanned. Infarct areas of each slice were traced, and the respective areas were calculated by pixel density analysis. *P < 0.05 for comparison of infarcted areas to control. #P < 0.05 for comparison of infarcted areas to α-MSH–pretreated (250 μg/kg) group.
Effects of α-MSH and SnPP on Tissue HO-1 Activity (Protocol II)
HO-1 enzyme activity was measured in myocardial tissue samples obtained from protocol II (pretreated) animals. No significant differences in activity of the enzyme were noted (data not shown). Nevertheless, HO-1 activity of MSH-treated animals was slightly increased compared with controls; SnPP pretreatment decreased the activity of the enzyme, whereas this reduction seemed to be less intense in the SnPP + MSH-treated group compared with SnPP treatment. Although measurement of the HO-activity corresponds to the results of the formerly mentioned methods, differences between the groups did not reach the level of statistical significance.
Western Blot Analysis (Protocol I)
Western blot method was performed by analyzation of frozen-stored myocardial tissue samples gained at the end of isolated working heart procedure of protocol I. Results of Western blot analyses, shown in Figure 8 demonstrate that the expression of HO-1 protein in myocardial tissue harvested from postischemic/reperfused, isolated working hearts excised from rats treated with 250 μg/kg α-MSH was significantly elevated relative to content of HO-1 protein in heart tissue from control rats treated with physiologic saline (P < 0.05).
FIGURE 8.
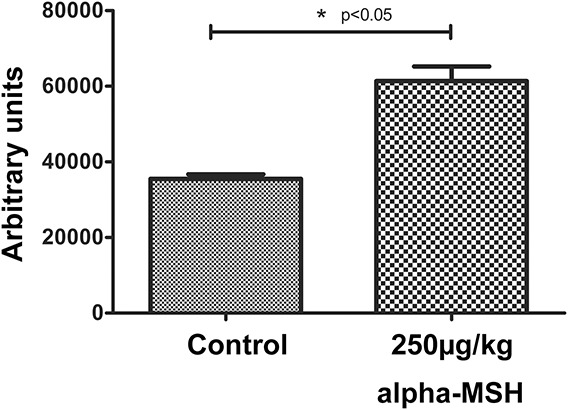
Western blot analysis for HO-1 protein expression of myocardial tissue from isolated working hearts (protocol I). Expression of HO-1 protein in rat myocardial tissue was measured in homogenized left ventricular cardiac tissue samples drawn from 2 test groups of rats, defined as follows: Rats in the control group were treated with 0.5 mL physiologic saline vehicle; a second group of animals were treated with 250 μg/kg body weight α-MSH. Treatments were administered by subcutaneous injection 24 hours before sacrifice and excision of hearts. Working hearts were mounted in a Langendorf apparatus and subjected to ischemia/reperfusion injury as described by protocol I. Homogenized myocardial tissue was evaluated for expression of HO-1 protein by Western blot analysis. The signal intensity of resulting bands corresponding to proteins of interest was evaluated as densitometry image intensity. Tissue content of HO-1 is shown in arbitrary units as the mean for each group of animals ± SEM. *P < 0.05 for comparison of average expression levels of HO-1 in myocardium to control.
DISCUSSION
Echocardiographic assessment of α-MSH effects on EF and FS, shown in Figures 2A, B respectively reveals that administration of the hormone correlated with significant increases in both parameters. These effects may reflect increased activation of sympathetic tone because it is well-known that α-MSH and its analogs are associated with mild sympathetic activation in the cardiovascular system, including increased HR, and thus may influence systolic activity.16 This increase of ejection fraction on α-MSH administration has also been shown by other authors in systemic inflammatory response syndrome in pigs.17 Effects on cardiac function in isolated hearts shown in Figure 3 include results shown in Figure 3A, demonstrating that α-MSH pretreatment according to protocol II increased the beat rate of isolated working hearts during the initial reperfusion period, as measured by ECG. Such HR-elevating effects of α-MSH have been confirmed on other animal models.19,20 The contribution of HO-1 activity to this effect was demonstrated by the observation that increased HR stimulated by the hormone was counteracted by SnPP, an inhibitor of HO-1. Nevertheless, it should be noted that the conspicuous low HRs in the SnPP + MSH group are partly due to the observed many severe arrhythmic events, as described in this report. Likewise, α-MSH pretreatment significantly increased CF (Fig. 3B) and AF (Fig. 3C), both in the preischemic periods and during reperfusion (Fig. 3B). This effect was also demonstrated to be significantly HO-1 dependent because CF and AF increases induced by the hormone were counteracted by SnPP. Increases in both of these outcome measures have been observed to correlate with improved cardiac function in isolated working heart studies21 and improved clinical prognoses for patients who have experienced ischemic events.22,23 These and related observations suggest possible use of α-MSH and agents that enhance expression of HO-1 in prevention of and therapy for ischemia/reperfusion-associated pathologies. Conversely, although α-MSH treatment significantly increased magnitude of CO (Fig. 3D) and SV (Fig. 3E), this effect was not or only partially HO-1 dependent, as evidenced by inability of SnPP to significantly counteract these increases.
