Abstract
Background
Follicular lymphoma is the second most common non-Hodgkin lymphoma in the United States and Europe. However, most of the prospective randomized studies have very little follow-up compared to the long natural history of the disease. The primary aim of this study was to investigate the long-term survival of our series of patients with follicular lymphoma.
Patients and methods
A total of 1074 patients with newly diagnosed FL were enrolled. Patients diagnosed were prospectively enrolled from 1980 to 2013.
Results
Median follow-up was 54.9 months and median overall survival is over 20 years in our series. We analyzed the patients who are still alive beyond 10 years from diagnosis in order to fully assess the prognostic factors that condition this group. Out of 166 patients who are still alive after more than 10 years of follow-up, 118 of them (73%) are free of evident clinical disease. Variables significantly associated with survival at 10 years were stage < II (p <0.03), age < 60 years (p <0.0001), low FLIPI (p <0.002), normal β2 microglobulin (p <0.005), no B symptoms upon diagnosis (p <0.02), Performance Status 0–1 (p <0.03) and treatment with anthracyclines and rituximab (p <0.001), or rituximab (p <0.0001).
Conclusions
A longer follow-up and a large series demonstrated a substantial population of patients with follicular lymphoma free of disease for more than 10 years.
Introduction
Follicular lymphomas (FL) are the second most common non-Hodgkin lymphoma (NHL) in Western Europe [1], accounting for approximately 20 percent to 30 percent of all NHLs. In the series from The Non-Hodgkin´s Lymphoma Classification Project they represent 22% [2, 3], and 22–40% of the NHL [4, 5] according to the WHO classification.
The annual incidence of this disease has increased rapidly in the last decades, from 2–3 cases in a population of 100,000 in 1950 to 5.7 cases/100,000 in 2009. The prevalence is of approximately 40/100.000 [6]. The incidence increases with age. Most cases occur in adults over 50 years old and the elderly. They are rare in the third and fourth decades and exceptional in children and adolescents. In Spain, between 3,000 and 5,000 new cases of follicular lymphoma are diagnosed each year. It is the second most common tumor of lymphoid lineage.
Despite recent improvements in survival, FL remains an incurable disease. About 80% of patients with FL are diagnosed in stages III / IV. The median of the overall survival (OS) is lengthy (about 10 years) and OS rate up to five years over 75% [7]. Additionally, transformation to more aggressive histological forms, usually diffuse large B-cell lymphoma (DLBCL), may also occur, which implies, in general, very poor prognosis [8]. The risk of transformation appears to be independent of the type of treatment used (or lack thereof) [9]. In autopsy series, most cases (95%) show some evidence of transformation [10].
The course of the disease can be highly variable [11]. This is a difficult task in such an uncommon disorder with a prolonged natural history, and almost impossible when complex treatments such as bone marrow transplantation are concerned. In order to facilitate clinical studies, a number of outcome biomarkers have been proposed, although none of them has a validated correlation with survival.
The Follicular Lymphoma International Prognostic Index (FLIPI), has been proven to have a better discriminatory power in assessing patient prognosis and seems to produce a more even patient distribution among different risk groups compared to the International Prognostic Index (IPI). The FLIPI is currently used for defining individual risk of death and the tumor grade [12, 13, 14]. More recently, FLIPI2 [15] was developed as a new model for prognostic definition of patients with FL. It will best fit the current reality of the problem, as it has been developed with patients treated with immunotherapy and discriminates in groups according to a progression-free interval of disease, which is a more appropriate variable for FL.
Patients usually have prolonged survival, with medians that can reach or exceed 10 years. They have high response rates to different treatments, although responses are followed by sequent relapses with a shrinking time interval. Despite this high chemosensitivity, historically, nor OS or disease free survival could be modified for decades, despite having employed different therapeutic strategies.
This situation has changed in the last decade due to the introduction of chemoimmunotherapy, which is already reflected in some population statistics [16]. The median survival has been increased up to 14 years and progression-free survival up to 5 years [17]. These increases in the patients´ survival have been observed since 2003 upon the introduction of the anti-CD20 monoclonal antibody, rituximab (R), in the chemotherapy regimens.
The purpose of the present study was to assess the clinical outcome of patients with FL included in a Spanish registry by the Spanish Lymphoma Oncology Group (GOTEL). This database is part of a prospective registry of all new lymphoma cases, regardless of their histological subtype, which tries to ascertain a possible clinical impact of different therapeutic strategies introduced in the last decades.
