Osteopontin (OPN), a major marker of osteogenic differentiation, suppresses osteoblast responses to mechanical stress and cytokines, including HGF and PDGF. These OPN-induced effects are mediated through focal adhesion kinase inactivation by the induction of low–molecular weight protein tyrosine phosphatase.
Abstract
Osteopontin (OPN) is an osteogenic marker protein. Osteoblast functions are affected by inflammatory cytokines and pathological conditions. OPN is highly expressed in bone lesions such as those in rheumatoid arthritis. However, local regulatory effects of OPN on osteoblasts remain ambiguous. Here we examined how OPN influences osteoblast responses to mechanical stress and growth factors. Expression of NO synthase 1 (Nos1) and Nos2 was increased by low-intensity pulsed ultrasound (LIPUS) in MC3T3-E1 cells and primary osteoblasts. The increase of Nos1/2 expression was abrogated by both exogenous OPN overexpression and recombinant OPN treatment, whereas it was promoted by OPN-specific siRNA and OPN antibody. Moreover, LIPUS-induced phosphorylation of focal adhesion kinase (FAK), a crucial regulator of mechanoresponses, was down-regulated by OPN treatments. OPN also attenuated hepatocyte growth factor–induced vitamin D receptor (Vdr) expression and platelet-derived growth factor–induced cell mobility through the repression of FAK activity. Of note, the expression of low–molecular weight protein tyrosine phosphatase (LMW-PTP), a FAK phosphatase, was increased in both OPN-treated and differentiated osteoblasts. CD44 was a specific OPN receptor for LWW-PTP induction. Consistently, the suppressive influence of OPN on osteoblast responsiveness was abrogated by LMW-PTP knockdown. Taken together, these results reveal novel functions of OPN in osteoblast physiology.
INTRODUCTION
Osteoblasts, which are derived from mesenchymal stem cells, have multiple roles in bone metabolism by producing various types of polypeptidic factors. Bone matrix proteins such as osteopontin (OPN), bone sialoprotein (BSP), and osteocalcin (OCN) are produced by mature osteoblasts in the process of bone formation and tissue mineralization (Staines et al., 2012; Neve et al., 2013). Osteoblasts also synthesize receptor activator of nuclear factor κ-B ligand (RANKL) and osteoprotegerin for the regulation of osteoclastic activities in bone resorption (Walsh and Choi, 2014). We have also showed that osteoblasts stably express several chemokines for guiding the migration and activation of immune cells (Bandow et al., 2007; Maeda et al., 2015).
OPN is a 33-kDa glycoprotein belonging to small integrin-binding ligand N-linked glycoprotein (SIBLING) family and is recognized as a major marker of osteogenic differentiation (Kruger et al., 2014). Increased expression of OPN is found in the lesions of bone metastasis (Carlinfante et al., 2003), rheumatoid arthritis (Ohshima et al., 2002), and periodontitis (Kido et al., 2001), and is suggested as a pathological predictor of bone disorders (Iwadate et al., 2014; Hou et al., 2015). The involvement of OPN in bone diseases has been partly explained by OPN-induced migration, differentiation, and functional activation of osteoclasts (Miyauchi et al., 1991; Faccio et al., 1998) and T-lymphocytes (Xu et al., 2005; Chen et al., 2010). Physiological functions of osteoblasts are influenced by a wide range of exogenous factors, such as growth factors, inflammatory cytokines, bacterial infections, oxygen concentration, and mechanical stress (Neve et al., 2011). The consequent dysfunctions of osteoblasts frequently lead to pathological processes of bone metabolic diseases through uncontrolled bone remodeling. On the other hand, reports have shown that osteoblast or osteocyte-derived SIBLING family proteins, including BSP and dentin matrix protein 1 (DMP1), modulate osteoblast functions through their receptor signaling (Karadag and Fisher, 2006; Martin et al., 2011). These studies raise the possibility that OPN may be considered as a local regulator of osteoblast physiology in autocrine/paracrine manners. However, the detailed modulatory effects of OPN on osteoblasts are still poorly elucidated.
In this study, we explore how OPN affects responses of osteoblasts to exogenous stimulants, including mechanical stress and cytokines. OPN inhibits osteoblast responsiveness via dephosphorylation of focal adhesion kinase (FAK) mediated by the increased expression of low–molecular weight protein tyrosine kinase (LMW-PTP).
RESULTS
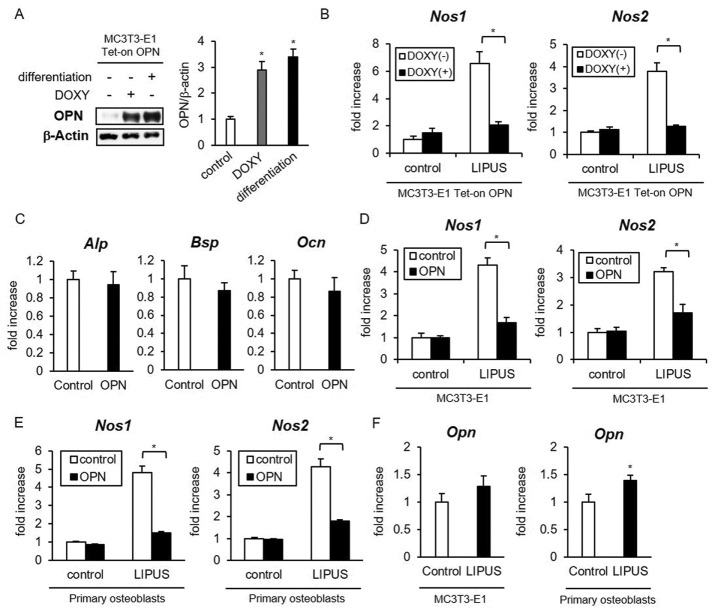
OPN suppresses the effects of mechanical stimulation on osteoblasts
To examine the functional effects of OPN on mechanical responses in osteoblasts, we prepared MC3T3-E1 cells, which inducibly overexpress OPN on doxycycline (DOXY) stimulation, using the Tet-on inducible expression system. The expression levels of OPN protein in DOXY-treated cells were very similar to those observed in differentiated osteoblasts (Figure 1A). Low-intensity pulsed ultrasound (LIPUS) is a mechanical stress that has been used as a clinical application to promote bone fracture healing (Padilla et al., 2014). Therapeutic potency of LIPUS is partly explained by nitric oxide (NO) production from osteoblasts, which orchestrates the balance of bone remodeling action between osteoblasts and osteoclasts (Reher et al., 2002; Wang et al., 2004). We found that LIPUS-induced NO synthase 1 (Nos1) and Nos2 mRNA expression is significantly inhibited by OPN overproduction (Figure 1B). We then examined the effects of recombinant OPN on LIPUS stimulation of MC3T3-E1 cells and primary osteoblasts. OPN treatment did not affect other osteogenic marker gene expressions, including alkaline phosphatase (Alp), bone sialoprotein (Bsp), and osteocalcin (Ocn), in undifferentiated MC3T3-E1 cells (Figure 1C). Pretreatment by recombinant OPN had suppressive effects on Nos1 and Nos2 inductions by LIPUS (Figure 1, D and E). In addition, we examined the effects of LIPUS on Opn expression in MC3T3-E1 cells and primary osteoblasts. LIPUS stimulation promoted Opn mRNA expression only in primary osteoblasts (Figure 1F).
FIGURE 1:
OPN attenuates the effects of LIPUS on osteoblasts. (A) MC3T3-E1 Tet-on cells, stably transfected with pTRE2Hyg-OPN, were incubated in regular medium with or without 2 μg/ml DOXY for 48 h or in osteogenic differentiation medium for 10 d. Cell lysates were analyzed for OPN protein expression by Western blotting. Significant difference from the control by Student’s t test (*p < 0.01). (B) Three independent MC3T3-E1 cell clones with inducible expression of OPN were incubated with or without 2 μg/ml DOXY for 48 h and either unstimulated or stimulated with LIPUS for 20 min. After additional incubation for 2 h, total RNA was isolated and reverse transcribed. The gene expressions of Nos1 and Nos2 were analyzed by real-time PCR. The same experiments were performed at least three times, with consistent results. Relative mRNA expression levels in comparison with ribosomal protein L 13a (Rpl13a) are shown. Error bars represent SD. Significant difference from the control by Student’s t test (*p < 0.01) (C–E) MC3T3-E1 cells (C, D) and mouse primary osteoblasts (E) were treated with 100 ng/ml recombinant OPN protein for 6 h (C), followed by LIPUS stimulation (D, E) and analysis as in B. (F) MC3T3-E1 cells and mouse primary osteoblasts were stimulated by LIPUS for 20 min, followed by incubation for 6 h and analysis as in B.