Additional suggestion that α-MSH holds potential for improvement of cardiovascular function through its vasoactive influence on cardiac function is shown by the effects of direct administration of hormone directly to healthy rat hearts in Figure 4. Administration of the hormone induced significant increases in CF by treated hearts versus controls. These outcomes reveal that α-MSH mediates an intensive dilatative effect on coronary vasculature, pointing to an additional physiologic effect that may be used by clinicians to design treatment strategies targeted specifically to pathological conditions that might be ameliorated by dilation of selected blood vessels. Such measures fall under the broad category of “biotherapeutic” interventions—which are strategies that target specific, root causes of a disease, ultimately resulting in true “cures” rather than providing only transitory palliative relief of symptoms.24
This investigation further revealed potential uses of agents such as α-MSH that modulate HO-1, in reducing susceptibility to ventricular arrhythmias. Here, ECG analysis was conducted to evaluate the involvement of α-MSH–induced HO-1 activity in antiarrhythmic cardioprotection. These results, shown in Figure 5, demonstrate that MSH treatment—possibly through HO-1 induction, inhibited the occurrence of arrhythmic events in comparison with a vehicle-treated control group. Treatment with SnPP in addition to the hormone resulted in a possible increase in susceptibility to ventricular arrhythmias, suggesting that HO-1 is cardioprotective in this respect, a finding that coincides with results of previous investigations on mice conducted by authors of this report.25 A possible major contributor to the biological basis for the higher occurrence of severe arrhythmias in hearts of animals receiving SnPP + MSH than in the SnPP-only group may be the HR-increasing effect of α-MSH. This property of the hormone, combined with the proarrhythmic effect of SnPP observed in the SnPP-treated group, may promote higher incidence of severe arrhythmic events. These outcomes are particularly intriguing in the context of previous work by the authors demonstrating that naturally occurring ginkgolide terpenes interact with platelet-activating factor receptors, calcineurin and the macrolide immunosuppressant FK506, to synergistically ameliorate ventricular arrhythmias in the same model as used in this report.26 Thus, it is likely that α-MSH may also exhibit clinically relevant synergism with these agents in management of cardiac arrhythmias.
Figure 6 shows the outcomes of experiments using an isolated, phenylephrine-precontracted aortic ring model conducted according to protocol V, to evaluate the antivasoconstrictive capacity of α-MSH. Results of these procedures demonstrate that treatment with α-MSH did not directly result in significant alteration of the resting vascular tone. Nevertheless conversely, the precontracted aortic rings exhibited significant relaxation in response to α-MSH. The maximal relaxing effect induced by 1 μM α-MSH concentration (in percentage of the initial contractile force, mean ± SEM) did not differ significantly among the naive, naive ET-, 200 μM NOARG, 3 μM INDO, and 3 μM INDO + 200 μM NOARG-treated groups. Thus, the effect of α-MSH on the increased vascular tone (due to α1 adrenoceptor stimulation) proved to be independent from the presence or absence of endothelium as well as from the ability of endothelium to produce prostacyclin (PGI2) or NO. These findings, along with the significant levels of relaxation induced by treatment with the hormone in phenylephrine-treated rings, demonstrate that α-MSH has potential for clinical use in treatment regimens where vasodilation is a desired outcome for improvement of patient prognosis.27,28 Moreover, the endothelium-independent α1-adrenoceptor–mediated effects on vascular tone, suggested by these outcomes, provide insight for future drug discovery efforts in which specific therapeutic targets may be identified and exploited.29
The capacity of α-MSH pretreatment to significantly inhibit the extent of ischemia/reperfusion-induced infarct zones shown in Figure 7 provides additional evidence of the range of cardioprotective effects mediated by the hormone. As shown in Figure 7, the mechanistic basis for this effect is substantially due to the cytoprotective action of HO-1 because inhibition of the enzyme with SnPP suppressed IR-injury–associated infarct area increases, not only in α-MSH-treated hearts, but also in the control groups. Authors of this report have extensively characterized processes by which ischemia/reperfusion injury promotes infarction-associated tissue damage and methods by which it may be counteracted by increasing HO-1 expression.30,31 Exploration by the authors, of mechanisms contributing to mechanisms by which infarcted zone expansion is augmented, strongly suggests that the antioxidant capacity of HO-1 reinforces compartmentalization of ionic species within cardiomyocytes and other cells composing cardiovascular tissue, the integrity of which is compromised by elevated levels of reactive oxygen molecules induced during the reoxygenation period associated with postischemic reperfusion.32,33
