Abstract
Objective
To compare the performance of waist-to-height ratio as a screening tool for cardiometabolic conditions – hypertension, prediabetes/diabetes, dyslipidemia, and subclinical inflammation – in 5 race/ethnic groups of mid-life women.
Methods
Waist-to-height ratio and 4 cardiometabolic conditions were assessed in 3033 premenopausal midlife women (249 Hispanic, 226 Chinese, 262 Japanese, 1435 European-American, and 861 African American). The areas under the receiver operating characteristic curve (AUROC) were compared across the five race/ethnic groups using waist-to-height ratio to determine the likelihood of the four cardiometabolic conditions.
Results
The performance of waist-to-height ratio to detect one or more cardiometabolic conditions was comparable among all race/ethnic groups (AUROC > 0.60, p = 0.252), and was good/fair (AUROC > 0.60) when hypertension, prediabetes/diabetes, dyslipidemia, or subclinical inflammation were analyzed separately. The performance of waist-to-height ratio of 0.50 was skewed towards higher specificity among groups with low prevalence of cardiometabolic conditions and lower median waist-to-height ratio, and towards higher sensitivity among groups with high prevalence of cardiometabolic conditions and higher median waist-to-height ratio.
Conclusions
Waist-to-height ratio can be used for community-based screening of mid-life women who may need secondary prevention for cardiometabolic conditions. A simple public health message: “Keep your waist to less than half of your height” applies to midlife women.
Keywords: waist circumference, metabolic syndrome, anthropometry, minority groups, middle-aged
1 INTRODUCTION
Indices of abdominal obesity, especially waist-to-height ratio (WHtR), appear to be superior to body mass index (BMI) to screen individuals for cardiometabolic conditions (hypertension, dyslipidemia, prediabetes/diabetes, and subclinical inflammation), cardiovascular disease, and years of life lost (Ashwell, Gunn, & Gibson, 2012; Ashwell, Mayhew, Richardson, & Rickayzen, 2014; Kodama et al., 2012). Moreover, WHtR has been advocated as a practical screening tool for cardiometabolic complications irrespective of age and sex (Ashwell et al., 2014). A universal boundary value aiming at WHtR < 0.5 has been proposed to simplify public health messages for prevention of obesity-related morbidity (Browning, Hsieh, & Ashwell, 2010).
Although the studies supporting WHtR use have included ethnically diverse populations globally, most data are available from cohorts of Asian-Pacific, African, and European descent (Evans et al., 2011). More research is needed to explore the associations of WHtR with the range of cardiometabolic conditions in American women of diverse race/ethnic backgrounds (Harris, Stevens, Thomas, Schreiner, & Folsom, 2000; Kotchen et al., 2008).
Our objective was to compare the WHtR as a screening tool for hypertension, dyslipidemia, prediabetes/diabetes, subclinical inflammation among Hispanic, Chinese, Japanese, European-American, and African American mid-life women. Our secondary objective was to explore the performance of a screening-relevant WHtR > 0.5 boundary value to screen for cardiometabolic outcomes across these 5 race/ethnic groups.
2 METHODS
Participants were enrolled in Study of Woman’s Health Across the Nation (SWAN), a longitudinal, multiethnic/racial, seven-site cohort study of the health determinants across the menopausal transition. SWAN’s recruitment methods, eligibility criteria, and design have been published (Matthews et al., 2005; Sowers et al., 2000). Briefly, women were 42–52 years old, non-pregnant, not-lactating; reported a menstrual period and no use of hormone therapy during the 3 months preceding enrollment, and self-identified as one of the five race/ethnic groups: African American (from Boston, Chicago, Pittsburgh, and the Detroit area), Chinese (from Oakland, CA), Hispanic (from Newark, NJ), Japanese (from Los Angeles, CA), and European-American (all sites). Each site recruited community-based cohorts of European-American women and women from one pre-specified minority group. This report includes study baseline data (1996–1997) from 3033 participants (92%) with a complete set of variables to define WHtR and cardiometabolic conditions (hypertension, dyslipidemia, prediabetes/diabetes, and subclinical inflammation).
