Abstract
Background
Myocardial infarction (MI) is one of the leading causes of morbidity and mortality worldwide. Dietary intervention on adverse cardiac remodeling after MI has significant clinical relevance. Rosemary leaves are a natural product with antioxidant/anti-inflammatory properties, but its effect on morphology and ventricular function after MI is unknown.
Methods and results
To determine the effect of the dietary supplementation of rosemary leaves on cardiac remodeling after MI, male Wistar rats were divided into 6 groups after sham procedure or experimental induced MI: 1) Sham group fed standard chow (SR0, n = 23); 2) Sham group fed standard chow supplemented with 0.02% rosemary (R002) (SR002, n = 23); 3) Sham group fed standard chow supplemented with 0.2% rosemary (R02) (SR02, n = 22); 4) group submitted to MI and fed standard chow (IR0, n = 13); 5) group submitted to MI and fed standard chow supplemented with R002 (IR002, n = 8); and 6) group submitted to MI and fed standard chow supplemented with R02 (IR02, n = 9). After 3 months of the treatment, systolic pressure evaluation, echocardiography and euthanasia were performed. Left ventricular samples were evaluated for: fibrosis, cytokine levels, apoptosis, energy metabolism enzymes, and oxidative stress. Rosemary dietary supplementation attenuated cardiac remodeling by improving energy metabolism and decreasing oxidative stress. Rosemary supplementation of 0.02% improved diastolic function and reduced hypertrophy after MI. Regarding rosemary dose, 0.02% and 0.2% for rats are equivalent to 11 mg and 110 mg for humans, respectively.
Conclusion
Our findings support further investigations of the rosemary use as adjuvant therapy in adverse cardiac remodeling.
Introduction
Myocardial infarction (MI) is one of the leading causes of morbidity and mortality worldwide. According to the 2015 update of A Report From the American Heart Association, approximately 635,000 Americans have a new coronary attack each year[1]. MI can be defined as a focus of necrosis resulting from poor tissue perfusion, with signs and symptoms resulting from cardiac cell death. The death of myocytes initiates a cascade of intracellular signaling, such as inflammation, oxidative stress, reabsorption of necrotic tissue, excessive deposition of collagen, and hypertrophy, that can result in adverse cardiac remodeling. These molecular, cellular and interstitial changes can clinically be manifested as changes in size, mass, geometry and heart function. Cardiac remodeling is an adaptation of the heart to aggression stimuli that may gradually lead to the development of heart failure (HF), responsible for the increased mortality after MI[2, 3].
Many factors can participate on MI pathophysiology. MI is started by myocardial ischemia and it is associated with increased generation of reactive oxygen species (ROS)[4]. In experimental studies, oxidative stress is identified as a major factor for the development of cardiac hypertrophy[5]. Oxidative stress can also activate the production of inflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-1β and IL-6[6], triggering inflammatory pathways, fibrosis and cell death[5]. ROS and cytokines also contribute to the activation of matrix metalloproteinases (MMPs) and collagen deposition that might lead to structural changes in the heart [6, 7].
Because oxidative stress can play a central pathophysiological role in cardiac remodeling after MI[4, 7], antioxidant supplements are beneficial after injury to the myocardium[8, 9]. In this context, the antioxidant properties of natural products have been examined[10]. Rosemary (Rosmarinus oficinallis Linn) is a popular culinary spice, but it is also known as a medicinal herb and a natural conservative in the food industry, with one of the highest levels of antioxidant compounds[11, 12]. Many compounds have been isolated from rosemary, including flavones and diterpenes. The phenolic diterpenes carnosic acid and carnosol are the major bioactive compounds in rosemary leaves related to the antioxidant activity [12, 13]. Rosmarinic acid is a caffeic acid ester with antioxidant and anti-inflammatory activity[14, 15]. In vitro studies described that rosemary compounds suppressed IL-β and TNF-α, and increased glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) activity in different models[16–18]. In vivo, rosemary extract supplementation improved the oxidative stress status in the heart of aged rats[19, 20]. Furthermore, rosmarinic acid reduced myocardial damage blood pressure in hypertensive rats fed a high fructose diet[15] and protected the heart against cardiac dysfunction and fibrosis after MI in rats[21]. However, little is known about the effect of rosemary leaves intake in morphology and ventricular function after myocardial injury. To our knowledge, the protective potential of rosemary has predominantly been studied in rosemary extract and/or its constituents, and information about rosemary intake as a whole food is limited. Evidence shows that focusing on an approach based on foods and dietary patterns instead of individual nutrients improves cardiometabolic health[22, 23]. Thus, the aim of the present study was to evaluate the effect of rosemary leaves dietary supplementation on cardiac remodeling after myocardial infarction.
Methods
Study design
All experiments and procedures were performed in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals and with the Ethical Principles in Animal Experimentation adopted by the Brazilian College of Animal Experimentation[24]. The study protocol (838/10) was submitted and approved by the Botucatu Medical School Animal Research Ethics Committee.
Male Wistar rats weighing 200 to 250 g were used in this study. MI was conducted by coronary artery ligation, as previously described[2, 25]. In brief, rats were anesthetized with ketamine (70 mg/kg) and xylazine (1 mg/kg), and after left thoracotomy, the heart was exteriorized by lateral compression of the thorax. The left atrium was retracted to facilitate ligation of the left coronary artery with wired polyvinyl (5–0 Ethicon). The left coronary artery was ligated approximately 2 mm between the border of the left atrium and the pulmonary outflow tract. The heart was then replaced in the thorax, the lungs were inflated by positive pressure, and thoracotomy closed. A sham group, in which animals were submitted to surgery but without coronary occlusion, was also created. After surgery rats were housed in a temperature-controlled room (24°C) with a 12-h light/12-h dark cycle. Water and food was supplied ad libitum after the procedure.
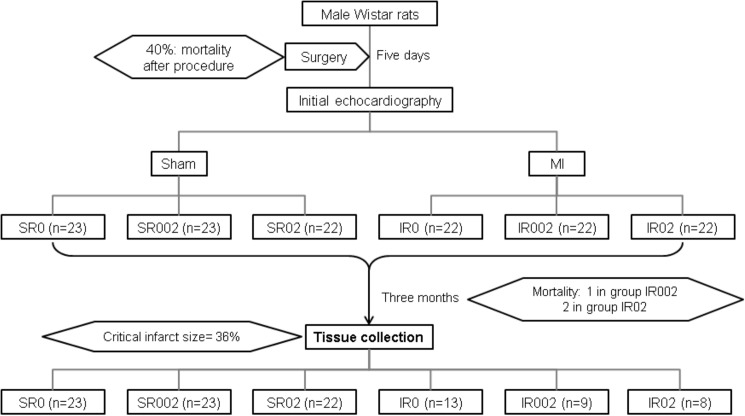
Two days after surgery, survivors were assigned to one of the six groups: 1) group SR0, sham animals fed standard chow only (n = 23); 2) group SR002, sham animals fed standard chow with 0.02% of rosemary leaves (n = 23); 3) group SR02, sham animals fed standard chow with 0.2% of rosemary leaves (n = 22); 4) IR0, infarcted animals fed standard chow only (n = 13); 5) IR002, infarcted animals fed standard chow with 0.02% of rosemary leaves (n = 8); and 6) IR02, infarcted animals fed standard chow with 0.2% of rosemary leaves (n = 9) (Fig 1). Treatment began 48 h after surgery because during this period mortality may be related to bleeding, pneumothorax, and anesthesia rather than to the infarction or treatment. Rosemary supplementation was provided for 90 days.
