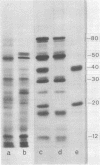
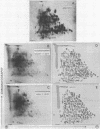
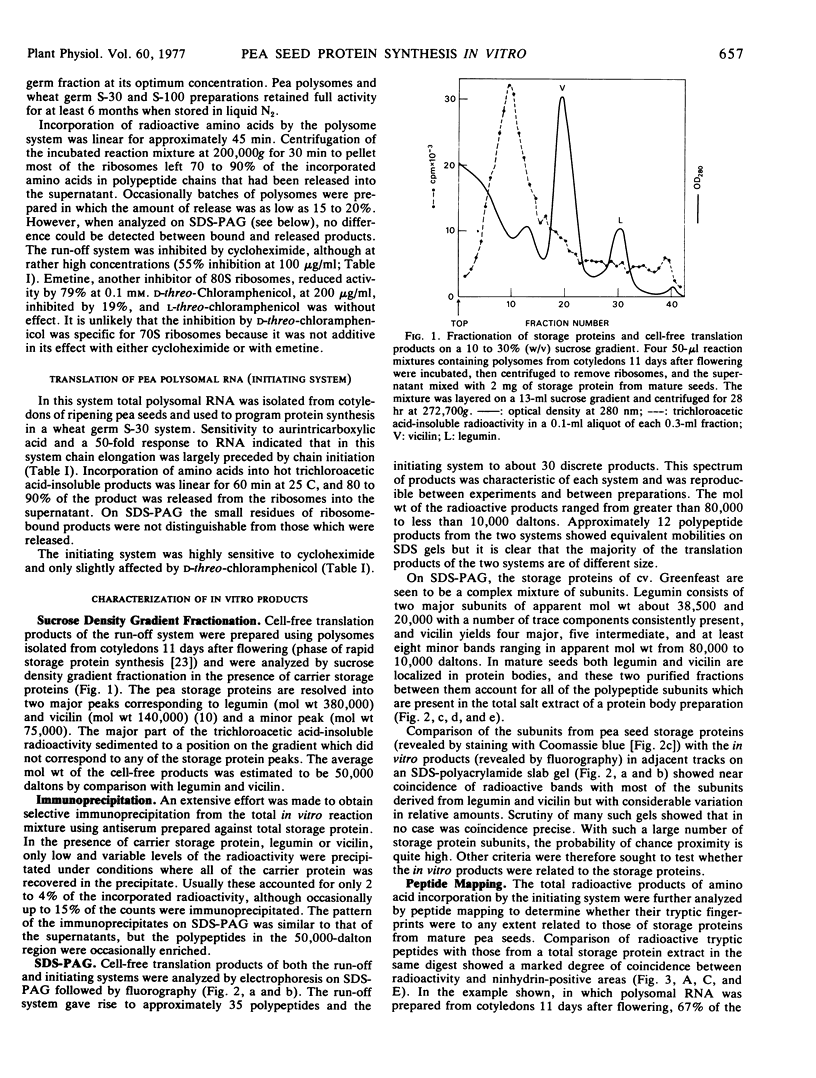
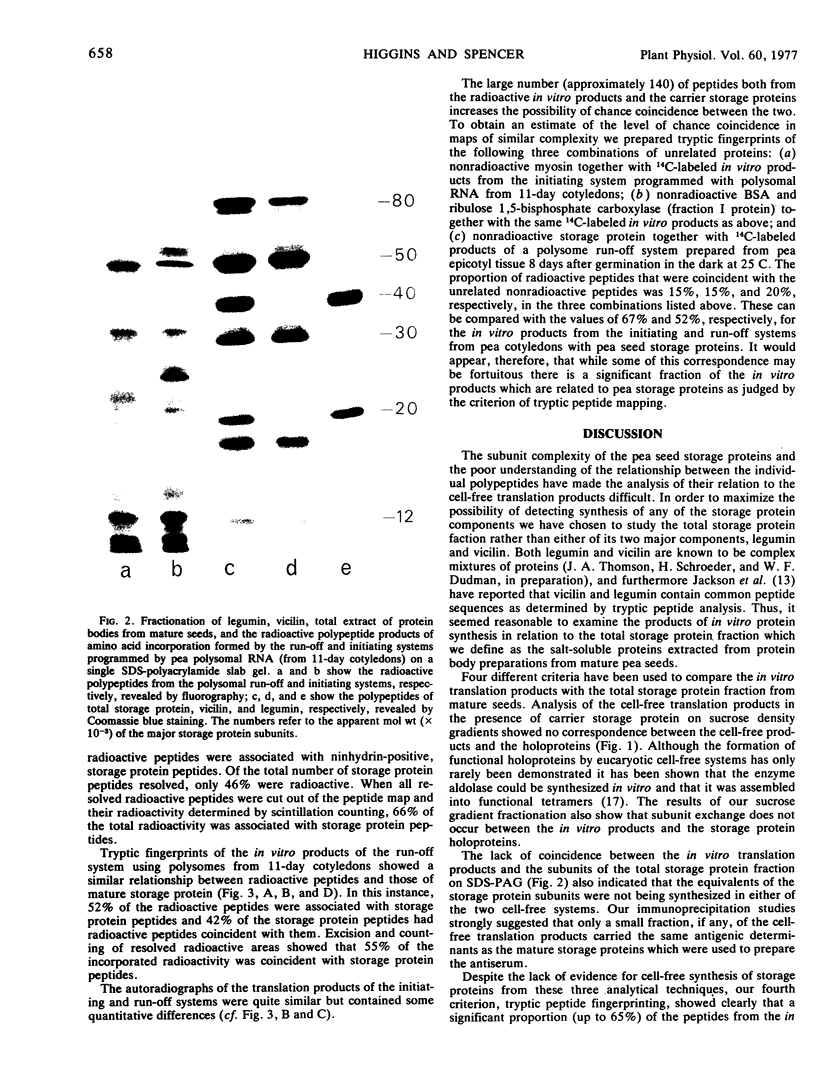
Abstract
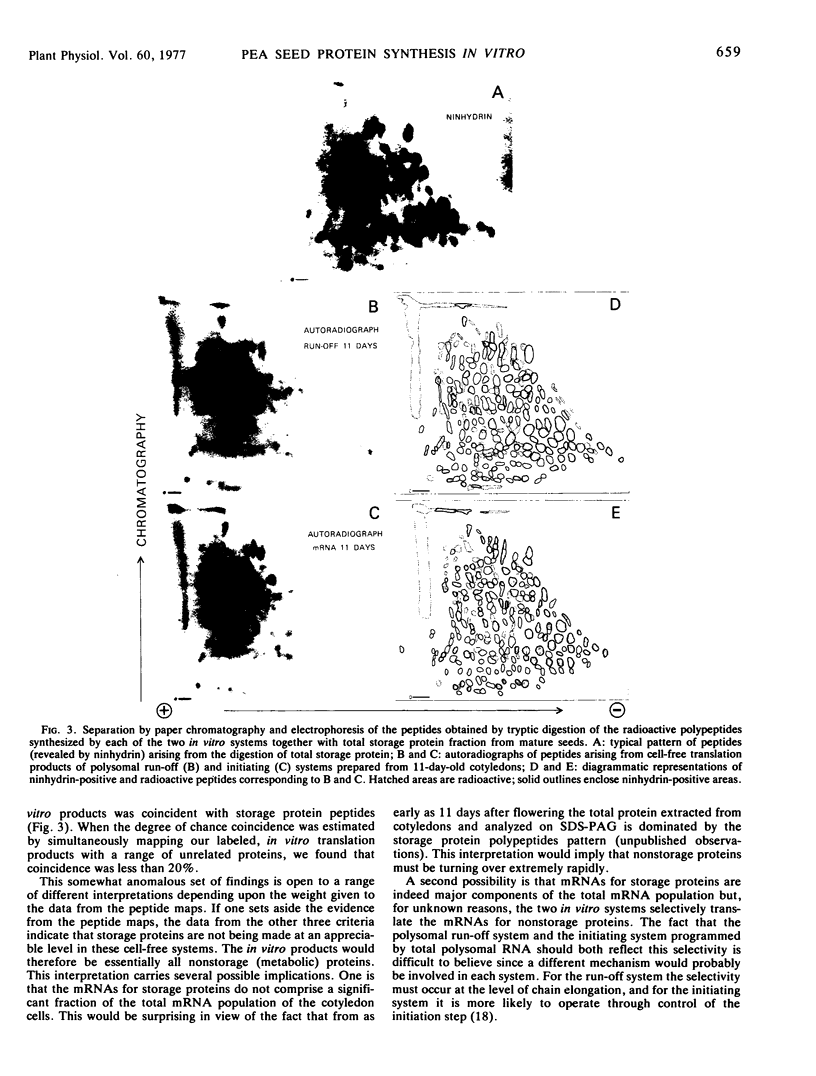
Both polysomes and polysomal RNA, isolated from cotyledons of ripening pea (Pisum sativum) seeds and supplemented respectively with wheat germ S-100 and S-30 fractions, were used to program the cell-free synthesis of polypeptides. The relationship of these polypeptide products to seed storage proteins has been investigated. When fractionated on sucrose density gradients the translation products did not coincide with native storage proteins, nor were they exactly coincident with the subunits of storage proteins on dissociating gels. Treatment with antiserum prepared against storage proteins precipitated only a very small proportion of these products. Nonetheless, tryptic peptide mapping showed that a significant proportion (up to 65%) of the in vitro products from cell-free systems were related to the storage proteins. Alternative interpretations of these results are that either the translatable mRNAs for storage proteins make up a small proportion of the total template isolated from pea cotyledon polysomes, or that storage protein polypeptides are made in significant amounts in vitro but lack major antigenic determinants which in vivo may be acquired during chain completion or post-translational modification.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Lewis J. B., Anderson C. W., Gesteland R. F. Enhanced differential synthesis of proteins in a mammalian cell-free system by addition of polyamines. J Biol Chem. 1975 Jul 25;250(14):5688–5695. [PubMed] [Google Scholar]
- Bailey C. J., Boulter D. The structure of legumin, a storage protein of broad bean (Vicia faba) seed. Eur J Biochem. 1970 Dec;17(3):460–466. doi: 10.1111/j.1432-1033.1970.tb01187.x. [DOI] [PubMed] [Google Scholar]