A standardized SWAN protocol was used to measure anthropometric indicators (waist circumference and height), medication use, diabetes history, and cardiometabolic risk indicators (blood pressure, fasting serum glucose and lipids), as previously described (Matthews et al., 2005; Sowers et al., 2000). Specifically, waist circumference was measured to the nearest 0.1 cm with a measuring tape placed horizontally around the narrowest part of the torso over undergarments or light clothing at the end of a normal exhalation. WHtR was calculated as waist circumference (cm)/height(cm). Three blood pressure measurements were taken with the participant seated after a minimum of 5 min of rest, and the last 2 values were averaged. The technicians were certified for their performance and compliance with the standard SWAN protocol before collecting physical measures. The venous blood samples collected after an 8-hour fast were used for analysis of fasting glucose, triglycerides, high-density lipoprotein cholesterol (HDL), and high-sensitivity C-reactive protein (hsCRP). If the participant admitted eating within 8 hours of blood collection, the non-fasting state was indicated (<5% of samples across all race/ethnic groups). Medical history and prescription medication use was assessed using standard SWAN questionnaires.
Using the data collected in the SWAN study at baseline, high cardiometabolic risk was defined as having at least one of the FOUR cardiometabolic conditions:
Prediabetes/diabetes, defined as a fasting blood glucose ≥5.56 mmol/L (or non-fasting of ≥7.78 mmol/L), self-reported diagnosis of diabetes, or use of glucose-lowering medications (American Diabetes Association, 2015).
Hypertension, defined as a blood pressure ≥140/90 mm Hg, or use of prescription antihypertensive medications (Go et al., 2014).
Dyslipidemia, defined as fasting triglyceride level ≥1.70 mmol/L (or non-fasting ≥2.26 mmol/L), HDL-cholesterol < 1.29 mmol/L, or use of prescription lipid-lowering medications (Stone et al., 2014).
Subclinical inflammation, defined as high-sensitivity C-reactive protein (hsCRP) level ≥2 mg/L (Blaha et al., 2011).
Three methods were used to compare the performance of the WHtR as a screening marker for cardiometabolic conditions among race/ethnic groups. First, the Area Under the Receiver Operating Characteristic Curves (AUROC) was calculated with WHtR as a classification variable and each of the cardiometabolic conditions as a reference variable. AUROCs for each race/ethnicity were compared using the method of DeLong (DeLong, DeLong, & Clarke-Pearson, 1988). The discriminative performance was considered good if AUROC was >0.70, fair if AUROC was 0.60–0.70, and poor if AUROC was <0.60 (Xia, Wilson, & Wishart, 2013). Second, we calculated the optimal WHtR boundary values for each cardiometabolic condition in each race/ethnic group using the maximum of the Youden index (J) based on the observed data (Bantis, Nakas, & Reiser, 2014). Third, the summary ROC curve were derived using the STATA midas module (Dwamena, Sylvester, & Calrlos, 2010) for WHtR = 0.50 in each condition and within each race/ethnic group. All analyses were performed using STATA®, version 14.1 (StataCorp, College Station, TX).
