Abstract
This work evaluated the impact of exogenous soil inoculation of beneficial fungal strain Piriformospora indica on phytochemical changes and the related genes expression of Chinese cabbage (Brassica campestris ssp. chinensis L.) by greenhouse pot experiments. High performance liquid chromatography (HPLC) affirmed that among the different combinations of fungal and organic fertilizer treatments, the phenolic acids and flavonoids were considerably enriched in organic fertilizer and fungi (OP) followed by organic fertilizer, biochar, fungi (OBP) treated plants. The antiradical activity was higher in OP (61.29%) followed by P (60%) and organic fertilizer (OF) (53.84%) inoculated plants which positively correlated with chlorophyll, carotenoids and flavonoids level (P<0.05). Furthermore, results showed that the exogenous application of P. indica significantly (P<0.05) enhanced plant growth, as well as stimulating the activation of chlorophyll, carotenoids and other antioxidant related pathways. The RT-qPCR analysis indicated that key FLS gene triggering the synthesis of kaemferol was up-regulated by the inoculation of P. indica. In conclusion, the results revealed that organic fertilizer and P. indica (OP) is the most appropriate combination for improving phytochemical and antiradical properties in Pakchoi.
Introduction
The consumption of fruits and vegetables could increase the human innate immunity against chronic diseases [1, 2]. The phytoconstituents including polyphenols, quercetin and flavonoids are largely demonstrated as important antioxidants and exhibit profound radical scavenging capabilities [3–7]. Chinese cabbage, which belongs to the Brassicaceae family is a predominantly consumed green leafy vegetable in China. It has the noteworthy health-promoting properties due to its high contents of fibers and phytochemicals [8].
The quality of fresh vegetables could be assessed based on their nutritional value, growing conditions and usage of fertilizer. Despite the fact that the genetic modification and agronomic manipulation methods are widely used to improve the nutritional value of plants, the inadequate public acceptance and soil specificity of genetically modified food are still the challenges. Alternatively, the modification of fertilization level brings a suitable method to improve the quality of edible plants, particularly in phytochemicals. To date, the use of beneficial microorganisms has become the sole alternative solution to ensure nutrient use efficiency and future food security because of the environmental concerns regarding excess utilization of chemical fertilizer. In general, the microorganisms exert positive effects on the growth characteristics by developing a holistic and functional relationship with plants [9]. Applying beneficial microbes in agriculture has a long history started from 60 years ago and becomes more supported as they were proven to reduce the biotic and abiotic stresses in plants [10]. Microorganisms that exist naturally in the soil are vital component of soil sub-ecosystem, since they play the key role in nutrient availability, reducing soil erosion and upgrading soil structure [11].
Mycorrhizae are associated with the majority of the plants under natural conditions [12]. Roots colonized by mycorrhizae are more efficient in nutrients acquisition, as its surface area can be extended up to several centimeters in soil [13]. Involvement of arbuscular mycorrhizal fungi(AMF) in micronutrient availability and mutualistic relationship construction with the roots have previously been deliberated [14]. The use of beneficial microbes and their products for agricultural purpose have many advantages such as bio-control agents without interrupting the ecological processes. On the other hand, the organism is chosen due to its resistance towards the specific chemical reagents. Furthermore, The self-replication of microbes may save the expenses of repeated applications [10, 15]. In addition to that, beneficial microbes can assist plants in transforming nutritional elements to available form and therefore holding a potential to ameliorate crop yields in an environmentally-friendlier manner [9, 16].
More than 90% of the plants establish a mutualistic relationship with AMF [17–19]. Previous studies supported the idea that AMF increases the level of secondary metabolites to assist the plant in resisting biotic and abiotic stresses [20]. Piriformospora indica, an axenically cultivable phytopromotional, biotrophic mutualistic root endosymbiont belongs to order Sebacinales (Basidiomycota). This fungus has a broad host range, which is not only confined to vascular plants but also to colonized mosses, implies that this fungus has evolved highly effective colonization strategies and provide plants multifaceted amenities (such as nutrient uptake, disease resistance, stress tolerance and growth- promotion involving value addition) [21–23]. In present study, the exogenous application of beneficial fungus Piriformospora indica on growth indices, phytochemical and health-promoting properties of Pakchoi was investigated by greenhouse experiments.
Materials and methods
Chemicals and reagents
Standard laboratory grade chemicals/reagents such as Folin-Ciocalteau reagent, 2, 2-diphenyl-1-picrylhydrazyl radical (DPPH), gallic acid, ascorbic acid, and (±)-6-Hydroxy-2, 5, 7, 8-tetramethylchromane-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich (USA). The phosphoric acid and DL-lactic acid were provided by Sangon Biotechnology; Biotin from Sinopharm chemical reagent Co., Ltd; whereas DTT (DL-Dithiothreitol), Trizma buffer, Nicotinamide (C6H6N2O), and Riboflavin (vitamin B2, C17H20N4O6) were procured from Shanghai Linfeng chemical reagent Co., Ltd. All other chemicals and solvents used were of analytical grade and used without any further purification.
Greenhouse experiments
In the greenhouse experiment, the following 9 treatments were applied: (1) CK (un-inoculated sterile soil), (2) CF (chemical fertilizer), (3) OF (organic fertilizer), (4) B (biochar), (5) OB (organic fertilizer and Biochar), (6) BP (biochar and fungi), (8) OP (organic fertilizer and fungi), (9) OBP (organic fertilizer, biochar and fungi). The soil collected from an organic farm which located in Shanghai, China (30°51′ N 121°30′E) was air-dried, grounded, and passed through a 2.0 mm sieve. Soil basic properties were determined as; pH, 7.32; Electrical conductivity (EC), 0.14 (dS/m); available nitrogen, 111.6 (ppm); available phosphate, 181.7 (ppm), available potassium, 306.8 (ppm), cation exchange capacity (CEC), 13.2 (cmol(+)/kg); NH4+, 7.86 (ppm); NO3-, 2.67 (ppm); total carbon, 1.92 (%); total nitrogen, 0.19 (%); and total potassium, 2063 (ppm).
Fertilizers and inoculation of soil
In treatment setup, analytical grade chemical fertilizers (urea and phosphorus for N source, and KH2PO4 as K source) were given with the following ratio; 0.6 g CO (NH2)2, 0.27 g KH2PO4 (N-P2O5-K2O = 0.28–0.14–0.13 g/pot). Organic fertilizer was prepared from chicken manure and mushroom waste and fermented for about 3 months with the following basic properties such as pH 7.9, water content 12.7%, OM 77.3%, total N 2.32%, P2O5 4.51%, and K2O 2.56% (23.5 g per pot). Whereas, the Biochar provided by Seek Biotechnology Co., Ltd. Shanghai, China was prepared through the pyrolysis process of bamboo material under 400–500°C for 24 h and then filtered through 2.0 mm sieve (35 g per pot). The complete treatment setup is summarized in Table 1.
Table 1. Design of pot experiment used in this study.
| Treatments | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Mycorrhizal fungi | None | None | None | None | None | Yes | Yes | Yes | Yes |
| Biochar | None | None | None | Yes | Yes | Yes | None | Yes | None |
| Fertilizer used | None | CF | OF | None | O.F | None | OF | OF | None |
| code | C | CF | OF | B | OB | BP | OP | OBP | P |
C Control. CF Chemical fertilizer. OF organic fertilizer. B Biochar. OB Organic fertilizer + Biochar. BP Biochar + Fungi. OP Organic fertilizer + Fungi. OBP Organic fertilizer + Biochar + Fungi. P Fungi
Fungal inoculum preparation
The fungus Pirimformospora indica (CBS 125645) was obtained from “Centraal bureau voorSchimmel cultures, Fungal Biodiversity Centre, Institute of the Royal Netherlands Academy of Arts and Sciences (KNAW)”. It was inoculated on sterile petri plates containing kaefer medium and followed by incubation at 28°C for 6 to 8 days. For inoculation of soil substrate, the fungus was propagated for 15 days in Erlenmeyer flasks containing liquid kaefer medium under shaking conditions (120 rpm) to obtain a dense culture of mycelia.
