Abstract
Introduction
Mounting evidence indicates that a disturbed Wnt–β-catenin signaling may be involved in the pathogenesis of chronic kidney disease-mineral and bone and mineral disorder (CKD-MBD). Data on the impact of CKD on circulating levels of the Wnt antagonists sclerostin and Dickkopf related protein 1 (DKK1) and the relationship with laboratory parameters of CKD-MBD are incomplete.
Methods
We analyzed serum sclerostin and DKK1 in 308 patients across the stages of chronic kidney disease (kDOQI stage 1–2 n = 41; CKD stage 3 n = 54; CKD stage 4–5 n = 54; hemodialysis n = 100; peritoneal dialysis n = 59) as well as in 49 healthy controls. We investigated associations with demographics, renal function, parameters of mineral metabolism including 25(OH) vitamin D, 1,25(OH)2 vitamin D, biointact fibroblast growth factor 23 (FGF23), and parathyroid hormone (PTH), and bone turnover markers.
Results
Serum sclerostin, but not DKK1, increases in more advanced stages of CKD and associates with PTH, phosphate, and 1,25(OH)2 vitamin D concentrations. Bone turnover markers are highest in hemodialysis patients presenting the combination of high PTH with low sclerostin level. Serum DKK1 levels are lower in CKD patients than in controls and are not associated with laboratory parameters of mineral metabolism. Interestingly, a direct association between DKK1 and platelet count was observed.
Conclusion
In CKD, serum levels of the Wnt inhibitors DKK1 and sclerostin are unrelated, indicating different sites of origin and/ or different regulatory mechanisms. Sclerostin, as opposed to DKK1, may qualify as a biomarker of CKD-MBD, particularly in dialysis patients. DKK1 serum levels, remarkably, correlate almost uniquely with blood platelet counts.
Introduction
The (canonical) Wnt–β-catenin pathway is increasingly recognized to play an important role in bone [1] and vascular biology [2]. This pathway is tightly regulated by several antagonists, of which the soluble Wnt inhibitors Dickkopf related protein 1 (DKK1, 26kD) and especially sclerostin (28kD) have been studied most intensively. While sclerostin expression is largely limited to bone [3] and calcifying vascular tissue [4], DKK1 is expressed in a number of other tissues and cells including platelets, the prostate and the kidneys [5]. Since sclerostin and DKK1 not only exert local (paracrine) effects, but are also released in the systemic circulation, inhibition of Wnt signaling in distant tissues and organs can also occur. In SOST-/- mice, for instance, it has been shown that kidney repair after unilateral urether obstruction is delayed [6] whilst in animal models of early CKD, incomplete recovery from acute kidney injury led to increased expression of Wnt inhibitors including DKK1 and sclerostin in the injured kidney and to increased levels in the systemic circulation [7]. Thus, DKK1 and sclerostin may also be involved in the many regulatory feedback loops that govern and fine-tune bone and mineral metabolism [8].
Circulating sclerostin levels increase with severity of chronic kidney disease (CKD) and are reported to reach levels that are 2 to 4-fold higher in patients with end stage renal disease as compared to individuals with normal renal function [9–15]. Data on circulating levels of DKK1 in CKD, conversely, are scarce and inconsistent with some investigators demonstrating increments already occurring in early stage CKD [16], while others showing levels in the normal range even in patients with advanced CKD [15, 17]. It is an ongoing debate to what extent sclerostin and DKK1 may serve as biomarkers of CKD-mineral and bone disorder (MBD) [18–20]. The purpose of this study was to evaluate circulating DKK1 and sclerostin levels in CKD and to describe for the first time the relationship between DKK1, sclerostin and prototypic laboratory parameters of mineral metabolism across stages of disease.
Materials and methods
Study population
The study population consisted of 308 prevalent CKD stage 1-5D patients and 49 controls. All patients were recruited from an ongoing observational study at the University Hospitals Leuven, Belgium, investigating uremic toxicity and bone and mineral metabolism in CKD patients (NCT 00441623). All patients were enrolled between February 2006 and July 2008. CKD stage 5D patients were treated either with thrice weekly conventional hemodialysis (n = 100) or peritoneal dialysis (PD, n = 59; continuous ambulatory PD: n = 30; Automated PD: n = 29). Dialysis adequacy was targeted in all patients according to the NKF K-DOQI guidelines. Controls, defined as individuals with no history of CKD and CKD-EPI estimated GFR > 60 ml/min 1.73 m2, were recruited from the dermatology outpatient clinic at the University Hospital Antwerp. All participants were 18 years of age or older and provided written informed consent. All studies were performed according to the Declaration of Helsinki, and approved by the Ethics Committees of the University Hospital Leuven and the University Hospital of Antwerp.