Fig 1. Study design.
Rosemary supplementation
Nuvilab chow (Nuvital®) was used for all experiments. Chow was initially chopped for the later addition of rosemary leaves. Fresh rosemary leaves were purchased in 2011 from Companhia de Entrepostos e Armazéns Gerais de São Paulo (CEAGESP), state of São Paulo, Brazil and were identified by a trained dietitian. The leaves were oven dried for 48 hours at a temperature of 50°C, ground in a domestic mixer (Walita, São Paulo, Brazil) for 30 seconds, and sieved using sieves of the Standard Tyler 32 (Bertel Industries, São Paulo, Brazil). Ground particles were stored under vacuum and maintained in a domestic freezer (Brastemp, São Paulo, Brazil) below –10°C. Rosemary powder was added to the crushed chow, and the mixture was pelletized. Food intake of all animals was measured every 24 hours. The mean daily intake for each rat was calculated. Rosemary supplementation doses of 0.02% and 0.2% were chosen based on the study of Posadas et al. (2009)[19].
Measurement of systolic arterial pressure
Systolic arterial pressure measurement was performed 2 weeks (corresponding to 2.5 months of rosemary supplementation) before euthanasia by tail plethysmograph, as described previously[26].
Echocardiographic study
After three months of supplementation, rats were weighed and evaluated by a transthoracic echocardiographic exam, as previously described[27, 28]. All measurements were made by the same observer blinded to individual animal treatments and according to the American Society of Echocardiography/European Association of Echocardiography[29].
After the echocardiographic study, the animals were euthanized with large dose of pentobarbital, and their hearts were removed. Left ventricle (LV) was isolated and LV samples were immediately frozen and stored at -80°C. One transverse section of the LV was separated and fixed in 10% buffered formalin and then was embedded in paraffin for histological study.
Morphometric analysis
Five-micrometer-thick sections were stained with hematoxylin and eosin (HE) for cardiomyocyte cross-sectional area (CSA) determination and with Pircrosirius red for Interstitial collagen fraction (ICF) and infarction size calculations. All animals were included in the morphometric analysis. First infarction size was calculated. To calculate infarction size, lengths of the infarcted and the viable muscle for both endocardial and epicardial circumferences were determined by planimetry, and then calculated by dividing endocardial and epicardial circumferences of the infarcted area by total epicardial and endocardial ventricular circumferences[28]. Measurements were performed on midventricular slices (5–6 mm from the apex), under the assumption that the left midventricular slice showed a close linear relation with the sum of the area measurements from all heart slices[30]. After infarction size calculation, infarcted animals with less than 36% of LV infarcted area were excluded of further analysis[31, 32]. Minicucci et al. (2011) showed that the infarct size cut-off value to induce cardiac remodeling in rats should be 36% of LV area[31].
The CSA measurements were obtained from at least 40 digital images(400 × magnification) with a digital pad, and the selected cells were transversely cut so that the nucleus was in the center of the myocyte[33, 34]. The CSA was considered to evaluate heart hypertrophy. ICF was determined in remote cardiac areas free from MI from at least 20 digital images (400 × magnification).
All images were collected with a video camera attached to a Leica microscope; the images were analyzed with the Image-Pro Plus 3.0 software program (Media Cybernetics; Silver Spring, MD).
Cytokine production
Tumor necrosis factor-α (TNF-α), IFN-γ and IL-10 concentrations in LV samples were determined by ELISA according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
MMP-2 and TIMP-1 evaluation
Matrix metalloproteinase (MMP)-2 activity was determined in LV samples by zimography, as previously reported[34, 35]. TIMP-1 levels were evaluated by ELISA according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN
Lipid hydroperoxide, antioxidant and energy metabolism enzymes
Eight LV samples of each experimental group were used for measurements of total protein and lipid hydroperoxide (LH) concentration and for enzyme activity determinations. Glutathione peroxidase (GSH-Px, E.C.1.11.1.9), superoxide dismutase (SOD, E.C.1.15.1.1) and catalase (CAT, E.C.1.11.1.6) activity was assessed as previously specified[36, 37]. Cardiac energy metabolism was assessed by β-hydroxyacyl coenzyme-A dehydrogenase (OHADH, E.C.1.1.1.35.), lactate dehydrogenase (LDH, E.C.1.1.1.27), citrate synthase (CS; E.C.4.1.3.7.), Complex I (NADH:ubiquinone oxidoreductase), Complex II (succinate dehydrogenase), and ATP synthase (EC 3.6.3.14) activities, as previously described[37, 38]. Spectrophotometric determinations were performed with a Pharmacia Biotech spectrophotometer UV/visible Ultrospec 5000 with Swift II Application software (Cambridge, England, UK) at 560 nm. All reagents were purchased from Sigma (St. Louis, Missouri, USA).
Western blot analysis
Briefly, left ventricular samples were extracted using RIPA Buffer to detect heme-oxygenase-1 (HO-1), caspase-3, Bcl2 and peroxisome proliferator-activated receptor-α coactivator (PGC)-1α expression. To determine nuclear erithroid factor 2 (Nrf-2), LV samples were extracted with Nuclear Extraction Buffer[39]. The following primary antibodies were used: HO-1-1 to heme oxygenase-1; ab13248 (Abcam Inc, Cambridge); Nrf2: C-20, rabbit Immunoglobulin G (Santa Cruz Biotechnology Inc, Europe); Cleaved Caspase-3 (Asp175) (5A002E) Rabbit mAb (Cell Signalling Technology Inc., USA); Bcl2 sc 492 IgG rabbit monoclonal (Santa Cruz Biotechnology, Inc, Europe); and PGC-1α Antibody H-300: sc-13067 (Santa Cruz Biotechnology, Inc, Europe). GAPDH (GAPDH (6C5), mouse monoclonal IgG1, (Santa Cruz Biotechnology, Inc., Europe, sc 32233) was used for normalization.
Statistical analysis
Data are presented as the mean±SEM. The results were tested for both normality (Kolmogorov–Smirnov test) and equal variance before statistical analyses, and all data passed these tests. Data were analyzed by 2-factor ANOVA, therefore this analysis gives three p values: 1) factor one: presence of myocardial infarction (I); 2) factor two: rosemary content (R); and 3) interaction between factors I and R. When an interaction was found to be significant, the mean values were compared using Holm-Sidak post hoc analysis. If an interaction was not found, the separated factors were analysed (marginal data). A χ2 test was used to evaluate mortality between infarcted animals. One-factor ANOVA was used to analyze infarction size in infarcted groups. Differences were to be considered statistically significant if P<0.05. Graphs and statistical analyses were performed using SigmaPlot for Windows version 12.0 (Systat Software Inc. San Jose, CA).
Results
Survival, food intake, body weight and systolic arterial pressure
The mortality rate within 48 hours after infarction was 43%. No death in the Sham animals was observed. In the infarcted groups, two animals in both the IR0 and IR02 groups died, and one animal in the IR002 group died (p = 0.765). No difference was observed in infarction size between infarcted groups (Table 1).
