Brief summary
Cardiopulmonary fitness is decreased in type 1 diabetes for reasons that are incompletely understood. In this study, leptin was associated with exercise capacity independent of insulin sensitivity (IS) and body mass index (BMI), suggesting that leptin may relate to cardiopulmonary fitness by mechanisms beyond IS and/or obesity.
Keywords: leptin, type 1 diabetes, cardiopulmonary fitness, adiposity, insulin sensitivity
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in type 1 diabetes (T1D) (1, 2) with abnormalities measurable in adolescence (3). In T1D youth we have reported an inverse relationship between insulin sensitivity (IS) and peak exercise capacity (VO2peak) (4). Adipocyte dysfunction is linked to insulin resistance (5), which is an important factor in CVD and cardio-renal disease in T1D (6). Leptin, an adipocytokine, is increased in obesity, and induces metabolic and cardiovascular actions that may contribute to CVD. Leptin is also associated with IS (7) and we previously demonstrated that higher IS relates to better peak exercise capacity (VO2peak) (8). Adiposopathy, as assessed by low plasma adiponectin/leptin ratio has also been proposed to contribute to cardiovascular disease (9). However, it is unknown whether leptin or adiponectin/leptin ratio are related to cardiopulmonary fitness itself, and whether this relationship is dependent or independent of adiposity and/or IS in youth with T1D. Accordingly, we tested the hypothesis that circulating leptin concentration is associated with VO2peak independent of IS and BMI, in youth with T1D.
Methods
Participants
Forty-one pubertal adolescents ages 12 to 21 years were included from the Effects of MEtformin on CardiovasculaR Function in AdoLescents with Type 1 Diabetes (EMERALD) study. Participants were recruited from the Barbara Davis Center and private practices with advertisements. The study was approved by the University of Colorado Denver Institutional Review Board, with appropriate consent and assent obtained. Pubertal development was assessed by a single pediatric endocrinologist (KJN) as previously described (8, 10). After 5 minutes of supine rest, 3 blood pressure measurements were obtained and averaged using a DynaPulse 5200A (Pulse Metric, San Diego, California). Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer; weight was measured to the nearest 0.1 kg using a Detecto scale (Detecto, Webb City, Missouri).
Laboratory testing was performed after an inpatient 12-h fast with an overnight intravenous insulin infusion to normalize glycemia (8, 10). Leptin and adiponectin were measured via radioimmunoassay (Millipore, Billerica MA). Other fasting laboratory evaluations included: total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein (HDL-C) cholesterol, triglycerides, glucose and HbA1c (DCCT-calibrated); assays were performed by standard methods in the CTRC laboratory. Body composition was assessed dual-energy x-ray absorptiometry (Hologic, Waltham, MA) (11). IS (glucose infusion rate [GIR] in mg/kg/min and mg/lean kg/min) was calculated from a hyperinsulinemic euglycemic clamp (80 mU/m2/min insulin) the morning following the inpatient stay (8, 10).
VO2peak was assessed using a graded bicycle protocol to exhaustion as previously reported (8). Oxygen consumption (VO2), carbon dioxide production (VCO2) and minute ventilation (VE) were measured breath-by-breath, at rest and during exercise. Respiratory exchange ratio (RER) was calculated as VCO2/VO2. To quantify the internal cardiac workload response, we calculated the Rate Pressure Product [ (RPP) = Heart Rate (HR) * Systolic Blood Pressure (SBP)].
Analyses were performed in SAS (version 9.4; SAS Institute, Cary, NC). Variables were checked for the distributional assumption of normality. ANOVA was used to test the mean of the dependent variable across tertiles of leptin. Univariable and multivariable linear regression models were employed to examine the relationships between leptin, VO2peak, unadjusted and adjusted for age, sex, BMI and IS. Linear regression results are presented as β estimate ± standard errors (SE). Significance is an α-level < 0.05.