Plant material and growth conditions
Pakchoi seeds were procured from ShouguangRenhe Seed Industry Co., Ltd. China. Uniform and healthy seeds were surface sterilized using 10% peroxide solution for 30 min [24] and then germinated in petri plates on sterilized wet filter paper. Two-day-old vigorous and healthy seedlings were transplanted to pots (top diameter 14 cm, bottom diameter 10 cm, height 12 cm) and sandwich layer model was applied to inoculate the soil with P. indica [25, 26]. Pots were randomly placed in green house at an optimum temperature of 15–17°C. Three plants per pot were grown with replication numbers of 12 for each treatment and harvested after 45 days.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from fresh leaves samples collected from six weeks old pakchoi plants using TaKaRa plant Mini kit according to the manufacture’s guidelines. The concentration of RNA was measured using a Nanodrop-2000 spectrophotometer (Thermo Scientific, USA), furthermore, the quality was confirmed by agarose gel electrophoresis. Prior to reverse transcription reaction, RNA samples were treated with DNase (TaKaRa, Japan) to remove genomic DNA. RNA was transcribed into cDNA using TaKaRa Reverse Transcription kit according to manufacture’s instructions (TaKaRa, Japan). The synthesized cDNA was quantified by a spectrophotometer (Eppendorf, Germany) at 260 nm.
Quantitative real-time PCR analysis
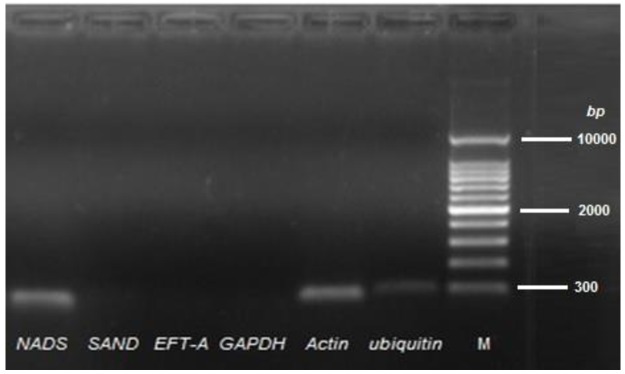
The expression levels of flavonoid biosynthetic pathway genes from Pakchoi were assessed by quantitative real-time PCR (qRT-PCR) using a Light Cycler® real-time PCR system (96 version 1.1.0-.1320, Roche Diagnostics international Ltd.) with SYBR® Premix Ex Taq™(TaKaRa, Japan). Two sets of gene-specific primers were designed for each gene (Table 2) by using GenScript Real-time PCR (TaqMan) online Primer Design tool (https://www.genscript.com/ssl-bin/app/primer). Referred to the relevant studies [22], five genes were selected and tested to be used as the internal control (Table 3). The qRT-PCR was performed in three independent experimental repeats using a 20 μl total volume, where each experimental repeat contains at least three samples. The assembly of reaction mixture and amplification were carried out as previously described by Hassani et al. [27]. The best gene-specific primers and internal control for qRT-PCR were selected based on their electrophoresis profiling (Figs 1 and 2). Calculation of relative expression level was performed by 2–ΔΔCT method [28].
Table 2. Primers designed for qRT-PCR analysis of flavonoid biosynthesis genes in Brassica campestris ssp. chinensis L.
| Gene | Forward primer (from 5’ to 3’) | Reverse primer (from 5’ to 3’) | Tm(°C) |
|---|---|---|---|
| CHS | CATCTGACACCCACCTTGAC | GAAGATGGGCTTCTCTCCAG | 58.5 |
| GAGAGAAGCCCATCTTCGAG | GTCCCACTTCCCTCAAGTGT | 57.2 | |
| CHI | TCCCTTTCTTCCGTGAAATC | TTCCATATCGCCACACAGTT | 54.7 |
| AACTGTGTGGCGATATGGAA | GAGAGCGAAGAGGATGGAAG | 55.7 | |
| F3H | GTCCCAAGGTTGCCTACAAT | CCAGTTCTCACAAGCCTCAA | 56.1 |
| ATAGCCACGTTCCAGAATCC | CTCCAAGATCGGCTTCTCTC | 57.0 | |
| ANS | GGGATCAGCTCTATCCCAAA | GGACTTGTGGACCGTCTTCT | 56.5 |
| GGTTTGCAGCTGTTCTACGA | CACCAATCCACGGTGAAGTA | 55.2 | |
| FLS | AATTACTATCCGCCGTGTCC | TTGACGTCGATCCAGTGATT | 55.5 |
| ACTTCCGGAATCATCGTCAT | AAACCGGCCATGATATTCTC | 56.2 |
Note: The primers which were finally selected for qRT-PCR analysis were shown in bold font.
Table 3. List of candidate housekeeping genes primers.
| Gene ID | Forward primer | Reverse primer |
|---|---|---|
| Ubiquitin | TCTGAGGCTTCGTGGTGGTA | AGGCGTGCATAACATTTGCG |
| Actin* | CTTGCATCCCTCAGCACCTT | TCCTGTGGACAATGGATGGA |
| GAPDH | TTCTCGTTGAGGGCTATTCCA | CCACAGACTTCATCGGTGACA |
| Eft-a | GAACTGGGTGCTTGATAGGC | AACCAAAATATCCGGAGTAAAAGA |
| SAND | CAACATCCTTTACCCATTGACAGA | GCATTTGATCCACTTGCAGATAAG |
| NADS | GATGCTTCTTGGGGCTTCTTGTT | CTCCAGTCACCAACATTGGCATAA |
*The selected primer has been bolded.
Fig 1. Electrophoresis profiles of the flavonoid biosynthesis pathway genes.
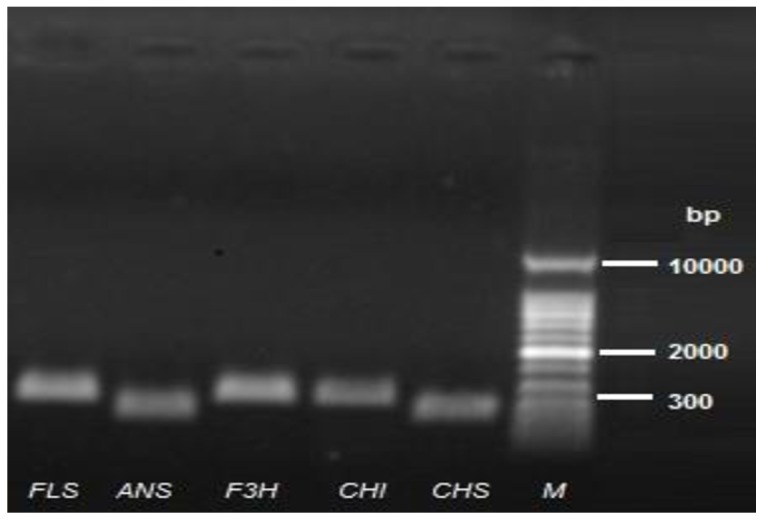
Fig 2. Electrophoresis profile of candidates for housekeeping genes.
Growth and root analysis
Growth attributes such as leaf number, leaf area, fresh and dry biomass were determined after harvesting the experimental samples under different treatments. The root colonization was assayed by using trypan blue staining kit [(protein and cell biology) life science products and services, Sangon Biotech (shanghai) Co., Ltd.] following the method of Smith et al. [29] and Newman’s intersection method with minor modifications [30].
Measurement of total phenolic compounds
Folin–Ciocalteau method was used to determine the total phenolic compounds (TPC) in sample extract [31]. Briefly, 500 μl of experimental sample in water was mixed with 2.0 ml of Folin–Ciocalteau reagent (0.2 N). After three min, 10 ml of Na2CO3 (10%, w/v) was added and the resulting mixture was allowed to stand for 30 min in the dark. The absorbance was measured at 725 nm against a blank and the results were expressed as mg gallic acid per gram of fresh weight of the sample.
