Abstract
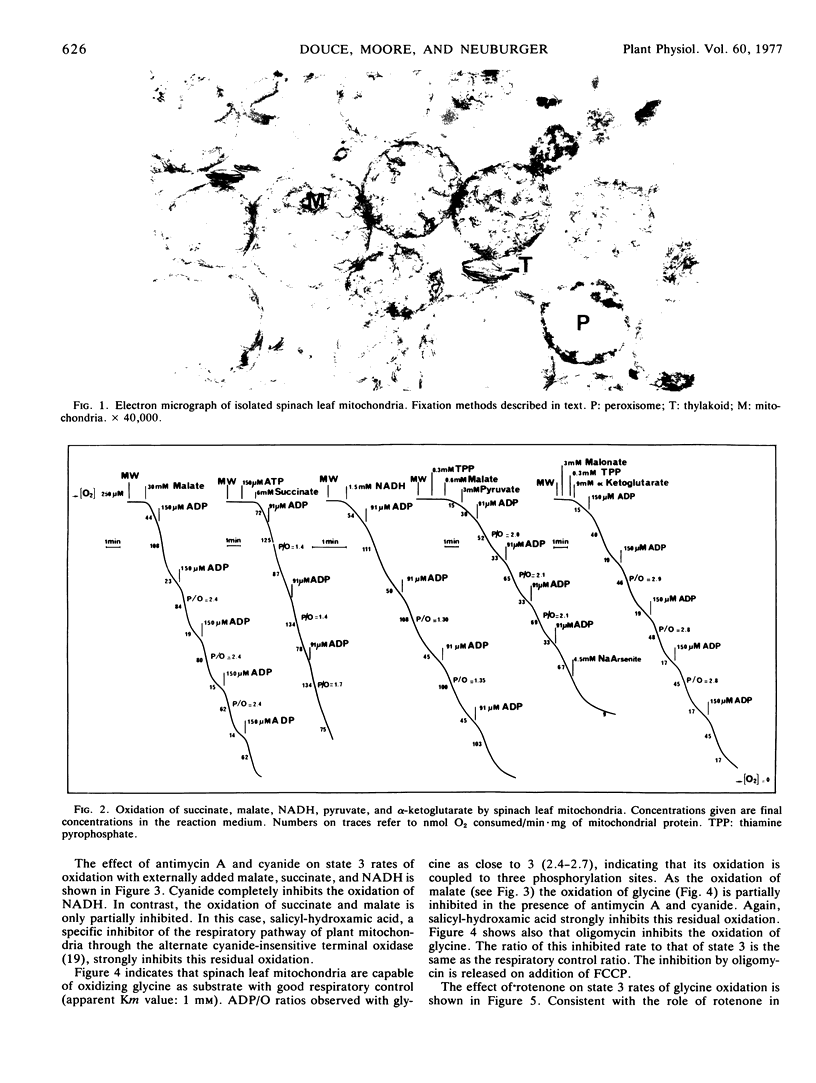
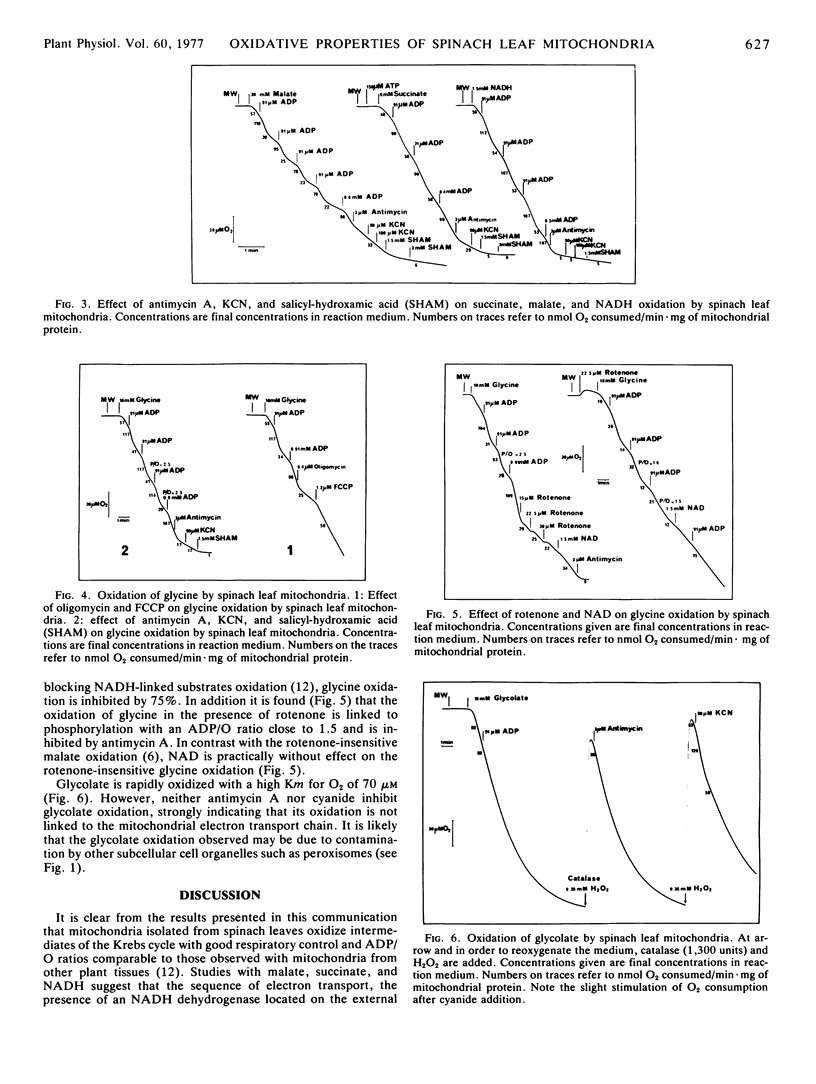
A procedure was described for preparing intact mitochondria from spinach (Spinacia oleracea L.) leaves. These mitochondria oxidized succinate, malate, pyruvate, α-ketoglutarate, and NADH with good respiratory control and ADP/O ratios comparable to those observed with mitochondria from other plant tissues. Glycine was oxidized by the preparations. This oxidation linked to the mitochondrial electron transport chain, was coupled to three phosphorylation sites and was sensitive to electron transport and phosphorylation inhibitors.
Cyanide completely inhibited the oxidation of NADH. The oxidation of succinate, malate, and glycine was only partially inhibited.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird I. F., Cornelius M. J., Keys A. J., Whittingham C. P. Adenosine triphosphate synthesis and the natural electron acceptor for synthesis of serine from glycine in leaves. Biochem J. 1972 Jun;128(1):191–192. doi: 10.1042/bj1280191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. O., Palmer J. M. The oxidation of malate by isolated plant mitochondria. Eur J Biochem. 1972 Apr 24;26(4):499–509. doi: 10.1111/j.1432-1033.1972.tb01792.x. [DOI] [PubMed] [Google Scholar]
- Collins N., Brown R. H., Merrett M. J. Oxidative phosphorylation during glycollate metabolism in mitochondria from phototrophic Euglena gracilis. Biochem J. 1975 Sep;150(3):373–377. doi: 10.1042/bj1500373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lance C., Hobson G. E., Young R. E., Biale J. B. Metabolic processes in cytoplasmic particles of the avocado fruit. IX. The oxidation of pyruvate and malate during the climacteric cycle. Plant Physiol. 1967 Apr;42(4):471–478. doi: 10.1104/pp.42.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]