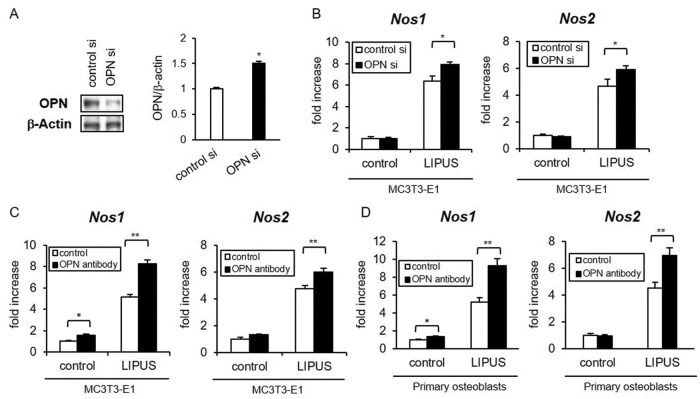
Conversely, we examined OPN effects on the mechanoresponsiveness of osteoblasts with the inhibition of endogenous OPN protein. MC3T3-E1 cells were cultured in osteogenic differentiation medium for sufficient secretion of OPN from osteoblasts. The OPN-specific small interfering RNA (siRNA) was transfected on day 10 to decrease OPN production (Figure 2A). The OPN knockdown significantly promoted LIPUS-induced Nos1 and Nos2 expression (Figure 2B). Moreover, treatment by neutralizing anti-OPN antibody efficiently increased the basal level of Nos1 and LIPUS-induced levels of Nos1 and Nos2 in both MC3T3-E1 cells (Figure 2C) and primary osteoblasts (Figure 2D). These results suggest that OPN negatively regulates LIPUS-induced expression of Nos1 and Nos2 in osteoblasts.
FIGURE 2:
LIPUS-induced gene expression of Nos1 and Nos2 was enhanced by blocking OPN. (A) MC3T3-E1 cells were induced to differentiate in osteogenic differentiation medium for 10 d. The differentiated cells were transiently transfected with either OPN or control siRNA. The knocking down of OPN protein expression was confirmed by immunoblotting. Significant difference from the control by Student’s t test (*p < 0.01). (B) After the treatment of OPN siRNA or control siRNA, LIPUS-induced Nos1 and Nos2 expressions were analyzed by real-time RT-PCR. The same experiments were performed at least three times, with consistent results. Relative mRNA expression levels in comparison with Rpl13a are shown. Error bars represent SD. Significant difference from the control by Student’s t test (*p < 0.05, **p < 0.01). (C, D) Differentiated MC3T3-E1 cells (C) or primary osteoblasts (D) were treated with 20 ng/ml neutralizing anti-OPN antibody for 3 h. After the pretreatment, cells were stimulated with LIPUS and analyzed as in B.
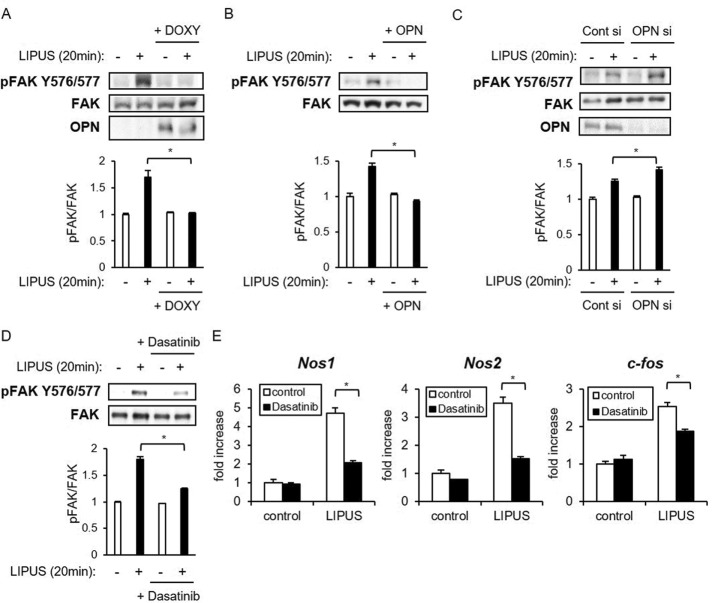
OPN attenuates LIPUS-induced FAK phosphorylation
FAK is a cytoplasmic protein tyrosine kinase that has been reported to play an important role in the mechanical stress signaling pathway of osteoblastic cell lines and primary cultured osteoblasts (Pommerenke et al., 2002; Boutahar et al., 2004; Young et al., 2009; Wang et al., 2011). FAK activation also has been reported in MC3T3-E1 cells treated with ultrasound stimulation (Wang et al., 2004). We confirmed significant LIPUS-induced phosphorylation of FAK (Y576/577) in the vector control MC3T3-E1 cells (Figure 3A). Of note, LIPUS-induced FAK phosphorylation was significantly inhibited by the induction of OPN overexpression (Figure 3A). Consistently, treatment with recombinant OPN also attenuated LIPUS-induced FAK phosphorylation in MC3T3-E1 cells (Figure 3B).
FIGURE 3:
OPN suppresses LIPUS-induced gene expression by inhibiting FAK Y576/577 phosphorylation. (A) MC3T3-E1 Tet-on OPN cell lines were incubated with or without 2 μg/ml DOXY for 48 h and either unstimulated or stimulated with LIPUS for 20 min. The prepared cell lysates were separated by SDS–PAGE, and Western blotting was performed with the indicated antibodies. Significant difference from the control by Student’s t test (*p < 0.01). (B) MC3T3-E1 cells were treated with 100 ng/ml recombinant OPN protein for 6 h. After OPN treatment, cells were stimulated by LIPUS and analyzed as in A. (C) MC3T3-E1 cells were cultured in osteogenic differentiation medium for 10 d. Cells were treated with anti-OPN antibodies for 2 h, followed by stimulation of LIPUS for 20 min and analyzed as in A. (D) MC3T3-E1 cells were pretreated with 10 μM dasatinib (a FAK Y576/577-specific inhibitor) for 6 h, followed by 20 min of LIPUS stimulation and analysis as in A. (E) After treatment with dasatinib and LIPUS as in D, cells were additionally incubated for 2 h. Total RNA was isolated for real-time PCR analysis. The same experiments were performed at least three times with consistent results. Relative mRNA expression levels in comparison with Rpl13a are shown. Error bars represent SD. Significant difference from the control by Student’s t test (*p < 0.01).
Conversely, transient knockdown of OPN expression in differentiated MC3T3-E1 cells increased the phosphorylated level of FAK after LIPUS treatment (Figure 3C). We then pretreated MC3T3-E1 cells with dasatinib, a specific Src inhibitor that reduces Y576/577 FAK phosphorylation, and found that it significantly decreased LIPUS-induced Nos1, Nos2, and c-fos mRNA expression (Figure 3D). These results raised the possibility that OPN might inhibit LIPUS-induced gene expression of Nos1 and Nos2 through the down-regulation of FAK activity.
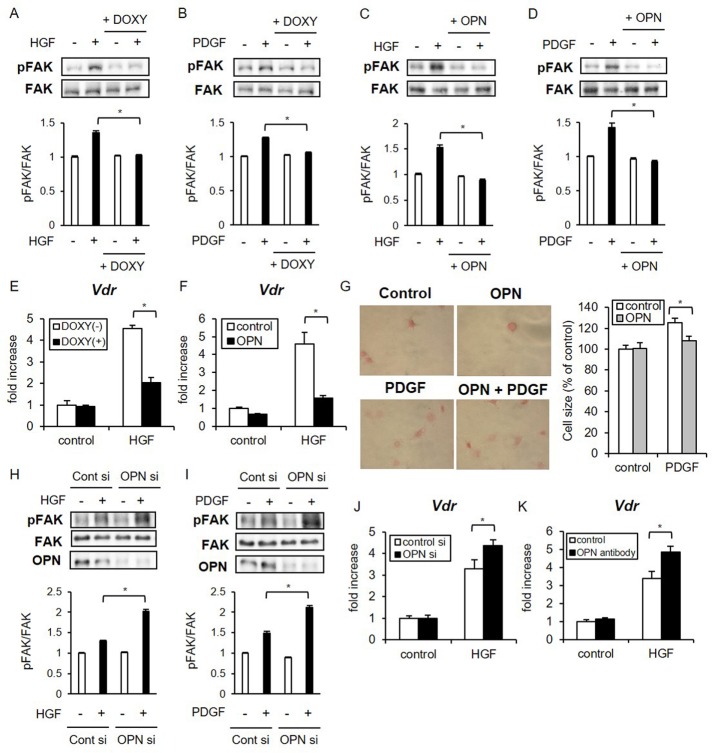
OPN inhibits osteoblast responses to hepatocyte growth factor and platelet-derived growth factor through the repression of FAK activity
Because OPN efficiently repressed FAK activation by LIPUS, we next examined whether OPN influences osteoblast responses to other types of stimuli. Because previous studies reported that hepatocyte growth factor (HGF; Tsai et al., 2012) and platelet-derived growth factor (PDGF; Ren et al., 2012) induced FAK phosphorylation in osteoblast cell lines, we analyzed the effects of OPN on HGF- and PDGF-induced FAK phosphorylation in osteoblasts. FAK was rapidly phosphorylated at Y576/577 by HGF and PDGF proteins, and the phosphorylation levels were significantly inhibited by the induction of OPN expression (Figure 4, A and B). Similarly, treatment with recombinant OPN protein suppressed the effects of HGF and PDGF on FAK phosphorylation (Figure 4, C and D). Although HGF and PDGF treatments did not induce Nos1 or Nos2 expression (unpublished data), a previous report that HGF facilitated the differentiation of human bone marrow–derived stem cells into the osteoblastic phenotype by the up-regulation of vitamin D receptor (VDR) expression (Chen et al., 2012) prompted us to analyze the effects of OPN on Vdr expression in immature osteoblasts stimulated with HGF. We found that HGF significantly elevated the mRNA level of Vdr in MC3T3-E1 cells, which was suppressed by DOXY-induced overexpression of OPN (Figure 4E). Consistent with this result, treatment with recombinant OPN also attenuated the Vdr gene expression induced by HGF (Figure 4F).