Biochemical measurements
In all participants but HD patients, blood samples were collected in the morning (random, non-fasted). In HD patients, blood samples were collected before the mid-week dialysis session. After standard centrifugation, serum was aliquoted and stored at -80°C pending further analysis. Creatinine, hemoglobin, calcium, phosphate, C-reactive protein (CRP), total alkaline phosphatase (tAP), and cholesterol were all measured using standard laboratory techniques. Serum C-terminal cross-linked telopeptide (CTX-I) was measured using an electrochemiluminescence immunoassay (Roche Diagnostics, Switzerland). Albumin was measured using the bromocresol green method. Bone specific alkaline phosphatase (Bone ALP), calcidiol (25(OH) D), calcitriol (1,25(OH)2D) and PTH (N-TACT II) (i.e. a 2nd generation PTH assay) were measured using a LIAISON XLautomated analyzer with the appropriate analyzer kits (DiaSorin, USA). Serum sclerostin (Biomedica, Austria), DKK1 (Biomedica, Austria), and biointact fibroblast growth factor 23 (FGF23, Kainos, Japan) were measured using ELISA kits according to the manufacturer’s instructions. As a complementary 3rd generation PTH assay, whole (1–84) PTH (CAP PTH) was also measured using the Scantibodies CAP assay (USA). Detection limits of the various assays were: Bone ALP (0.1 μg/l); sclerostin (8.9 pmol/l); DKK1 (0.38 pmol/l); 25(OH)D (4.0 ng/ml); 1,25(OH)2D (< 2.0 pg/ml); CAP PTH (1.0 pg/ml); N-TACT PTH (1.7 pg/ml); FGF23 (3 pg/ml). Available reference values for healthy subjects are: sclerostin (11.9–47.9 pmol/l); DKK1 (47.7±20 pmol/l); calcitriol (25.1–66.1 pg/ml); CAP PTH (5–39 pg/ml); N-TACT PTH (14.5–87.1 pg/ml); FGF23 (8.2–54.3 pg/ml). All assays used report intra- and inter-assay variations below 15%. The eGFR was calculated using the CKD-EPI equation. Single pool Kt/V (spKt/V), a measure of dialysis efficacy was calculated using the second-generation logarithmic formula of Daugirdas [21]. Anuria was defined as a urine output <100 ml.
Statistical analysis
Data are expressed as mean (standard deviation) for normally distributed variables or median (IQR) for non-normally distributed variables. Differences between groups were tested using parametric ANOVA, Kruskal-Wallis or chi-squared test as appropriate. Correlations between circulating levels of DKK1 and sclerostin and other variables were calculated by Spearman’s rank correlation coefficients. Multivariate linear regression analysis was performed including all univariately associated variables (p<0.2) to identify independent determinants of serum DKK1 and sclerostin. After excluding collinearity, the best subset of variables was selected by backward elimination on p <0.2. This subset was then subjected to a final elimination procedure on p <0.05. Inspection of residual plots assured that the a priori assumptions for linear regression were justified. For all statistical analysis, p-values less than 0.05 were considered significant. All statistical analyses were performed using SAS (version 9.3, the SAS institute, Cary, NC, USA).
Results
Demographics
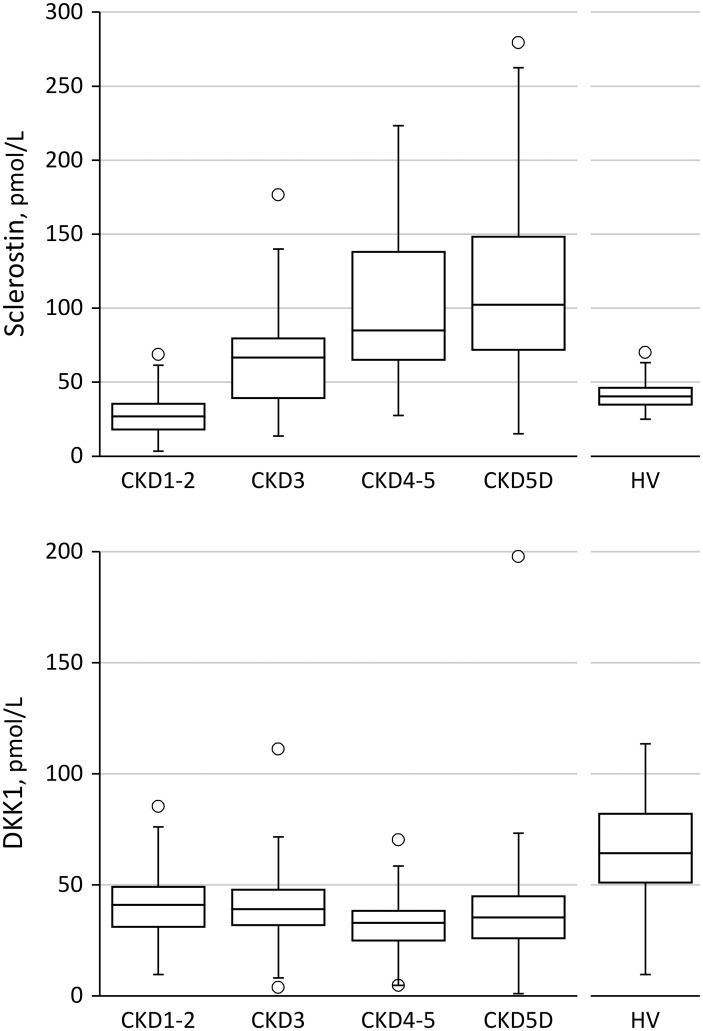
Relevant clinical and biochemical characteristics of the healthy controls and CKD patients, categorized according to stage of disease, are summarized in Table 1. Serum sclerostin levels were higher and serum DKK1 levels were lower in CKD patients as compared to controls. Fig 1 shows serum sclerostin and DKK1 levels across CKD stages. In patients with chronic kidney disease treated with dialysis, sclerostin was approximately 2.5-fold higher than in non-CKD controls. Serum DKK1 levels, conversely were approximately 2-fold lower in dialysis patients as compared to non-CKD controls. S1 Fig shows temporal aspects of disordered mineral metabolism and sclerostin in CKD stage 1-5D.