Results
In youth with T1D we evaluated the relationships between leptin concentration, adiponectin and adiponectin/leptin ratio and VO2peak. Stratifying data by tertiles of leptin, participants in the high leptin group had significantly lower IS and VO2peak and higher blood pressure percentiles (Table 1). Leptin concentrations were significantly higher in females and individuals with elevated BMI (data not shown). After adjusting for sex and BMI or sex and IS, the relationship between leptin and VO2peak remained (Figure 1). HbA1c and LDL-C did not differ by leptin tertiles. Leptin correlated negatively with VO2peak and positively with RPPwhich remained significant after adjusting for age, sex, BMI and IS (Table 2). Whereas adiponectin concentration was not associated with VO2peak or RPP in univariable or multivariable models, adiponectin/leptin ratio was positively associated with cardiopulmonary fitness and inversely associated with RPR (Table 2).
Table 1.
Clinical characteristics of adolescents with type 1 diabetes across tertiles of leptin
| Variables | Low Leptin Tertile (<10.6ng/mL) [n=13] | Mid Leptin Tertile (10.6–26ng/mL) [n=13] | High Leptin Tertile (>26ng/mL) [n=14] | p-value |
|---|---|---|---|---|
| Age (years) | 16.9±1.6 | 17.4±2.4 | 15.5±2.1* | 0.051 |
| Weight (kg) | 81.8±41.4 | 72.5±13.7 | 79.4±13.7 | 0.64 |
| Height (cm) | 158.7±36.3 | 165.3±7.2 | 162. ±16.8 | 0.77 |
| Tanner Stage | 5 (4–5) | 5 (5–5) | 5 (5–5) | 0.53 |
| Female (%) | 15% | 62% | 79% | 0.003 |
| Fasting glucose (mg/dL) | 114±16 | 110±19 | 112±17 | 0.80 |
| HbA1c (%) | 9.2±1.6 | 8.3±0.9 | 8.8±1.7 | 0.30 |
| BMI (%tile) | 59±26 | 81±21* | 92±8‡ | 0.0005 |
| Resting HR (min−1) | 65±11 | 72±14 | 76±8 | 0.01 |
| Rate Pressure Product (mm Hg * min−1) | 7614±1461 | 8887±1352 | 9407±1492 | 0.008 |
| SBP (mm Hg) | 117±8 | 122±10 | 124±10 | 0.19 |
| DBP (mm Hg) | 68±6 | 74±5 | 74±8 | 0.03 |
| SBP (%tile) | 51±26 | 77±19* | 80±20‡ | 0.002 |
| DBP (%tile) | 48±20 | 74±14* | 72±20‡ | 0.001 |
| LDL-C (mg/dL) | 78±18 | 81±20 | 94±17‡ | 0.07 |
| HDL-C (mg/dL) | 43±9 | 46±10 | 48±10 | 0.34 |
| Total cholesterol (mg/dL) | 136±24 | 142±24 | 160±27 | 0.04 |
| Triglycerides (mg/dL) | 74±26 | 73±27 | 90±52 | 0.42 |
| Lean mass (kg) | 49.8±9.0 | 45.7±8.5 | 45.9±7.6† | <0.0001 |
| Fat mass (kg) | 15.5±5.4 | 24.9±5.9 | 31.3±6.9 | 0.37 |
| Leptin (ng/mL) | 4.6±2.7 | 17.1±4.9 | 39.2±12.0† | <0.0001 |
| Adiponectin | 9.4±4.4 | 11.8±4.5 | 10.6±5.6 | 0.47 |
| Adiponectin/leptin ratio¥ | 2.3 (1.6–3.2) | 0.7 (0.5–1.0) | 0.2 (0.2–0.3)† | <0.0001 |
| IS (mg/kg/min) | 9.5±3.1 | 7.2±3.3 | 4.5±2.1† | 0.0007 |
| IS (mg/lean kg/min) | 12.8±4.1 | 12.3±4.6 | 7.8±3.6† | 0.009 |
| Average insulin at steady state | 180.2±48.3 | 156.5±26.5 | 223.0±89.8* | 0.03 |
| IS (mg/kg/mn)/steady state insulin | 0.05±0.02 | 0.05±0.03 | 0.02±0.01 | 0.0006 |
| IS (mg/lean kg/mn)/steady state insulin | 0.07±0.02 | 0.09±0.04 | 0.04±0.03 | 0.001 |
| VO2peak (mL/kg/min) | 33.5±6.7 | 22.9±3.4‡ | 20.9±3.9‡ | <0.0001 |
| VO2peak (mL/lean kg/min) | 44.6±6.5 | 36.3±6.9‡ | 35.9±6.5‡ | 0.002 |
p<0.05 compared to mid tertile
p<0.05 compared to mid and low tertiles
p<0.05 compared to low tertile
Geometric means and 95% CI
Figure 1.