Methods
Patients
The study was performed as a prospective multicenter study [18]. It was conducted in compliance with the Declaration of Helsinki, and was approved by Puerta de Hierro-Majadahonda Ethics Committee.
Patients referred to the Oncology Department of 18 Spanish hospitals between January 1980 and December 2013, diagnosed with FL were included. Information concerning demographic and clinical-pathological features of each patient as well as prognostic factors, type of treatment and treatment outcome was collected. Patients were staged according to the Ann Arbor system, the FLIPI was calculated, and systemic symptoms were regarded as present when the patient had unexplained fever, night sweats, or weight loss of >10% of initial body weight. Treatment information such as type of therapy (radiation therapy, chemotherapy, combined therapy, or no therapy), response to therapy, and the patient’s survival status, was collected and was assessed in months.
All patients included required a certain diagnosis of follicular lymphoma and were staged according to the Ann Arbor system. Treatment information had to be fully documented, including treatment modality, and initial and final doses. Patients who did not meet any of these criteria were excluded.
The authors served as the advisory board for this study, and participated in all phases of the study, including protocol design, data collection and analysis, and consideration of participating sites.
Statistical analysis
Overall survival was the end point of all statistical analyses. Survival rates and corresponding standard errors were estimated using Kaplan and Meier estimators. Survival curves were compared applying the log-rank test.
According to the external evaluator’s criteria and due to a lack of sufficient diagnostic, survival or follow up data, 104 patients out of the 1178 patients diagnosed with FL were excluded from the analysis. Therefore we reviewed 1074 patients treated in our country, investigating the use of R and exploring the association between this treatment and survival compared with other patients treated without R in first line or watchful waiting approach in our series with long-follow, and with a median survival of 234 months.
Statistical analyses were performed firstly in all patients and secondly in the treatment group, where efficacy endpoints were analyzed. Overall survival times were estimated with the Kaplan-Meier method and compared with a two-sided log-rank test. Univariate and multivariate Cox regression analysis were used to assess the association between each potential prognostic factor and overall survival and calculate the relative risk (RR). All analyses were two-sided with a 5% significance level and were performed with SPSS version 19 and STATA version 12.
Death hazard risk (HR) according to year of treatment and to age upon diagnosis was calculated. Correlation between group and HR was obtained with the Cox model.
Results
Patient characteristics
A total of 1178 patients diagnosed with grade I-IIIa FL between January 1980 and December 2013, in the Oncology Department of 18 Spanish hospitals, were enrolled in the FL Registry, a prospective registry promoted by GOTEL (Spanish Lymphoma Oncology Group) that includes all new lymphoma cases, regardless of their histological subtype. All diagnoses were confirmed by a study hematopathologist. Exclusion criteria were: initial diagnosis of grade IIIb, primary cutaneous FL and HIV positive.
According to the external evaluator´s criteria and due to a lack of sufficient diagnostic, survival or follow up data, 104 patients out of the 1178 patients diagnosed with LF were excluded from the analysis.
The median follow up in the entire series was 54.9 months (1–365.0), 63.7 months (0.1–365.3) only considering patients alive free of disease, 43.7 months (0.1–365.4) for patients alive with the disease, and 32.5 months (0.4–228.4) for those patients who died.
The main clinical variables registered at the time of diagnosis with potential prognostic relevance are reported in Table 1 and the median follow-up of surviving patients and the OS of these patients are shown in Fig 1.
Table 1. Patient characteristics.