Table 1. Demographics, biochemistry and therapy in healthy volunteers and CKD patients across stages.
| HV | CKD 1–2 | CKD3 | CKD 4–5 | CKD5D | P (CKD) | |
|---|---|---|---|---|---|---|
| N | 49 | 41 | 54 | 54 | 159 | |
| Age | 50.88 ± 16.19 | 45.37 ± 14.69 | 64.07 ± 14.04 | 66.95 ± 11.47 | 63.47 ± 15.25 | <0.0001 |
| BMI | 23.99 ± 33.64 | 25.84 ± 4.92 | 27.04 ± 5.60 | 27.59 ± 5.59 | 23.56 ± 4.14 | <0.0001 |
| Renal dx Diabetes (%) | - | 0 | 1.9 | 1.9 | 17.6 | <0.0001 |
| Glomerular (%) | - | 63.4 | 29.6 | 13.0 | 28.9 | |
| Interstitial (%) | - | 0 | 5.6 | 3.7 | 4.4 | |
| Vascular (%) | - | 2.4 | 7.4 | 20.4 | 13.8 | |
| Cystic Heriditary (%) | - | 9.8 | 11.1 | 14.8 | 4.4 | |
| Miscellaneous, unknown (%) | - | 24.4 | 44.4 | 46.3 | 30.8 | |
| Male gender, % | 41 | 32 | 56 | 70 | 60 | 0.002 |
| CVD, % | - | 14.6 | 31.5 | 44.4 | 40.9 | 0.21 |
| DM, % | - | 10 | 13 | 20 | 26 | <0.05 |
| Smoking, % (never/previous/current) | NA | 62/22/16 | 66/20/14 | 45/37/18 | 46/37/18 | 0.14 |
| Anti-platelet agents, % | 0 | 10 | 35 | 52 | 51 | <0.0001 |
| Non-calcium PB, % | 0 | 0 | 0 | 0 | 24.4 | |
| Phosphate binder, % | 0 | 12.2 | 14.8 | 35.2 | 86.5 | <0.0001 |
| Nutritional VitD, % | 0 | 4.9 | 18.5 | 31.5 | 48.4 | <0.0001 |
| Active VitD, % | 0 | 2.4 | 7.4 | 14.8 | 53.2 | <0.0001 |
| Calcimimetics, % | 0 | 0 | 0 | 0 | 9 | 0.003 |
| Bisphosphonates, % | 0 | 5 | 15 | 6 | 4 | <0.05 |
| Hb, g/dL | - | 14.1 ± 1.56 | 13.6 ± 1.5 | 12.5 ± 1.4 | 11.8 ± 1.3 | <0.0001 |
| Platelets, ×103/mm3 | - | 280 ± 73 | 227 ± 68 | 208 ± 67 | 245 ± 83 | <0.0001 |
| Tchol, mg/dL | - | 187 ± 32 | 181± 38 | 178 ± 35 | 163± 37 | 0.0009 |
| CRP, mg/L | - | 3.66 ± 6.15 | 3.55 ± 4.85 | 10.89 ± 26.93 | 8.30 ± 12.73 | <0.0001 |
| Albumin, g/L | - | 44.79 ± 3.33 | 45.59 ± 2.11 | 44.48 ± 3.04 | 39.42 ± 3.78 | <0.0001 |
| Urea Nitrogen, mg/dL | - | 33.5 ± 10,6 | 62.7± 21.2 | 110.3 ± 42.5 | 116.2± 32.4 | <0.0001 |
| Creatinine, mg/dL | 0.94 ± 0.13 | 0.86 ± 0.14 | 1.49± 0.22 | 3.04 ± 1.43 | 7.25± 2.75 | <0.0001 |
| eGFR, mL/min 1.73m2 | 81.25± 14.45 | 79.75± 18.90 | 38.71± 7.67 | 19.07± 6.53 | - | <0.0001 |
| Ca, mg/dL | 10.18± 1.16 | 9.18± 0.45 | 9.24± 0.35 | 9.09± 0.54 | 9.35± 0.73 | <0.0001 |
| Phos, mg/dL | 4.02± 0.87 | 3.05± 0.58 | 3.13± 0.62 | 3.71± 0.83 | 4.51± 1.31 | <0.0001 |
| Bicarbonate, mmol/L | - | 25.6 ±2.0 | 25.0 ±2.5 | 23.6 ± 2.6 | 25.0 ± 2.8 | 0.002 |
| tAP, U/L | - | 167.34 ± 45.77 | 182.23 ± 60.64 | 220.26 ± 111.55 | 254.25 ± 150.09 | <0.0001 |
| Bone ALP, μg/L | - | 10.9 ± 4.7 | 12.1 ± 6.0 | 16.3 ± 14.5 | 18.7 ±15.0* | 0.0003 |
| CTX-I, ng/L | - | - | - | - | 2539 ± 2392 | - |
| 25(OH)D, ng/mL | 22.8 (15.7–27.5) | 18.2 (12.1–26.2) | 17.3 (13.3–25.0) | 14.6 (10.9–19.3) | 14.8 (10.3–20.6)* | 0.08 |
| 1,25(OH)2D, pg/mL | - | 82.0 (47.2–111.1) | 54.0 (29.1–92.0) | 30.5 (23.0–43.6) | - | <0.0001 |