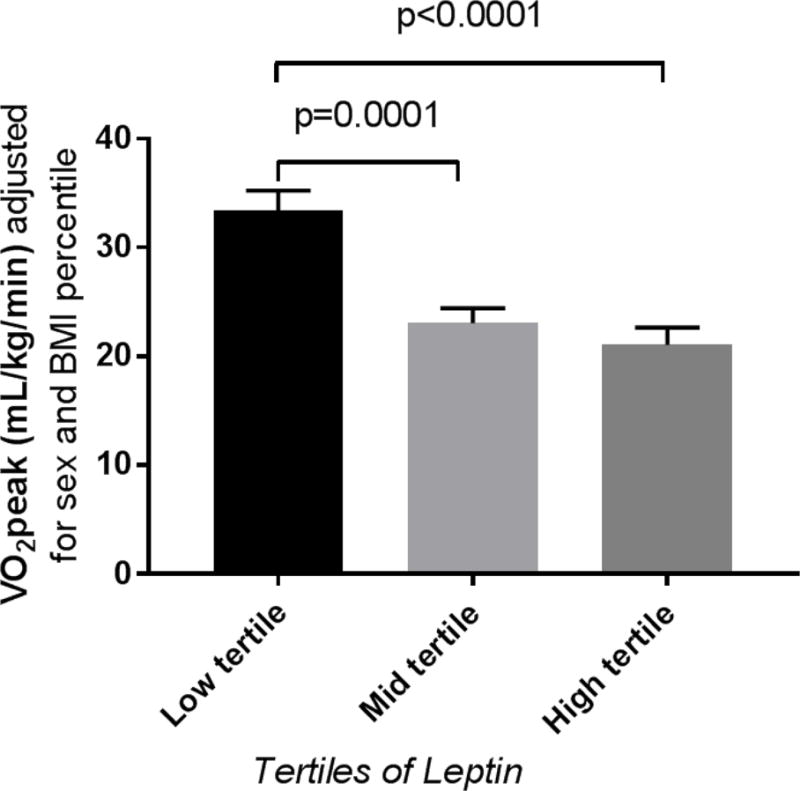
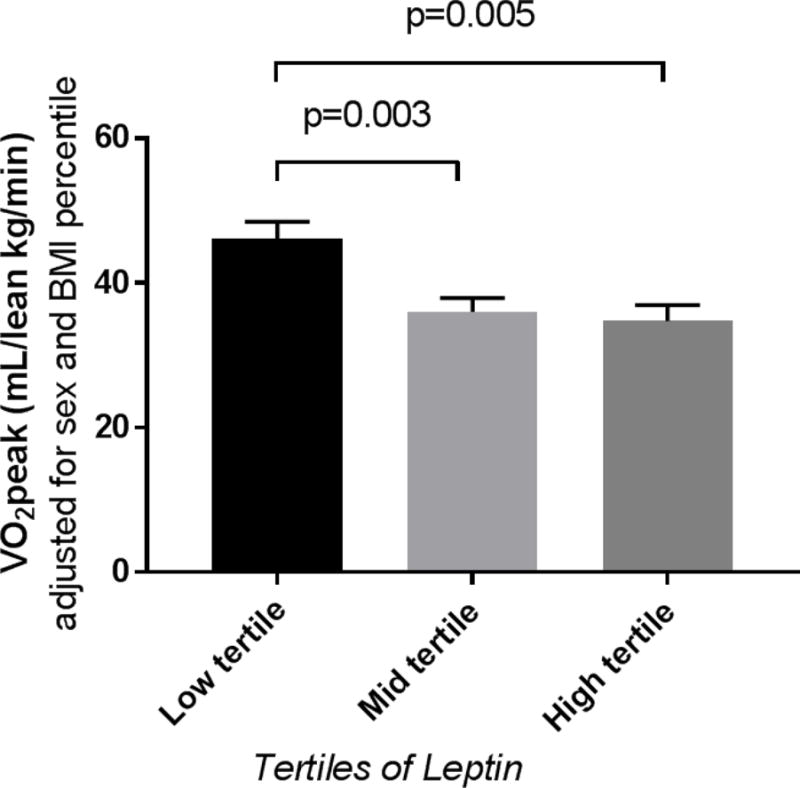
Least square means and standard errors for VO2peak across tertiles of leptin
Table 2.