3 RESULTS
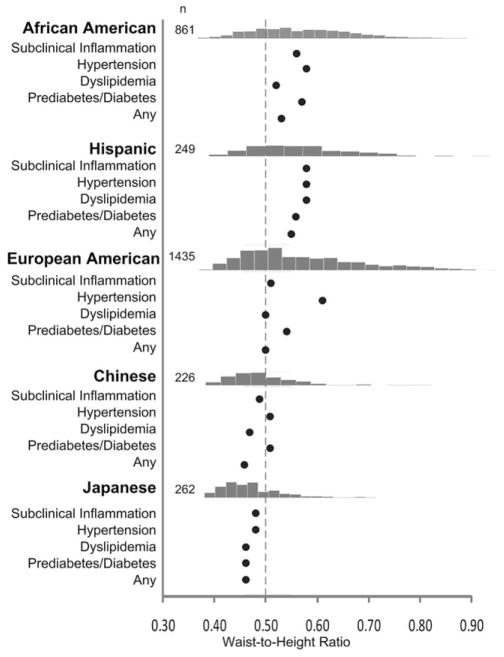
By design, almost half of the cohort was non-Hispanic European-American, a quarter was African American, whereas Hispanic, Chinese, and Japanese women represented 8.2, 7.5, and 8.6%, respectively (Supporting Information Table S1). Age at baseline was 45.9±2.7 years, similar across race/ethnic groups. Of note, African American and European-American women were >6 cm taller on average than Hispanic, Chinese, and Japanese women. More than 57% of African American and Hispanic women had WHtR >0.5, compared to less than one fifth of Chinese and Japanese women (Figure 1). The prevalence of high cardiometabolic risk (≥1 cardiometabolic condition) ranged 50–85% across race/ethnicities, with lowest prevalence in Chinese and Japanese women (Supporting Information Table S2).
FIGURE 1.
The estimated boundary values (the maximum of Youden index) for waist-to-height ratio in each race/ethnic group to screen for subclinical inflammation, hypertension, dyslipidemia, prediabetes/diabetes, or any (≥1) of these cardiometabolic outcomes. The bar graphs present the frequencies of waist-to-height ratios in each race/ethnic group. The dashed line denotes the waist-to-height ratio boundary value of 0.50. Subclinical inflammation was defined as high-sensitivity C-reactive protein ≥2 mg/L (Blaha et al., 2011). Hypertension was defined as blood pressure ≥140/90 mm Hg, or use of prescription antihypertensive medications (Go et al., 2014). Dyslipidemia was defined as fasting triglyceride level ≥1.70 mmol/L (or non-fasting ≥2.26 mmol/L – less than 5% of samples were non-fasting), or HDL-cholesterol <1.29 mmol/L or use of any prescription lipid-lowering medications (Stone et al., 2014). Prediabetes/diabetes was defined as fasting blood glucose ≥5.56 mmol/L (or non-fasting ≥7.78 mmol/L), self-reported diagnosis of diabetes, or use of glucose-lowering medications (American Diabetes Association, 2015)
The association of WHtR with cardiometabolic conditions was good/fair in all 5 race/ethnic groups (Table 1). Although the discriminative performance was significantly different among race/ethnic groups in models for individual cardiometabolic conditions (except for hypertension model), the performance of WHtR was similar in high cardiometabolic risk (≥1 condition) models. This suggests a good/fair performance of WHtR as a screening marker for cardiometabolic health in women of all 5 races/ethnicities.
TABLE 1.
The discriminative performance of weight-to-height ratio to screen for cardiometabolic conditions
| Cardiometabolic conditions | Area under the receiver operating characteristic curve (95% confidence interval) | p-valuee | ||||
|---|---|---|---|---|---|---|
| African American | Hispanic | European American | Chinese | Japanese | ||
| Subclinical inflammationa | 0.78 (0.75–0.82) | 0.68 (0.62–0.75) | 0.80 (0.78–0.82) | 0.74 (0.66–0.82) | 0.78 (0.69–0.86) | 0.020 |
| Hypertensionb | 0.63 (0.59–0.67) | 0.67 (0.58–0.77) | 0.71 (0.67–0.75) | 0.70 (0.58–0.82) | 0.68 (0.57–0.80) | 0.123 |
| Dyslipidemiac | 0.65 (0.61–0.68) | 0.64 (0.57–0.71) | 0.77 (0.75–0.80) | 0.78 (0.72–0.84) | 0.80 (0.75–0.87) | <0.001 |
| Prediabetes/diabetesd | 0.69 (0.65–0.72) | 0.65 (0.58–0.73) | 0.75 (0.71–0.78) | 0.70 (0.62–0.79) | 0.76 (0.69–0.83) | 0.036 |
| Overall high risk (≥1 condition) | 0.80 (0.76–0.83) | 0.73 (0.66–0.81) | 0.81 (0.79–0.83) | 0.68 (0.61–0.76) | 0.73 (0.66–0.80) | 0.252 |
Subclinical inflammation was defined as high-sensitivity C-Reactive protein ≥2 mg/L (Blaha et al., 2011).