FIGURE 4:
OPN inhibits HGF- and PDGF-induced cellular events associated with FAK phosphorylation. (A, B) MC3T3-E1 Tet-on OPN cell lines were incubated with or without 2 μg/ml DOXY for 48 h and untreated or treated with either HGF (15 ng/ml) for 20 min or PDGF (10 ng/ml) for 10 min. The prepared cell lysates were separated by SDS–PAGE, and Western blotting was performed with the indicated antibodies. Significant difference from the control by Student’s t test (*p < 0.01). (C, D) MC3T3-E1 cells were treated with 100 ng/ml recombinant OPN protein for 6 h, followed by stimulation and analysis as in A and B. (E) MC3T3-E1 Tet-on OPN cell lines were treated with DOX for 48 h, followed by stimulation with HGF for 2 h. HGF-induced Vdr expression was evaluated by real-time RT-PCR. The same experiments were performed at least three times, producing consistent results. Relative mRNA expression levels in comparison with Rpl13a are shown. Error bars represent SD. Significant difference from the control by Student’s t test (*p < 0.01). (F) MC3T3-E1 cells were pretreated with OPN for 6 h and analyzed as in E. (G) MC3T3-E1 cells were treated with OPN for 3 h, followed by stimulation with 10 ng/ml mouse recombinant PDGF for 30 min. Cells were seeded on fibronectin-coated dishes and fixed with 10% formaldehyde at 60 min. Cells were stained to visualize the cytoplasm by eosin solution. Ten attached cells were randomly selected to measure the cell-spreading area using ImageJ software. Error bars represent SD. Statistical significance was determined by Student’s t test (*p < 0.01). (H, I) MC3T3-E1 cells were cultured in osteogenic differentiation medium for 10 d. The differentiated cells were transfected with OPN siRNA, stimulated by HGF or PDGF, and analyzed as in A and B. (J, K) Differentiated MC3T3-E1 cells were treated with OPN-specific siRNA or anti-OPN antibody, followed by HGF stimulation and analysis as in E and F.
We subsequently measured suppressive effects of OPN on PDGF in a cell-spreading assay. PDGF has been reported to stimulate cell spreading and migration of osteoblasts through FAK-induced G-protein–coupled receptor kinase–interacting protein 1 (GIT-1) activation (Ren et al., 2012). MC3T3-E1 cells were pretreated with PDGF for 30 min and seeded on fibronectin-coated dishes to quantify cell areas at 60 min. PDGF stimulation significantly increased the eosin-stained cytoplasmic area of osteoblasts, which was abrogated by OPN treatment (Figure 4G). These results were consistent with the reduced FAK phosphorylation in the presence of OPN protein.
Conversely, functional OPN inhibition by OPN-specific siRNA or anti-OPN neutralizing antibody significantly up-regulated HGF and PDGF-induced FAK phosphorylation (Figure 4, H and I). It also up-regulated HGF-induced Vdr expressions in mature osteoblasts (Figure 4, J and K). Taken together, these data indicate that OPN suppresses cellular responses of osteoblasts not only to mechanical stress but also to cytokines such as HGF and PDGF via inhibition of FAK activity.
OPN inactivates FAK through the induction of LMW-PTP
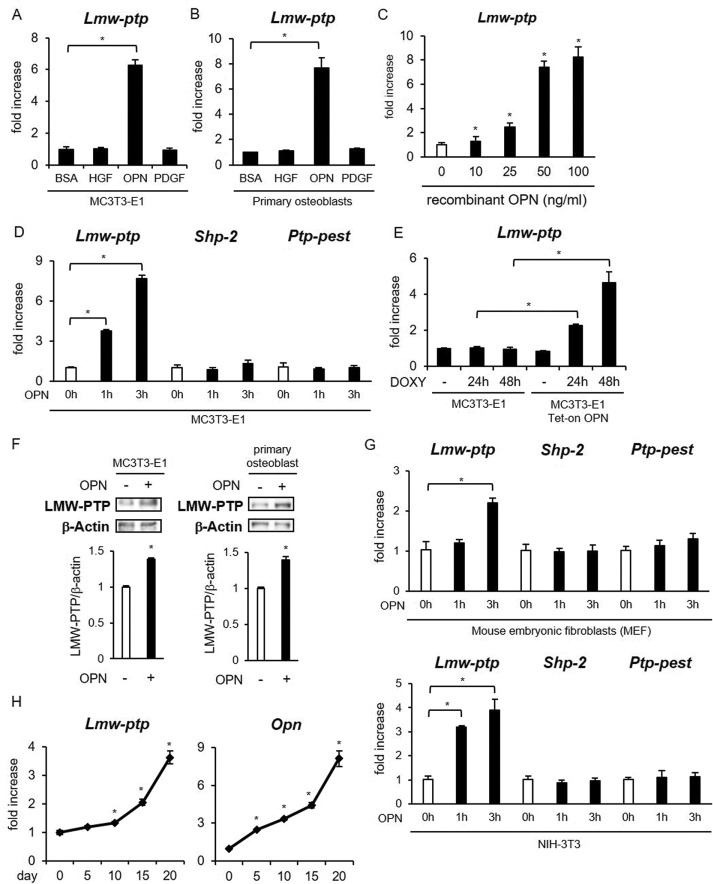
FAK activity is down-regulated by various tyrosine phosphatases, including LMW-PTP, src homology region 2 domain–containing phosphatase-2 (SHP-2), and protein tyrosine phosphatase containing proline-glutamine-serine-threonine–rich motifs (PTP-PEST; Miao et al., 2000; Lyons et al., 2001; Rigacci et al., 2002; Giannoni et al., 2003; Zheng et al., 2011; Lee et al., 2015). LMW-PTP is a ubiquitously expressed 18-kDa tyrosine-specific phosphatase (Raugei et al., 2002), which dephosphorylates FAK in fibroblasts (Rigacci et al., 2002) and T-cells (Giannoni et al., 2003). We found that Lmw-ptp mRNA expression was highly induced by treatment with OPN recombinant protein in MC3T3-E1 cells (Figure 5A) and primary osteoblasts (Figure 5B). We also analyzed the OPN dose response of Lmw-ptp mRNA expression in MC3T3-E1 cells (Figure 5C). On the other hand, HGF and PDGF, both of which are activators of FAK, did not induce Lmw-ptep mRNA (Figure 5, A and B). Shp-2 and Ptp-pest expressions were not affected by the OPN treatments (Figure 5D). DOXY-induced overexpression of OPN also increased Lmw-ptp mRNA expression (Figure 5E). We also confirmed that the protein level of LMW-PTP was significantly increased by treatment with recombinant OPN protein in osteoblasts (Figure 5F). OPN also induced Lmw-ptp mRNA expression in fibroblasts, including mouse embryonic fibroblasts (MEFs) and NIH-3T3 cells (Figure 5G).
FIGURE 5:
Stimulation with OPN increased the expression of LMW-PTP in osteoblasts. (A–D) MC3T3-E1 cells (A, C, D) and primary osteoblasts (B) were stimulated with HGF, OPN, or PDGF for 1 or 3 h. Gene expression was analyzed by real-time RT-PCR. The same experiments were performed at least three times with consistent results. Relative mRNA expression levels in comparison with Rpl13a are shown. Error bars represent SD. Significant difference from the control by Student’s t test (*p < 0.01). (E) MC3T3-E1 and MC3T3-E1 Tet-on OPN cell lines were incubated with or without 2 μg/ml DOXY for 24 or 48 h. The gene expression of Lmw-ptp was analyzed as in A. (F) MC3T3-E1 cells and primary osteoblasts were stimulated by OPN for 6 h. LMW-PTP protein expression was analyzed by Western blotting. Significant difference from the control by Student’s t test (*p < 0.01). (G) MEFs and NIH-3T3 cells were stimulated with OPN for 1 or 3 h. The same analysis was performed as in D. (H) MC3T3-E1 cells were induced to differentiate by the addition of ascorbic acid and β-glycerophosphate for the indicated days. Gene expression of Lmw-ptp and Opn during osteogenic differentiation was examined by real-time RT-PCR.
We next cultured MC3T3-E1 cells in the induction medium of osteogenesis and analyzed Lmw-ptp expression during osteogenic differentiation. The gene expression of Lmw-ptp was gradually elevated from day 15 of differentiation (Figure 5H). Of interest, the time course of Lmw-ptp mRNA induction was similar to that of Opn (Figure 5H). These results raised the possibility that OPN might down-regulate FAK activity by dephosphorylation via induction of LMW-PTP expression in osteoblasts.