Univariable and multivariable linear regression models
| Variables | VO2peak (mL/lean kg/min) β±SE | RPP (mm Hg * min−1) β±SE |
|---|---|---|
| Leptin (ng/dL) | −0.20±0.07 (R2 = 18.4%) p=0.006 | 47.49±13.68 (R2 = 24.1%) p=0.001 |
| Adiponectin | −0.18±0.45 (R2 = 1.4%) p=0.46 | −45.59±51.73 (R2 = 1.9%) p=0.38 |
| Ln adiponectin/leptin | 3.13±1.00 (R2 = 20.4%) p=0.003 | −848.24±189.25 (R2 = 34.6%) p<0.0001 |
| Leptin* (ng/dL) | −0.23±0.11 p=0.04 | 61.63±22.26 p=0.009 |
| Adiponectin* | −0.01±0.25 p=0.98 | −57.42±54.07 p=0.30 |
| Ln adiponectin/leptin* | 4.25±1.45 p=0.006 | −1125.46±289.02 p=0.0004 |
| Leptin** (ng/dL) | −0.22±0.10 p=0.04 | 50.78±19.77 p=0.01 |
| Adiponectin** | −0.04±0.26 p=0.89 | −33.35±54.35 p=0.54 |
| Ln adiponectin/leptin** | 3.36±1.25 p=0.01 | −849.27±245.02 p=0.002 |
Adjusted for age, sex and BMI percentile
Adjusted for age, sex and IS
Similar relationships also observed with VO2peak (mL/kg/min)
Discussion
We found that in T1D, elevated leptin may relate to decreased cardiopulmonary fitness by mechanisms other than IS and/or obesity alone. CVD-related morbidity and mortality are markedly increased in individuals with T1D (1, 2), with 17 years of life lost when T1D is diagnosed at age 10 (12). Obesity is an increasingly prevalent problem in youth with T1D (13) and linked to CVD development (14). Obesity induces a progressive resistance to the anorexigenic effects of leptin, which promotes further weight gain and metabolic dysfunction (15). However, not all tissues are prone to leptin resistance (16). The pathophysiology underlying the relationship between elevated leptin and poor cardiopulmonary fitness remains unclear. Huby et al. demonstrated that leptin contributes to CVD by upregulating aldosterone synthesis leading to increased blood pressures, and by promoting endothelial dysfunction and the expression of profibrotic markers in the heart (16). Other possible mechanisms include adiposopathy (17) and leptin related mitochondrial dysfunction (18), but these pathways have to our knowledge not been examined in T1D.
The literature is conflicting regarding whether circulating leptin concentrations are affected by T1D status (19–23), and measurements of leptin in relation to VO2peak in T1D youths have to our knowledge never previously been reported.
We previously demonstrated reduced VO2peak, cardiac and vascular dysfunction in otherwise healthy, non-obese adolescents with T1D, compared with well-matched nondiabetic controls of similar BMI, pubertal stage, and habitual level of physical activity (8). Low fitness levels in adults with and without diabetes are associated with increased CVD mortality and decreased longevity (24). Furthermore, we previously found that IS correlated strongly with VO2peak in youth with T1D (8), however the relationship between leptin, adiponectin/leptin ratio and VO2peak in this analysis was independent of IS.