Measurement of total flavonoid content
Analysis of total flavonoid content was carried out following the method as reported earlier [32]. One ml of aluminum chloride (2.0%, w/v) was thoroughly mixed with 1.0 ml of crude sample, followed by incubation at room temperature for 10 min. Results of total flavonoids were calculated by measuring optical density at 430 nm and expressed in mg quercetin per gram of fresh weight of the sample.
Measurement of phenolic acid content
A previously reported method was adopted for measuring the phenolic acid contents (PACs) in the extracted sample [33]. To this end, 1.0 mL of sample was thoroughly mixed with a combination of 5.0 mL of sterilized distilled water, 1.0 mL HCl (0.5 M), Arnov reagent (100 ml H2O, 10 g sodium nitrite and 10 g sodium molybdate) and NaOH (1.0 M) followed by OD measurement at 590 nm. A calibration curve was constructed to measure the total PACS and the results were presented equivalent as caffeic acid in micro gram per gram of fresh weight of the sample.
HPLC-MS analysis
Quantitative assessment of phenolic compounds was carried out through HPLC-MS (LTQ XL, Thermo Fisher Scientific, San Jose, CA, USA) using a C18 column (2.1 mm×150 mm, 3.5 μm; Waters) [34]. The column temperature was maintained at 35°C. The mobile phase A (0.1% formic acid/water) and B (100% acetonitrile) was used; the gradient program was as follows: 0–2 min 5.0% B; 4–11 min 15%-35% B; 15–17 min, 100% B; 17.5–22 min, 5.0% B; flow rate was 0.30 ml min-1, the injection volume was 10 μl. MS was scanned in ESI source in negative mode, mass range: m/z 92 to 1000; source voltage 3.5 kV, capillary temperature 350°C, sheath gas flow 35, aux gas flow 15.0, sweep gas flow 1.0, and a capillaryvoltage of 43V. The dependent scan was performed with collision-induced dissociation (CID) at collision energy of 35 eV. Data acquisition, handling, and instrument control was performed using X calibur 2.3.1 software.
Chlorophylls and carotenoids measurement
Chlorophyll and carotenoid levels were evaluated as reported [35, 36]. Briefly, a 2.0 ml acetone (80%) was used overnight at 4°C to elute chlorophyll and carotenoids from 0.05 g freeze-dried leaves. Supernatants were collected after centrifugation of the sample at 13,000 rpm for 5.0 min. The absorbance was recorded at wavelengths of 663, 645, and 470 nm for chl a, chl b, and carotenoids, respectively and concentrations of chl a, chl b and carotenoids were measured using the equations given below:
Total antioxidant activity
Free radical scavenging assay was carried out to determine antioxidant activity using 1, 1-diphenyl-2-picrylhydrazyl (DPPH) as reported earlier [37]. An 80 μl of methanolic sample extract was mixed with 1.92 ml DPPH solution, and absorbance was noted at 515 nm.
Statistical analysis of data
All the analytic determinations were carried out at least in three times, and results are expressed as mean ± SD of triplicate samples. Data were statistically analyzed by one-way analysis of variance (ANOVA), followed by Duncan’s multiple range (DMR) tests (SPSS Inc., Chicago, IL, USA). Differences were denoted statistically significant at P<0.05.
Results and discussion
Morphological indices
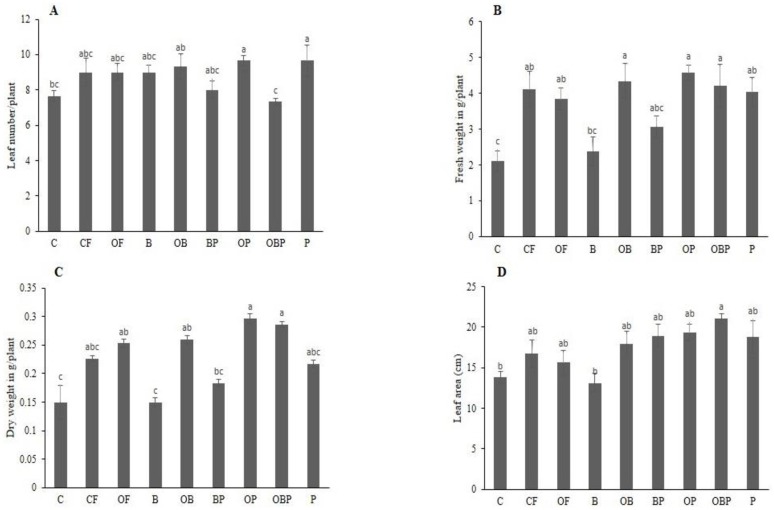
The morphological indices of crop Pakchoi were evaluated following nine selected treatments and results are illustrated in (Fig 3A–3D). The plant characteristics such as leaf number, fresh weight, dry weight and leaf area were significantly (P<0.05) varied between the treatments. Notably, the leaf numbers were found higher with treatment P (55.8%) and lowest in treatment OBP. Fresh and dry weights were increased up to 68.18% and 65.9% respectively in OP compared to control treatment. The leaf area was maximum in OBP (60.48%) and minimum in B (48.77%) treatments. In summary, the results showed that P. indica-inoculated plants exhibited excellent growth as compared to control and other treatments (S1 Fig).
Fig 3. Effect of given treatments on growth of Brassica campestris ssp. chinensis L.
A) Leaf number, B) Shoot fresh weight, C) Shoot dry weight, D) Leaf area. Values are means and bars indicate SDs (n = 8). Columns with different letters indicate significant difference at P < 0.05 (Duncan test). Treatments Control (C), Chemical fertilizer (CF), organic fertilizer (OF), Biochar (B), Organic fertilizer + Biochar (OB), Biochar + Fungi (BP), Organic fertilizer + Fungi (OP), Organic fertilizer + Biochar + Fungi (OBP), Fungi (P).
Similar findings representing the growth enhancing features of P. indica inoculation have been documented earlier [25, 38, 39]. A considerable increase in the fresh weight of seedlings was recorded in P. indica inoculated Arabidopsis plants [40]. Similarly, the P. inidica treated C. forskohlii resulted a substantial increase in biomass including aerial growth, leaf area and the average length of the branches [41] which might be due to the higher expression of genes responsible for development [42]. Moreover, the growth-promoting effects could also be associated with high nutrient uptake especially nitrogen and phosphorus from the soil [43, 44]. Several researchers demonstrated that P. indica assists in phosphorus uptake and involves in phosphorus shipping through PiPT transporter [45–47].
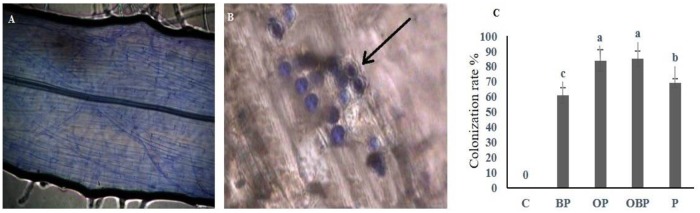
Roots colonization assay
Roots colonization in treated plants was monitored under a microscope. Colonization in the form of mycelia, hyphae, and mature piriform shaped chlamydo spores was observed in primary and secondary roots (Fig 4). The use of staining technique thus confirmed the presence of beneficial endophytic fungus inside the inoculated sample roots (Fig 4). Comparable results were observed previously with Arabidopsis thaliana, Zea mays, Hordeumvulgare, Oryza sativa and many other monocots and dicots etc. [29].
Fig 4. Root colonization and sporulation of P. indica in Brassica campestris ssp. chinensis L., A) Control, B) Chlamydospores inside the root cells, C) Plant root infection rate by P. indica in different treatments.
Alphabets on bars significantly differ at p <0.05. Treatments Control (C), Biochar + Fungi (BP), Organic fertilizer + Fungi (OP), Organic fertilizer + Biochar + Fungi (OBP), Fungi (P).