- Basha S. M., Beevers L. Glycoprotein Metabolism in the Cotyledons of Pisum sativum during Development and Germination. Plant Physiol. 1976 Jan;57(1):93–97. doi: 10.1104/pp.57.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Poulson R. Protein Synthesis in Cotyledons of Pisum sativum L: I. Changes in Cell-Free Amino Acid Incorporation Capacity during Seed Development and Maturation. Plant Physiol. 1972 Apr;49(4):476–481. doi: 10.1104/pp.49.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E., Kafatos F. C. Immunochemistry of an insect protease, cocoonase, and its zymogen. Immunochemistry. 1971 May;8(5):391–403. doi: 10.1016/0019-2791(71)90502-7. [DOI] [PubMed] [Google Scholar]
- Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus mRNA. 3. Discrete polypeptides translated from a monocistronic messenger in vitro. Arch Biochem Biophys. 1972 Dec;153(2):706–713. doi: 10.1016/0003-9861(72)90389-x. [DOI] [PubMed] [Google Scholar]
- Breen M. D., Whitehead E. I., Kenefick D. G. Requirement for extraction of polyribosomes from barley tissue. Plant Physiol. 1972 May;49(5):733–739. doi: 10.1104/pp.49.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr B., Burr F. A. Zein synthesis in maize endosperm by polyribosomes attached to protein bodies. Proc Natl Acad Sci U S A. 1976 Feb;73(2):515–519. doi: 10.1073/pnas.73.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Goodwin P. B., Whitfeld P. R. Occurrence of short particles in beans infected with the cowpea strain of TMV. II. Evidence that short particles contain the cistron for coat-protein. Virology. 1976 Jun;71(2):486–497. doi: 10.1016/0042-6822(76)90376-7. [DOI] [PubMed] [Google Scholar]
- Jackson R., Hunter T. Role of methionine in the initiation of haemoglobin synthesis. Nature. 1970 Aug 15;227(5259):672–676. doi: 10.1038/227672a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Dalby A. In vitro synthesis of zein-like protein by maize polyribosomes. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1048–1054. doi: 10.1016/0006-291x(75)90746-9. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Jones R. A., Tsai C. Y. Isolation and in vitro translation of zein messenger ribonucleic acid. Biochemistry. 1976 Dec 14;15(25):5506–5511. doi: 10.1021/bi00670a014. [DOI] [PubMed] [Google Scholar]