There are limitations to the present study, including the cross-sectional design, and the lack of a non-diabetic control group which prohibit determination of causality and whether the findings are specific to T1D Furthermore, fasting leptin concentrations may not capture the pulsatile nature of leptin. Although we adjusted for a variety of important confounding variables, we cannot rule out the presence of unknown factors that may have biased the present analyses.
In sum, these data suggest that leptin may influence VO2peak by mechanisms independent of IR and adiposity. Further research and especially longitudinal studies are needed to better understand the role of leptin in cardiopulmonary fitness in youth with T1D.
Acknowledgments
Special thanks to the EMERALD Study Group and the research participants. This project was supported by the following: NIH T32 DK063687; NCRR K23 RR020038-01; NIH BIRCWH K12 5K12HD057022, R56 DK088971; ADA 7-11-CD-08, ADA 1-11-JF-23; JDRF Award #11-2010-343; K23DK107871; NIH/NCATS Colorado CTSI UL1 TR001082, Center for Women’s Health Research, VA Merit. Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
PB researched, wrote, formulated analytic plan, contributed to discussion and analytic plan, and reviewed/edited the manuscript; MCG contributed to discussion, and reviewed/edited the manuscript; AB contributed to discussion, and reviewed/edited the manuscript; GC contributed to discussion, and reviewed/edited the manuscript; YGR contributed to discussion, and reviewed/edited the manuscript; MS contributed to discussion, and reviewed/edited the manuscript; LP contributed to discussion, and reviewed/edited the manuscript; JGR contributed to discussion, and reviewed/edited the manuscript; JEBR contributed to discussion, and reviewed/edited the manuscript; KN researched, formulated analytic plan, contributed to the discussion and analytic plan, and reviewed/edited the manuscript.
None of the authors have any conflicts of interest to disclose.
References
- 1.Krolewski AS, Kosinski EJ, Warram JH, Leland OS, Busick EJ, Asmal AC, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59(8):750–5. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 2.de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 Diabetes Mellitus and Cardiovascular Disease: A Scientific Statement From the American Heart Association and American Diabetes Association. Circulation. 2014 doi: 10.1161/CIR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 3.Bjornstad P, Pyle L, Nguyen N, Snell-Bergeon JK, Bishop FK, Wadwa RP, et al. Achieving International Society for Pediatric and Adolescent Diabetes and American Diabetes Association clinical guidelines offers cardiorenal protection for youth with type 1 diabetes. Pediatr Diabetes. 2015;16(1):22–30. doi: 10.1111/pedi.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95(2):513–21. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–77. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjornstad P, Maahs DM, Duca LM, Pyle L, Rewers M, Johnson RJ, et al. Estimated insulin sensitivity predicts incident micro- and macrovascular complications in adults with type 1 diabetes over 6 years: the coronary artery calcification in type 1 diabetes study. J Diabetes Complications. 2016;30(4):586–90. doi: 10.1016/j.jdiacomp.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jimenez-Pavon D, Sese MA, Valtuena J, Cuenca-Garcia M, Gonzalez-Gross M, Gottrand F, et al. Leptin, vitamin D, and cardiorespiratory fitness as risk factors for insulin resistance in European adolescents: gender differences in the HELENA Study. Appl Physiol Nutr Metab. 2014;39(5):530–7. doi: 10.1139/apnm-2013-0250. [DOI] [PubMed] [Google Scholar]