Hypertension was defined as blood pressure ≥140/90 mm Hg, or use of prescription antihypertensive medications (Go et al., 2014).
Dyslipidemia was defined as fasting triglyceride level ≥1.70 mmol/L (or non-fasting ≥2.26 mmol/L – less than 5% of samples were non-fasting), or HDL-cholesterol <1.29 mmol/L or use of any prescription lipid-lowering medications (Stone et al., 2014).
Prediabetes/diabetes (abnormal glucose metabolism) was defined as fasting blood glucose ≥5.56 mmol/L (or non-fasting ≥7.78 mmol/L – less than 5% of samples were non-fasting), self-reported diagnosis of diabetes, or use of glucose-lowering medications (American Diabetes Association, 2015).
Testing for an overall difference across ethnic groups (DeLong et al., 1988).
The determination of optimal WHtR boundary value using the maximum of the Youden index (J) suggests heterogeneity across race/ethnic groups (Figure 1). The heterogeneity of the optimal WHtR boundary values to predict each cardiometabolic condition was lower within the race/ethnicity, perhaps due to overlap of cardiometabolic conditions within the groups.
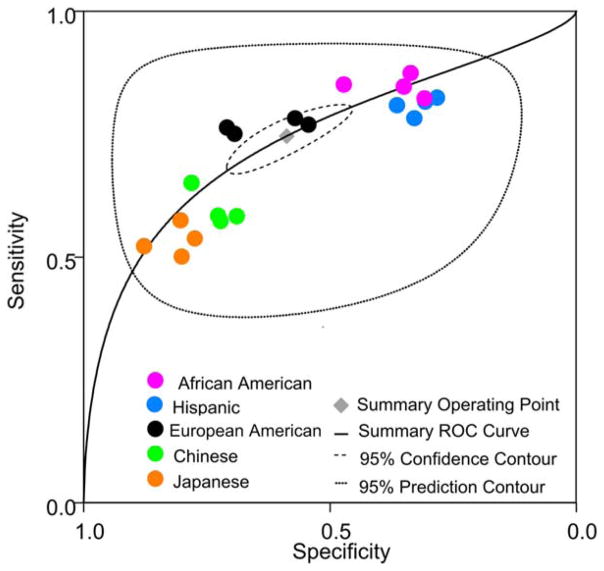
The previously proposed “WHtR global boundary value of 0.50” (Browning, Hsieh, & Ashwell, 2010) was tested across the 4 individual cardiometabolic conditions in 5 race/ethnic groups (condition × group data points n = 20) by constructing a summary ROC curve (Figure 2). Significant heterogeneity (χ2 likelihood ratio test Q = 582.7, dF = 2.0, p <0.001) was observed among the 20 data points (Dwamena et al., 2010). The performance of WHtR global boundary value of 0.50 was skewed towards higher specificity in Chinese and Japanese women (with lower prevalence of cardiometabolic conditions and lower median WHtR); skewed towards higher sensitivity in African American and Hispanic women (with high prevalence of cardiometabolic conditions and high median WHtR); and clustered around the summary operating point (with sensitivity of 0.75, 95% CI 0.69–0.79, and specificity of 0.59, 95% CI 0.49–0.68) in European–American women. The performance characteristics of WHtR were similar within each racial/ethnic group, perhaps due to overlap of cardiometabolic conditions. Yet, the analysis indicates residual variability in adiposity thresholds among race/ethnic groups, possibly related to the spectrum of WHtR distribution (Figure 1) and prevalence of cardiometabolic conditions (Supporting Information Table S2), both higher in African American and Hispanic women and lower in Chinese and Japanese women.
FIGURE 2.