Table 1. Food intake, body weight, infarction size, echocardiographic and morphometric studies in Sham and infarcted rats with and without rosemary supplementation.
| SHAM groups | Myocardial infarction groups | p values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SR0 | SR002 | SR02 | IR0 | IR002 | IR02 | p (I) | p (R) | p (IxR) | |
| Food intake (g) | 25.8±0.2 | 24.6±0.2 | 25.7±0.2 | 25.4±0.2 | 25.0±0.2 | 25.5±0.2 | 0.187 | 0.101 | 0.143 |
| Weight gain (g) | 143±8.2 | 157±8.0 | 148±8.6 | 152±8.8 | 156±8.8 | 159±10 | 0.385 | 0.552 | 0.773 |
| Infarction size (%) | - | - | - | 41.9±4.5 | 40.1±4.2 | 43.6±4,7 | - | 0.244 | - |
| LVDD/BW (mm/kg) | 17.9±0.41 | 17.1±0.39 | 17.9±0.29 | 24.6±0.74 | 24.2±0.68 | 24.1±0.93 | <0.001 | 0.504 | 0.582 |
| E wave (cm/s) | 75.7±1.5 | 77.2±2.1 | 78.9±1.8 | 98.4±5.5 | 87.5±8.5 | 92.7±9.4 | 0.014 | 0.482 | 0.246 |
| Diastolic area (mm2) | 44.4±1.6 | 44.5±1.8 | 43.3±1.6 | 92.2±3.9 | 91.7±6.9 | 85.2±4.5 | <0.001 | 0.359 | 0.557 |
| FAC (%) | 73.3±0.9 | 74.3±1.2 | 75.6±1.0 | 27.0±2.3 | 28.2±1.5 | 27.2±3.5 | <0.001 | 0.697 | 0.757 |
| Ejection fraction | 0.91±0.01 | 0.92±0.01 | 0.93±0.01 | 0.47±0.02 | 0.49±0.02 | 0.46±0.02 | <0.001 | 0.571 | 0.612 |
| E/E’ ratio | 19.1±0.6 | 19.6±0.9 | 18.8±0.6 | 24.4±1.7 | 25.6±2.8 | 23.6±2.6 | <0.001 | 0.898 | 0.523 |
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation; Weight gain: Final body weight–initial body weight (g); BW: body weight; LVDD/BW: left ventricular diastolic diameter indexed for body weight; E wave: peak velocity of early ventricular filling; FAC: fractional area change; E/E’ ratio: early diastolic mitral inflow velocity to early mitral annular velocity ratio. Data are expressed as the mean ± SEM. Bold numbers represent the significant effects that were considered.
No difference was observed for food intake, weight gain (Table 1) and systolic arterial pressure (Fig 2D) among all groups.
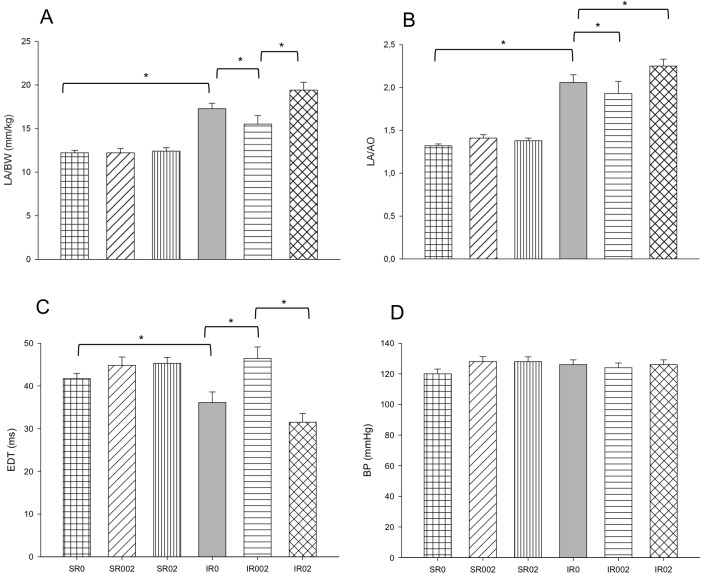
Fig 2. Echocardiographic study and blood systolic pressure in Sham and infarcted rats with and without rosemary supplementation.
A. LA/BW: left atrial diameter indexed for body weight (p = 0.001); B. LA/AO: left atrial diameter indexed for aortic diameter (p = 0.024); C. EDT: E wave deceleration time (p = 0.004); D. BP: blood systolic pressure (p = 0.343). Data are expressed as the mean ± SEM. Asterisks (*) represent significant difference between groups (p<0.05). Sample size: SR0 = 23; SR002 = 23; SR02 = 22; IR0 = 13; IR002 = 8; and IR02 = 9.
Effect of MI in rat hearts
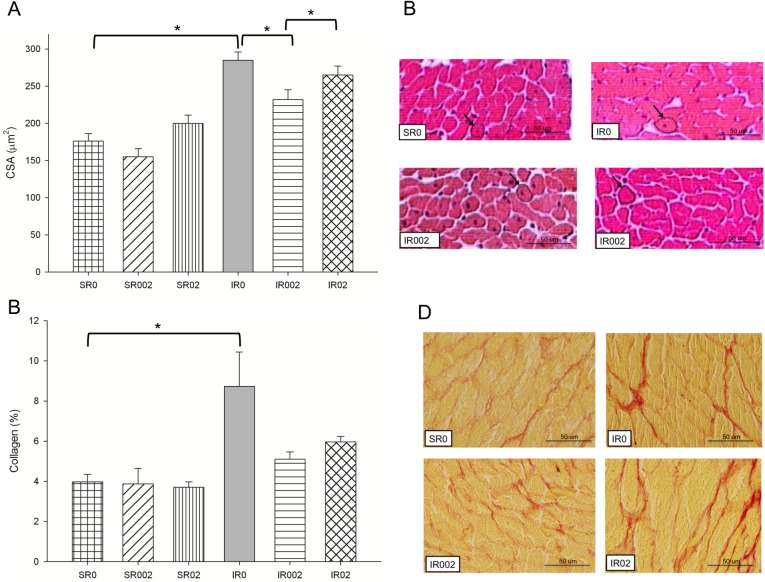
MI led to adverse cardiac remodeling. Regarding morphological data, MI led to higher left ventricular end-diastolic diameter adjusted for body weight, higher diastolic area (Table 1) and higher left atrium (Fig 2A and 2B), CSA (Fig 3A and 3B) and percentage of collagen (Fig 3C and 3D). MI impaired diastolic heart function, as shown by increased shorter E wave deceleration time (EDT) (Fig 2C) and increased E/E' ratio (Table 1), and systolic function showed by lower fractional area change (Table 1).
Fig 3. Morphometric study in Sham and infarcted rats with and without rosemary supplementation.
A. CSA: cardiomyocyte cross-sectional area (p<0.001); B. Microscopic images of CSA H&E stained (evidenced with arrows) of groups SR0, IR0, IR002 and IR02; C. % collagen: p = 0.035; D. Microscopic images of myocytes Picrosirius red stained for collagen (red marks in images) of SR0, IR0, IR002 and IR02. Data are expressed as the mean ± SEM. Asterisks (*) represent significant difference between groups (p<0.05). Sample size: SR0 = 23; SR002 = 23; SR02 = 22; IR0 = 13; IR002 = 8; and IR02 = 9.