- 8.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin Resistance in Adolescents with Type 1 Diabetes and Its Relationship to Cardiovascular Function. Journal of Clinical Endocrinology & Metabolism. 2010;95(2):513–21. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57(25):2461–73. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 10.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, et al. Insulin Resistance in Adolescents with Type 2 Diabetes Is Associated with Impaired Exercise Capacity. Journal of Clinical Endocrinology & Metabolism. 2009;94(10):3687–95. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamel EG, McNeill G, Han TS, Smith FW, Avenell A, Davidson L, et al. Measurement of abdominal fat by magnetic resonance imaging, dual-energy X-ray absorptiometry and anthropometry in non-obese men and women. Int J Obes Relat Metab Disord. 1999;23(7):686–92. doi: 10.1038/sj.ijo.0800904. [DOI] [PubMed] [Google Scholar]
- 12.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 13.DuBose SN, Hermann JM, Tamborlane WV, Beck RW, Dost A, DiMeglio LA, et al. Obesity in Youth with Type 1 Diabetes in Germany, Austria, and the United States. J Pediatr. 2015;167(3):627–32. e1–4. doi: 10.1016/j.jpeds.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 14.Kelsey MM, Zaepfel A, Bjornstad P, Nadeau KJ. Age-related consequences of childhood obesity. Gerontology. 2014;60(3):222–8. doi: 10.1159/000356023. [DOI] [PubMed] [Google Scholar]
- 15.Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301(4):E567–84. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, et al. Adipocyte-Derived Hormone Leptin Is a Direct Regulator of Aldosterone Secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis. Circulation. 2015;132(22):2134–45. doi: 10.1161/CIRCULATIONAHA.115.018226. [DOI] [PubMed] [Google Scholar]
- 17.Huth C, Pigeon E, Riou ME, St-Onge J, Arguin H, Couillard E, et al. Fitness, adiposopathy, and adiposity are independent predictors of insulin sensitivity in middle-aged men without diabetes. J Physiol Biochem. 2016;72(3):435–44. doi: 10.1007/s13105-016-0488-2. [DOI] [PubMed] [Google Scholar]
- 18.Yehuda-Shnaidman E, Nimri L, Tarnovscki T, Kirshtein B, Rudich A, Schwartz B. Secreted human adipose leptin decreases mitochondrial respiration in HCT116 colon cancer cells. PLoS One. 2013;8(9):e74843. doi: 10.1371/journal.pone.0074843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soliman AT, Omar M, Assem HM, Nasr IS, Rizk MM, El Matary W, et al. Serum leptin concentrations in children with type 1 diabetes mellitus: relationship to body mass index, insulin dose, and glycemic control. Metabolism. 2002;51(3):292–6. doi: 10.1053/meta.2002.30502. [DOI] [PubMed] [Google Scholar]
- 20.Morales A, Wasserfall C, Brusko T, Carter C, Schatz D, Silverstein J, et al. Adiponectin and leptin concentrations may aid in discriminating disease forms in children and adolescents with type 1 and type 2 diabetes. Diabetes Care. 2004;27(8):2010–4. doi: 10.2337/diacare.27.8.2010. [DOI] [PubMed] [Google Scholar]
- 21.McCormick KL, Mick GJ, Butterfield L, Ross H, Parton E, Totka J. Leptin in children with newly diagnosed type 1 diabetes: effect of insulin therapy. Int J Exp Diabetes Res. 2001;2(2):121–7. doi: 10.1155/EDR.2001.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luna R, Garcia-Mayor RV, Lage M, Andrade MA, Barreiro J, Pombo M, et al. High serum leptin levels in children with type 1 diabetes mellitus: contribution of age, BMI, pubertal development and metabolic status. Clin Endocrinol (Oxf) 1999;51(5):603–10. doi: 10.1046/j.1365-2265.1999.00848.x. [DOI] [PubMed] [Google Scholar]
- 23.Bjornstad P, Truong U, Pyle L, Dorosz JL, Cree-Green M, Baumgartner A, et al. Youth with type 1 diabetes have worse strain and less pronounced sex differences in early echocardiographic markers of diabetic cardiomyopathy compared to their normoglycemic peers: A RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) Study. Journal of diabetes and its complications. 2016;30(6):1103–10. doi: 10.1016/j.jdiacomp.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165(18):2114–20. doi: 10.1001/archinte.165.18.2114. [DOI] [PubMed] [Google Scholar]