Health-promoting compounds analysis
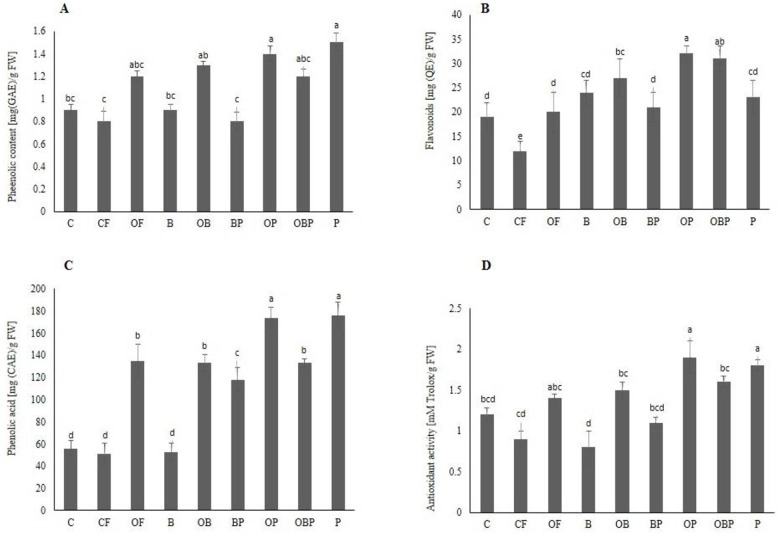
In order to determine the nutritional quality of Pakchoi, it is of profound significance to investigate the content and the activity of health-promoting phytochemicals. Thus, in the present study, the quality of the Pakchoi was determined by analyzing the concentration of different secondary metabolites especially antioxidants (total phenolics, flavonoids, and phenolic acid) under given treatments. It was observed that phytochemicals were significantly augmented in fungal-inoculated plant samples in combination with organic fertilizer and biochar in comparison to control and other treatments (Fig 5A–5D). It is worth to mention that the elevated synthesis of phenolic content, as well as flavonoids, correspond perfectly with the results obtained by Kilam et al. [48]. Treatments such as OB (59.09%), OP (60.86%) and P (62.5%) presented the most promising effects on the plant phenolic contents. The concentration of flavonoids increased considerably as a consequence of OBP (62%) and OP (62.7%) treatments as compared to control and other treatments (Fig 5A–5D). Likewise, plants treated with OP (75.6%) and P (75.86%) accumulated higher concentrations of phenolic acid. Analogous effects have been observed for some other plants such as Hordeumvulgare, Stevia rebaudiana, Oryza sativa L., and Bacopamonniera [48–51].
Fig 5. Influence of different treatments on A) phenolic contents as gallic acid equivalent (GAE) in mg per g of fresh weight (FW) B) Flavonoids as Quercetin equivalent (QE) in μg per g of fresh weight (FW)., C) phenolic acid as caffeic acid equivalent (CAE) in μg per g of fresh weight (FW)., D) Antioxidant activity.
Treatments Control (C), Chemical fertilizer (CF), organic fertilizer (OF), Biochar (B), Organic fertilizer + Biochar (OB), Biochar + Fungi (BP), Organic fertilizer + Fungi (OP), Organic fertilizer + Biochar + Fungi (OBP), Fungi (P).
Antioxidants exhibit strong curative or preventive activities by inhibiting many cellular pathways which are crucial for chronic ailments such as neurodegenerative, cardiovascular diseases and cancer [52–54]. Key pathways such as inflammatory, detoxification, immune response, cell division and proliferation, growth and differentiation are regulated by the action of specific enzymes that can be induced or inhibited by flavonoids. A vast array of biological functions have been attributed to flavonoids since it can modify immune system, influence cancer at any stage and homeostasis in cell system [55, 56]. The antioxidant capacity in Brassica species might be ascribed to the presence of flavonoids and phenolic contents as compared to vitamins and carotenoids [57]. This has been demonstrated that Pakchoi is rich in beneficial phytochemicals that are correlated with environmental biotic and abiotic factors [34].
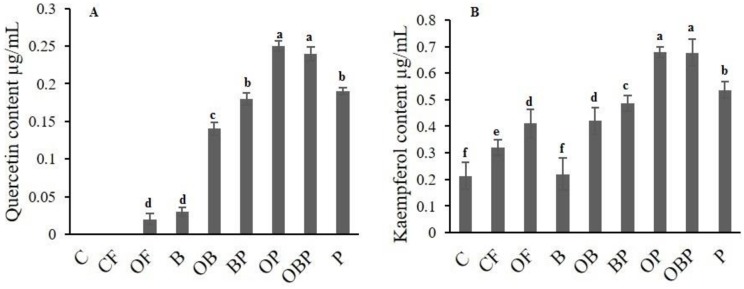
The possible compounds were identified by HPLC-MS and compared with the reported literatures about Brassica campestris ssp. chinensis L. (S2 and S3 Figs). The retention time, m/z in negative mode, MS2 fragments, and the possible chemical name are detailed in Table 4. Results showed that the dominant fractions in Pakchoi were found to be Ferulic acid, Caffeic acid, Kaempferol and Luteolin. Noticeably, one of the most important flavonoids “Quercetin” was not detected in control and under chemical fertilizer treatment while it was present in OP associated plants (Table 4). Additionally, the quantities of Caffeoyltartaric acid, Phillyrin, Isorhamnetin-3-Gentiobioside-7-glucoside, Kaempferol, Chlorogenic acid, Caffeic acid and Ferulic acid were found to be higher in OBP and followed by OP inoculated plants. Moreover, the level of Ferulic acid was increased at high degree with OP, OBP, and P, similarly, the Caffeic acid enhancement was evident in plants elicited with OP, B and P treatments. Previous studies also supported that kaempferol, isorhamnetin. quercetin, flavonoid derivatives and other imperative polyphenols were relatively higher in different varieties of Pakchoi following fungal inoculation [58, 59]. [60]. An improved biosynthesis of phenolic content and flavonoids might be related to the symbiosis which involves a molecular dialogue between beneficial fungus and plant [61, 62]. Such an induction of potentially beneficial compounds in lettuce associated with fungi has also previously been reported [17]. In another study, an elevated level of phenolic compounds (chlorogenic acid, gallic acid, Hydroxy benzoic acid) was recorded in Valerianajatamansi Jones when treated with mycorrhiza as compared to non-inoculated one’s [63].
Table 4. Quantitative analysis of phenolic compounds in the leaves of Brassica campestris ssp. chinensis L. after treatments.