| Clinical Variables | N | |
|---|---|---|
| Sex | Male | 506 |
| Female | 568 | |
| Grade | I | 341 |
| II | 316 | |
| III | 129 | |
| IIIa | 131 | |
| IIIb | 23 | |
| Centrofollicular cutaneous variant | 12 | |
| Centrofollicular diffuse variant | 50 | |
| NA | 72 | |
| Origin | Nodal | 907 |
| Extranodal | 167 | |
| Ann Arbor stage | I | 141 |
| II | 157 | |
| III | 273 | |
| IV | 500 | |
| NA | 3 | |
| ECOG | 0 | 609 |
| 1 | 366 | |
| 2 | 68 | |
| 3 | 25 | |
| 4 | 4 | |
| NA | 2 | |
| Bone marrow involvement | No | 654 |
| Yes | 417 | |
| NA | 3 | |
| B Symptoms | No | 847 |
| Yes | 224 | |
| NA | 3 | |
| Bulky mass | No | 820 |
| Yes | 254 | |
| Number of extranodal localizations | 0 | 602 |
| 1 | 357 | |
| 2 | 115 | |
| HIV | No | 1072 |
| Yes | 2 | |
| Treatment | CT with anthracyclines | 164 |
| CT w/o anthracyclines | 98 | |
| CT with anthracyclines and R | 633 | |
| CT w/o anthracyclines or R | 110 | |
| R monotherapy | 37 | |
| Cx | 13 | |
| Observation | 19 | |
| RT | No | 826 |
| Yes | 248 | |
| ASCT | No | 1017 |
| Yes | 57 | |
| Transformation | No | 1040 |
| Yes | 34 | |
| Type of transformation | Diffuse large B cell | 20 |
| MALT | 1 | |
| Hodgkin´s Lymphoma | 1 | |
| Mantle B cells lymphoma | 1 | |
| Follicular lymphoma grade 3 | 2 | |
| Burkitt like lymphoma | 2 | |
| Angiocentric high grade peripheral T cells lymphoma | 1 | |
| High grade follicular lymphoma | 1 | |
| Unknown | 5 | |
| Cause of death | Primary tumour | 135 |
| Secondary tumour | 21 | |
| Other | 68 | |
| Hb | <12 | 257 |
| >12 | 817 | |
| LDH | average | 812 |
| high | 362 | |
| FLIPI | 0 | 171 |
| 1 | 279 | |
| 2 | 337 | |
| 3 | 174 | |
| 4 | 88 | |
| 5 | 25 | |
| B2microglobulin | average | 644 |
| high | 345 | |
| NA | 85 |
CT: chemotherapy; R: rituximab; ECOG: Eastern Cooperative Oncology Group; HIV: human immunodeficiency virus; FLIPI: Follicular Lymphoma International Prognostic Index; Hb: hemoglobin; LDH: lactate dehydrogenase; ASCT: autologous stem cell transplantation; RT: radiotherapy; Cx: surgery
Fig 1. Overall survival (in months) according to performance status ECOG (B) and FLIPI score at diagnosis (C) for all patients (n = 1074).
Follow-up and survival
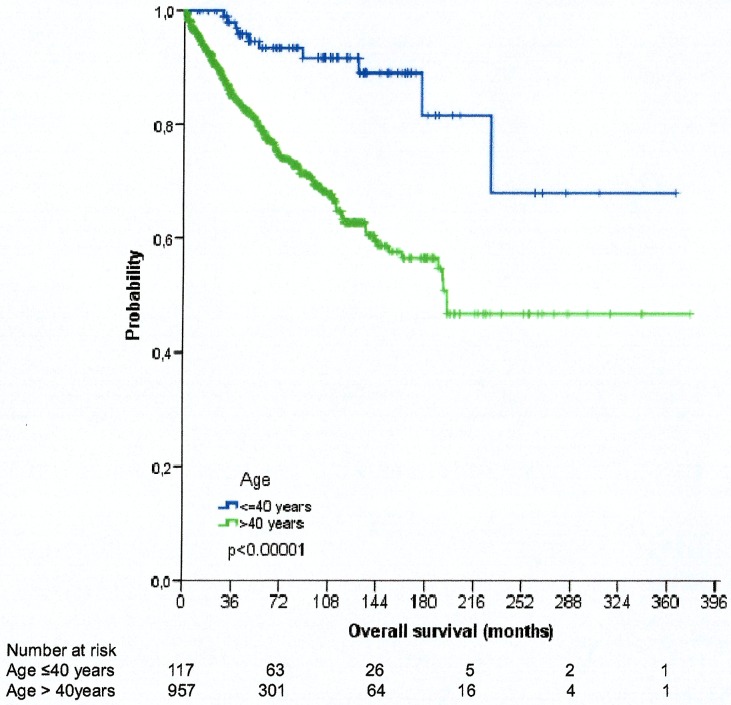
Significant variables in univariate analysis (Table 2) for OS in our series were incorporated in a multivariate model (Table 3). The multivariate analysis in our series shows greater significance at 60 years of age, as well as performance status (PS), transformation and FLIPI. A cutpoint at 40 years of age was also analyzed to estimate an influence in survival. Our series shows an increase in OS in patients under 40 years old without reaching the median survival, and of 16.3 years in patients over 40 years old, with significant statistical difference (p <0.00001) between the curves of OS (Fig 2).
Table 2. Results of univariate analysis of different prognostic factors in the whole population of 1074 patients with follicular lymphoma (FL).