| N-TACT PTH, pg/mL | 19.7 (14.0–24.7) | 18.4 (11.4–27.6) | 29.9 (22.6–44.3) | 62.95 (46.0–137.0) | 80.95 (45.2–154.0) * | <0.0001 |
| CAP PTH, pg/mL | 45.7 (39.2–63.3) | 20.4 (12.4–38.0) | 32.1 (25.4–47.6) | 77.6 (48.7–123.4) | 166.5 (67.7–341.4) | <0.0001 |
| Sclerostin, pmol/L | 40.5 (34.8–46.2) | 27.0 (18.1–35.45) | 66.7 (39.1–79.6) | 85.0 (63.8–138.2) | 102.4 (72.0–148.9) | <0.0001 |
| DKK1, pmol/L | 64.3 (51.0–82.0) | 41.0 (31.0–49.1) | 39.1 (31.9–47.9) | 32.9 (24.9–38.4) | 35.30 (25.6–45.0) | 0.02 |
| FGF23, ng/L | 41.54 (35.1–49.1) | 35.9 (30.3–45.9) | 65.2 (49.1–91.4) | 155.7 (93.0–279.2) | 3725.0 (824.4–9963.1) | <0.0001 |
Fig 1. Serum sclerostin (A) and DKK1 (B) levels according to CKD stages and in healthy volunteers (HV).
Serum sclerostin in CKD
In CKD patients not yet on dialysis (S1 Table), male gender, history of CVD, higher age, phosphate, FGF23, PTH (both assays), and lower eGFR, bicarbonate, calcitriol, blood platelets all were significantly associated with higher serum sclerostin levels. In multivariable analysis, only gender, age, eGFR and calcitriol independently associated with serum sclerostin levels, explaining 54% of its variability (p<0.0001).
In CKD stage 5D patients (S2 Table), male gender, hemodialysis as modality, history of cardiovascular disease (CVD), higher age and phosphate and lower bicarbonate, PTH (both assays), residual renal function, and blood platelets all were significantly associated with higher serum sclerostin levels. In multivariate analysis, only gender, phosphate and N TACT PTH independently associated with serum sclerostin levels, explaining 16% of its variability (p<0.0001).
Serum DKK1 in CKD
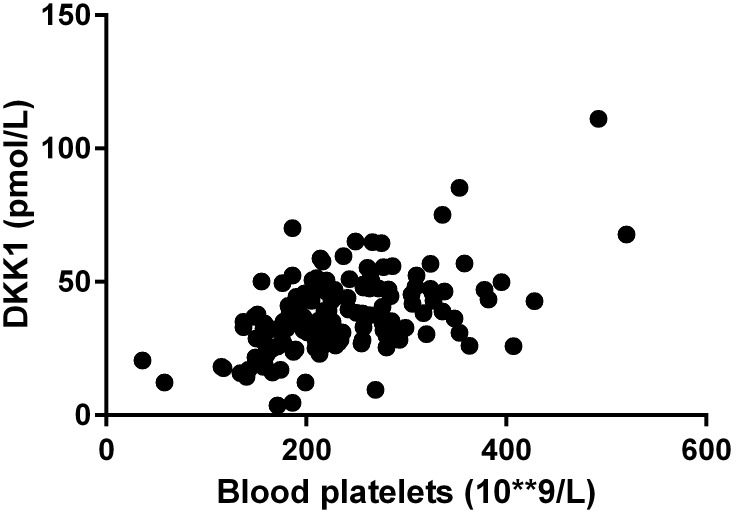
In CKD patients not yet on dialysis (S1 Table), higher bicarbonate, eGFR, blood platelets, and lower FGF23 all are significantly associated with higher serum DKK1 levels. In multivariable analysis, only bicarbonate and blood platelets independently associated with serum DKK1 levels, explaining 25% of its variability (p<0.0001). Fig 2 shows the correlation between blood platelet count and serum DKK1 concentration in CKD patients not yet on dialysis.
Fig 2. Correlation between blood platelet count and serum DKK levels in CKD patients not yet on dialysis (R2 = 0.28, p<0.0001, Spearman).
In CKD stage 5D patients (S2 Table), higher calcium, CRP, and blood platelets and lower PTH all were significantly associated with higher serum DKK1 levels. In multivariable analysis, only calcium and blood platelets independently associated with serum DKK1 levels, explaining 14% of its variability (p<0.0001). Of note, no association was observed between serum DKK1 levels and use of antiplatelet agents. Serum sclerostin and DKK1 levels did not correlate, neither in the overall cohort nor in subgroups.