The summary ROC curve for discriminative performance of WHtR boundary value of >0.50 (Browning et al., 2010) to screen for patients with high risk of cardiometabolic conditions. Each marker represents one of the 4 cardiometabolic outcomes: subclinical inflammation, hypertension, dyslipidemia, prediabetes/diabetes, whereas the marker colors indicate race/ethnic groups
4 DISCUSSION
This is the first study directly comparing the performance of WHtR as a screening marker for cardiometabolic conditions among mid-life women from 5 race/ethnic backgrounds residing in the United States. The overall discriminative performance of WHtR was good to fair across all five race/ethnic groups to screen for hypertension, dyslipidemia, pre-diabetes/diabetes, or subclinical inflammation. In general, our study supports the previously advocated public health message “Keep your waist less than half of your height” for adiposity-related primary prevention of non-communicable diseases (Ashwell et al., 2012; Ware et al., 2014). However, the “global WHtR boundary value of 0.50” (Browning et al., 2010) has higher specificity in Chinese and Japanese women, and higher sensitivity in African American and Hispanic women. It is worth noting that our primary objective is to evaluate the WHtR as a screening test in the community-based settings to identify whether one may need further diagnostic testing for cardiometabolic conditions in a health care setting. WHtR does not replace the standard testing to diagnose individual cardiometabolic conditions. Abdominal obesity (with high WHtR) is one of the several related conditions contributing to cardiometabolic risk, and the other conditions (such as hypertension, dyslipidemia, prediabetes, and subclinical inflammation) require more specific diagnostic testing.
Adjustment for height intends to normalize WHtR for body build, related to race/ethnicity (Katzmarzyk et al., 2011). However, residual race/ethnic variation in WHtR boundary values persists after adjustment for height, and correlates with race/ethnicity-specific prevalence of cardiometabolic outcomes (Supporting Information Table S2) and the spectrum of WHtR (Figure 1), suggesting the influence of adipose tissue distribution, plasticity, expandability or other biological processes in the common pathogenesis of cardiometabolic conditions (Wells, 2012).
The principal strength of our study is the large and diverse sample of mid-life women which allows a 5-race/ethnic comparison of WHtR in screening for 4 common cardiometabolic conditions. For the purpose of race/ethnic comparisons in our analyses, the age homogeneity in our cohort offered an advantage, as cardiometabolic conditions are also related to aging.
The principal limitation of our study was the inability to assess the longitudinal relationship of WHtR with incident mortality and cardiovascular outcomes in this racially/ethnically diverse cohort. However, hypertension, dyslipidemia, prediabetes/diabetes, and subclinical inflammation are known to predict global cardiovascular risk regardless of race/ethnicity. The narrow age range and only women participants limit the generalizability of our findings to the other age groups and to men.
Midlife is a vulnerable period in women’s life for progression of abdominal adiposity and related cardiometabolic conditions. A simple public health message: “Keep your waist to less than half of your height” applies to midlife women.
Supplementary Material
Table S1. Participant characteristics stratified by race/ethnicity.
Table S2. Cardiometabolic conditions stratified by race/ethnicity.
Acknowledgments
Funding Information
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). This support for this work was also provided by the National Institute of Heart, Lung, and Blood (NHLBI U01 HL097894).
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA –Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 –2004; and the University of Pittsburgh, Pittsburgh, PA –Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 – present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor –Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 – present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA – Sonja McKinlay, PI 1995 – 2001. Steering Committee: Susan Johnson, Current Chair. Chris Gallagher, Former Chair
The authors thank the study staff at each study site and all the women who participated in SWAN.
Footnotes
CONFLICT OF INTERESTS
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, NHLBI, ORWH or the NIH. There are no competing financial interests in relation to the work described.
AUTHOR CONTRIBUTIONS
RK and IJ drafted the manuscript. HL and EFAM analyzed the data. LHP and HMK directed implementation. CPC and HMK edited the manuscript for intellectual content and provided critical comments on the manuscript.