The table shows the statistically significant prognostic factors.
| Overall Survival | N | Median (95% CI) | % | p |
|---|---|---|---|---|
| Age >60 | 455 | 117,2(85.2–149.1) | 38 | <0.0001 |
| Ann Arbor stage >II | 730 | 192.1(148.7–235.5) | 85,9 | 0.003 |
| ECOG >1 | 671 | NR | 5,1 | <0.0001 |
| Bone marrow involvement | 387 | 152.1(117.2–187.1) | 38,6 | 0.006 |
| B symptoms (NO) | 767 | NR | 81,5 | 0.02 |
| Nodal sites>4 | 426 | 176.1(149.9–202.3) | 10,4 | 0.03 |
| Bulky mass (YES) | 239 | 170.4(118.2–222.7) | 20,3 | 0.02 |
| Extranodal sites (=1) | 334 | 192.7(—) | 32,1 | 0.002 |
| RT (NO) | 782 | NR | 69,8 | 0.001 |
| ASCT (NO) | 952 | 228.4(—) | 91,8 | 0.07 |
| Transformation (NO) | 974 | NR | 95,8 | 0.003 |
| Hb<12 | 235 | 192.1(—) | 76,6 | 0.02 |
| normal LDH | 757 | NR | 76,8 | <0.0001 |
| FLIPI 0 | 157 | NR | 20,5 | <0.0001 |
| β2-microglobulin (normal) | 598 | NR | 71,7 | <0.0001 |
| Treatment with anthracyclines | 199 | 192.1(113.5–270.7) | 17,7 | <0.0001 |
RT: radiotherapy; ECOG: Eastern Cooperative Oncology Group; FLIPI: Follicular Lymphoma International Prognostic Index; Hb: hemoglobin; LDH: lactate dehydrogenase; ASCT: autologous stem cell transplantation; NR: not representative.
Table 3. Multivariate analysis: Characteristics associated with overall survival.
| Variables | HR (95% CI) | p |
|---|---|---|
| ECOG | 1 ref. | |
| ECOG (1) | 1.8(1.3–2.5) | <0.0001 |
| ECOG (2) | 3.8(2.5–5.8) | <0.0001 |
| ECOG (3) | 8.3(4.6–14.8) | <0.0001 |
| ECOG (4) | 15.5(4.7–51.1) | <0.0001 |
| B Symptoms | 1.4(1.1–1.9) | 0.015 |
| Transformation | 2.6(1.5–4.5) | <0.0001 |
| FLIPI | 1 ref. | |
| FLIPI 1 | 2.2(1.1–4.3) | 0.03 |
| FLIPI 2 | 2.4(1.2–4.9) | 0.01 |
| FLIPI 3 | 3.6(1.8–7.5) | 0.001 |
| FLIPI 4 | 3.5(1.6–7.5) | 0.001 |
| FLIPI 5 | 4.1(1.6–10.4) | 0.001 |
| Age>60 | 1.9(1.5–2.7) | <0.0001 |
ECOG: Eastern Cooperative Oncology Group; FLIPI: Follicular Lymphoma International Prognostic Index
Fig 2. Kaplan-Meier estimates of survival (in years) according to age (over or under 40 years of age).
Taking as reference group the young adults group, aged 20–29 years, the risk increases in every group, as shown in Table 4. When calculating HR in Cox model, the reference value always corresponds to minimum risk group, aged ≤19 years, which is why HR increases from 50 years old. Correlation between group of age and HR obtained with Cox model is presented in Fig 3. There is no linear correlation with age, it’s a quadratic correlation. R2 shows a great correlation between HR and age, being 94.7% the variability of HR explained by age, with less than a 5% error.
Table 4. Age distribution at time of diagnosis of follicular lymphoma for all patients.
Correlation between age, mortality, and risk of death, taking as reference group the group aged 20–29 years.
| Age | Alive | Dead | N | RR | OR | HR | p |
|---|---|---|---|---|---|---|---|
| ≤29 | 20 | 1 | 21 | 0.05 | 1 | Ref. | |
| 30–39 | 64 | 7 | 71 | 0.09 | 1.8 | 0.47 | 0.54 |
| 40–49 | 122 | 12 | 134 | 0.09 | 1.8 | 0.99 | 0.99 |
| 50–59 | 128 | 22 | 150 | 0.15 | 3 | 1.87 | 0.54 |
| 60–69 | 128 | 28 | 156 | 0.18 | 3.6 | 3.23 | 0.25 |
| 70–79 | 52 | 30 | 82 | 0.36 | 7.2 | 7.04 | 0.06 |
| ≥80 | 10 | 3 | 13 | 0.23 | 4.6 | 5.69 | 0.15 |
RR: relative risk (of death); OR: overall risk; HR: hazard risk.