Sclerostin, DKK1 and bone turnover biomarkers in HD patients
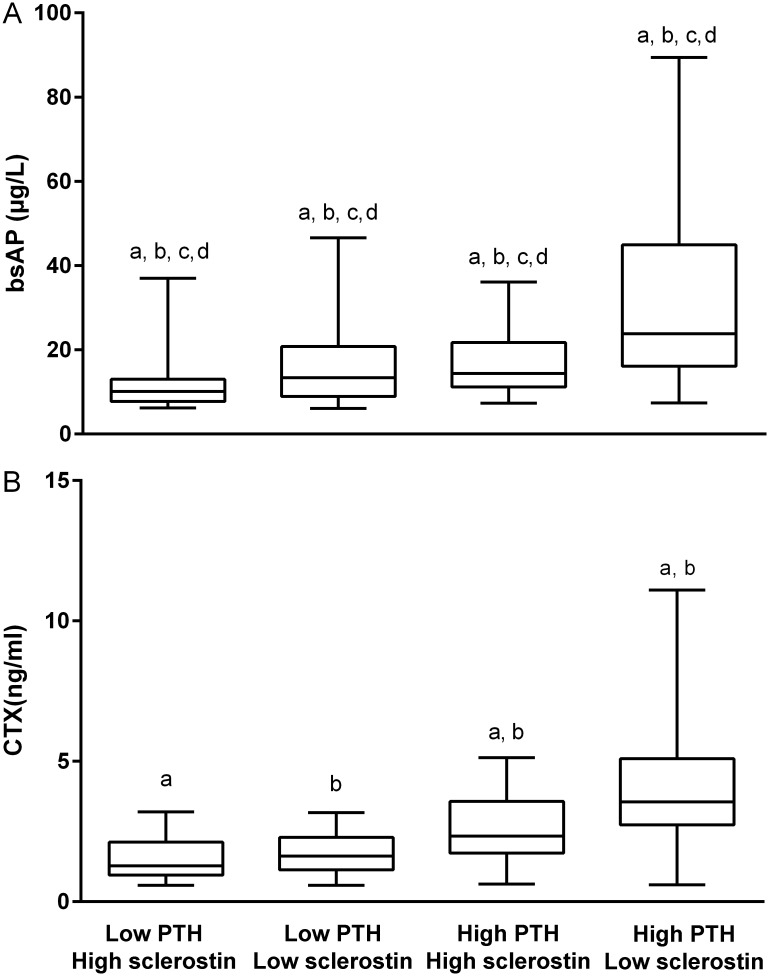
As eGFR turned out to be a major confounder of the relationship between Wnt inhibitors, in particular sclerostin, and bone metabolism, we further investigated the correlation of sclerostin, DKK1, laboratory parameters of mineral metabolism and markers of bone formation (Bone ALP) and resorption (CTX-I) in hemodialysis patients only (Table 2). PTH (both assays) strongly and directly correlated with Bone ALP and CTX-I. Sclerostin, as opposed to DKK1, tended (p ≤ 0.1) to correlate inversely with bone formation and resorption. Fig 3 shows the mean Bone ALP and CTX-I levels in patients categorized according to PTH and sclerostin levels above or below the median. Patients with high (above the median) PTH in combination with low sclerostin (below the median) had the highest Bone ALP and CTX-I levels. In regression analyses, low sclerostin independently associated with high Bone ALP levels (but not CTX-I levels), independent of PTH.
Table 2. Spearman correlation matrix HD patients.
| Ca | Phos | N-TACT PTH | CAP PTH | Sclerostin | DKK1 | FGF23 | Bone ALP | CTX-I | |
| Ca | 1 | 0.05 | -0.04 | 0.05 | -0.05 | 0.15 | 0.36Y | 0.08 | -0.03 |
| Phos | 1 | 0.37 Y | 0.33X | 0.16 | -0.06 | 0.57 Z | 0.06 | 0.41 Z | |
| N-TACT PTH | 1 | 0.99Z | -0.19 (p = 0.07) | -0.10 | 0.32 Y | 0.57 Z | 0.73 Z | ||
| CAP PTH | 1 | -0.20 (p = 0.06) | -0.09 | 0.31 Y | 0.63 Z | 0.70 Z | |||
| Sclerostin | 1 | -0.05 | 0.06 | -0.18(p = 0.10) | -0.18 (p = 0.08) | ||||
| DKK1 | 1 | -0.02 | -0.13 | -0.09 | |||||
| FGF23 | 1 | 0.03 | 0.32 X | ||||||
| Bone ALP | 1 | 0.58 Z | |||||||
| CTX-I | 1 |
X: p < 0.05;
Y: p < 0.01;
Z: p < 0.001
Fig 3. Bone-specific alkaline phosphatase level (Bone ALP) (A) and C-terminal telopeptide of collagen type 1 (CTX-I) (B), categorized according to PTH and sclerostin levels above [high] or below [low] the median.
Groups with same indices differ significantly.
Discussion
A first finding of the present study is that circulating levels of sclerostin, as opposed to DKK1, increase with severity of CKD, reaching levels that are 2–3 fold higher than in non-CKD controls.
This increase is likely the result of an increased production of sclerostin, since a recent clinical study in 120 patients with CKD stage 1–5 showed an increased rather than a decreased absolute and fractional urinary excretion of sclerostin with declining kidney function [10]. Furthermore, in jck mouse, a genetic model of polycystic kidney disease that exhibits progressive renal disease, a transient increase in bone sclerostin was observed already in early stage disease [22]. Till today, it is not clear which mechanism underlies signaling to the skeleton to increase the production of sclerostin in the setting of CKD.