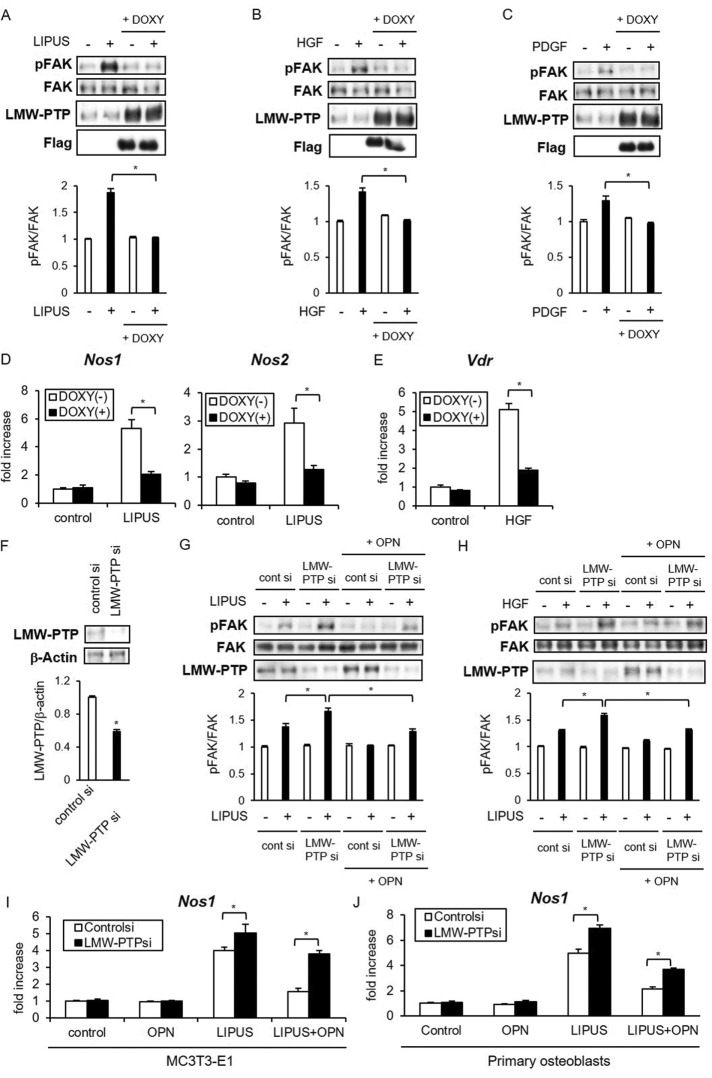
To examine how the increased expression of LMW-PTP affects osteoblast responses to mechanical stress and cytokines, we established MC3T3-E1 Tet-on Flag-tagged LMW-PTP cells. In this inducible cell line, LIPUS-induced FAK phosphorylation was strongly inhibited in the presence of DOXY (Figure 6A). Similarly to LIPUS stimulation, HGF- and PDGF-induced FAK phosphorylation was significantly suppressed by LMW-PTP overexpression (Figure 6, B and C). Furthermore, LIPUS-induced Nos1/Nos2 expression and HGF-induced Vdr expression were significantly attenuated by induced expression of LMW-PTP (Figure 6, D and E).
FIGURE 6:
LMW-PTP is involved in OPN-induced inactivation of osteoblast reactions. (A–C) MC3T3-E1 Tet-on Flag-LMW-PTP cell lines were incubated with or without 2 μg/ml DOXY for 48 h and either untreated or treated with LIPUS, recombinant HGF (15 ng/ml), or PDGF (10 ng/ml) for the indicated time. The levels of total and phosphorylated FAK were evaluated by Western blotting. Significant difference from the control by Student’s t test (*p < 0.01). (D) MC3T3-E1 Tet-on Flag-LMW-PTP cells were incubated with DOXY for 48 h and stimulated with LIPUS for 20 min. After additional incubation for 2 h, total RNA was isolated and reverse transcribed. Gene expression of Nos1 and Nos2 was analyzed by real-time PCR. The same experiments were performed at least three times with consistent results. Relative mRNA expression levels in comparison with Rpl13a are shown. Error bars represent SD. Significant difference from the control by Student’s t test (*p < 0.01). (E) MC3T3-E1 Tet-on Flag-LMW-PTP cell lines were treated with DOXY for 48 h, followed by stimulation by HGF for 2 h. Real-time PCR analyses were performed as in D. (F–J) MC3T3-E1 cells (F–I) and primary mouse osteoblasts (J) were transiently transfected with either LMW-PTP siRNA or control siRNA. Inhibitory effects of LMW-PTP siRNA on protein expression (F) were examined by Western blotting. After transfection, cells were treated with recombinant OPN for 6 h, followed by stimulation by LIPUS (G, I, J) or HGF (H). Phosphorylation of FAK (G, H) and Nos1 mRNA expression (I, J) was analyzed as in A and D.
We then prepared siRNA against LMW-PTP to explore its involvement in OPN-induced suppression of FAK activation. Transient transfection with LMW-PTP siRNA efficiently inhibited LMW-PTP protein expression in MC3T3-E1 cells (Figure 6F). At 24 h after siRNA transfection, we treated cells with recombinant OPN, followed by 6 h incubation and LIPUS stimulation. We found that LIPUS-induced FAK phosphorylation was enhanced, whereas the inhibitory effect of OPN on FAK phosphorylation was negated by the LMW-PTP knockdown (Figure 6G). LMW-PTP siRNA transfection similarly abrogated the suppressive effect of OPN on HGF-induced FAK phosphorylation (Figure 6H). We also found that LIPUS-induced Nos1 mRNA levels were further increased by LMW-PTP knockdown in MC3T3-E1 cells (Figure 6I) and primary osteoblasts (Figure 6J). Of note, the suppressive influence of OPN on the LIPUS-induced Nos1 expression was abrogated by LMW-PTP knockdown (Figure 6, I and J). These results indicated that LMW-PTP is an OPN-inducible phosphatase that down-regulates the FAK-induced cellular responses in osteoblasts.
CD44 is a specific OPN receptor for LMW-PTP induction
OPN interacts with a variety of cell surface receptors, including several integrins (αVβ1, αVβ3, αVβ5, αVβ6, and α5β1) and CD44 (Kazanecki et al., 2007; Wang and Denhardt, 2008). Histologically, OPN and its receptors, α− and β-integrin subunits (Lim et al., 2005), and CD44 (Zoller, 2011) are coexpressed in osteoblasts. To determine which OPN receptor is functionally involved in LMW-PTP induction in osteoblasts, we used cyclic RGD peptide, which blocks the binding of OPN at αV integrin, as well as neutralizing antibodies against CD29 (integrin α5β1) and CD44. We found that the blockade of CD44 specifically suppressed OPN-induced Lmw-ptp mRNA and protein expression, whereas the two other blockers had no significant effects on LWM-PTP (Figure 7, A and B). We also confirmed the binding of OPN to CD44 by immunoprecipitation (Figure 7C). This result suggests that OPN-induced LMW-PTP expression is especially mediated through CD44.
FIGURE 7:
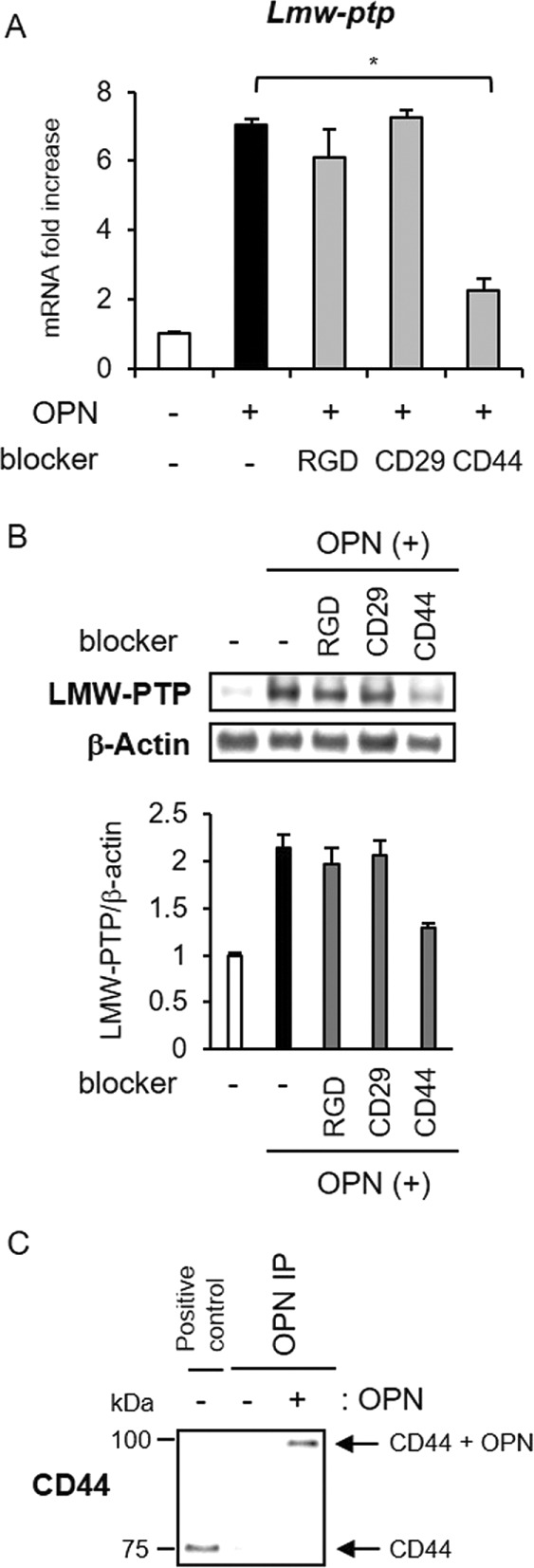
CD44 is involved in the induction of LMW-PTP expression by OPN. (A) MC3T3-E1 cells were treated with 10 μM cyclic RGD peptide (an integrin αVβ3–specific antagonist), anti-CD29 neutralizing antibody, or anti-CD44 neutralizing antibody for 6 h, followed by OPN stimulation at 100 ng/ml for 3 h. Lmw-ptp mRNA induction was analyzed by real-time RT-PCR. The same experiments were performed at least three times with consistent results. Relative mRNA expression levels in comparison with Rpl13a are shown. Error bars represent SD. Significant difference from the control by Student’s t test (*p < 0.01). (B) After treatment as in A, LMW-PTP protein expression was analyzed by Western blotting. (C) MC3T3-E1 cells were stimulated by 100 ng/ml OPN for 1 h. Cells were further incubated in PBS with 50 mM BS3 for 20 min at room temperature. Immunoprecipitation was performed with anti-OPN antibody. Precipitates and lysate from MC3T3-E1 cells (positive control) were separated by SDS–PAGE, followed by detection using anti-CD44 antibody.