| μg/mL | C | CF | OF | B | OB | BP | OP | OBP | P |
|---|---|---|---|---|---|---|---|---|---|
| Ferulic acid | 0.66 ± 0.02 cde | 0.54 ± 0.01 de | 0.57 ± 0.15 de | 0.73 ± 0.007 abc | 0.65 ± 0.50 bcd | 0.48 ± 0.8 e | 0.83 ± 0.10 a | 0.8 ± 0.13 ab | 0.74 ± 0.05 abc |
| Caffeic acid | 0.7 ± 0.05 i | 2.68 ± 0.39 h | 4.87 ± 0.19 g | 7.42 ± 0.50 b | 6.63 ± 0.32 d | 6.44 ± 0.009 e | 9.23 ± 0.001 a | 5.81 ± 0.22 f | 7.1 ± 0.01 c |
| Chlorogenic acid | 3.45 ± 0.09 a | 4.85 ± 0.10 a | 4.26 ± 0.12 a | 4.09 ± 0.76 a | 6.3 ± 0.39 a | 5.66 ± 0.17 a | 6.21 ± 0.05 a | 5.69 ± 0.005 a | 6.45 ± 0.10 a |
| Kaempferol | 0.21 ± 0.15 e | 0.32 ± 0.67 d | 0.41 ± 0.05 c | 0.22 ± 0.39 e | 0.42 ± 0.40 c | 0.48 ± 0.25 b | 0.68 ± 0.67 a | 0.67 ± 0.02 a | 0.53 ± 0.33 b |
| Luteolin | 0.02 ± 0.004 d | ND | 0.05 ± 0.39 cd | 0.02 ± 0.04 d | ND | 0.08 ± 0.005 ab | 0.09 ± 0.04 a | 0.07 ± 0.05 abc | 0.06 ± 0.44 bc |
| Caffeoylmalic acid | 8.81 ± 0.5 b | 10.69 ± 0.05 ab | 10.95 ± 0.18 ab | 11.73 ± 0.25 ab | 10.31 ± 0.08 ab | 10.74 ± 0.03 ab | 12.22 ± 0.44 ab | 13.96 ± 0.16 ab | 11.08 ± 0.16 ab |
| Coumaric acid | 0.19 ± 0.13 d | 0.18 ± 0.005 d | ND | 0.26 ± 0.44 bc | 0.18 ± 0.16 d | 0.21 ± 0.44 cd | 0.31 ± 0.007 ab | 0.34 ± 0.25 a | 0.26 ± 0.54 bc |
| Quercetin-3-gentiobioside-7-glucoside | ND | 0.03 ± 0.02 e | 0.08 ± 0.31 cd | 0.1 ± 0.14 cd | 0.1 ± 0.04 cd | 0.06 ± 0.16 de | 0.28 ± 0.52 a | 0.15 ± 0.03 b | 0.11 ± 0.22 bc |
| Isorhamnetin-3-Gentiobioside-7-glucoside | 0.13 ± 0.03 de | 0.13 ± 0.18 de | 0.08 ± 0.67 e | 0.2 ± 0.06 d | 0.27 ± 0.03 bc | 0.22 ± 0.007 c | 0.33 ± 0.13 b | 0.43 ± 0.02 a | 0.26 ± 0.005 c |
| Quercetin | ND | ND | 0.02 ± 0.18 d | 0.03 ± 0.22 d | 0.14 ± 0.01 c | 0.18 ± 0.05 b | 0.25 ± 0.008 a | 0.24 ± 0.14 a | 0.19 ± 0.02 b |
| 5-p-coumaroylquinic acid | 6.24 ± 0.17 ab | 4.77 ± 0.76 b | 4.46 ± 0.001 b | 6.62 ± 0.05 ab | 8.01 ± 0.005 a | 6.84 ± 0.01 ab | 9 ± 0.11 a | 6.77 ± 0.10 ab | 9.41 ± 1.59 a |
| Schisantherin D | 0.29 ± 0.20 a | ND | 0.17 ± 0.42 cd | 0.27 ± 0.18 ab | 0.25 ± 0.18 abc | 0.24 ± 0.32 abc | 0.2 ± 0.42 bc | 0.28 ± 0.42 ab | 0.12 ± 0.05 d |
| Phillyrin | 0.12 ± 0.07 e | 0.16 ± 0.02 de | 0.18 ± 0.03 bc | 0.24 ± 0.01 a | 0.24 ± 0.33 a | 0.12 ± 0.02 e | 0.22 ± 0.22 ab | 0.26 ± 0.05 a | 0.17 ± 0.15 bcd |
| Caffeoyltartaric acid | 0.58 ± 0.43 c | 0.73 ± 0.13 bc | 0.72 ± 0.04 | 0.63 ± 0.005 bc | 0.8 ± 0.15 b | 0.76 ± 0.005 bc | 1.01 ± 0.03 d | 1.28 ± 0.03 a | 0.8 ± 0.03 b |
Treatments Control (C), Chemical fertilizer (CF), organic fertilizer (OF), Biochar (B), Organic fertilizer + Biochar (OB), Biochar + Fungi (BP), Organic fertilizer + Fungi (OP), Organic fertilizer + Biochar + Fungi (OBP), Fungi (P). Values are means and bars indicate SDs. Columns with different letters indicate significant difference at P < 0.05 (Duncan test).
Chlorophylls and carotenoids
Like other green leafy vegetables, pakchoi is a persuasive source of dietary chlorophylls and carotenoids that play a striking role in reducing the risk of heart disease, cancer, cataract, stroke and in-vitro anti-inflammatory effects [64, 65]. Carotenoids are also secondary metabolites with profound antioxidant activities, whereas some studies revealed that chlorophyll causes the inhibition of Cox-1 and Cox-2 enzymes [65, 66]. The results in Table 5 showed that chlorophyll contents were markedly increased as a result of CF and OP treatments followed by P and OBP treatments as compared to control, however, no significant difference (P>0.05) was found between control and all other treatments. Chlorophyll b contents were higher with P and OP treatments followed by OB treatment in comparison to control and other treatments. Likewise, chlorophyll a, b were recorded highest in chemical fertilizer and OP treatments. While there was no significant difference (P>0.05) among other treatments, The results were in consonance with previous investigations that P. indica possess beneficial influence on chlorophyll content and photosynthetic efficiency [67, 68]. Some reports also showed that P. indica confers stress resistance and enhance fresh weight and chlorophyll content in the model plant, Arabidopsis thaliana. Data regarding carotenoids evaluation showed that Pakchoi treated with OP was categorized to have significantly higher (P<0.05) total carotenoids followed by B and OBP treatment with respect to other treatments. Improvement of carotenoid content in the P. indica associated plants has previously been explicated in several studies [69, 70]. Similarly, biosynthesis of a higher level of carotenoids and photosynthetic pigments (chl a, b) have also been reported in P. indica-inoculated rice seedlings as compared to non-inoculated seedlings [71].
Table 5. Influence of given treatments on chlorophyll and carotenoids contents.
| Treatment | Constituents (mg/100 g dw) | |||
|---|---|---|---|---|
| Chl a | Chl b | Chl a + b | Car | |
| C | 338.45±3.7 d | 121.31 ± 21.17 de | 459.76 ± 24.87 f | 25.74 ± 0.8 c |
| C.F | 473.22 ± 17.45 a | 208.20 ± 8.60 a | 681.42 ± 26.05 a | 30.96 ± 1.76 bc |
| O.F | 341.32 ± 7.62 cd | 131.74 ± 2.42 bc | 473.06 ± 10.04 e | 18.45 ± 3.08 d |
| B | 340.28 ± 13.40 cd | 120.74 ± 2.42 e | 461.02 ± 15.62 f | 34.51 ± 1.54 b |
| OB | 344.44 ± 17.22 cd | 136.81 ± 11.03 b | 481.25 ± 28.25 d | 24.21 ± 1.24 cd |
| BP | 348.81 ± 17.22 c | 128.83 ± 6.25 cd | 477.64 ± 23.47 de | 27.32 ± 2.12 c |
| OP | 468.25 ± 21.40 a | 215.44 ± 17.08 a | 683.69 ± 38.48 a | 41.23 ± 2.32 7 a |
| OBP | 434.71 ± 21.40 b | 127.28 ± 13.40 cde | 561.99 ± 34.8 c | 34.51 ± 1.55 b |
| P | 439.23 ± 22.44 b | 214.62 ± 17.04 a | 653.85 ± 39.48 b | 29.32 ± 2.14 bc |
Abbreviations: Chl a, chlorophyll a; Chl b, chlorophyll b; Car, carotenoids. Treatments Control (C), Chemical fertilizer (CF), organic fertilizer (OF), Biochar (B), Organic fertilizer + Biochar (OB), Biochar + Fungi (BP), Organic fertilizer + Fungi (OP), Organic fertilizer + Biochar + Fungi (OBP), Fungi (P). Values are means and bars indicate SDs. Columns with different letters indicate significant difference at P < 0.05 (Duncan test).