Besides an association with kidney function, we observed significant associations between circulating sclerostin levels and various laboratory parameters of CKD-MBD. These associations were most pronounced in CKD stage 5D patients. Most probably, the overwhelming impact of eGFR on circulating sclerostin levels obscured associations with laboratory parameters of mineral metabolism in CKD patients not yet on dialysis.
Serum 1,25(OH)2D levels negatively associated with circulating sclerostin levels in CKD patients not yet on dialysis, independent of eGFR, 25(OH)D, PTH and FGF23. This observation is in line with recent experimental evidence by Ryan et al. [8]. These investigators showed increased 25-hydroxyvitamin D 1α-hydroxylase cytochrome P450 (cyp27B1) mRNA in kidneys of SOST KO mice as compared to their wild types. Moreover, treatment of cultured proximal tubule cells with mouse recombinant sclerostin decreased cyp27B1 mRNA transcripts. Whether vitamin D, reciprocally, affects SOST expression and circulating sclerostin levels remains to be investigated. Of note, circulating sclerostin in the present study did not differ between patients on and off therapy with active and/or nutritional vitamin D (data not shown).
In agreement with previous studies, we observed a positive and independent association between serum phosphate and sclerostin levels [9, 13, 14]. Additional studies are required to unravel the underlying regulatory mechanisms. In this context it is worth to be mentioned that cross-sectional studies investigating the association between sclerostin and FGF23, yielded conflicting results with some studies (including present study) reporting no association [11] and other studies observing a positive association [23].
In agreement with previous studies [11, 24], we observed an inverse relationship between sclerostin and PTH concentrations in dialysis patients. These data confirm and extend clinical observations in patients with non-renal parathyroid disorders [25–27] and are consistent with experimental data demonstrating downregulation of SOST by PTH [28]. Of note, high sclerostin levels coexist with high PTH levels in patients with advanced CKD. This observation suggests skeletal resistance to the action of PTH [29–31], similar to FGF23 resistance explaining the coexistence of high FGF23 and PTH levels in advanced stage CKD [32]. Of note, gender, serum phosphate and PTH levels determined only 16% of the variability of sclerostin levels in CKD stage 5D patients, implying that many other systemic and local determinants remain to be identified.
Consistent with the biological effects of sclerostin on bone, we observed an inverse relationship between serum sclerostin and Bone ALP, a bone formation marker, and between serum sclerostin and CTX-I, a bone resorption marker. As such, our data in HD patients confirm and extend previous clinical data [11, 12, 33]. Of interest, levels of the bone turnover biomarkers were highest in patients with high PTH levels in combination with low sclerostin levels.
Contrary to sclerostin, circulating DKK1 levels were not or only marginally associated with kidney function and parameters of mineral metabolism. Previous studies investigating the association between DKK1 and kidney function yielded conflicting results with some investigators observing unaltered [15, 17], while others reported increased [16] DKK1 levels in CKD. Of note, serum levels of DKK1 and sclerostin were unrelated in the present study, pointing to different origin and different regulatory mechanisms.
Importantly, the expression of sclerostin and DKK1 is not restricted to bone. Substantial evidence indicates that platelets may be a major source of circulating levels of DKK1. Moreover, it has been demonstrated that during clotting ex vivo, DKK1 is released from platelets to a significant but variable extent [34]. In a cohort of healthy volunteers, levels of DKK1 levels were 2.7-fold higher in serum samples as compared to plasma samples [34]. Whether the degree of this ex vivo release is a random phenomenon or relates to the in vivo activation state of the platelets, as suggested by some investigators, is a matter of ongoing discussion [35]. Of interest, in the present study, only platelet count and calcium, playing a crucial role in platelet activation [36], were found to be independently associated with serum DKK1 levels in dialysis patients and CKD patients not yet on dialysis. As opposed to others [37], we failed to demonstrate lower DKK1 levels in CKD patients receiving antiplatelet drugs compared with those not on antiplatelet therapy.
Significant but often complex associations have been reported between circulating levels of sclerostin and DKK1 and indices of vascular health [13, 15, 20, 38, 39]. Both sclerostin and DKK1 may be considered mediators and markers of cardiovascular disease (CVD). In the present study, and opposite to recent studies in non-CKD patients [35, 40], we failed to find higher circulating DKK1 and sclerostin levels in CKD patients with CVD as compared to counterparts free of CVD.
In conclusion, serum levels of the Wnt inhibitors DKK1 and sclerostin are unrelated in CKD, reflecting a different origin and different regulatory mechanisms. Sclerostin, as opposed to DKK1, may qualify as a biomarker of CKD-MBD, particularly in dialysis patients. DKK1 serum levels mainly relate to platelet count and/or activity. Additional experimental and clinical studies are required to elucidate the (path)physiological role of circulating sclerostin and DKK1 in bone disease and beyond. This information is mandatory as anti-sclerostin and anti-DKK1 monoclonal antibodies emerge as very promising pharmaceuticals in the treatment of osteoporosis.
