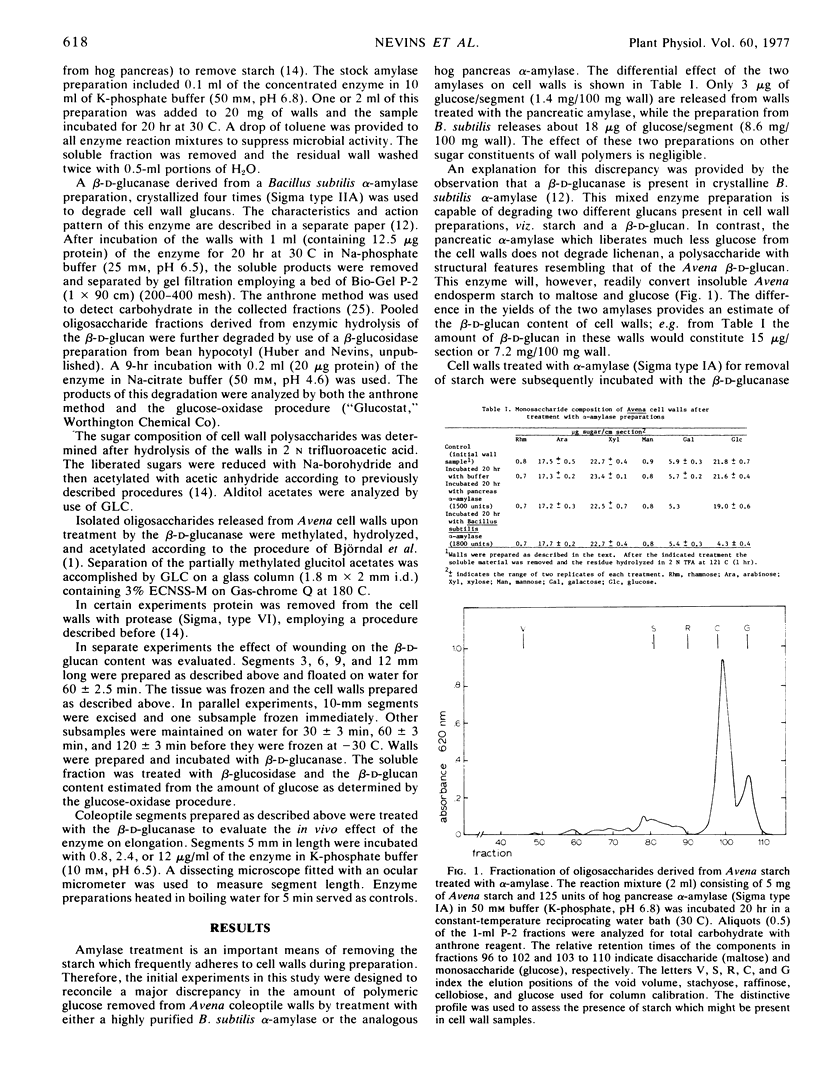
Abstract
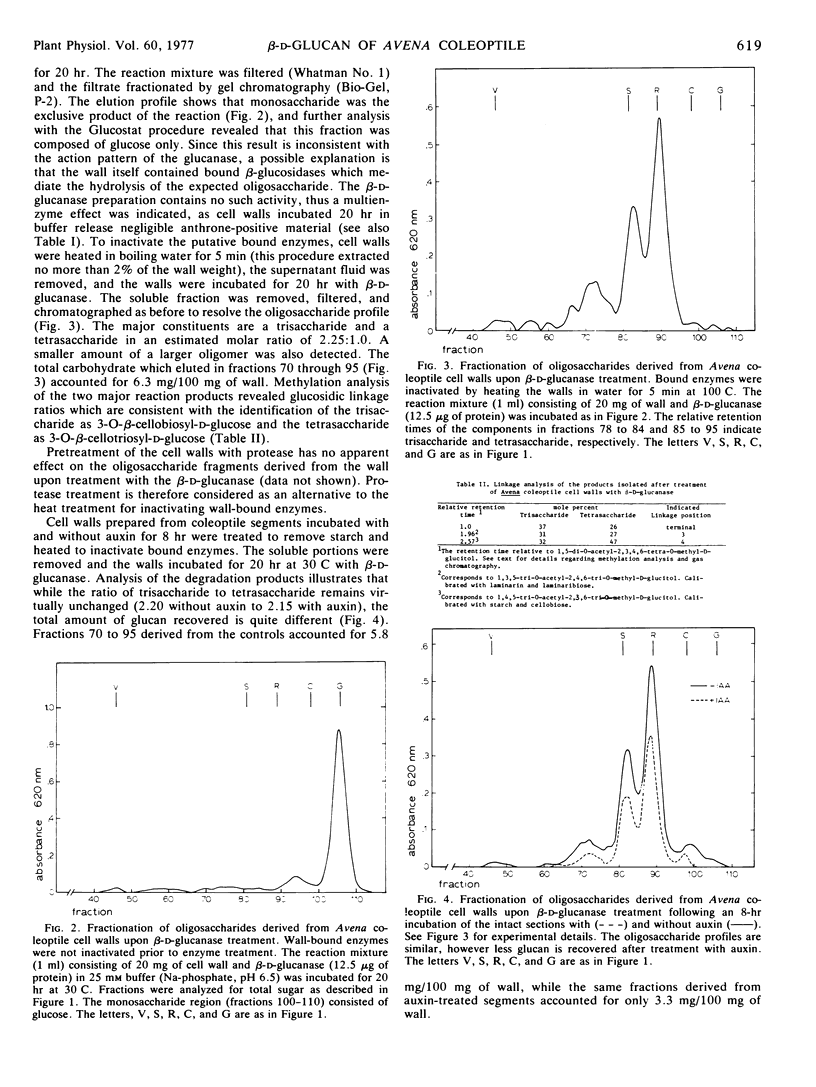
A specific glucanase was used to liberate a noncellulosic β-d-glucan from isolated cell walls of Avena sativa coleoptile tissue. Cell walls of this tissue contain as much as 7 to 9 mg of glucan/100 mg of dry wall. Because of the specific action pattern of the enzyme, a linkage sequence of.. 1 → 4 Glc 1 → 3 Glc 1 → 4 Glc.. is indicated and the predominance of trisaccharide and tetrasaccharide as hydrolytic products suggests a rather regular repeating pattern in the polysaccharide. The trisaccharide and the tetrasaccharide are tentatively identified as 3-O-β-cellobiosyl-d-glucose and 3-O-β-cellotriosyl-d-glucose, respectively. Recovery of these oligosaccharides following glucanase treatment of native wall material was feasible only after wall-bound glucosidases were inactivated. In the absence of enzyme inactivation the released fragments were recovered as glucose. The β-d-glucan was not extracted from walls by either hot water or protease treatment.