Expression level of flavonoid pathway genes
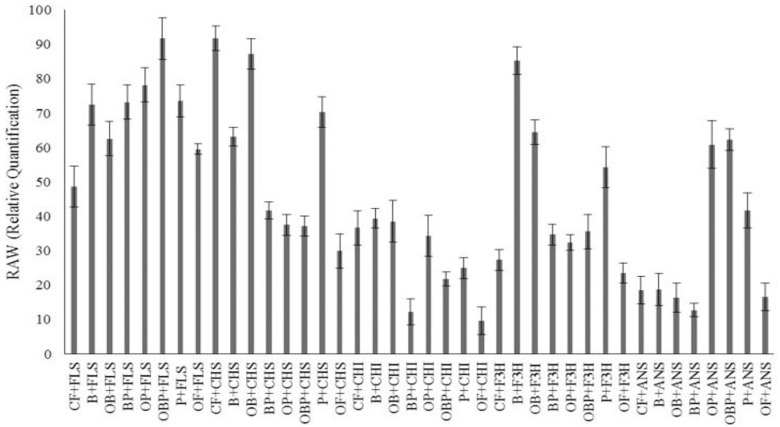
To date, seventy-three anthocyanin biosynthetic pathway genes (ABGs) have been characterized in the genome of Brassica campestris ssp. chinensis L. Structural genes accompanying this pathway can be categorized into(1) early flavonoid biosynthesis genes, including CHS, CHI and F3H, and (2) late flavonoid biosynthesis genes, such as FLS and ANS [72]. In flavonoid biosynthetic pathway, the synthesis of flavonoids begins with the precursor, 4-coumaroyl-CoA. Enzymes encoded by the genes CHS, CHI, F3H, FLS and ANS convert this precursor to several intermediate compounds which are used as a substrate for the next step of the pathway. Kaempferol as a product of FLS is known to be the major flavonoid in Brassica campestris ssp. chinensis L. In this experiment, the expression level of five important structural genes of flavonoid biosynthetic pathway in pakchoi was determined and quantified using RT-qPCR in nine different treatments (Table 1). Results proposed that the expression level of early genes of the flavonoid pathway including CHS, CHI and F3H were considerably higher in treatment by Biochar followed by OB. On the other hand, the expression level of late flavonoid pathway genes including FLS and ANS was recorded to be significantly higher in OBP and OP treatments. The expression profile of individual genes under each treatment has been illustrated in Fig 6.
Fig 6. QRT-PCR analysis of the expression levels of the FLS, CHI, ANS, CHS and F3’H genes from flavonoids biosynthesis pathway.
Data from qRT-PCR analysis revealed that OBP and OP significantly (P<0.05) affected the expression level of FLS and ANS which are involved in major flavonoids biosynthesis (Fig 7A and 7B). More importantly, the spectrophotometric results, HPLC and expression level of late genes in flavonoids biosynthesis pathway (FLS, ANS) coincided well and therefore, it is concluded that P. indica in combination with organic fertilizer and Biochar can induce the synthesis of flavonoids in the host plant. Similar results have been reported earlier [51, 73–75].
Fig 7. Major flavonoids detected by HPLC A) Quantitative assessment of quercetin and B) Kaemferol by HPLC.
Treatments Control (C), Chemical fertilizer (CF), organic fertilizer (OF), Biochar (B), Organic fertilizer + Biochar (OB), Biochar + Fungi (BP), Organic fertilizer + Fungi (OP), Organic fertilizer + Biochar + Fungi (OBP), Fungi (P).
Free radical scavenging activity
DPPH free-radical scavenging assay was used to determine the antioxidant activity which was increased by P, OP, OBP and OB treated crop as compared to control and other treatments. The highest capacity reached to 1.9 and 1.8 mM of TE/g FW for OP and P treated which constitute up to 61.29% and 60%, respectively in comparison to control. The higher antioxidant capacity might be attributed to the flavonoids and phenolic contents, which were significantly influenced by fungi whose positive effect was evident from the above-mentioned data (Table 4, Fig 5A–5D). Besides antioxidant properties, phenolic compounds also possess the anti-inflammatory activity by inhibiting enzymes involved in inflammation process [65, 76]. Previous reports elaborated that B. monniera has shown a drastic increase in antioxidant activity when treated with P. indica. The level of major antioxidants (i.e., flavonols and caffeic acid derivatives) can be amplified by AMF in lettuce plant when studied comparatively to the non-inoculated plants [17]. Extrapolating all the results achieved after supplying nine different treatments along with previous results, it is summarized that overall positive change could be achieved in Pakcoi by intervention with P. indica.
Conclusions
In conclusion, the results showed that OP and OBP treatments have a favorable effect on the concentration of beneficial nutrients and therefore can improve the nutritional quality of pakchoi. In addition to that, the elevated levels of phenolics, flavonoids, and phenolic acid were found in co-cultivated plants with P. indica compared to non-inoculated ones. These findings reveal that treatment with beneficial fungus as bio-fertilizer can be a cost-effective and environmentally-friendlier approach for enhancing the quality and health properties of fresh vegetables, which may act as an alternative to conventional chemical-based strategies. In-depth studies on unraveling the contribution of beneficial fungi and its relationship with different host plants would be important for future sustainable agriculture
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The technical and analytical help provided by the Instrumental Analysis Center of Shanghai Jiao Tong University is also thankfully acknowledged.
Abbreviations
- B
Biochar
- BP
Biochar + Fungi
- C
Control
- CF
Chemical fertilizer
- chla
chlorophyll a
- chlab
chlorophyll ab
- Chlb
chlorophyll b
- DW
dry weight
- FW
fresh weight
- OB
Organic fertilizer + Biochar
- OBP
Organic fertilizer + Biochar + Fungi
- OF
organic fertilizer
- OP
Organic fertilizer + Fungi
- P
Fungi
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported in part by the National High-tech R&D Program of China (863 Program) (Grant No. 2013AA103000) and Shanghai Agriculture Applied Technology Development Program, China (Grant No. T20140502).
References
- 1.Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. American Journal of Epidemiology. 1999;149(10):943–9. [DOI] [PubMed] [Google Scholar]
- 2.Bagchi D, Sen CK, Ray SD, Das DK, Bagchi M, Preuss HG, et al. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2003;523:87–97. [DOI] [PubMed] [Google Scholar]
- 3.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutrition reviews. 1998;56(11):317–33. [DOI] [PubMed] [Google Scholar]
- 4.Middleton E Jr, Kandaswami C. The impact of plant flavonoids on mammalian biology: implications for immunity, inflammation and cancer. The flavonoids London: Chapman and Hall; 1994. [Google Scholar]
- 5.Duthie GG, Duthie SJ, Kyle JA. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutrition Research Reviews. 2000;13(01):79–106. [DOI] [PubMed] [Google Scholar]
- 6.Gil MI, Ferreres F, Tomás-Barberán FA. Effect of postharvest storage and processing on the antioxidant constituents (flavonoids and vitamin C) of fresh-cut spinach. Journal of agricultural and food chemistry. 1999;47(6):2213–7. [DOI] [PubMed] [Google Scholar]
- 7.Chu YH, Chang CL, Hsu HF. Flavonoid content of several vegetables and their antioxidant activity. Journal of the Science of Food and Agriculture. 2000;80(5):561–6. [Google Scholar]
- 8.Pant AP, Radovich TJ, Hue NV, Talcott ST, Krenek KA. Vermicompost extracts influence growth, mineral nutrients, phytonutrients and antioxidant activity in pak choi (Brassica rapa cv. Bonsai, Chinensis group) grown under vermicompost and chemical fertiliser. Journal of the Science of Food and Agriculture. 2009;89(14):2383–92. [Google Scholar]
- 9.Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant and soil. 2003;255(2):571–86. [Google Scholar]
- 10.Shen D. Microbial diversity and application of microbial products for agricultural purposes in China. Agriculture, ecosystems & environment. 1997;62(2):237–45. [Google Scholar]
- 11.Shetty K, Hetrick B, Figge D, Schwab A. Effects of mycorrhizae and other soil microbes on revegetation of heavy metal contaminated mine spoil. Environmental Pollution. 1994;86(2):181–8. [DOI] [PubMed] [Google Scholar]