Fig 3. Correlation between group of age and HR value, obtained by Cox model.
HR indicates hazard risk and R2explains the great correlation between HR and age, with less than a 5% error.
An analysis of patients who are still alive beyond 10 years upon diagnosis was conducted, to fully assess the prognostic factors that condition this group. In a group of 166 patients who are still alive after more than 10 years of follow-up, 118 of them (73%) are free of evident clinical disease.
The following variables were not significant: gender, number of nodal areas affected, bone marrow involved or not, lactate dehydrogenase (LDH) levels, nodal or extranodal origin, presence of bulky mass, hemoglobin or transformation to a more aggressive lymphoma. However, stage<II (p <0.03), age under 60 years (p <0.0001), low FLIPI (p <0.002), average B2 microglobulin (p <0.005), no B symptoms upon diagnosis (p <0.02), PS 0–1 (p <0.03) and combined treatment with anthracyclines and R were the variables that were significantly associated with survival at 10 years (p <0.0001) (Table 5).
Table 5. Variables significantly associated with survival at 10 years.
| N | % | p value | |
|---|---|---|---|
| Ann Arbor Stage<II | 44 | 33.3 | 0.03 |
| Age<60 | 96 | 72.7 | 0.0001 |
| Low FLIPI | 71 | 53.8 | 0.002 |
| Normal β2 microglobulin | 89 | 73.6 | 0.05 |
| No B symptoms | 115 | 87.1 | 0.02 |
| PS 0–1 | 129 | 97.7 | 0.03 |
| Treatment CT+R | 45 | 39.1 | 0.001 |
| R monotherapy | 60 | 45.5 | <0.0001 |
CT: chemotherapy; R: rituximab; FLIPI: Follicular Lymphoma International Prognostic Index; PS: performance status;
The HR of death among patients has been declining progressively over the years as can be observed in Fig 4.
Fig 4. Adjusted death hazard rate to year of diagnosis for the Spanish Lymphoma Study.
Discussion
Several prognostic factors have been identified in an effort to predict the outcome in patients with FL, including FLIPI, which divides FL cases into three groups with distinct survival probabilities. FLIPI is an internationally validated prognostic index, which provides some hope for improved categorization for patients in clinical studies. Unfortunately, it is solely based on demographic and clinical data and is somehow limited. Alternatively, prognostic models that incorporate biological information, such as those based on gene expression profiles, are potentially much more powerful, although they require validation. Still, and despite such limitations, progress has been made and improvements during the past decade in survival of FL patients have been repeatedly demonstrated in randomized studies and meta-analyses.
In our series, we found significant difference between low/intermediate (<3), and high-risk FLIPI groups (≥3) in terms of OS. Although currently the FLIP2 score is used, we were unable to apply this type of score in our cases due to incomplete availability of some clinical and biological parameters, which were not routinely collected at the time of diagnosis.
The prognostic factors in our series are similar to other series [19, 20, 21] and have already been mentioned: age, B symptoms, stage and tumor burden, extent of bone marrow infiltration, infiltration of specific organs, levels of LDH and β2 microglobulin.
Also, we investigated alternative cutpoints of age other than the traditional 60 years. Thereby, our series shows an increase in overall survival in patients under 40 years of age, without reaching the median survival and those over 40 years of age of 16.3 years, with great statistical difference (p <0.00001) between the curves of overall survival. These results are similar to those obtained in a recent study at Princess Margaret Hospital on 61 patients under 40 years of age, showing that the classic cutpoint at 60 years might not be entirely adequate; an earlier age may reflect better prognostic factors and later ages may serve to identify causes of competitive mortality. We looked into this aspect, and our findings suggest that age gives an increased risk of death regardless of the cut point that we use. As a matter of fact, a comparison of clinical features at diagnosis between patients ≤40 and >40 years was carried out in a European series of 1002 patients from 4 different institutions indicated that prognostic factors are useful in the whole population of patients with FL and also apply to younger patients, and that lymphoma-specific survival is similar in young adults and in patients aged 40–60 [22].