Supporting information
(TIF)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
FB is an employee of Diasorin Inc. The funder provided support in the form of salaries for author FB and also supplied research materials for the current study. PCD has received previous research grants from Diasorin SAP.
References
- 1.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92. 10.1038/nm.3074 [DOI] [PubMed] [Google Scholar]
- 2.Thompson B, Towler DA. Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol. 2012;8(9):529–43. 10.1038/nrendo.2012.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6):805–14. 10.1084/jem.20031454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandenburg VM, Kramann R, Koos R, Kruger T, Schurgers L, Muhlenbruch G, et al. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol. 2013;14:219 10.1186/1471-2369-14-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev. 2012;33(5):747–83. 10.1210/er.2011-1060 [DOI] [PubMed] [Google Scholar]
- 6.Wang LF, Wu H, Xu Y, Deng M, Han XL, Bai D. Effect of SOST gene deletion on the progression of renal interstitial fibrosis in obstructive kidney injury. Ren Fail. 2015;37(9):1514–7. 10.3109/0886022X.2015.1077323 [DOI] [PubMed] [Google Scholar]
- 7.Hruska KA, Sugatani T, Agapova O, Fang Y. The chronic kidney disease—Mineral bone disorder (CKD-MBD): Advances in pathophysiology. Bone. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan ZC, Ketha H, McNulty MS, McGee-Lawrence M, Craig TA, Grande JP, et al. Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci U S A. 2013;110(15):6199–204. 10.1073/pnas.1221255110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere MC, Diarra D, et al. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol. 2011;6(4):877–82. 10.2215/CJN.06550810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cejka D, Marculescu R, Kozakowski N, Plischke M, Reiter T, Gessl A, et al. Renal elimination of sclerostin increases with declining kidney function. J Clin Endocrinol Metab. 2014;99(1):248–55. 10.1210/jc.2013-2786 [DOI] [PubMed] [Google Scholar]
- 11.Delanaye P, Krzesinski JM, Warling X, Moonen M, Smelten N, Medart L, et al. Clinical and biological determinants of sclerostin plasma concentration in hemodialysis patients. Nephron Clin Pract. 2014;128(1–2):127–34. 10.1159/000366449 [DOI] [PubMed] [Google Scholar]
- 12.Ishimura E, Okuno S, Ichii M, Norimine K, Yamakawa T, Shoji S, et al. Relationship between serum sclerostin, bone metabolism markers, and bone mineral density in maintenance hemodialysis patients. J Clin Endocrinol Metab. 2014;99(11):4315–20. 10.1210/jc.2014-2372 [DOI] [PubMed] [Google Scholar]
- 13.Kanbay M, Siriopol D, Saglam M, Kurt YG, Gok M, Cetinkaya H, et al. Serum sclerostin and adverse outcomes in nondialyzed chronic kidney disease patients. J Clin Endocrinol Metab. 2014;99(10):E1854–61. 10.1210/jc.2014-2042 [DOI] [PubMed] [Google Scholar]
- 14.Pelletier S, Dubourg L, Carlier MC, Hadj-Aissa A, Fouque D. The relation between renal function and serum sclerostin in adult patients with CKD. Clin J Am Soc Nephrol. 2013;8(5):819–23. 10.2215/CJN.07670712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thambiah S, Roplekar R, Manghat P, Fogelman I, Fraser WD, Goldsmith D, et al. Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness. Calcif Tissue Int. 2012;90(6):473–80. 10.1007/s00223-012-9595-4 [DOI] [PubMed] [Google Scholar]
- 16.Fang Y, Ginsberg C, Seifert M, Agapova O, Sugatani T, Register TC, et al. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J Am Soc Nephrol. 2014;25(8):1760–73. 10.1681/ASN.2013080818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malluche HH, Davenport DL, Cantor T, Monier-Faugere MC. Bone mineral density and serum biochemical predictors of bone loss in patients with CKD on dialysis. Clin J Am Soc Nephrol. 2014;9(7):1254–62. 10.2215/CJN.09470913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandenburg VM, D'Haese P, Deck A, Mekahli D, Meijers B, Neven E, et al. From skeletal to cardiovascular disease in 12 steps-the evolution of sclerostin as a major player in CKD-MBD. Pediatr Nephrol. 2016;31(2):195–206. 10.1007/s00467-015-3069-7 [DOI] [PubMed] [Google Scholar]
- 19.Evenepoel P, D'Haese P, Brandenburg V. Sclerostin and DKK1: new players in renal bone and vascular disease. Kidney Int. 2015;88(2):235–40. 10.1038/ki.2015.156 [DOI] [PubMed] [Google Scholar]
- 20.Pelletier S, Confavreux CB, Haesebaert J, Guebre-Egziabher F, Bacchetta J, Carlier MC, et al. Serum sclerostin: the missing link in the bone-vessel cross-talk in hemodialysis patients? Osteoporos Int. 2015;26(8):2165–74. 10.1007/s00198-015-3127-9 [DOI] [PubMed] [Google Scholar]
- 21.Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4(5):1205–13. [DOI] [PubMed] [Google Scholar]