- 12.Smith SE, Read DJ. Mycorrhizal symbiosis: Academic press; 1996. [Google Scholar]
- 13.Khan A, Kuek C, Chaudhry T, Khoo C, Hayes W. Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere. 2000;41(1):197–207. [DOI] [PubMed] [Google Scholar]
- 14.Krishna K, Bagyaraj D. Role of vesicular arbuscular mycorrhiza in the uptake of micronutrient by groundnut plants. Curr Res. 1991;20:173–5. [Google Scholar]
- 15.Gould W, Nakas J, Hagedorn C. Biological control of plant root diseases by bacteria. Biotechnology of plant-microbe interactions. 1990:287–317. [Google Scholar]
- 16.Hegde D, Dwivedi B, Sudhakara Babu S. Biofertilizers for cereal production in India: A review. Indian journal of agricultural science. 1999;69(2):73–83. [Google Scholar]
- 17.Baslam M, Garmendia I, Goicoechea N. Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown lettuce. Journal of agricultural and food chemistry. 2011;59(10):5504–15. 10.1021/jf200501c [DOI] [PubMed] [Google Scholar]
- 18.Hause B, Fester T. Molecular and cell biology of arbuscular mycorrhizal symbiosis. Planta. 2005;221(2):184–96. 10.1007/s00425-004-1436-x [DOI] [PubMed] [Google Scholar]
- 19.Gianinazzi-Pearson V. Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. The Plant Cell. 1996;8(10):1871 10.1105/tpc.8.10.1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeram NP. Berry fruits: compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. Journal of agricultural and food chemistry. 2008;56(3):627–9. [DOI] [PubMed] [Google Scholar]
- 21.Unnikumar K, Sree KS, Varma A. Piriformospora indica: a versatile root endophytic symbiont. Symbiosis. 2013;60(3):107–13. [Google Scholar]
- 22.Sarwat M, Hashem A, Ahanger MA, Abd_Allah EF, Alqarawi A, Alyemeni MN, et al. Mitigation of NaCl stress by arbuscular mycorrhizal fungi through the modulation of osmolytes, antioxidants and secondary metabolites in mustard (Brassica juncea L.) plants. Frontiers in Plant Science. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Vahabi K, Dorcheh SK, Monajembashi S, Westermann M, Reichelt M, Falkenberg D, et al. Stress promotes Arabidopsis-Piriformospora indica interaction. Plant signaling & behavior. 2016;11(5):e1136763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu S, Cao Z, Li Z, Cheung K, Wong M. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trial. Geoderma. 2005;125(1):155–66. [Google Scholar]
- 25.Varma A, Verma S, Sahay N, Bütehorn B, Franken P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Applied and Environmental Microbiology. 1999;65(6):2741–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varma A, Schuepp H. Positive influence of arbuscular mycorrhizal fungus on in vitro raised hortensia plantlets. Angewandte Botanik (Germany). 1994. [Google Scholar]
- 27.Hassani D, Liu H, Chen Y, Wan Z, Zhuge Q, Li S. Analysis of biochemical compounds and differentially expressed genes of the anthocyanin biosynthetic pathway in variegated peach flowers. Genetics and Molecular Research. 2015;14(4):13425–36. 10.4238/2015.October.28.4 [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods. 2001;25(4):402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 29.Michal Johnson J, Sherameti I, Ludwig A, Nongbri PL, Sun C, Lou B, et al. Protocols for Arabidopsis thaliana and Piriformospora indica co-cultivation–A model system to study plant beneficial traits. Endocytobiosis and Cell Research. 2011:101–13. [Google Scholar]
- 30.GIOVANNETTI M, Mosse B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New phytologist. 1980;84(3):489–500. [Google Scholar]
- 31.Singleton VL, Orthofer R, Lamuela-Raventos RM. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in enzymology. 1999;299:152–78. [Google Scholar]
- 32.Lamaison J, Carnat A. Levels of principal flavonoids in flowers and leaves of Crataegus-Monogyna Jacq and Crataegus-Laevigata (Poiret) Dc (Rosaceae). Pharmaceutica Acta Helvetiae. 1990;65(11):315–20. [Google Scholar]
- 33.Szaufer-Hajdrych M. Phenolic acids in leaves of species of the Aquilegia L. genus. Herba Polonica. 2004;50(2). [Google Scholar]
- 34.Świeca M, Gawlik-Dziki U, Kowalczyk D, Złotek U. Impact of germination time and type of illumination on the antioxidant compounds and antioxidant capacity of Lens culinaris sprouts. Scientia Horticulturae. 2012;140:87–95. [Google Scholar]
- 35.Porra R, Thompson W, Kriedemann P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1989;975(3):384–94. [Google Scholar]
- 36.Holm G. Chlorophyll mutations in barley. Acta Agriculturae Scandinavica. 1954;4(1):457–71. [Google Scholar]
- 37.Złotek U, Świeca M, Jakubczyk A. Effect of abiotic elicitation on main health-promoting compounds, antioxidant activity and commercial quality of butter lettuce (Lactuca sativa L.). Food chemistry. 2014;148:253–60. 10.1016/j.foodchem.2013.10.031 [DOI] [PubMed] [Google Scholar]
- 38.Oelmüller R, Sherameti I, Tripathi S, Varma A. Piriformospora indica, a cultivable root endophyte with multiple biotechnological applications. Symbiosis. 2009;49(1):1–17. [Google Scholar]
- 39.Achatz B, von Rüden S, Andrade D, Neumann E, Pons-Kühnemann J, Kogel K-H, et al. Root colonization by Piriformospora indica enhances grain yield in barley under diverse nutrient regimes by accelerating plant development. Plant and soil. 2010;333(1–2):59–70. [Google Scholar]
- 40.Sherameti I, Shahollari B, Venus Y, Altschmied L, Varma A, Oelmüller R. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. Journal of Biological Chemistry. 2005;280(28):26241–7. 10.1074/jbc.M500447200 [DOI] [PubMed] [Google Scholar]
- 41.Das A, Kamal S, Shakil NA, Sherameti I, Oelmüller R, Dua M, et al. The root endophyte fungus Piriformospora indica leads to early flowering, higher biomass and altered secondary metabolites of the medicinal plant, Coleus forskohlii. Plant signaling & behavior. 2012;7(1):103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waller F, Mukherjee K, Deshmukh SD, Achatz B, Sharma M, Schäfer P, et al. Systemic and local modulation of plant responses by Piriformospora indica and related Sebacinales species. Journal of plant physiology. 2008;165(1):60–70. 10.1016/j.jplph.2007.05.017 [DOI] [PubMed] [Google Scholar]
- 43.Varma A, Singh A, Sahay NS, Sharma J, Roy A, Kumari M, et al. Piriformospora indica: an axenically culturable mycorrhiza-like endosymbiotic fungus. Fungal Associations: Springer; 2001. p. 125–50. [Google Scholar]
- 44.Kumar M, Yadav V, Kumar H, Sharma R, Singh A, Tuteja N, et al. Piriformospora indica enhances plant growth by transferring phosphate. Plant signaling & behavior. 2011;6(5):723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yadav V, Kumar M, Deep DK, Kumar H, Sharma R, Tripathi T, et al. A phosphate transporter from the root endophytic fungus Piriformospora indica plays a role in phosphate transport to the host plant. Journal of Biological Chemistry. 2010;285(34):26532–44. 10.1074/jbc.M110.111021 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Vadassery J, Ritter C, Venus Y, Camehl I, Varma A, Shahollari B, et al. The role of auxins and cytokinins in the mutualistic interaction between Arabidopsis and Piriformospora indica. Molecular Plant-Microbe Interactions. 2008;21(10):1371–83. 10.1094/MPMI-21-10-1371 [DOI] [PubMed] [Google Scholar]
- 47.Lee Y-C, Johnson JM, Chien C-T, Sun C, Cai D, Lou B, et al. Growth promotion of Chinese cabbage and Arabidopsis by Piriformospora indica is not stimulated by mycelium-synthesized auxin. Molecular plant-microbe interactions. 2011;24(4):421–31. 10.1094/MPMI-05-10-0110 [DOI] [PubMed] [Google Scholar]