OPN suppresses the effects of mechanical stimulation on human mesenchymal stem cells
Finally, we examined the suppressive effects of OPN on the response to mechanical stress in UE6E7-16, a human mesenchymal stem cell line. Pretreatment by recombinant human OPN had suppressive effects on FAK phosphorylation by LIPUS (Figure 8A). Consistently, OPN treatment increased LMW-PTP expression in UE6E7-16 cells. Next UE6E7-16 cells were differentiated into osteoblastic cells in osteogenic differentiation medium to induce OPN secretion. After differentiation for 10 d, UE6E7-16 cells highly expressed Opn and Lmw-ptp mRNAs (Figure 8B). Treatment of the differentiated UE6E7-16 cells by neutralizing anti-OPN antibody efficiently increased the both basal and LIPUS-induced levels of Nos1 (Figure 8C). These results suggest that a negative regulatory effect of OPN is observed in human mesenchymal stem cells as in mouse counterparts.
FIGURE 8:
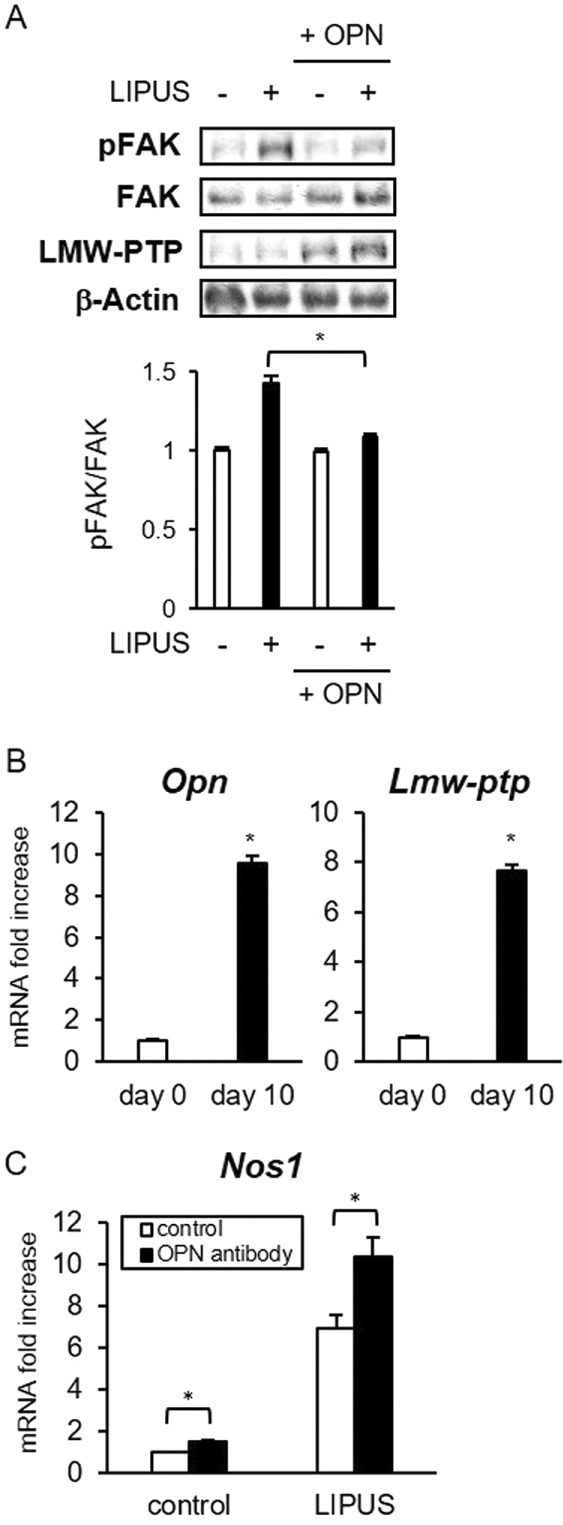
OPN attenuates the effects of LIPUS on human mesenchymal stem cells. (A) UE6E7-16 cells were incubated with recombinant human OPN for 6 h and stimulated by LIPUS for 20 min. Cell were lysed in PLC lysis buffer. Cell lysates were separated by SDS–PAGE, and Western blotting was performed with the indicated antibodies. Significant difference from the control by Student’s t test (*p < 0.01). (B) UE6E7-16 cells were induced to differentiate in osteogenic differentiation medium for 10 d. Gene expression was analyzed by real-time PCR. The same experiments were performed at least three times with consistent results. Relative mRNA expression levels compared with RPL13a are shown. Error bars represent SD. Significant difference from the control by Student’s t test (*p < 0.01). (C) UE6E7-16 cells were induced to differentiate as in B. Cells were treated with anti-OPN antibodies for 2 h, followed by LIPUS stimulation for 20 min, and incubated for 2 h. Nos1 mRNA expression was analyzed by real-time PCR.
DISCUSSION
OPN, also called secreted phosphoprotein 1 (SPP1), was initially identified as an extracellular matrix protein that had inhibitory effects on the formation and growth of hydroxyapatite crystals in bone matrix and other organs (Sodek et al., 2000). Osteoblasts are the major source of OPN in bone tissue. Although the expression of OPN is generally recognized as a middle-stage marker of osteogenic differentiation (Kruger et al., 2014), skeletal analysis of Opn-knockout mice showed that OPN is not essential for normal mouse development and osteogenesis (Rittling et al., 1998). Of note, however, a more detailed study using Fourier-transformed infrared microspectroscopy and infrared imaging indicated that bone mineral content and size were significantly increased in Opn-knockout mice (Boskey et al., 2002), indicating an essential regulatory role of OPN in bone metabolism. The precise roles of OPN in osteoblastic functions remain ambiguous. Our present findings indicate that OPN has suppressive effects on osteoblast physiology in autocrine/paracrine manners by increasing the expression of LMW-PTP.
Various regulatory factors are involved in the proper development and maintenance of bone structure. It was previously reported that PDGF stimulates osteoblast migration and bone formation by inducing the tyrosine phosphorylation of GIT1 (Ren et al., 2012). Moreover, Chen et al. (2012) demonstrated that HGF induces osteoblastic differentiation of MSCs by increasing the expression of VDR. Our findings show that OPN negatively regulates the osteoblast responses to these two cytokines. Furthermore, we demonstrated that OPN also negatively regulates osteoblast responses to a mechanical stress by LIPUS, which is an efficient promoter of osteogenic differentiation (Kusuyama et al., 2014), raising the possibility that OPN might be considered as a general inhibitory cytokine of osteoblastic functions.
In this regard, note that OPN is also recognized as an inflammatory cytokine (Wang and Denhardt, 2008). OPN is produced by various types of immune cells, including lymphocytes, macrophages, NK cells, and dendritic cells. Several studies reported that OPN expression is increased in the bone tissues of chronic bone inflammatory diseases such as rheumatoid arthritis (Ohshima et al., 2002), periodontitis (Kido et al., 2001), and metastatic cancer in bone (Carlinfante et al., 2003), suggesting the possibility that immune cell-derived OPN might exert negative effects on osteoblastic functions in the inflamed bone tissue.
FAK, also referred to as protein tyrosine kinase 2 (PTK2), is widely involved in the attachment and sensitivity of cells to the extracellular matrix organization (Bellido, 2010). Interaction between integrin and extracellular structure induces FAK autophosphorylation and activation, which in turn activates Src and ERK signaling pathways. FAK also plays an important role in the cellular responses to mechanical stress (Thompson et al., 2012). Although osteoblast-specific FAK-knockout mice exhibited normal skeletal phenotype and osteogenic differentiation, bone fracture healing was delayed and disrupted (Kim et al., 2007), suggesting that FAK might be essentially in maintaining the integrity of bone tissues. Indeed, our present data confirm that FAK becomes phosphorylated by various exogenous stimuli, including mechanical stress, HGF, and PDGF (Figures 3A and 4, A and B). Furthermore, a specific FAK inactivation significantly inhibited LIPUS-induced mRNA expression of Nos1, Nos2, and c-fos (Figure 3D), indicating that FAK is an essential signal mediator of LIPUS.