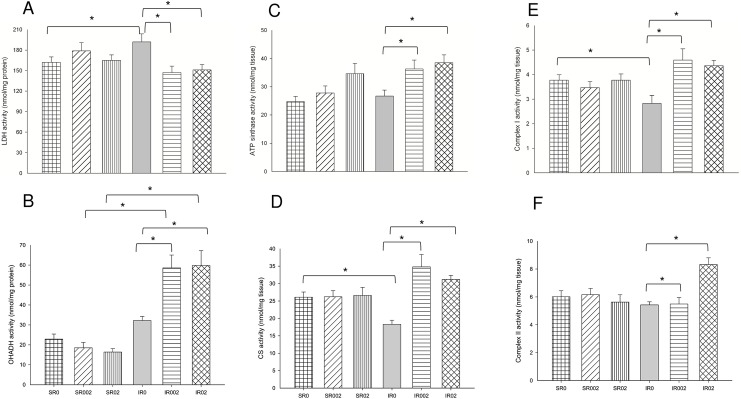
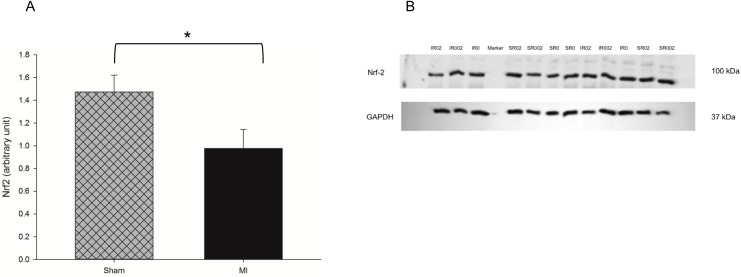
A greater oxidation of carbohydrates and impaired energy metabolism was observed as shown by higher activity of LDH (Fig 4A and S2 Table) and ATP synthase (Fig 4C and S2 Table), and lower activity of CS (Fig 4D and S2 Table) and Complex I (Fig 4E and S2 Table). MI also increased oxidative stress, as presented with higher LH concentration (Fig 5A and S2 Table) and SOD activity (Fig 5B and S2 Table), lower GSH-Px activity (Fig 5C and S3 Table), and lower expression of Nrf-2 (Fig 6A and 6B). No difference was observed for HO-1 (S3 Table and S1 Fig) and PGC-1α expression (S3 Table and S2 Fig).
Fig 4. Energy metabolism enzymes in Sham and infarcted rats with and without rosemary supplementation.
LDH activity: lactate dehydrogenase activity (p = 0.014); CS activity: citrate synthase activity (p<0.001); OHADH activity: 3-hydroxyacyl coenzyme-A dehydrogenase activity (p<0.001); ATP sinthase activity (p = 0.039); Complex I activity: p = 0.004; Complex II activity: p<0.001. Data are expressed as the mean ± SEM. Asterisks (*) represent significant difference between groups (P<0.05). Sample size: SR0 = 8; SR002 = 8; SR02 = 8; IR0 = 10; IR002 = 7; and IR02 = 8.
Fig 5. Oxidative stress enzymes in Sham and infarcted rats with and without rosemary supplementation.
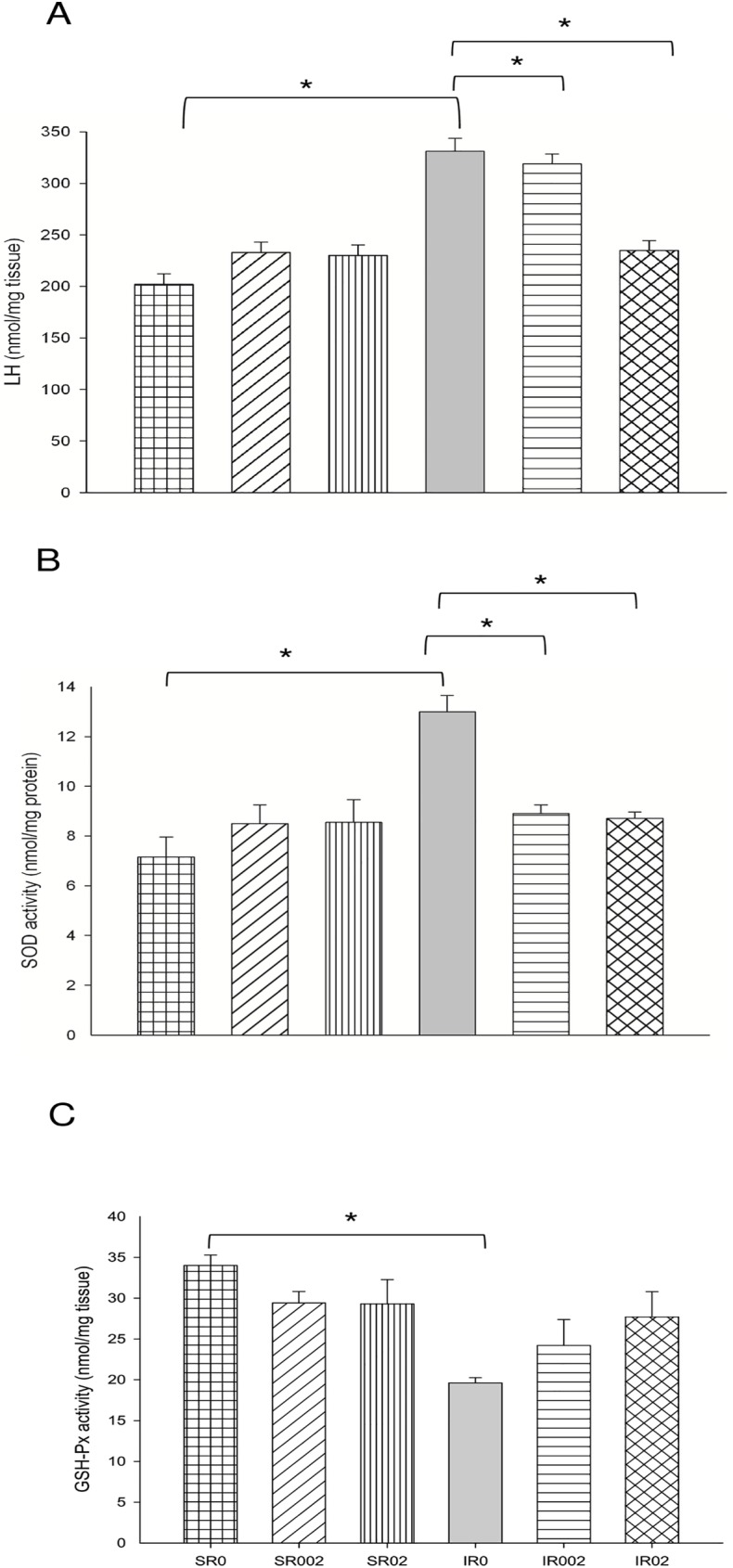
LH: lipid hydroperoxide concentration (p<0.001); SOD activity: superoxide dismutase activity (p<0.001); GSH-Px activity: glutathione peroxidase activity (p = 0.031). Data are expressed as the mean ± SEM. Asterisks (*) represent significant difference between groups (P<0.05). Sample size: SR0 = 8; SR002 = 8; SR02 = 8; IR0 = 10; IR002 = 7; and IR02 = 8.