The significant increases in both protein expression, shown in Figure 8, and activity of HO-1 in myocardial tissue stimulated by pretreatment with α-MSH demonstrate that the hormone is capable of amplifying this major adaptive response at levels that further underscore the therapeutic value of its use. Pharmacological amplification of HO-1 has been demonstrated to powerfully remediate cardiovascular disease, along with disorders of the lung, kidney, and central nervous system.8
CONCLUSIONS
This investigation is consistent with results of other studies demonstrating a diverse range of cardioprotective effects mediated by α-MSH. The results provide insight as to the relevance of these findings in the “biotherapeutic” management of cardiovascular diseases through specific effects on aspects of vasodilation, which may be manipulated to improve patient prognoses in treatment regimens that use the compound. Major findings of this study include echocardiographic outcomes that suggest its effects on systolic parameters of the heart (ejection fraction), along with outcomes of cardiac function experiments demonstrating favorable influences on HR, CF, and AF. The hormone showed potent antiarrhythmic properties and further promotes intensive dilatative effects on coronary vasculature—a finding that will potentially allow development of interventions that specifically target selected blood vessels for dilation. Such precise targeting of the underlying causes of a particular disease process is the core concept of “biotherapeutic” approaches which have emerged as a major policy current emphasis by the US National Institutes of Health (NIH) on developing medical strategies that attack disease at its roots, rather than merely providing analgesic and palliative relief from symptoms. This encouraging trend is reflected by the NIH's current policy directive.34 Also a major finding of this investigation is that most of the cellular and tissue processes studied required the activity of HO-1 as a downstream effector of α-MSH. Nevertheless, some of the potentially beneficial effects, such as changes in CO and SV, were observed to occur independently of HO-1 activity, as evidenced by insensitivity of these processes to treatment with the HO-1 inhibitor, SnPP. These findings are particularly significant to ongoing work by the authors, who have developed numerous preventive and therapeutic applications for phytochemical HO-1 inducers.24,18
ACKNOWLEDGMENTS
The authors are sincerely grateful to Stephanie C. Fox, of QueenBeeEdit in Bloomfield, CT, USA, for her hard work in organizing, formatting, and editing this article.
Footnotes
Supported in part by the TÁMOP-4.2.2.D-15/1/KONV-2015-0016 project, implemented through the New Széchenyi Plan, and co-financed by the European Social Fund, and in part by KUTEGY (B. Juhasz), University of Debrecen (B. Juhasz), OTKA PD-78223, NKFI-K—104017, GINOP- 2.3.2-15-2016-00043. Further support is derived from AGR-PIAC-13-1-2013-0008 (D. Priksz, M. Bombicz, R. Gesztelyi, B. Varga, B. Juhasz) and the TÁMOP-4.2.2.A-11/1/KONV-2012-0045 and TÁMOP-4.2.6.-15/1-2015-0001 (D. Priksz, M. Bombicz, R. Gesztelyi, B. Varga, B. Juhasz, A. Tosaki) projects co-financed by the European Union and the European Social Fund. This research was also supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP-4.2.4.A/2–11/1-2012-0001 “National Excellence Program” (M. Bombicz, B. Varga).
The authors report no conflicts of interest.
M. Vecsernyes: study design, isolated working heart preparations, isolated aortic ring, treatments, data analysis, and manuscript preparation. M. Szokol: study design, treatments, and echocardiography. M. Bombicz: isolated working heart method, protein isolation, enzyme activity measurements, and Western blot analysis. D. Priksz: echocardiography, isolated working heart method, and manuscript preparation. B. Varga: treatments, isolated working heart preparations, and manuscript preparation. R. Gesztelyi: isolated aortic ring experiments, data analysis, and statistical analysis. G. A. Fulop: echocardiography and data analysis. D. Haines: data interpretation and manuscript preparation. A. Tosaki: isolated working heart preparations and manuscript preparation, B. Juhasz: study design and set up, echocardiography, and is the corresponding author.