The median OS of patients diagnosed with FL is over 20 years in our series. We observed a constant decrease in the HR of death as the years have passed, showing a best prognosis for these patients. Of interest, we wanted to see if a percentage of PFS patients beyond 10 years were sustained. The skill of the therapeutic group seems unlikely to be the explanation of these results, since it is a multicenter study, and also with one of the largest published series that gives a clear consistency to the results. Our data support the initial combined treatment with R and anthracyclines could be considered key factors versus observation.
In 2012 a data analysis from the F2 Study Registry from the International Follicular Lymphoma Prognostic Factor was published. Data from this cohort was compared to patients within the same study but initially treated with regimens that would contain R, in order to know if an initial expectant attitude could influence the effectiveness of these treatments. The 5-year survival was similar in both groups, 87% in the group of patients under observation versus 88% in the group of patients initially treated, concluding that in the R era the strategy of "wait and watch" remains valid for patients with favorable prognostic factors and low-grade tumors (GELF criteria) [23, 24].
First line treatment with R was introduced in Spain only since 2004. However, many patients may have received this treatment during the course of the disease and benefit from it. It seems that the addition of R to chemotherapy schemes has managed to modify the OS of these patients.
Aiming to clarify it, two phase III studies were launched; the National Lympho Care Study [25] and FOLL-05 [26]. No difference was observed in either of them between R-CHOP (cyclophosphamide, doxorubicine, vincristine, prednisone), R-CVP (cyclophosphamide, vincristine, prednisone) and R-FM (fludarabine and mitoxantrone), in terms of OS or progression-free survival. Increased time to progression (27 months vs 7 months) and longer survival at 4 years (83% vs 77%) was also observed [27].
We observed an increase in OS of patients over the years and diagnostic times, which are identifiable in all age and sex groups, including advanced stages. This finding is consistent with data from American [28, 29] and European [30] studies, ours being the largest study of all the ones published in our continent that can be related to the introduction of R. It has been speculated whether the improvements achieved in support care or even high doses of chemotherapy may have influenced this.
According to our results, especially taking into account the study of patients alive for more than 10 years, we believe that the weight of the introduction of R in a young population, associated with chemotherapy, has given these high rates of survival in an unselected population. The median OS of patients diagnosed of FL is over 20 years in our series, which suggest that increase in survival might be due to the use of anthracyclines, R and radiotherapy.
The development of national registries such as the Spanish Follicular Lymphoma Registry, promoted by GOTEL, help us identify the clinical-pathological characteristics of the patients in our area and therefore try to develop the best treatment program for our patients, improving the effectiveness of our clinical practice.
Acknowledgments
All of the authors who contributed in this work are members of GOTEL; Dr Mariano Provencio from Puerta de Hierro Teaching Hospital is the Chair of the group; Pilar Sabín on behalf of GOTEL (Spanish Lymphoma Oncology Group); Jose Gomez-Codina on behalf of GOTEL (Spanish Lymphoma Oncology Group); Maria Torrente on behalf of GOTEL (Spanish Lymphoma Oncology Group); Virginia Calvo on behalf of GOTEL (Spanish Lymphoma Oncology Group); Marta Llanos on behalf of GOTEL (Spanish Lymphoma Oncology Group); Josep Gumá on behalf of GOTEL (Spanish Lymphoma Oncology Group); Cristina Quero on behalf of GOTEL (Spanish Lymphoma Oncology Group); Ana Blasco on behalf of GOTEL (Spanish Lymphoma Oncology Group); Miguel Angel Cruz on behalf of GOTEL (Spanish Lymphoma Oncology Group); David Aguiar on behalf of GOTEL (Spanish Lymphoma Oncology Group); Francisco García-Arroyo on behalf of GOTEL (Spanish Lymphoma Oncology Group); Javier Lavernia on behalf of GOTEL (Spanish Lymphoma Oncology Group); Natividad Martinez on behalf of GOTEL (Spanish Lymphoma Oncology Group); Manuel Morales on behalf of GOTEL (Spanish Lymphoma Oncology Group); Alvaro Saez-Cusi on behalf of GOTEL (Spanish Lymphoma Oncology Group); Delvys Rodriguez on behalf of GOTEL (Spanish Lymphoma Oncology Group); Luis de la Cruz on behalf of GOTEL (Spanish Lymphoma Oncology Group); Jose Javier Sanchez on behalf of GOTEL (Spanish Lymphoma Oncology Group), and Antonio Rueda on behalf of GOTEL (Spanish Lymphoma Oncology Group).