Fig 6. Nrf2 expression in Sham and infarcted rats by Western blot.
A. Nrf2 expression; B. representative western blot of Nrf2 expression. Nrf2: nuclear erithroid factor 2; GAPDH glyceraldehyde-3-phosphate dehydrogenase. Since no interaction between factors was observed, the effect of myocardial infarction is represented. Data are expressed as the mean ± SEM. Asterisks (*) represent significant difference between groups. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
Higher intermediate and total MMP-2 activity (S4 Table and S3 Fig), lower IL-10, TNF-α and INF-γ concentration (S4 Table), and lower Bcl2 expression (S4 Table and S4 Fig) was observed after MI. No difference was observed for caspase-3 expression (S4 Table).
Effect of rosemary supplementation after MI
Rosemary supplementation improved diastolic function (lower left atrial diameter [Fig 2A and 2B and S1 Table] and higher EDT [Fig 2C and S1 Table]) and reduced hypertrophy (Fig 3A and S1 Table) after MI.
Morphologic and functional changes were accompanied by increased β-oxidation of fatty acids, reduced lactate oxidation and improved respiratory chain activity, as shown by lower LDH activity (Fig 4A and S2 Table), and higher CS (Fig 4C and S2 Table), OHADH (Fig 4E and S2 Table), ATP synthase (Fig 4B and S2 Table) and Complex I (Fig 4D and S2 Table) activities. Rosemary supplementation also decreased oxidative stress, with lower concentration of LH (Fig 5A and S2 Table) and SOD activity (Fig 5B and S2 Table). No differences were observed for GSH-Px (Fig 5C and S2 Table) and catalase (S1 Table) activities, and Nrf-2, HO-1 and PGC-1α expression (S3 Table and S3 Fig).
Differences among the doses were also observed in diastolic function and hypertrophy. Group IR002 presented lower left atrium (Fig 2A and 2B and S1 Table) and CSA (Fig 3A and S1 Table). Group IR02 presented higher Complex II activity (Fig 4F and S2 Table).
No difference was observed for percentage of collagen with rosemary supplementation (Fig 3B and S1 Table), cytokines concentration, MMP-2 activity (S4 Table and S3 Fig), caspase-3 and Bcl2 expression (S4 Table and S4 Fig).
Discussion
The aim of the present study was to analyze the influence of rosemary supplementation of rat chow on adverse cardiac remodeling after myocardial infarction. Cardiac remodeling has significant clinical relevance as it can lead to complex changes in ventricular architecture, potentially evolving into chronic heart failure [7, 40]. For this reason, several strategies have been used to mitigate this process, including compounds found in rosemary leaves[17, 41]. It is well known that lifestyle factors, including nutrition, play a key role in the etiology of Cardiovascular Diseases (CVD), evidencing the importance to promote health diet including selected foods rather than individual nutrients[23, 42]. In this context, the use of rosemary leaves instead of one isolated compound might lead to a synergistic effect, since adjuvant substances in the plant might enhance the activity of benefic components[43]. To our knowledge, this is the first study that assessed the supplementation with whole rosemary leaves on the morphology and function of the infarcted heart.
Myocardial infarction in the rat is an ideal model to study adverse cardiac remodeling post-infarction[44]. Cardiac remodeling can be defined as molecular, cellular, and interstitial modifications that are clinically manifested as changes in size, mass, geometry and heart function after cardiac injury. Heart dysfunction is the final sign of cardiac remodeling and is an important prognostic factor after MI, increasing the risk of death[45, 46]. Diastolic dysfunction after MI refers to mechanical and functional abnormalities during relaxation and filling of the LV and is associated with hypertrophy and increased LA[47]. In the present study, rosemary supplementation after MI improved diastolic function, LV hypertrophy and LA diameters, evidencing the protective effect of rosemary leaves in the heart. Our results are in accordance with previous studies describing improved cardiac function in cardiac injury models by doxorubicin and ischemia/reperfusion after treatment with compounds found in rosemary[48, 49].
The improvement of hypertrophy and diastolic function observed after rosemary intake was associated with changes in energy metabolism and decreased oxidative stress after MI in the present study. As clinical manifestations can be the result of changes to the heart’s cellular and molecular components[3], energy metabolism and antioxidant pathways could represent the mechanisms of action of rosemary leaves supplementation. In injury situations such as MI, the preferential use of fatty acids that are observed in the normal hearts may be shifted for glucose use[50, 51]. In this case, the heart starts to form large amounts of lactate, increasing anaerobic metabolism of carbohydrates and LDH activity[52, 53]. Rosemary supplementation after MI led to higher fatty acid oxidation and respiratory chain improvement, similar to the energy metabolism of normal hearts.
In addition to metabolic changes, oxidative stress and redox signaling are important contributors to cardiac remodeling[54]. Increased oxidative stress and cardiac oxidation has also been associated with diastolic dysfunction[47]. The toxic effects of ROS can be prevented in part by the antioxidant enzyme system including GSH-Px, SOD and catalase[55]. SOD is considered the first line of defense in protecting the mitochondria against deleterious effects of increased superoxide production, as described in cardiac remodeling and HF[56] and observed in the present study. Our results showed that rosemary supplementation decreased oxidative stress, which is in agreement with previous studies reporting antioxidant effect of rosemary and its compounds[11, 19, 57]. Rosemary can mimic SOD by removing superoxide radicals[58], which could also explain the lower enzyme activity in cardiac tissue of the supplemented groups. No effect of rosemary was observed on Nrf-2 and HO-1 expression, different from what we expected and from previous reports showing Nrf2 activation after treatment with rosemary compounds[59, 60]. One possible explanation is that in some pathological situations, as in hypoxia, Nrf-2 does not increase. Regulatory mechanisms such as the E3 ubiquitin (Siah2) protein ligase 2, which is activated in hypoxia, binds to Nrf-2 and increases its degradation, preventing it from acting on AREs and increasing the expression of antioxidant proteins[61].
In the present study, rosemary doses led to different effect in heart diastolic function and hypertrophy, similar to a J-shape response. The smallest dose of rosemary supplementation caused greater improvement in post-MI heart function. [62]. Forman and collaborators (2014) describes that antioxidants in fruits and vegetables maintain a cellular defense and adaptation response. The authors exemplifies that supplementation of phytochemicals to levels that exceed saturation of the antioxidant system will hardly exert any beneficial effect, by a mechanism that can be called “para-hormesis”[62]. In the present study, 0.2% of rosemary supplementation led to a greater antioxidant response (lower LH and higher antioxidant enzymes activities) which could impair the cellular healthy response signaling by ROS[63]. Also our finding of higher Complex II after 0.2% rosemary supplementation could indicate the para-hormesis mechanism. In the mithocondria, Complex II is part of the antioxidant system by controlling the ubiquinone pool and superoxide scavenging activity of the respiratory chain (RC). Complex II might also be activated upon reduction of the RC[64]. So in the present study, the supplementation with the highest dose of rosemary might have lost the protective effect exhibited with 0.02% of rosemary.
The limitation of the present study lies in its experimental type, not allowing us to extrapolate the findings to humans. However, it is important to highlight the clinical relevance of the results described. Our findings support further investigations of rosemary use as adjuvant therapy in adverse cardiac remodeling. Regarding rosemary dose in the present study, 0.02% and 0.2% in the rat chow is equivalent to 11 mg/day and 110 mg/day in humans, respectively[65].