Of note, our present data indicate that the inhibitory effects of OPN on osteoblast functions are specifically mediated by the inactivation of FAK signaling (Figures 3 and 4). FAK is dephosphorylated by various protein tyrosine phosphatases, such as SHP-2 (Lee et al., 2015), PTP-PEST (Lyons et al., 2001), and LMW-PTP (Rigacci et al., 2002). We showed that endogenous and exogenous OPN stimulation significantly increased gene and protein expression of LMW-PTP (Figure 5). On the other hand, Shp-2 and Ptp-pest mRNA expression was not affected by OPN treatment (Figure 5C). FAK inactivation by LMW-PTP has been reported to impair fibroblast migration (Rigacci et al., 2002) and T-cell adhesion (Giannoni et al., 2003). To our knowledge, the present study is the first report showing the relationship between OPN and LMW-PTP in a broad range of osteoblast functions. Of interest, Lmw-ptp expression was gradually increased and correlated well with Opn expression during osteogenic differentiation (Figure 5, D and E), indicating a possible regulatory involvement of LMW-PTP in the process of osteoblast differentiation. A previous study reported that Src is inactivated by increased expression and activity of LMW-PTP during osteoblast differentiation (Zambuzzi et al., 2008). The Src-FAK signaling pathway might be regulated by OPN-induced LMW-PTP expression in differentiated osteoblasts.
OPN treatment also increased Lmw-ptp mRNA in fibroblasts similarly to in osteoblasts (Figure 5G), demonstrating that this OPN effect is not limited to osteoblasts. Several studies showed that the elevated OPN expression affects physiological functions of fibroblasts. For example, increased OPN expression modified adhesive properties and integrin-mediated signal transduction in NIH-3T3 cells (Chambers et al., 1992). Paracrine signaling by tumor-derived OPN reprogramed normal fibroblasts into tumor-promoting inflammatory fibroblasts (Sharon et al., 2015). Given that FAK signaling complexes are known to play an integral role in fibroblastic functions such as cell proliferation, collagen synthesis, and wound healing (Rustad et al., 2013), OPN-induced LMW-PTP expression might regulate FAK-associated molecular events in fibroblasts.
We found that PDGF-induced FAK phosphorylation (Figure 6C) and cell spreading (unpublished data) were significantly impaired by LMW-PTP overexpression, indicating that LMW-PTP might negatively regulate PDGF-induced physiological functions of osteoblasts through the dephosphorylation of FAK. However, a previous study indicated that LMW-PTP dephosphorylates PDGF receptor and reduces the growth rate of fibroblasts in response to PDGF stimulation (Chiarugi et al., 1998). Therefore it is possible that LMW-PTP inhibits osteoblast responses to PDGF via direct dephosphorylation of PDGF receptor rather than FAK.
Expressional regulation of LMW-PTP was reported in myogenic and neurogenic differentiation of C2C12 myoblasts and PC12 phenochromocytoma cells, respectively (Fiaschi et al., 2001). Cell differentiation up-regulated the expression and enzymatic activity of LMW-PTP in each cell line (Fiaschi et al., 2001). Consistent with this previous report, we found that LMW-PTP expression gradually increased in osteoblastic differentiation (Figure 5E). LMW-PTP expression also increased by cell–cell contact in C2C12 (Fiaschi et al., 2001) and NIH-3T3 cell lines (Berti et al., 1994). Our and previous results strongly suggest that LMW-PTP plays important roles in physiological processes that require cell growth arrest, including cell confluency and differentiation. Further investigation of regulatory mechanisms of LMW-PTP expression, including its gene regulatory region, is needed for better understanding of the relationship between LMW-PTP expression and cell differentiation.
Finally, our present findings might provide insight into the clinical effectiveness of LIPUS for bone diseases. We showed that both endogenous overexpression and exogenous addition of OPN significantly suppressed LIPUS-induced expression of Nos1 and Nos2, which are regulatory synthases for NO production (Figure 1). Because the therapeutic effect of LIPUS has partly been explained by ultrasound-induced NO synthesis by osteoblasts (Reher et al., 2002), our results suggest that osteoblast responses to mechanical stress such as LIPUS might vary, depending on the local tissue concentration of OPN. This might explain why LIPUS treatment has variable clinical effects on bone fracture healing.
In summary, we demonstrated that OPN suppresses osteoblast responses to mechanical stress and cytokines, including HGF and PDGF. These OPN-induced effects are mediated through FAK inactivation by the induction of LMW-PTP. These results suggest possible new OPN-targeted clinical approaches to chronic bone metabolic disorders such as osteoporosis. This study also provides new insights into the molecular mechanisms by which OPN affects osteoblast physiology.
MATERIALS AND METHODS
Reagents and antibodies
Recombinant mouse and human OPN was purchased from Peprotech (Rocky Hill, NJ). Recombinant mouse HGF was provided by Mitsubishi Tanabe Pharma (Osaka, Japan). Recombinant mouse PDGF-AA was purchased from Prospec (Rehovot, Israel). Dasatinib, a specific FAK Y576/577 inhibitor, was purchased from Focus Biomolecules (Plymouth Meeting, PA). Cyclo αVβ3-integrin binding cyclic RGD peptide was from Anaspec (Fremont, CA). Anti-mouse CD29 neutralizing antibody was from Biolegend (San Diego, CA). Anti-mouse CD44 neutralizing antibody was from BD Biosciences (San Jose, CA). Antibodies recognizing total and phosphorylated (Y575/576) forms of FAK were obtained from Cell Signaling Technology (Danvers, MA). Antibodies against OPN and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA). M2 anti-Flag antibody was from Sigma-Aldrich (St. Louis, MO). The antibody against LMW-PTP was purchased from R&D systems (Minneapolis, MN).
Cell culture
MC3T3-E1, a mouse osteoblastic cell line, was obtained from RIKEN Cell Bank (Tsukuba, Japan) and maintained in Eagle’s α-MEM (WAKO) containing 10% fetal bovine serum (FBS), 50 U/ml penicillin, and 50 mg/ml streptomycin. NIH-3T3, a mouse fibroblastic cell line, was obtained from RIKEN Cell Bank and maintained in D-MEM (WAKO) containing 10% FBS, 50 U/ml penicillin, and 50 mg/ml streptomycin. UE6E7-16, a human mesenchymal stem cell line, was obtained from RIKEN Cell Bank and maintained in Poweredby10 medium (Glycotechnica, Yokohama, Japan). Primary osteoblasts were isolated from newborn mouse calvariae. Briefly, calvariae were excised under aseptic condition, rinsed twice with ice-cold phosphate-buffered saline (PBS), and incubated in enzyme solution (PBS containing 0.25% collagenase I and 0.125% trypsin) with agitation. After consecutive enzyme treatment (6 × 20 min), the fourth, fifth, and sixth supernatants were centrifuged. The pellets were resuspended in α-MEM for cell culture. MEFs were prepared from 14–d postcoital C57BL/6 mouse embryos as previously described (Coats et al., 1999). Osteogenic differentiation of MC3T3-E1, primary osteoblasts, and UE6E7-16 was induced by the addition of 280 μM l-ascorbic acid 2-phosphate trisodium and 5 mM β-glycerophosphate in the culture medium.
Tet-on inducible expression system
The establishment of the MC3T3-E1 pEF1α-Tet-on cell line has been described (Matsuguchi et al., 2009). The coding cDNAs for mouse OPN and LMW-PTP were amplified by reverse transcription PCR (RT-PCR) with specific primers using total RNA from C57BL/6 mouse primary osteoblasts as the template. After the confirmation of the DNA sequences, these cDNAs were cloned into pEFBOS-Flag (Matsuguchi et al., 2001) vector, producing pEFBOS-Flag-mOPN and pEFBOS-Flag-mLMW-PTP, respectively. The cDNA inserts of these plasmids were recloned into pTRE2-Hyg vector (Clontech Laboratories), producing inducible expression plasmids pTRE2-Hyg-Flag-OPN and pTRE2-Hyg-Flag-LMW-PTP, respectively. pTRE2-Hyg-Flag-OPN and pTRE2-Hyg-Flag-LMW-PTP were stably transfected into the MC3T3-E1 pEF1α-Tet-on cell line by HilyMax (Dojindo, Kumamoto, Japan). After selection with 0.15 mg/ml hygromycin B, the isolated resistant clones were tested for inducible protein expression from the inserted cDNAs with 2 μg/ml DOXY. Three individual cell lines with good inducible protein expression were analyzed for each construct.
Ultrasound application
Cells were stimulated using a LIPUS-generating system (Teijin Pharma, Tokyo, Japan) as previously described (Kusuyama et al., 2014). The LIPUS signal consisted of a series of 1.5-MHz, 200-μs burst sine waves at 1.0 kHz and was delivered at 30 mW/cm2. The pattern and intensity of the LIPUS signal were essentially the same as used in clinical practice and animal model experiments.
RNA interference
Chemically synthesized siRNA duplexes specific for murine LMW-PTP: r(CCAUUGAGCAGCUCACUCA)dTdT and Ur(GAGUGAGCUGCUCAAUGG)dTdT were purchased from Sigma-Aldrich. OPN-specific and nontargeting control siRNA duplexes (Control siRNA-A) were from Santa Cruz Biotechnology. The duplex siRNAs were transfected using HilyMax according to manufacturer’s instructions.
Quantitative PCR analysis
The isolation of total RNA and quantitative PCR were conducted as previously described (Kusuyama et al., 2014). The primer sequences are listed in Table 1. The unlisted primers were previously described (Bandow et al., 2010; Kusuyama et al., 2014).