- 22.Sabbagh Y, Graciolli FG, O'Brien S, Tang W, dos Reis LM, Ryan S, et al. Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res. 2012;27(8):1757–72. 10.1002/jbmr.1630 [DOI] [PubMed] [Google Scholar]
- 23.Moyses RM, Jamal SA, Graciolli FG, dos Reis LM, Elias RM. Can we compare serum sclerostin results obtained with different assays in hemodialysis patients? Int Urol Nephrol. 2015;47(5):847–50. 10.1007/s11255-015-0971-7 [DOI] [PubMed] [Google Scholar]
- 24.Drechsler C, Evenepoel P, Vervloet MG, Wanner C, Ketteler M, Marx N, et al. High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant. 2015;30(2):288–93. 10.1093/ndt/gfu301 [DOI] [PubMed] [Google Scholar]
- 25.Ardawi MS, Al-Sibiany AM, Bakhsh TM, Rouzi AA, Qari MH. Decreased serum sclerostin levels in patients with primary hyperparathyroidism: a cross-sectional and a longitudinal study. Osteoporos Int. 2012;23(6):1789–97. 10.1007/s00198-011-1806-8 [DOI] [PubMed] [Google Scholar]
- 26.Costa AG, Cremers S, Rubin MR, McMahon DJ, Sliney J Jr., Lazaretti-Castro M, et al. Circulating sclerostin in disorders of parathyroid gland function. J Clin Endocrinol Metab. 2011;96(12):3804–10. 10.1210/jc.2011-0566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Lierop AH, Witteveen JE, Hamdy NA, Papapoulos SE. Patients with primary hyperparathyroidism have lower circulating sclerostin levels than euparathyroid controls. Eur J Endocrinol. 2010;163(5):833–7. 10.1530/EJE-10-0699 [DOI] [PubMed] [Google Scholar]
- 28.Kramer I, Loots GG, Studer A, Keller H, Kneissel M. Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res. 2010;25(2):178–89. 10.1359/jbmr.090730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berdud I, Martin-Malo A, Almaden Y, Tallon S, Concepcion MT, Torres A, et al. Abnormal calcaemic response to PTH in the uraemic rat without secondary hyperparathyroidism. Nephrol Dial Transplant. 1996;11(7):1292–8. [PubMed] [Google Scholar]
- 30.Massry SG, Coburn JW, Lee DB, Jowsey J, Kleeman CR. Skeletal resistance to parathyroid hormone in renal failure. Studies in 105 human subjects. Ann Intern Med. 1973;78(3):357–64. [DOI] [PubMed] [Google Scholar]
- 31.Wesseling-Perry K, Harkins GC, Wang HJ, Elashoff R, Gales B, Horwitz MJ, et al. The calcemic response to continuous parathyroid hormone (PTH)(1–34) infusion in end-stage kidney disease varies according to bone turnover: a potential role for PTH(7–84). J Clin Endocrinol Metab. 2010;95(6):2772–80. 10.1210/jc.2009-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K, et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77(3):232–8. 10.1038/ki.2009.414 [DOI] [PubMed] [Google Scholar]
- 33.Cejka D, Jager-Lansky A, Kieweg H, Weber M, Bieglmayer C, Haider DG, et al. Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrol Dial Transplant. 2012;27(1):226–30. 10.1093/ndt/gfr270 [DOI] [PubMed] [Google Scholar]
- 34.Voorzanger-Rousselot N, Goehrig D, Facon T, Clezardin P, Garnero P. Platelet is a major contributor to circulating levels of Dickkopf-1: clinical implications in patients with multiple myeloma. Br J Haematol. 2009;145(2):264–6. 10.1111/j.1365-2141.2009.07587.x [DOI] [PubMed] [Google Scholar]
- 35.Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(8):1228–34. 10.1161/ATVBAHA.109.189761 [DOI] [PubMed] [Google Scholar]
- 36.Vemana HP, Karim ZA, Conlon C, Khasawneh FT. A critical role for the transient receptor potential channel type 6 in human platelet activation. PLoS One. 2015;10(4):e0125764 10.1371/journal.pone.0125764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lattanzio S, Santilli F, Liani R, Vazzana N, Ueland T, Di Fulvio P, et al. Circulating dickkopf-1 in diabetes mellitus: association with platelet activation and effects of improved metabolic control and low-dose aspirin. Journal of the American Heart Association. 2014;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claes KJ, Viaene L, Heye S, Meijers B, d'Haese P, Evenepoel P. Sclerostin: Another vascular calcification inhibitor? J Clin Endocrinol Metab. 2013;98(8):3221–8. 10.1210/jc.2013-1521 [DOI] [PubMed] [Google Scholar]
- 39.Register TC, Hruska KA, Divers J, Bowden DW, Palmer ND, Carr JJ, et al. Plasma Dickkopf1 (DKK1) concentrations negatively associate with atherosclerotic calcified plaque in African-Americans with type 2 diabetes. J Clin Endocrinol Metab. 2013;98(1):E60–5. 10.1210/jc.2012-3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Martin A, Reyes-Garcia R, Garcia-Fontana B, Morales-Santana S, Coto-Montes A, Munoz-Garach M, et al. Relationship of Dickkopf1 (DKK1) with cardiovascular disease and bone metabolism in Caucasian type 2 diabetes mellitus. PLoS One. 2014;9(11):e111703 10.1371/journal.pone.0111703 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.