In conclusion, dietary rosemary supplementation attenuated adverse cardiac remodeling caused by myocardial infarction in rats. The mechanism could involve improved energy metabolism and reduced oxidative stress. Rosemary supplementation may serve as a promising approach to attenuate adverse cardiac remodeling after MI.
Supporting information
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
(TIF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
(TIF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 5; IR002 = 5; and IR02 = 4.
(TIF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
(TIF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation; BW: body weight; LVDD/BW: left ventricular diastolic diameter indexed for body weight; AO: aortic diameter; LA: left atrial diameter; LA/BW: left atrial diameter indexed for body weight; LA/AO: left atrial diameter indexed for aortic diameter; E wave: peak velocity of early ventricular filling; FAC: fractional area change; EDT: E wave deceleration time; E/E’ ratio: early diastolic mitral inflow velocity to early mitral annular velocity ratio; CSA: cardiomyocyte cross-sectional area. Data are expressed as the mean ± SEM. Bold numbers represent the significant effects that were considered. *IxR: when interactions are observed, same superscript letters represent differences (p<0.05) in a row (a = IR0≠SR0; b = IR002≠SR002; c = IR02≠SR02; A = IR0≠IR002; B = IR002≠IR02; C = IR0≠IR02). Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
(PDF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation; LDH activity: lactate dehydrogenase activity (nmol/mg protein); PIDH activity: pyruvate dehydrogenase activity; CS activity: citrate synthase activity; OHADH activity: 3-hydroxyacyl coenzyme-A dehydrogenase activity; ATP sinthase activity; Complex I activity; Complex II activity; LH: lipid hydroperoxide concentration; SOD activity: superoxide dismutase activity; GSH-Px activity: glutathione peroxidase activity. Data are expressed as the mean ± SEM. Bold numbers represent the significant effects that were considered. *IxR: when interactions are observed, same superscript letters represent differences (p<0.05) in a row (a = SR0≠IR0; b = SR002≠IR002; c = IR02≠SR02; A = IR0≠IR002; B = IR002≠IR02; C = IR0≠IR02). Sample size: SR0 = 8; SR002 = 8; SR02 = 8; IR0 = 10; IR002 = 7; and IR02 = 8.
(PDF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation; Nrf-2: nuclear erithroid factor 2; HO-1: heme-oxygenase-1; PGC1α: peroxisome proliferator-activated receptor-α coactivator. Data are expressed as the mean ± SEM. Bold numbers represent the significant effects that were considered. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
(PDF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation; PFK activity: Phosphofructokinase activity; TIMP-1: Metaloprotease inhibitor-1; MMP-2: metaloprotease-2; IL-10: interleukin-10; ICAM-1: intercellular adhesion mollecule-1; TNF-α: tumor necrosis factor-α; INF-γ: interferon-γ. Data are expressed as mean ± SEM. Bold numbers represents significant effects considered. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
(PDF)
Acknowledgments
We thank Mario B. Bruno, José Georgette, Antonio Carlos de Lalla, Elenize Jamas, and Corina Corrêa for animal management and technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by National Council of Technological and Scientific Development (CNPq 140262/2011-3) and São Paulo Research Foundation (FAPESP 2011/08956-0 and 2011/15059-5), Brazil. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 2.Zornoff LA, Paiva SA, Minicucci MF, Spadaro J. Experimental myocardium infarction in rats: analysis of the model. Arq Bras Cardiol. 2009;93:434–40. [DOI] [PubMed] [Google Scholar]
- 3.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–82. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res. 2009;81:482–90. doi: 10.1093/cvr/cvn333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou S, Sun W, Zhang Z, Zheng Y. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid Med Cell Logev. 2014;2014:260429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–64. doi: 10.1093/cvr/cvn335 [DOI] [PubMed] [Google Scholar]
- 7.Zornoff LA, Paiva SA, Minicucci MF, Spadaro J. Ventricular remodeling after myocardial infarction: concepts and clinical implications. Arq Bras Cardiol. 2009;92:157–64. [DOI] [PubMed] [Google Scholar]
- 8.Punithavathi VR, Prince PS. Pretreatment with a combination of quercetin and α-tocopherol ameliorates adenosine triphosphatases and lysosomal enzymes in myocardial infarcted rats. Life Sci. 2010;86:178–84. doi: 10.1016/j.lfs.2009.11.021 [DOI] [PubMed] [Google Scholar]
- 9.Buttros JB, Bergamaschi CT, Ribeiro DA, Fracalossi ACC, Campos RR. Cardioprotective actions of ascorbic acid during isoproterenol-induced acute myocardial infarction in rats. Pharmacology. 2009;84:29–37. doi: 10.1159/000222245 [DOI] [PubMed] [Google Scholar]
- 10.Zhu YZ, Huang SH, Tan BK, Sun J, Whitemanb M, Zhu YC. Antioxidants in Chinese herbal medicines: a biochemical perspective. Nat Prod Rep. 2004;21:478–89. doi: 10.1039/b304821g [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Fons L, Garzón MT, Micol V. Relationship between the antioxidant capacity and effect of rosemary (Rosmarinus officinalis L.) polyphenols on membrane phospholipid order. J Agric Food Chem. 2010;58:161–71. doi: 10.1021/jf9026487 [DOI] [PubMed] [Google Scholar]
- 12.Genena AK, Hense H, Junior AS, Souza SM. Rosemary (Rosmarinus officinalis)–a study of the composition, antioxidant and antimicrobial activities of extracts obtained with supercritical carbon dioxide. Food Sci Tech. 2008;28:463–9. [Google Scholar]
- 13.Bai N, He K, Roller M, Lai CS, Shao X, Pan MH, et al. Flavonoids and phenolic compounds from Rosmarinus officinalis. Journal of agricultural and food chemistry. 2010;58(9):5363–7. doi: 10.1021/jf100332w [DOI] [PubMed] [Google Scholar]
- 14.Scheckel K, Degner S, Romagnolo D. Rosmarinic acid antagonizes activator protein-1-dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J Nutr. 2008;138:2098–105. doi: 10.3945/jn.108.090431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karthik D, Viswanathan P, Anuradha CV. Administration of rosmarinic acid reduces cardiopathology and blood pressure through inhibition of p22phox NADPH oxidase in fructose-fed hypertensive rats. J Cardiovasc Pharmacol. 2011;58(5):514–21. doi: 10.1097/FJC.0b013e31822c265d [DOI] [PubMed] [Google Scholar]
- 16.Haraguchi H, Sait T, Okamura N, Yagi A. Inhibition of lipid peroxidation and superoxide generation by diterpenoids from Rosmarinus officinalis. Planta Med. 1995;61:333–6. doi: 10.1055/s-2006-958094 [DOI] [PubMed] [Google Scholar]
- 17.Peng C, Su J, Chyau C, Sung T, Ho S, Peng C, et al. Supercritical fluid extracts of rosemary leaves exhibit potent anti-inflammation and anti-tumor effects. Biosci Biotechnol Biochem. 2007;71:2223–32. doi: 10.1271/bbb.70199 [DOI] [PubMed] [Google Scholar]
- 18.Lian K-C, Chuang J-J, Hsieh C-W, Wung B-S, Huang G-D, Jian T-Y, et al. Dual mechanisms of NF-κB inhibition in carnosol-treated endothelial cells. Toxicol Appl Pharm. 2010;245:21–35. [DOI] [PubMed] [Google Scholar]
- 19.Posadas SJ, Caz V, Largo C, Gándara BDl, Matallanas B, Reglero G, et al. Protective effect of supercritical fluid rosemary extract, Rosmarinus officinalis, on antioxidants of major organs of aged rats. Exp Gerontol. 2009;44:383–9. doi: 10.1016/j.exger.2009.02.015 [DOI] [PubMed] [Google Scholar]
- 20.Botsoglou N, Taitzoglou I, Zervos I, Botsoglou E, Tsantarliotou M, Chatzopoulou PS. Potential of long-term dietary administration of rosemary in improving the antioxidant status of rat tissues following carbon tetrachloride intoxication. Food Chem Toxicol. 2010;48(3):944–50. doi: 10.1016/j.fct.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Tian J, Xu Y, Li C, Meng X, Fu F. Protective Effect of RA on Myocardial Infarction-Induced Cardiac Fibrosis via AT1R/p38 MAPK Pathway Signaling and Modulation of the ACE2/ACE Ratio. Journal of agricultural and food chemistry. 2016;64(35):6716–22. doi: 10.1021/acs.jafc.6b03001 [DOI] [PubMed] [Google Scholar]
- 22.Retelny VS, Neuendorf A, Roth JL. Nutrition protocols for the prevention of cardiovascular disease. Nutr Clin Pract. 2008;23(5):468–76. doi: 10.1177/0884533608323425 [DOI] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation. 2011;123(24):2870–91. doi: 10.1161/CIRCULATIONAHA.110.968735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guide for the Care and Use of Laboratory Animals. Animals NRCUCftUotGftCaUoL, editor. Washington, DC: National Academies Press; 2011.