- Lebherz H. G., Doyle D. Synthesis of functional aldolase tetramers in a heterologous cell-free system. J Biol Chem. 1976 Jul 25;251(14):4355–4358. [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Peterson D. M. Cell-free Synthesis of Globulin by Developing Oat (Avena sativa L.) Seeds. Plant Physiol. 1977 May;59(5):836–841. doi: 10.1104/pp.59.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Solubilization of Escherichia coli nitrate reductase by a membrane-bound protease. J Bacteriol. 1975 Mar;121(3):1102–1110. doi: 10.1128/jb.121.3.1102-1110.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Osborn M. The rate of polypeptide chain elongation in a cell-free system from Krebs II ascites cells. Biochim Biophys Acta. 1974 Mar 8;340(2):147–152. doi: 10.1016/0005-2787(74)90107-5. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Differential rates of initiation of conalbumin and ovalbumin messenger ribonucleic acid in reticulocyte lysates. J Biol Chem. 1974 Nov 10;249(21):6779–6787. [PubMed] [Google Scholar]
- RAACKE I. D. Protein synthesis in ripening pea seeds. I. Analysis of whole seeds. Biochem J. 1957 May;66(1):101–110. doi: 10.1042/bj0660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna E., Boime I. mRNA-dependent synthesis of authentic precursor to human placental lactogen: conversion to its mature hormone form in ascites cell-free extracts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1179–1183. doi: 10.1073/pnas.73.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse T. P., Taylor J. M. Translation of albumin messenger RNA in a cell-free protein-synthesizing system derived from wheat germ. J Biol Chem. 1977 Feb 25;252(4):1272–1278. [PubMed] [Google Scholar]