TABLE 1:
Primers used in this study.
| Gene symbol | Primer (5′–3′) |
|---|---|
| mNos1 | TATGTGGCAGAAGCTCCAGA |
| TCGATCGGCTGGATTTAGGA | |
| mNos2 | ATGGAGCATCCCAAGTACGA |
| TGCCCATGTACCAACCATTG | |
| mc-fos | GGCAATAGCGTGTTCCAATTA |
| AATGAACATTGACGCTGAAGG | |
| mVdr | CGTGCAGACGTAAGTACAGG |
| CGGTTCCATCATGTCCAGTG | |
| mLmw-ptp | GCCCATAAGGCAAGACAGATTA |
| TCATAGCTCCCAAGTAGCTCA | |
| mShp-2 | GTGGAGAGAGGGAAGAGCAA |
| CCGACCTTAGAGAGTTTGAGC | |
| mPtp-pest | CAATGGGGAGGACAACTTCG |
| AACTCGGCTGTGATCAAATGG | |
| hRpl13a | AGGTGCAGGTCCTGGTG |
| GTTGATGCCTTCACAGCGTA | |
| hNos1 | CCACACCCATAAACCAGTCG |
| GTAGGGACTGAGCCGTGAG | |
| hOpn | CATCACCTGTGCCATACCAG |
| TTGGAAGGGTCTGTGGGG | |
| hLmw-ptp | GCCCGGCAATTACCAAA |
| TGTGGATCATAGCTCCCAAG |
Protein cross-linking and immunoprecipitation
MC3T3-E1 cells were stimulated by 100 ng/ml OPN for 1 h. Cells were further incubated in PBS with 50 mM bis(sulfosuccinimidyl)suberate (BS3), an amine-to-amine cross-linker, for 20 min at room temperature. The cell lysates were prepared in PLC lysis buffer and incubated with OPN antibody for 2 h at 4°C, followed by incubation with protein A–Sepharose beads (GE Healthcare Biosciences, Piscataway, NJ) for an additional 1 h. The beads were washed three times in ice-cold PLC lysis buffer, suspended in SDS sample buffer, and heated at 95°C for 10 min. The eluted proteins were applied to SDS–PAGE and analyzed by Western blotting.
Western blot analysis
For Western blot analyses, total cellular lysate preparation and immunoblotting procedures were performed as previously described (Kusuyama et al., 2014). The same experiments were performed three times, producing consistent results. Relative protein expression levels in comparison with β-actin were quantitatively analyzed using ImageJ software. Error bars represent SD.
Cell-spreading assays
Cells were incubated with normal medium for 12 h and serum-starved medium for 6 h and then suspended in normal medium. Cells were seeded on fibronectin-coated dishes with or without PDGF and OPN treatment and fixed with 10% formaldehyde at 60 min. Cells were stained to visualize the cytoplasm by eosin solution. Ten attached cells were randomly selected for measurement of cell-spreading area using ImageJ software. Statistical analysis was performed using Student’s t test.
Acknowledgments
We thank Yoko Amita and Yuka Kajiya for secretarial assistance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Kanazawa Medical Research Foundation, the Nakatomi Foundation, the Japan Foundation for Aging and Health, and Teijin Pharma, which supplied the LIPUS device.
Abbreviations used:
- DOXY
doxycycline
- FAK
focal adhesion kinase
- HGF
hepatocyte growth factor
- LIPUS
low-intensity pulsed ultrasound
- LMW-PTP
low–molecular weight protein tyrosine phosphatase
- OPN
osteopontin
- PDGF
platelet-derived growth factor
- PTP-PEST
protein tyrosine phosphatase containing proline-glutamine-serine-threonine–rich motifs
- SHP-2
src homology region 2 domain–containing phosphatase-2
- VDR
vitamin D receptor.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E16-10-0716) on March 22, 2017.
REFERENCES
- Bandow K, Maeda A, Kakimoto K, Kusuyama J, Shamoto M, Ohnishi T, Matsuguchi T. Molecular mechanisms of the inhibitory effect of lipopolysaccharide (LPS) on osteoblast differentiation. Biochem Biophys Res Commun. 2010;402:755–761. doi: 10.1016/j.bbrc.2010.10.103. [DOI] [PubMed] [Google Scholar]
- Bandow K, Nishikawa Y, Ohnishi T, Kakimoto K, Soejima K, Iwabuchi S, Kuroe K, Matsuguchi T. Low-intensity pulsed ultrasound (LIPUS) induces RANKL, MCP-1, and MIP-1beta expression in osteoblasts through the angiotensin II type 1 receptor. J Cell Physiol. 2007;211:392–398. doi: 10.1002/jcp.20944. [DOI] [PubMed] [Google Scholar]
- Bellido T. Antagonistic interplay between mechanical forces and glucocorticoids in bone: a tale of kinases. J Cell Biochem. 2010;111:1–6. doi: 10.1002/jcb.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti A, Rigacci S, Raugei G, Degl’Innocenti D, Ramponi G. Inhibition of cellular response to platelet-derived growth factor by low M(r) phosphotyrosine protein phosphatase overexpression. FEBS Lett. 1994;349:7–12. doi: 10.1016/0014-5793(94)00620-2. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem. 2004;279:30588–30599. doi: 10.1074/jbc.M313244200. [DOI] [PubMed] [Google Scholar]
- Carlinfante G, Vassiliou D, Svensson O, Wendel M, Heinegard D, Andersson G. Differential expression of osteopontin and bone sialoprotein in bone metastasis of breast and prostate carcinoma. Clin Exp Metastasis. 2003;20:437–444. doi: 10.1023/a:1025419708343. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Behrend EI, Wilson SM, Denhardt DT. Induction of expression of osteopontin (OPN; secreted phosphoprotein) in metastatic, ras-transformed NIH 3T3 cells. Anticancer Res. 1992;12:43–47. [PubMed] [Google Scholar]
- Chen G, Zhang X, Li R, Fang L, Niu X, Zheng Y, He D, Xu R, Zhang JZ. Role of osteopontin in synovial Th17 differentiation in rheumatoid arthritis. Arthritis Rheum. 2010;62:2900–2908. doi: 10.1002/art.27603. [DOI] [PubMed] [Google Scholar]
- Chen K, Aenlle KK, Curtis KM, Roos BA, Howard GA. Hepatocyte growth factor (HGF) and 1,25-dihydroxyvitamin D together stimulate human bone marrow-derived stem cells toward the osteogenic phenotype by HGF-induced up-regulation of VDR. Bone. 2012;51:69–77. doi: 10.1016/j.bone.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Cirri P, Marra F, Raugei G, Fiaschi T, Camici G, Manao G, Romanelli RG, Ramponi G. The Src and signal transducers and activators of transcription pathways as specific targets for low molecular weight phosphotyrosine-protein phosphatase in platelet-derived growth factor signaling. J Biol Chem. 1998;273:6776–6785. doi: 10.1074/jbc.273.12.6776. [DOI] [PubMed] [Google Scholar]
- Coats S, Whyte P, Fero ML, Lacy S, Chung G, Randel E, Firpo E, Roberts JM. A new pathway for mitogen-dependent cdk2 regulation uncovered in p27(Kip1)-deficient cells. Curr Biol. 1999;9:163–173. doi: 10.1016/s0960-9822(99)80086-4. [DOI] [PubMed] [Google Scholar]
- Faccio R, Grano M, Colucci S, Zallone AZ, Quaranta V, Pelletier AJ. Activation of alphav beta3 integrin on human osteoclast-like cells stimulates adhesion and migration in response to osteopontin. Biochem Biophys Res Commun. 1998;249:522–525. doi: 10.1006/bbrc.1998.9180. [DOI] [PubMed] [Google Scholar]
- Fiaschi T, Chiarugi P, Buricchi F, Giannoni E, Taddei ML, Talini D, Cozzi G, Zecchi-Orlandini S, Raugei G, Ramponi G. Low molecular weight protein-tyrosine phosphatase is involved in growth inhibition during cell differentiation. J Biol Chem. 2001;276:49156–49163. doi: 10.1074/jbc.M107538200. [DOI] [PubMed] [Google Scholar]
- Giannoni E, Chiarugi P, Cozzi G, Magnelli L, Taddei ML, Fiaschi T, Buricchi F, Raugei G, Ramponi G. Lymphocyte function-associated antigen-1-mediated T cell adhesion is impaired by low molecular weight phosphotyrosine phosphatase-dependent inhibition of FAK activity. J Biol Chem. 2003;278:36763–36776. doi: 10.1074/jbc.M302686200. [DOI] [PubMed] [Google Scholar]
- Hou X, Wu X, Huang P, Zhan J, Zhou T, Ma Y, Qin T, Luo R, Feng Y, Xu Y, et al. Osteopontin is a useful predictor of bone metastasis and survival in patients with locally advanced nasopharyngeal carcinoma. Int J Cancer. 2015;137:1672–1678. doi: 10.1002/ijc.29540. [DOI] [PubMed] [Google Scholar]
- Iwadate H, Kobayashi H, Kanno T, Asano T, Saito R, Sato S, Suzuki E, Watanabe H, Ohira H. Plasma osteopontin is correlated with bone resorption markers in rheumatoid arthritis patients. Int J Rheum Dis. 2014;17:50–56. doi: 10.1111/1756-185X.12115. [DOI] [PubMed] [Google Scholar]
- Karadag A, Fisher LW. Bone sialoprotein enhances migration of bone marrow stromal cells through matrices by bridging MMP-2 to alpha(v)beta3-integrin. J Bone Miner Res. 2006;21:1627–1636. doi: 10.1359/jbmr.060710. [DOI] [PubMed] [Google Scholar]
- Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem. 2007;102:912–924. doi: 10.1002/jcb.21558. [DOI] [PubMed] [Google Scholar]
- Kido J, Nakamura T, Asahara Y, Sawa T, Kohri K, Nagata T. Osteopontin in gingival crevicular fluid. J Periodontal Res. 2001;36:328–333. doi: 10.1034/j.1600-0765.2001.360509.x. [DOI] [PubMed] [Google Scholar]