- 25.Pfeffer JM, Finn PV, Zornoff LA, Pfeffer MA. Endothelin-A receptor antagonism during acute myocardial infarction in rats. Cardiovasc Drugs Ther. 2000;14:579–87. [DOI] [PubMed] [Google Scholar]
- 26.Santos PP, Rafacho BP, Goncalves Ade F, Jaldin RG, Nascimento TB, Silva MA, et al. Vitamin D induces increased systolic arterial pressure via vascular reactivity and mechanical properties. PLoS One. 2014;9:e98895 doi: 10.1371/journal.pone.0098895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zornoff LA, Matsubara BB, Matsubara LS, Azevedo PS, Minicucci MF, Campana AO, et al. b-carotene supplementation results in adverse ventricular remodeling after acute myocardial infarction. Nutrition. 2006;22:146–51. doi: 10.1016/j.nut.2005.05.008 [DOI] [PubMed] [Google Scholar]
- 28.Paiva SA, Novo R, Matsubara BB, Matsubara LS, Azevedo PS, Minicucci MF, et al. beta-carotene attenuates the paradoxical effect of tobacco smoke on the mortality of rats after experimental myocardial infarction. J Nutr. 2005;135(9):2109–13. [DOI] [PubMed] [Google Scholar]
- 29.Lang RM, Bierig M, Devereaux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 30.Spadaro J, Fishbein M, Hare C, Pfeffer M, Maroko P. Characterization of myocardial infarcts in the rat. Arch Pathol Lab Med. 1980;104:179–83. [PubMed] [Google Scholar]
- 31.Minicucci MF, Azevedo PS, Martinez PF, Lima AR, Bonomo C, Guizoni DM, et al. Critical infarct size to induce ventricular remodeling, cardiac dysfunction and heart failure in rats. Int J Cardiol. 2011;151:242–3. doi: 10.1016/j.ijcard.2011.06.068 [DOI] [PubMed] [Google Scholar]
- 32.Csonka C, Kupai K, Kocsis GF, Novak G, Fekete V, Bencsik P, et al. Measurement of myocardial infarct size in preclinical studies. J Pharmacol Toxicol Methods. 2010;61:163–70. doi: 10.1016/j.vascn.2010.02.014 [DOI] [PubMed] [Google Scholar]
- 33.Paiva SAR, Zornoff LAM, Okoshi MP, Okoshi K, Matsubara LS, Matsubara BB, et al. Ventricular remodeling induced by retinoic acid supplementation in adult rats. Am J Physiol Heart Circ Physiol. 2003;284(6):2242–6. [DOI] [PubMed] [Google Scholar]
- 34.Goncalves AF, Congio LH, dos Santos PP, Rafacho BP, Pereira BL, Claro RF, et al. Pamidronate attenuates diastolic dysfunction induced by myocardial infarction associated with changes in geometric patterning. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2015;35(1):259–69. [DOI] [PubMed] [Google Scholar]
- 35.Weber KT, Sun Y, Tyagi SC, Cleutjens JP. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994;26:279–92. doi: 10.1006/jmcc.1994.1036 [DOI] [PubMed] [Google Scholar]
- 36.Ewing JF, Janero DR. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal Biochem. 1995;232(2):243–8. doi: 10.1006/abio.1995.0014 [DOI] [PubMed] [Google Scholar]
- 37.Burneiko RC, Diniz YS, Galhardi CM, Rodrigues HG, Ebaid GM, Faine LA, et al. Interaction of hypercaloric diet and physical exercise on lipid profile, oxidative stress and antioxidant defenses. Food Chem Toxicol. 2006;44(7):1167–72. doi: 10.1016/j.fct.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 38.Assalin HB, Rafacho BP, dos Santos PP, Ardisson LP, Roscani MG, Chiuso-Minicucci F, et al. Impact of the Length of Vitamin D Deficiency on Cardiac Remodeling. Circulation-Heart Failure. 2013;6(4):809–16. doi: 10.1161/CIRCHEARTFAILURE.112.000298 [DOI] [PubMed] [Google Scholar]
- 39.Lustosa BB, Polegato B, Minicucci M, Rafacho B, Santos PP, Fernandes AA, et al. Green tea (Cammellia sinensis) attenuates ventricular remodeling after experimental myocardial infarction. International journal of cardiology. 2016;225:147–53. doi: 10.1016/j.ijcard.2016.09.092 [DOI] [PubMed] [Google Scholar]
- 40.Idikio HA. Postmyocardial infarct remodeling and heart failure: potential contributions from pro- and antiaging factors. Cardiol Res Pract. 2011;2011:836806 doi: 10.4061/2011/836806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheung S, Tai J. Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol Rep. 2007;17:1525–31. [PubMed] [Google Scholar]
- 42.Eilat-Adar S, Sinai T, Yosefy C, Henkin Y. Nutritional recommendations for cardiovascular disease prevention. Nutrients. 2013;5(9):3646–83. doi: 10.3390/nu5093646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilbert B, Alves LF. Synergy in plant medicines. Current medicinal chemistry. 2003;10(1):13–20. [DOI] [PubMed] [Google Scholar]
- 44.Pfeffer M, Pfeffer J, Fishbein M, Fletcher P, Spadaro J, Cloner R, et al. Myocardial infarct size and ventricular function in rats. Circ Res. 1979;44:503–12. [DOI] [PubMed] [Google Scholar]
- 45.Minicucci MF, Farah E, Fusco DR, Cogni AL, Azevedo PS, Okoshi K, et al. Infarct Size as Predictor of Systolic Functional Recovery after Myocardial Infarction. Arquivos brasileiros de cardiologia. 2014;102(6):549–56. doi: 10.5935/abc.20140051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polegato BF, Minicucci MF, Azevedo PS, Gonçalves AF, Lima AF, Martinez PF, et al. Association between Functional Variables and Heart Failure after Myocardial Infarction in Rats. Arquivos brasileiros de cardiologia. 2016;106(2):105–12. doi: 10.5935/abc.20160015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeong EM, Dudley SC Jr. Diastolic dysfunction. Circ J. 2015;79(3):470–7. doi: 10.1253/circj.CJ-15-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadzuka Y, Sugiyama T, Shimoi K, Kinae N, Hirota S. Protective effect of flavonoids on doxorubicin-induced cardiotoxicity. Toxicol Lett. 1997;92:1–7. [DOI] [PubMed] [Google Scholar]
- 49.Sun D, Huang J, Zhang Z, Gao H, Li J, Shen M, et al. Luteolin limits infarct size and improves cardiac function after myocardium ischemia/reperfusion injury in diabetic rats. PLoS One. 2012;7:e33491 doi: 10.1371/journal.pone.0033491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saks V, Favier R, Guzun R, Schlattner U, Wallimann T. Molecular system bioenergetics: regulation of substrate supply in response to heart energy demands. J Physiol. 2006;577:769–77. doi: 10.1113/jphysiol.2006.120584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–129. doi: 10.1152/physrev.00006.2004 [DOI] [PubMed] [Google Scholar]