References
- American Diabetes Association. Classiffication and diagnosis of diabetes. Diabetes Care. 2015;38:S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- Ashwell M, Mayhew L, Richardson J, Rickayzen B. Waist-to-height ratio is more predictive of years of life lost than body mass index. PLoS One. 2014;9:e103483. doi: 10.1371/journal.pone.0103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantis LE, Nakas CT, Reiser B. Construction of confidence regions in the ROC space after the estimation of the optimal Youden index-based cut-off point. Biometrics. 2014;70:212–223. doi: 10.1111/biom.12107. [DOI] [PubMed] [Google Scholar]
- Blaha MJ, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, O’leary DH, … Szklo M. Association between obesity, high-sensitivity C-reactive protein >/=2 mg/L, and subclinical atherosclerosis: implications of JUPITER from the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:1430–1438. doi: 10.1161/ATVBAHA.111.223768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutrition Research Reviews. 2010;23:247–269. doi: 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Dwamena B, Sylvester R, Calrlos R. Midas: meta-analysis of diagnostic accuracy studies 2010 [Google Scholar]
- Evans J, Micklesfield L, Jennings C, Levitt NS, Lambert EV, Olsson T, Goedecke JH. Diagnostic ability of obesity measures to identify metabolic risk factors in South African women. Metab Syndr Relat Disord. 2011;9:353–360. doi: 10.1089/met.2011.0034. [DOI] [PubMed] [Google Scholar]
- Go AS, Bauman MA, Coleman King SM, Fonarow GC, Lawrence W, Williams KA, Sanchez E. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension. 2014;63:878–885. doi: 10.1161/HYP.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MM, Stevens J, Thomas N, Schreiner P, Folsom AR. Associations of Fat Distribution and Obesity with Hypertension in a Bi-ethnic Population: The ARIC Study. Obesity Research. 2000;8:516–524. doi: 10.1038/oby.2000.64. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Ravussin E, Bouchard C. Ethnic-specific BMI and waist circumference thresholds. Obesity (Silver Spring) 2011;19:1272–1278. doi: 10.1038/oby.2010.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Horikawa C, Fujihara K, Heianza Y, Hirasawa R, Yachi Y, … Iida KT. Comparisons of the strength of associations with future type 2 diabetes risk among anthropometric obesity indicators, including waist-to-height ratio: a meta-analysis. Am J Epidemiol. 2012;176:959–969. doi: 10.1093/aje/kws172. [DOI] [PubMed] [Google Scholar]
- Kotchen TA, Grim CE, Kotchen JM, Krishnaswami S, Yang H, Hoffmann RG, McGinley EL. Altered relationship of blood pressure to adiposity in hypertension. Am J Hypertens. 2008;21:284–289. doi: 10.1038/ajh.2007.48. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Sowers MF, Derby CA, Stein E, Miracle-McMahill H, Crawford SL, Pasternak RC. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women’s Health Across the Nation (SWAN) Am Heart J. 2005;149:1066–1073. doi: 10.1016/j.ahj.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Sowers MR, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews KA. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Kelsey J, Marcus R, editors. Menopause: biology and pathobiology. San Diego, CA: Academic Press; 2000. pp. 175–188. [Google Scholar]
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, … Lloyd-Jones DM. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Ware LJ, Rennie KL, Kruger HS, Kruger IM, Greeff M, Fourie CM, Kruger R. Evaluation of waist-to-height ratio to predict 5 year cardiometabolic risk in sub-Saharan African adults. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD. 2014;24:900–907. doi: 10.1016/j.numecd.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Wells JC. Ethnic variability in adiposity, thrifty phenotypes and cardiometabolic risk: addressing the full range of ethnicity, including those of mixed ethnicity. Obes Rev. 2012;13(Suppl 2):14–29. doi: 10.1111/j.1467-789X.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics. 2013;9:280–299. doi: 10.1007/s11306-012-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Participant characteristics stratified by race/ethnicity.
Table S2. Cardiometabolic conditions stratified by race/ethnicity.