- 48.Kilam D, Saifi M, Abdin M, Agnihotri A, Varma A. Combined effects of Piriformospora indica and Azotobacter chroococcum enhance plant growth, antioxidant potential and steviol glycoside content in Stevia rebaudiana. Symbiosis. 2015;66(3):149–56. [Google Scholar]
- 49.Prasad R, Kamal S, Sharma PK, Oelmüller R, Varma A. Root endophyte Piriformospora indica DSM 11827 alters plant morphology, enhances biomass and antioxidant activity of medicinal plant Bacopa monniera. Journal of basic microbiology. 2013;53(12):1016–24. 10.1002/jobm.201200367 [DOI] [PubMed] [Google Scholar]
- 50.Bagheri AA, Saadatmand S, Niknam V, Nejadsatari T, Babaeizad V. Effect of endophytic fungus, Piriformospora indica, on growth and activity of antioxidant enzymes of rice (Oryza sativa L.) under salinity stress. International Journal of Advanced Biological and Biomedical Research. 2013;1(11):1337–50. [Google Scholar]
- 51.Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytologist. 2008;180(2):501–10. 10.1111/j.1469-8137.2008.02583.x [DOI] [PubMed] [Google Scholar]
- 52.Ackland ML, Van De Waarsenburg S, Jones R. Synergistic antiproliferative action of the flavonols quercetin and kaempferol in cultured human cancer cell lines. In vivo. 2005;19(1):69–76. [PubMed] [Google Scholar]
- 53.Kim J-D, Liu L, Guo W, Meydani M. Chemical structure of flavonols in relation to modulation of angiogenesis and immune-endothelial cell adhesion. The Journal of nutritional biochemistry. 2006;17(3):165–76. 10.1016/j.jnutbio.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 54.Cartea ME, Francisco M, Soengas P, Velasco P. Phenolic compounds in Brassica vegetables. Molecules. 2010;16(1):251–80. 10.3390/molecules16010251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aron PM, Kennedy JA. Flavan-3-ols: Nature, occurrence and biological activity. Molecular nutrition & food research. 2008;52(1):79–104. [DOI] [PubMed] [Google Scholar]
- 56.Fresco P, Borges F, Marques M, Diniz C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Current pharmaceutical design. 2010;16(1):114–34. [DOI] [PubMed] [Google Scholar]
- 57.Podsędek A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Science and Technology. 2007;40(1):1–11. [Google Scholar]
- 58.Rochfort SJ, Imsic M, Jones R, Trenerry VC, Tomkins B. Characterization of flavonol conjugates in immature leaves of pak choi [Brassica rapa L. Ssp. chinensis L.(Hanelt.)] by HPLC-DAD and LC-MS/MS. Journal of Agricultural and Food Chemistry. 2006;54(13):4855–60. 10.1021/jf060154j [DOI] [PubMed] [Google Scholar]
- 59.Francisco M, Velasco P, Moreno DA, García-Viguera C, Cartea ME. Cooking methods of Brassica rapa affect the preservation of glucosinolates, phenolics and vitamin C. Food Research International. 2010;43(5):1455–63. [Google Scholar]
- 60.Harbaum B, Hubbermann EM, Zhu Z, Schwarz K. Free and bound phenolic compounds in leaves of pak choi (Brassica campestris L. ssp. chinensis var. communis) and Chinese leaf mustard (Brassica juncea Coss). Food chemistry. 2008;110(4):838–46. 10.1016/j.foodchem.2008.02.069 [DOI] [PubMed] [Google Scholar]
- 61.Ceccarelli N, Curadi M, Martelloni L, Sbrana C, Picciarelli P, Giovannetti M. Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower heads two years after field transplant. Plant and Soil. 2010;335(1–2):311–23. [Google Scholar]
- 62.Schliemann W, Ammer C, Strack D. Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry. 2008;69(1):112–46. 10.1016/j.phytochem.2007.06.032 [DOI] [PubMed] [Google Scholar]
- 63.Jugran A, Bahukhandi A, Dhyani P, Bhatt I, Rawal R, Nandi S, et al. The effect of inoculation with mycorrhiza: AM on growth, phenolics, tannins, phenolic composition and antioxidant activity in Valeriana jatamansi Jones. Journal of soil science and plant nutrition. 2015;15(4):1036–49. [Google Scholar]
- 64.Caldwell CR, Britz SJ. Effect of supplemental ultraviolet radiation on the carotenoid and chlorophyll composition of green house-grown leaf lettuce (Lactuca sativa L.) cultivars. Journal of Food Composition and Analysis. 2006;19(6):637–44. [Google Scholar]
- 65.Mulabagal V, Ngouajio M, Nair A, Zhang Y, Gottumukkala AL, Nair MG. In vitro evaluation of red and green lettuce (Lactuca sativa) for functional food properties. Food chemistry. 2010;118(2):300–6. [Google Scholar]
- 66.Kim H-J, Chen F, Wang X, Choi J-H. Effect of methyl jasmonate on phenolics, isothiocyanate, and metabolic enzymes in radish sprout (Raphanus sativus L.). Journal of agricultural and food chemistry. 2006;54(19):7263–9. 10.1021/jf060568c [DOI] [PubMed] [Google Scholar]
- 67.Sherameti I, Tripathi S, Varma A, Oelmüller R. The root-colonizing endophyte Pirifomospora indica confers drought tolerance in Arabidopsis by stimulating the expression of drought stress-related genes in leaves. Molecular Plant-Microbe Interactions. 2008;21(6):799–807. 10.1094/MPMI-21-6-0799 [DOI] [PubMed] [Google Scholar]
- 68.Strasser RJ, Tsimilli-Michael M, Dangre D, Rai M. Biophysical phenomics reveals functional building blocks of plants systems biology: a case study for the evaluation of the impact of mycorrhization with Piriformospora indica. Advanced Techniques in Soil Microbiology: Springer; 2007. p. 319–41. [Google Scholar]
- 69.Baishya D, Deka P, Kalita MC. In vitro co-cultivation of Piriformospora indica filtrate for improve biomass productivity in Artemisia annua (L.). Symbiosis. 2015;66(1):37–46. [Google Scholar]
- 70.Abadi VAJM, Sepehri M. Effect of Piriformospora indica and Azotobacter chroococcum on mitigation of zinc deficiency stress in wheat (Triticum aestivum L.). Symbiosis. 2016;69(1):9–19. [Google Scholar]
- 71.Jogawat A, Saha S, Bakshi M, Dayaman V, Kumar M, Dua M, et al. Piriformospora indica rescues growth diminution of rice seedlings during high salt stress. Plant signaling & behavior. 2013;8(10):e26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei Y-Z, Hu F-C, Hu G-B, Li X-J, Huang X-M, Wang H-C. Differential expression of anthocyanin biosynthetic genes in relation to anthocyanin accumulation in the pericarp of Litchi chinensis Sonn. PloS one. 2011;6(4):e19455 10.1371/journal.pone.0019455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun C, Johnson JM, Cai D, Sherameti I, Oelmüller R, Lou B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. Journal of plant physiology. 2010;167(12):1009–17. 10.1016/j.jplph.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 74.Camehl I, Drzewiecki C, Vadassery J, Shahollari B, Sherameti I, Forzani C, et al. The OXI1 kinase pathway mediates Piriformospora indica-induced growth promotion in Arabidopsis. PLoS Pathog. 2011;7(5):e1002051 10.1371/journal.ppat.1002051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vadassery J, Tripathi S, Prasad R, Varma A, Oelmüller R. Monodehydroascorbate reductase 2 and dehydroascorbate reductase 5 are crucial for a mutualistic interaction between Piriformospora indica and Arabidopsis. Journal of plant physiology. 2009;166(12):1263–74. 10.1016/j.jplph.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 76.Gawlik-Dziki U, Swieca M, Sugier D, Cichocka J. Comparison of in vitro lipoxygenase, xanthine oxidase inhibitory and antioxidant activity of Arnica montana and Arnica chamissonis tinctures. Acta Scientiarum Polonorum, Hortorum Cultus. 2011;10(3):15–27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.