REFERENCES
- 1.Vecsernyes M, Julesz J. Specific radioimmunoassay of alpha-melanocyte-stimulating hormone in rat plasma. Exp Clin Endocrinol. 1989;93:45–51. [DOI] [PubMed] [Google Scholar]
- 2.Juhasz B, Der P, Szodoray P, et al. Adrenocorticotrope hormone fragment (4–10) attenuates the ischemia/reperfusion-induced cardiac injury in isolated rat hearts. Antioxid Redox Signal. 2007;9:1851–1861. [DOI] [PubMed] [Google Scholar]
- 3.Petervari E, Szabad AO, Soos S, et al. Central alpha-MSH infusion in rats: disparate anorexic vs. metabolic changes with aging. Regul Pept. 2011;166:105–111. [DOI] [PubMed] [Google Scholar]
- 4.Varga B, Gesztelyi R, Bombicz M, et al. Protective effect of alpha-melanocyte-stimulating hormone (α-MSH) on the recovery of ischemia/reperfusion (I/R)-induced retinal damage in a rat model. J Mol Neurosci. 2013;50:558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catania A, Lonati C, Sordi A, et al. The peptide NDP-MSH induces phenotype changes in the heart that resemble ischemic preconditioning. Peptides. 2010;31:116–122. [DOI] [PubMed] [Google Scholar]
- 6.Ottani A, Giuliani D, Neri L, et al. NDP-alpha-MSH attenuates heart and liver responses to myocardial reperfusion via the vagus nerve and JAK/ERK/STAT signaling. Eur J Pharmacol. 2015;769:22–32. [DOI] [PubMed] [Google Scholar]
- 7.Ottani A, Neri L, Canalini F, et al. Protective effects of the melanocortin analog NDP-alpha-MSH in rats undergoing cardiac arrest. Eur J Pharmacol. 2014;745:108–116. [DOI] [PubMed] [Google Scholar]
- 8.Haines DD, Lekli I, Teissier P, et al. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol (Oxf). 2012;204:487–501. [DOI] [PubMed] [Google Scholar]
- 9.Wu ML, Ho YC, Yet SF. A central role of heme oxygenase-1 in cardiovascular protection. Antioxid Redox Signal. 2011;15:1835–1846. [DOI] [PubMed] [Google Scholar]
- 10.Czibik G, Derumeaux G, Sawaki D, et al. Heme oxygenase-1: an emerging therapeutic target to curb cardiac pathology. Basic Res Cardiol. 2014;109:450. [DOI] [PubMed] [Google Scholar]
- 11.Juhasz B, Varga B, Czompa A, et al. Postischemic cardiac recovery in heme oxygenase-1 transgenic ischemic/reperfused mouse myocardium. J Cell Mol Med. 2011;15:1973–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csiki Z, Papp-Bata A, Czompa A, et al. Orally delivered sour cherry seed extract (SCSE) affects cardiovascular and hematological parameters in humans. Phytotherapy Res. 2015;29:444–449. [DOI] [PubMed] [Google Scholar]
- 13.Tosaki A, Braquet P. DMPO and reperfusion injury: arrhythmia, heart function, electron spin resonance, and nuclear magnetic resonance studies in isolated working guinea pig hearts. Am Heart J. 1990;120:819–830. [DOI] [PubMed] [Google Scholar]
- 14.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vecsernyes M, Juhasz B, Der P, et al. The administration of alpha-melanocyte-stimulating hormone protects the ischemic/reperfused myocardium. Eur J Pharmacol. 2003;470:177–183. [DOI] [PubMed] [Google Scholar]
- 16.Rinne P, Tikka S, Makela S, et al. Hemodynamic actions and mechanisms of systemically administered alpha-MSH analogs in mice. Peptides. 2012;38:150–158. [DOI] [PubMed] [Google Scholar]
- 17.Kristensen J, Jonassen TE, Rehling M, et al. The alpha-MSH analogue AP214 attenuates rise in pulmonary pressure and fall in ejection fraction in lipopolysaccharide-induced systemic inflammatory response syndrome in pigs. Clin Physiol Funct Imaging 2011;31:54–60. [DOI] [PubMed] [Google Scholar]