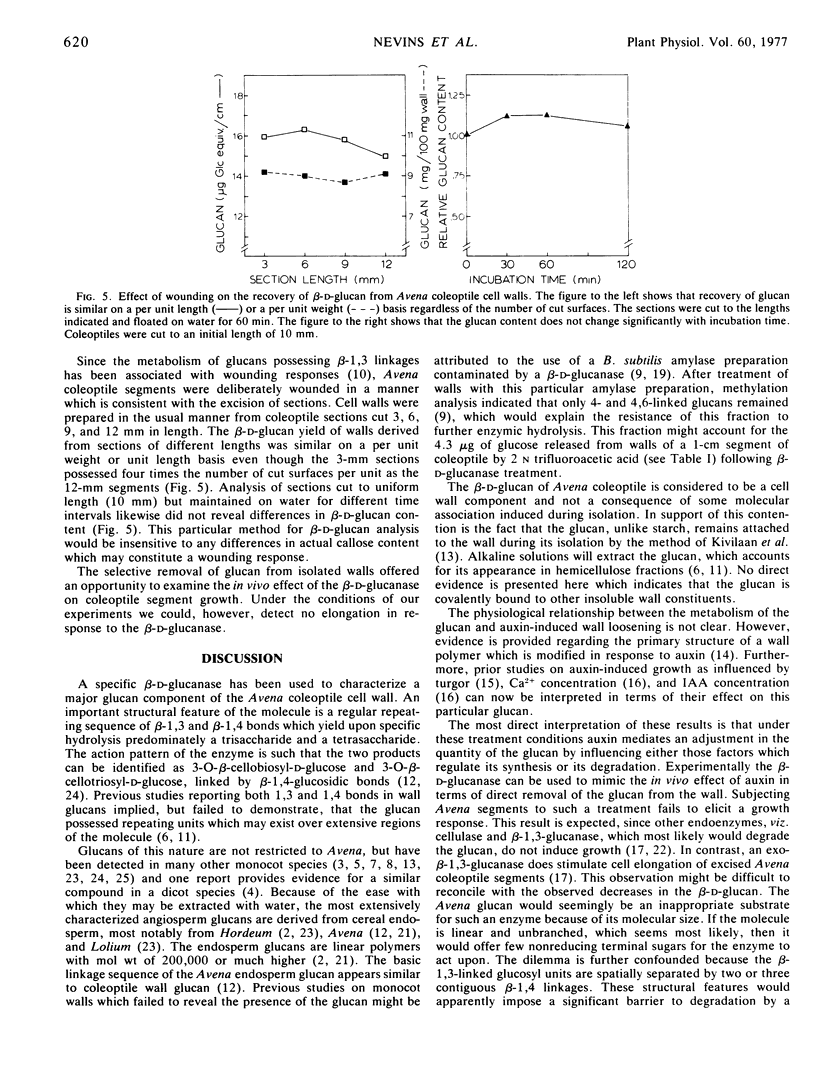
Cell walls prepared from auxin-treated Avena coleoptile segments yielded less glucan than did segments incubated in buffer suggesting an auxin effect on the quantity of this wall component. No IAA-induced change in the ratio of the trisaccharide and tetrasaccharide could be detected, suggesting no shift in the 1,3 to 1,4 linkage ratio. While the enzyme acts directly on the β-d-glucan, no elongation response was apparent when Avena sections were treated with the purified glucanase. The presence of the glucan was not associated with any wound response which could be attributed to the preparation of coleoptile segments. The relationship of glucan metabolism to auxin growth responses is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke D., Kaufman P., McNeil M., Albersheim P. The Structure of Plant Cell Walls: VI. A Survey of the Walls of Suspension-cultured Monocots. Plant Physiol. 1974 Jul;54(1):109–115. doi: 10.1104/pp.54.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D. J., Nevins D. J. Preparation and Properties of a beta-d-Glucanase for the Specific Hydrolysis of beta-d-Glucans. Plant Physiol. 1977 Aug;60(2):300–304. doi: 10.1104/pp.60.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivilaan A., Bandurski R. S., Schulze A. A partial characterization of an autolytically solubilized cell wall glucan. Plant Physiol. 1971 Oct;48(4):389–393. doi: 10.1104/pp.48.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loescher W. H., Nevins D. J. Turgor-dependent Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1973 Sep;52(3):248–251. doi: 10.1104/pp.52.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loescher W., Nevins D. J. Auxin-induced Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1972 Nov;50(5):556–563. doi: 10.1104/pp.50.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Albersheim P. The Structure of Plant Cell Walls: VII. Barley Aleurone Cells. Plant Physiol. 1975 Jan;55(1):64–68. doi: 10.1104/pp.55.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. M., Stone B. A. Beta-glucan synthesis by cell-free extracts from Lolium multiflorum endosperm. Biochim Biophys Acta. 1973 Jun 20;313(1):72–94. doi: 10.1016/0304-4165(73)90189-x. [DOI] [PubMed] [Google Scholar]