- 52.Nelly JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Ann Rev Physiol. 1974;36:413–59. [DOI] [PubMed] [Google Scholar]
- 53.Wolfe CL, Sievers RE, Visseren FL, Donnelly TJ. Loss of myocardial protection after preconditioning correlates with the time course of glycogen recovery within the preconditioned segment. Circulation. 1993;87:881–92. [DOI] [PubMed] [Google Scholar]
- 54.Madamanchi NR, Runge MS. Redox signaling in cardiovascular health and disease. Free Radic Biol Med. 2013;61C:473–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Deel ED, Lu Z, Xu X, Zhu G, Hu X, Oury TD, et al. Extracellular SOD protects the heart against oxidative stress and hypertrophy after myocardial infarction. Free Radic Biol Med. 2008;44:1305–13. doi: 10.1016/j.freeradbiomed.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu T, Chen L, Kim E, Tran D, Phinney BS, Knowlton AA. Mitochondrial proteome remodeling in ischemic heart failure. Life Sci. 2014;101:27–36. doi: 10.1016/j.lfs.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamaki Y, Tabuchi T, Takahashi T, Kosaka K, Satoh T. Activated glutathione metabolism participates in protective effects of carnosic acid against oxidative stress in neuronal HT22 cells. Planta Med. 2010;76:683–8. doi: 10.1055/s-0029-1240622 [DOI] [PubMed] [Google Scholar]
- 58.Chohan M, Naughton DP, Opara EI. Determination of superoxide dismutase mimetic activity in common culinary herbs. SpringerPlus. 2014;3:578 doi: 10.1186/2193-1801-3-578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun GB, Sun X, Wang M, Ye JX, Si JY, Xu HB, et al. Oxidative stress suppression by luteolin-induced heme oxygenase-1 expression. Toxicol Appl Pharmacol. 2012;265:229–40. doi: 10.1016/j.taap.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 60.Sahu BD, Putcha UK, Kuncha M, Rachamalla SS, Sistla R. Carnosic acid promotes myocardial antioxidant response and prevents isoproterenol-induced myocardial oxidative stress and apoptosis in mice. Mol Cell Biochem. 2014;394:163–76. doi: 10.1007/s11010-014-2092-5 [DOI] [PubMed] [Google Scholar]
- 61.Baba K, Morimoto H, Imaoka S. Seven in absentia homolog 2 (Siah2) protein is a regulator of NF-E2-related factor 2 (Nrf2). J Biol Chem. 2013;288(25):18393–405. doi: 10.1074/jbc.M112.438762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forman HJ, Davies KJ, Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ristow M, Schmeisser K. Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS). Dose-Response. 2014;12:288–341. doi: 10.2203/dose-response.13-035.Ristow [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rustin P, Munnich A, Rötig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur J Hum Genet. 2002;10:289–91. doi: 10.1038/sj.ejhg.5200793 [DOI] [PubMed] [Google Scholar]
- 65.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
(TIF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
(TIF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 5; IR002 = 5; and IR02 = 4.
(TIF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
(TIF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation; BW: body weight; LVDD/BW: left ventricular diastolic diameter indexed for body weight; AO: aortic diameter; LA: left atrial diameter; LA/BW: left atrial diameter indexed for body weight; LA/AO: left atrial diameter indexed for aortic diameter; E wave: peak velocity of early ventricular filling; FAC: fractional area change; EDT: E wave deceleration time; E/E’ ratio: early diastolic mitral inflow velocity to early mitral annular velocity ratio; CSA: cardiomyocyte cross-sectional area. Data are expressed as the mean ± SEM. Bold numbers represent the significant effects that were considered. *IxR: when interactions are observed, same superscript letters represent differences (p<0.05) in a row (a = IR0≠SR0; b = IR002≠SR002; c = IR02≠SR02; A = IR0≠IR002; B = IR002≠IR02; C = IR0≠IR02). Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
(PDF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation; LDH activity: lactate dehydrogenase activity (nmol/mg protein); PIDH activity: pyruvate dehydrogenase activity; CS activity: citrate synthase activity; OHADH activity: 3-hydroxyacyl coenzyme-A dehydrogenase activity; ATP sinthase activity; Complex I activity; Complex II activity; LH: lipid hydroperoxide concentration; SOD activity: superoxide dismutase activity; GSH-Px activity: glutathione peroxidase activity. Data are expressed as the mean ± SEM. Bold numbers represent the significant effects that were considered. *IxR: when interactions are observed, same superscript letters represent differences (p<0.05) in a row (a = SR0≠IR0; b = SR002≠IR002; c = IR02≠SR02; A = IR0≠IR002; B = IR002≠IR02; C = IR0≠IR02). Sample size: SR0 = 8; SR002 = 8; SR02 = 8; IR0 = 10; IR002 = 7; and IR02 = 8.
(PDF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation; Nrf-2: nuclear erithroid factor 2; HO-1: heme-oxygenase-1; PGC1α: peroxisome proliferator-activated receptor-α coactivator. Data are expressed as the mean ± SEM. Bold numbers represent the significant effects that were considered. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
(PDF)
I: infarction; S: Sham; R: Rosemary; R0: no supplementation; R002: 0.02% of rosemary supplementation; R02: 0.2% of rosemary supplementation; PFK activity: Phosphofructokinase activity; TIMP-1: Metaloprotease inhibitor-1; MMP-2: metaloprotease-2; IL-10: interleukin-10; ICAM-1: intercellular adhesion mollecule-1; TNF-α: tumor necrosis factor-α; INF-γ: interferon-γ. Data are expressed as mean ± SEM. Bold numbers represents significant effects considered. Sample size: SR0 = 10; SR002 = 10; SR02 = 10; IR0 = 10; IR002 = 8; and IR02 = 9.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.