- Kim JB, Leucht P, Luppen CA, Park YJ, Beggs HE, Damsky CH, Helms JA. Reconciling the roles of FAK in osteoblast differentiation, osteoclast remodeling, and bone regeneration. Bone. 2007;41:39–51. doi: 10.1016/j.bone.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger TE, Miller AH, Godwin AK, Wang J. Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers. Crit Rev Oncol Hematol. 2014;89:330–341. doi: 10.1016/j.critrevonc.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuyama J, Bandow K, Shamoto M, Kakimoto K, Ohnishi T, Matsuguchi T. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem. 2014;289:10330–10344. doi: 10.1074/jbc.M113.546382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Yoo YH, Kim MS, Yadav BK, Kim Y, Lim D, Hwangbo C, Moon KW, Kim D, Jeoung D, et al. CD99 inhibits CD98-mediated beta1 integrin signaling through SHP2-mediated FAK dephosphorylation. Exp Cell Res. 2015;336:211–222. doi: 10.1016/j.yexcr.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Lim JY, Taylor AF, Li Z, Vogler EA, Donahue HJ. Integrin expression and osteopontin regulation in human fetal osteoblastic cells mediated by substratum surface characteristics. Tissue Eng. 2005;11:19–29. doi: 10.1089/ten.2005.11.19. [DOI] [PubMed] [Google Scholar]
- Lyons PD, Dunty JM, Schaefer EM, Schaller MD. Inhibition of the catalytic activity of cell adhesion kinase beta by protein-tyrosine phosphatase-PEST-mediated dephosphorylation. J Biol Chem. 2001;276:24422–24431. doi: 10.1074/jbc.M011080200. [DOI] [PubMed] [Google Scholar]
- Maeda A, Bandow K, Kusuyama J, Kakimoto K, Ohnishi T, Miyawaki S, Matsuguchi T. Induction of CXCL2 and CCL2 by pressure force requires IL-1beta-MyD88 axis in osteoblasts. Bone. 2015;74:76–82. doi: 10.1016/j.bone.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, Quarles LD. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25:2551–2562. doi: 10.1096/fj.10-177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuguchi T, Chiba N, Bandow K, Kakimoto K, Masuda A, Ohnishi T. JNK activity is essential for Atf4 expression and late-stage osteoblast differentiation. J Bone Miner Res. 2009;24:398–410. doi: 10.1359/jbmr.081107. [DOI] [PubMed] [Google Scholar]
- Matsuguchi T, Musikacharoen T, Johnson TR, Kraft AS, Yoshikai Y. A novel mitogen-activated protein kinase phosphatase is an important negative regulator of lipopolysaccharide-mediated c-Jun N-terminal kinase activation in mouse macrophage cell lines. Mol Cell Biol. 2001;21:6999–7009. doi: 10.1128/MCB.21.20.6999-7009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- Miyauchi A, Alvarez J, Greenfield EM, Teti A, Grano M, Colucci S, Zambonin-Zallone A, Ross FP, Teitelbaum SL, Cheresh D, et al. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991;266:20369–20374. [PubMed] [Google Scholar]
- Neve A, Corrado A, Cantatore FP. Osteoblast physiology in normal and pathological conditions. Cell Tissue Res. 2011;343:289–302. doi: 10.1007/s00441-010-1086-1. [DOI] [PubMed] [Google Scholar]
- Neve A, Corrado A, Cantatore FP. Osteocalcin: skeletal and extra-skeletal effects. J Cell Physiol. 2013;228:1149–1153. doi: 10.1002/jcp.24278. [DOI] [PubMed] [Google Scholar]
- Ohshima S, Yamaguchi N, Nishioka K, Mima T, Ishii T, Umeshita-Sasai M, Kobayashi H, Shimizu M, Katada Y, Wakitani S, et al. Enhanced local production of osteopontin in rheumatoid joints. J Rheumatol. 2002;29:2061–2067. [PubMed] [Google Scholar]
- Padilla F, Puts R, Vico L, Raum K. Stimulation of bone repair with ultrasound: a review of the possible mechanic effects. Ultrasonics. 2014;54:1125–1145. doi: 10.1016/j.ultras.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Pommerenke H, Schmidt C, Durr F, Nebe B, Luthen F, Muller P, Rychly J. The mode of mechanical integrin stressing controls intracellular signaling in osteoblasts. J Bone Miner Res. 2002;17:603–611. doi: 10.1359/jbmr.2002.17.4.603. [DOI] [PubMed] [Google Scholar]
- Raugei G, Ramponi G, Chiarugi P. Low molecular weight protein tyrosine phosphatases: small, but smart. Cell Mol Life Sci. 2002;59:941–949. doi: 10.1007/s00018-002-8481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reher P, Harris M, Whiteman M, Hai HK, Meghji S. Ultrasound stimulates nitric oxide and prostaglandin E2 production by human osteoblasts. Bone. 2002;31:236–241. doi: 10.1016/s8756-3282(02)00789-5. [DOI] [PubMed] [Google Scholar]
- Ren Y, Yu L, Fan J, Rui Z, Hua Z, Zhang Z, Zhang N, Yin G. Phosphorylation of GIT1 tyrosine 321 is required for association with FAK at focal adhesions and for PDGF-activated migration of osteoblasts. Mol Cell Biochem. 2012;365:109–118. doi: 10.1007/s11010-012-1249-3. [DOI] [PubMed] [Google Scholar]
- Rigacci S, Rovida E, Dello Sbarba P, Berti A. Low Mr phosphotyrosine protein phosphatase associates and dephosphorylates p125 focal adhesion kinase, interfering with cell motility and spreading. J Biol Chem. 2002;277:41631–41636. doi: 10.1074/jbc.M201709200. [DOI] [PubMed] [Google Scholar]
- Rittling SR, Matsumoto HN, McKee MD, Nanci A, An XR, Novick KE, Kowalski AJ, Noda M, Denhardt DT. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J Bone Miner Res. 1998;13:1101–1111. doi: 10.1359/jbmr.1998.13.7.1101. [DOI] [PubMed] [Google Scholar]
- Rustad KC, Wong VW, Gurtner GC. The role of focal adhesion complexes in fibroblast mechanotransduction during scar formation. Differentiation. 2013;86:87–91. doi: 10.1016/j.diff.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Sharon Y, Raz Y, Cohen N, Ben-Shmuel A, Schwartz H, Geiger T, Erez N. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Cancer Res. 2015;75:963–973. doi: 10.1158/0008-5472.CAN-14-1990. [DOI] [PubMed] [Google Scholar]
- Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- Staines KA, MacRae VE, Farquharson C. The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J Endocrinol. 2012;214:241–255. doi: 10.1530/JOE-12-0143. [DOI] [PubMed] [Google Scholar]
- Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503:179–193. doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Huang YL, Yang WH, Tang CH. Hepatocyte growth factor-induced BMP-2 expression is mediated by c-Met receptor, FAK, JNK, Runx2, and p300 pathways in human osteoblasts. Int Immunopharmacol. 2012;13:156–162. doi: 10.1016/j.intimp.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014;5:511. doi: 10.3389/fimmu.2014.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Du T, Wang Y, Yang C, Zhang S, Cao X. Focal adhesion kinase signaling pathway is involved in mechanotransduction in MG-63 cells. Biochem Biophys Res Commun. 2011;410:671–676. doi: 10.1016/j.bbrc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- Wang FS, Kuo YR, Wang CJ, Yang KD, Chang PR, Huang YT, Huang HC, Sun YC, Yang YJ, Chen YJ. Nitric oxide mediates ultrasound-induced hypoxia-inducible factor-1alpha activation and vascular endothelial growth factor-A expression in human osteoblasts. Bone. 2004;35:114–123. doi: 10.1016/j.bone.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Xu G, Nie H, Li N, Zheng W, Zhang D, Feng G, Ni L, Xu R, Hong J, Zhang JZ. Role of osteopontin in amplification and perpetuation of rheumatoid synovitis. J Clin Invest. 2005;115:1060–1067. doi: 10.1172/JCI23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SR, Gerard-O’Riley R, Kim JB, Pavalko FM. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J Bone Miner Res. 2009;24:411–424. doi: 10.1359/JBMR.081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambuzzi WF, Granjeiro JM, Parikh K, Yuvaraj S, Peppelenbosch MP, Ferreira CV. Modulation of Src activity by low molecular weight protein tyrosine phosphatase during osteoblast differentiation. Cell Physiol Biochem. 2008;22:497–506. doi: 10.1159/000185506. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Yang W, Xia Y, Hawke D, Liu DX, Lu Z. Ras-induced and extracellular signal-regulated kinase 1 and 2 phosphorylation-dependent isomerization of protein tyrosine phosphatase (PTP)-PEST by PIN1 promotes FAK dephosphorylation by PTP-PEST. Mol Cell Biol. 2011;31:4258–4269. doi: 10.1128/MCB.05547-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]