Data Availability
All relevant data are within the paper.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Marcos-Gragera R, Gumà J, De Sanjose S. Análisis de la incidencia, la supervivencia y la mortalidad según las principales localizaciones tumorales, 1985–2019: linfomas no Hodgkin. Med Clin (Barc) 2008; 131 (Supl 1):72–77. [DOI] [PubMed] [Google Scholar]
- 2.The Non-Hodgkin´s Lymphoma Classification Project: A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin´s lymphoma. Blood 1997; 89 (11): 3909–18. [PubMed] [Google Scholar]
- 3.Armitage JO, Weisenburger DD: New approach to classifying non-Hodgkin´s lymphomas: clinical features of the major histologic subtypes. J Clin Oncol 1998; 16:2780–95. 10.1200/JCO.1998.16.8.2780 [DOI] [PubMed] [Google Scholar]
- 4.Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H. et al. WHO classification of tumors of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. ISBN 9283224310. Pp. 200–226. [Google Scholar]
- 5.Dreyling M, Guielmini M, Marcus R, Salles G,Vitolo U, Ladetto M, on behalf of the ESMO Guidelines Working Group. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann of Oncol 2014; 25 (suppl 3): iii76–iii82 [DOI] [PubMed] [Google Scholar]
- 6.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M et al. , Cancer incidence in five continents. Vol. IX Lyon: IARC; 2009. [Google Scholar]
- 7.Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U et al. Follicular Lymphoma International Prognostic Index 2: A New Prognostic Index for Follicular Lymphoma Developed by the International Follicular Lymphoma Prognostic Factor Project. J Clin Oncol 2009; 27:27, 4555–62. 10.1200/JCO.2008.21.3991 [DOI] [PubMed] [Google Scholar]
- 8.Sarkozy C, Trneny M, Xerri L, Wickham N, Feugier P, Leppa S, et al. Risk Factors and Outcomes for Patients With Follicular Lymphoma Who Had Histologic Transformation After Response to First-Line Immunochemotherapy in the PRIMA Trial. J Clin Oncol. 2016;34(22):2575–82. 10.1200/JCO.2015.65.7163 [DOI] [PubMed] [Google Scholar]
- 9.Cullen MH, Lister TA, Brearly RL, Shand WS and Stansfeld AG. Histological transformation of non-Hodgkin´s Lymphomas. A prospective study. Cancer 1979; 44:645–51. [DOI] [PubMed] [Google Scholar]
- 10.Garvin AJ, Simon RM, Osborne CK, Merrill J, Young RC and Berard CW.An autopsy study of histologic progression in non-Hodgkin´s lymphomas.192 cases from the National Cancer Institute. Cancer 1983; 52:393–8. [DOI] [PubMed] [Google Scholar]
- 11.Horning SJ, Rosenberg SA. The natural history of initially untreated low-grade non-Hodgkin's lymphomas.NEngl J Med 1984; 311:1471. [DOI] [PubMed] [Google Scholar]
- 12.Chau I, Jones R, Cunningham D, Wotherspoon A., Maisey N., Norman A. et al. Outcome of follicular lymphoma grade 3: is anthracycline necessary as front-line therapy? British Journal of Cancer. 2003;89(1):36–42. 10.1038/sj.bjc.6601006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Relander T, Johnson NA, Farinha P, Connors JM, Sehn LH, and Gascoyne RD. Prognostic factors in follicular lymphoma. J ClinOncol 2010; 28:17, 2902–2913 [DOI] [PubMed] [Google Scholar]
- 14.van de Schans SA, Steyerberg EW, Nijziel MR, Creemers GJ, Janssen-Heijnen ML, and van Spronsen DJ; Validation, revision and extension of the Follicular Lymphoma International Prognostic Index (FLIPI) in a population-based setting. Ann Oncol 2009; 20 (10): 1697–1702 10.1093/annonc/mdp053 [DOI] [PubMed] [Google Scholar]
- 15.Swenson WT, Wooldridge JE, Lynch CF, Forman-Hoffman VL, Chrischilles E, and Link BK. Improved survival of follicular lymphoma patients in United States. J Clin Oncol 2005; 23:5019–26. 10.1200/JCO.2005.04.503 [DOI] [PubMed] [Google Scholar]