- 18.Bombicz M, Priksz D, Varga B, et al. Anti-atherogenic properties of Allium ursinum liophylisate: impact on lipoprotein homeostasis and cardiac biomarkers in hypercholesterolemic rabbits. Int J Mol Sci. 2016;17(8):1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rinne P, Harjunpaa J, Makela S, et al. Genetic and pharmacological mouse models of chronic melanocortin activation show enhanced baroreflex control of heart rate. Regul Pept. 2013;182:19–27. [DOI] [PubMed] [Google Scholar]
- 20.Eerola K, Rinne P, Penttinen AM, et al. Alpha-MSH overexpression in the nucleus tractus solitarius decreases fat mass and elevates heart rate. J Endocrinol. 2014;222:123–136. [DOI] [PubMed] [Google Scholar]
- 21.Skrzypiec-Spring M, Grotthus B, Szelag A, et al. Isolated heart perfusion according to Langendorff—still viable in the new millennium. J Pharmacol Toxicol Methods. 2007;55:113–126. [DOI] [PubMed] [Google Scholar]
- 22.Broyd CJ, Sen S, Mikhail GW, et al. Myocardial ischemia in aortic stenosis: insights from arterial pulse-wave dynamics after percutaneous aortic valve replacement. Trends Cardiovasc Med. 2013;23:185–191. [DOI] [PubMed] [Google Scholar]
- 23.Piciche M, Kingma JJ, Fadel E, et al. Enhancement of non-coronary collateral blood flow from the internal thoracic arteries: the theoretical and practical basis of an alternative method of myocardial blood supply. J Cardiovasc Surg. 2011;52:127–131. [PubMed] [Google Scholar]
- 24.Mahmoud FF, Al-Awadhi AM, Haines DD. Amelioration of human osteoarthritis symptoms with topical “biotherapeutics”: a phase I human trial. Cell Stress Chaperones. 2015;20:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bak I, Czompa A, Juhasz B, et al. Reduction of reperfusion-induced ventricular fibrillation and infarct size via heme oxygenase-1 overexpression in isolated mouse hearts. J Cell Mol Med. 2010;14:2268–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haines DD, Bak I, Ferdinandy P, et al. Cardioprotective effects of the calcineurin inhibitor FK506 and the PAF receptor antagonist and free radical scavenger, EGb 761, in isolated ischemic/reperfused rat hearts. J Cardiovasc Pharmacol. 2000;35:37–44. [DOI] [PubMed] [Google Scholar]
- 27.Rinne P, Nordlund W, Heinonen I, et al. Alpha-melanocyte-stimulating hormone regulates vascular NO availability and protects against endothelial dysfunction. Cardiovasc Res. 2013;97:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadowitz PJ, Chapnick BM, Kastin AJ. Comparison of alpha-MSH and several vasoactive substances on vascular resistance in the feline mesenteric vascular bed. Pharmacol Biochem Behav. 1976;5:219–221. [DOI] [PubMed] [Google Scholar]
- 29.Rinne P, Penttinen AM, Nordlund W, et al. Alpha-MSH analogue attenuates blood pressure elevation in DOCA-salt hypertensive mice. PLoS One. 2013;8:e72857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czompa A, Gyongyosi A, Czegledi A, et al. Cardioprotection afforded by sour cherry seed kernel: the role of heme oxygenase-1. J Cardiovasc Pharmacol. 2014;64:412–419. [DOI] [PubMed] [Google Scholar]
- 31.Csepanyi E, Czompa A, Haines D, et al. Cardiovascular effects of low versus high-dose beta-carotene in a rat model. Pharmacol Res. 2015;100:148–156. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee S, Ray D, Lekli I, et al. Effects of Longevinex (modified resveratrol) on cardioprotection and its mechanisms of action. Can J Physiol Pharmacol. 2010;88:1017–1025. [DOI] [PubMed] [Google Scholar]
- 33.Lekli I, Ray D, Mukherjee S, et al. Co-ordinated autophagy with resveratrol and gamma-tocotrienol confers synergetic cardioprotection. J Cell Mol Med. 2010;14:2506–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NIH. National Institute of Health Precision Medicine Initiative Cohort Program. 2016. Available at: https://www.nih.gov/precision-medicine-initiative-cohort-program. Accessed August 31, 2016.