- 16.Fisher RI, Leblanc M, Press OW, Maloney DG, Unger JM, and Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. J ClinOncol 2005;23 (33):8447–52. [DOI] [PubMed] [Google Scholar]
- 17.Provencio M, Sabín P, Gómez Codina J, Rueda A, Llanos M, Gumá J, et al. Are there any significant variations in the clinical or histological presentation of lymphoid pathologies over the course of time in Spain? Clin Transl Oncol 2012; 14 (5):386–90. [DOI] [PubMed] [Google Scholar]
- 18.Denham JW, Denham E, Dear KB, and Hudson GV. The follicular non-Hodgkin´s lymphomas II. Prognostic factors: what do they mean? Eur J Cancer 1996; 32A(3):480–90. [DOI] [PubMed] [Google Scholar]
- 19.Coiffer B, Bastion Y, Berger F, Felman P, and Bryon PA.: Prognostic factors in follicular lymphomas. Semin Oncol 1993; 20:89–95. [PubMed] [Google Scholar]
- 20.Young RC, Longo DL, Glatstein E, Ihde DC, Jaffe ES, and DeVita VT Jr. The treatment of indolent lymphomas: Watchful waiting versus aggressive combined modality treatment. Semin Hematol 1988; 25(Supl.2):11–6. [PubMed] [Google Scholar]
- 21.Soubeyran P, Eghbali H, Bonichon F, Trojani M, Richaud P, and Hoerni B. Low-grade follicular lymphomas: Analysis of prognosis in a series of 281 patients. Eur J Cancer 1991; 27:1606–13. [DOI] [PubMed] [Google Scholar]
- 22.Conconi A, Lobetti-Bodoni C, Montoto S, Lopez-Guillermo A, Coutinho R, Matthews J, et al. Life expectancy of young adults with follicular lymphoma. Ann Oncol. 2015. November;26(11):2317–22. 10.1093/annonc/mdv376 [DOI] [PubMed] [Google Scholar]
- 23.Solal-Céligny P, Bellei M, Marcheselli L, Pesce EA, Pileri S, McLaughlin P et al. Watchful waiting in low-tumor burden follicular lymphoma in the rituximab era: results of an F2-study database. J Clin Oncol. 2012; 30:3848–3853. 10.1200/JCO.2010.33.4474 [DOI] [PubMed] [Google Scholar]
- 24.Nastoupil LJ, Sinha R, Byrtek M, Zhou X., Taylor M. D., Friedberg J. W. The use and effectiveness of rituximab maintenance in patients with follicular lymphoma diagnosed between 2004 and 2007 in the United States. Cancer. 2014;120(12):1830–1837. 10.1002/cncr.28659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Federico M, Luminari S, Dondi A, Tucci A, Vitolo U, Rigacci L et al. R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-state follicular lymphoma: results of the FOLL05 trial conducted by the Fondazione Italiana Linfomi. J ClinOncol 2013;31:1506–1513. [DOI] [PubMed] [Google Scholar]
- 26.Sebban C, Brice P, Delarue R, Haioun C, Souleau B, Mounier N, et al. Impact of rituximab and or high-dose therapy with autotransplant at time of relapse in patients with follicular lymphomas: a GELA study. J Clin Oncol 2008; 26: 3614–3620. 10.1200/JCO.2007.15.5358 [DOI] [PubMed] [Google Scholar]
- 27.Gangatharan SA, Maganti M, Kuruvilla JG, Kukreti V, Tiedemann RE, Gospodarowicz MK, et al. Clinical characteristics and early treatment outcomes of follicular lymphoma in young adults. Br J Haematol 2015; 170(3):384–90 10.1111/bjh.13451 [DOI] [PubMed] [Google Scholar]
- 28.Swenson WT, Wooldridge JE, Lynch CF, Forman-Hoffman VL, Chrischilles E, Link BK. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol. 2005. August 1; 23(22):5019–26. 10.1200/JCO.2005.04.503 [DOI] [PubMed] [Google Scholar]
- 29.Nabhan C, Aschebrook-Kilfoy B, Chiu BC, Kruczek K, Smith SM, Evens AM. The impact of race, age, and sex in follicular lymphoma: A comprehensive SEER analysis across consecutive treatments. Am J Hematol. 2014. June; 89(6):633–8. 10.1002/ajh.23708 [DOI] [PubMed] [Google Scholar]
- 30.Conconi A, Motta M, Bertoni F, Piona C, Stathis A, Wannesson L, et al. Patterns of survival of follicular lymphomas at a single institution through three decades. Leukemia and Lymphoma 2010; 51 (6): 1028–1034. 10.3109/10428191003743460 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.