Abstract
Background:
Amniotic membrane is tissue obtained from human placenta rich in cytokines, growth factors, and stem cells that possess the ability to inhibit infection, improve healing, and stimulate regeneration.
Methods:
A meta-analysis was performed examining randomized controlled trials comparing amniotic tissue products with standard of care in nonhealing diabetic foot ulcers including PubMed, Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews.
Results:
A search of 3 databases identified 596 potentially relevant articles. Application of selection criteria led to the selection of 5 randomized controlled trials. The 5 selected randomized controlled trials represented a total of 311 patients. The pooled relative risk of healing with amniotic products compared with control was 2.7496 (2.05725–3.66524, P < 0.001).
Conclusions:
The current meta-analysis indicates that the treatment of diabetic foot ulcers with amniotic membrane improves healing rates in diabetic foot ulcers. Further studies are needed to determine whether these products also decrease the incidence of subsequent complications, such as amputation or death, in diabetic patients.
Since the adoption of the Affordable Care Act in 2010, Congress has mandated medical institutions deliver quality- and value-based healthcare. This is premised on value-based purchasing, decreased infection rates, hospitalizations, and even readmissions. Today in the United States, there are over 29.3 million diabetics, or 9.3% of the population, a number that has steadily increased over the past 10–15 years.1 Nearly 25% of diabetics will develop a diabetic foot ulcer (DFU) in their lifetime, amounting to approximately two-thirds of all nontraumatic amputations performed in the United States, and resulting in a 3-year mortality rate approaching 28%.2 The average 1 year per patient medical cost for DFUs approaches $28,000.3 DFUs not only contribute significantly to morbidity and mortality of individual patients, but impose a substantial financial burden on both taxpayers and private payers.4
Despite the enormous morbidity and cost associated with DFUs, DFUs are notoriously difficult to effectively treat. Several methods have been proposed to augment healing in patients with nonhealing DFUs, including negative pressure therapy, hyperbaric oxygen, growth factor injection, and the use of human or bioengineered skin substitutes; these adjunct treatments have had variable results. Several companies have recently started marketing amniotic tissue products for use in nonhealing DFUs. Treatment with various forms of amniotic tissue has been intermittently attempted since the early 20th century for patients with chronic wounds and burns and has been utilized in ocular surgeries.5–14 Amniotic tissue is rich in cytokines, growth factors, and stem cells that are thought to play a role in improved healing and regeneration and decreased immunogenicity.15–22 Despite evidence that amniotic tissue contains molecules that can significantly impact healing, utilization has been limited by the fear of infectious disease transmission and a need for the technology necessary to properly process and store amniotic-derived products for commercial use. Products that are now becoming available contain amniotic tissue that is either cryopreserved or dehydrated and can be easily implemented in a general practice setting.
Although a wide variety of case studies have been published detailing the use of amniotic tissue in chronic wounds, far fewer randomized controlled trials have been conducted to compare commercially available amniotic tissue products with standard wound care for use in the treatment of diabetic ulcers.23–27 The purpose of this review is to describe commercially available products that have been compared with standard wound care in randomized controlled trials and to synthesize the results of those studies with a meta-analysis. We present a meta-analysis of 5 prospective trials that compared amniotic products with standard of care (SOC) in patients with nonhealing DFUs and cost analysis for the use of amniotic products in these patients.
METHODS
Selection Criteria
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Randomized controlled trials comparing amniotic tissue products with SOC for use in nonhealing DFUs published in peer-reviewed English language journals were included in the review. Studies solely comparing amniotic tissue products with bioengineered skin substitutes were excluded. All publications were limited to the use of human subjects.
Literature Search Strategy
Electronic searches were performed using PubMed, Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews. The following keywords were used: “dehydrated amnion/chorion membrane,” “amniotic membrane diabetic foot ulcers,” “Grafix,” “Epifix,” and “amniotic membrane wound healing.” The reference lists of all retrieved articles were reviewed for identification of potentially relevant studies.
Data Extraction
All data were extracted from article texts and tables. Two investigators reviewed each retrieved article. Assessment for risk of bias was performed based on guidelines proposed by the Meta-Analysis of Observational Studies in Epidemiology Collaboration. Discrepancies between the 2 reviewers were resolved by discussion and consensus and final results were reviewed by a senior investigator.
Outcome Measure
The outcome measure was the proportion of patients with healed ulcers after a set time period. All papers included in this analysis defined healing as complete re-epithelization of the wound. Papers included in this meta-analysis had varied endpoints at which they assessed whether wounds had healed completely. The trial endpoint for selected studies was either 6 weeks or 12 weeks. For studies that included data at various time points, we chose to include the data from the latest possible date of follow-up.
Patients were seen at least once a week by a physician in all 5 of the studies included and the amniotic membrane product was applied in clinic after an initial debridement if necessary and covered with nonadherent and/or moisture-retentive dressings. The product was reapplied as needed or at each weekly visit.
Some studies included comparison of bioengineered skin substitutes to amniotic tissue or SOC. We chose to focus solely on data that compared amniotic tissue products with SOC and therefore only extracted some of the data provided by these studies. Some papers also presented data on average time to heal, but we chose to focus solely on proportion healed, because that measure was consistently reported across all studies.
Statistical Analysis
The relative risk (RR) of healing was used as a summary statistic. A random effects model was tested based on the assumption that study populations were representative of a random sample from the general population rather than identical to one another. Variations in methodology and type of amniotic tissue used also necessitated the use of a random effects model. Heterogeneity was assessed using Q and I2 statistics. The I2 statistic can be calculated as: I2 = 100% × (Q − df)/Q, with Q defined as Cochrane’s heterogeneity statistics and df defined as degree of freedom. The I2 value estimates the amount of variance in a pooled sample that can be accounted for by heterogeneity in the sample of studies. I2 values of 25%, 50%, and 75% were considered indicative of a low, moderate, and high amount of heterogeneity, respectively. The weights assigned to each study sample in our meta-analysis were based on the size of the population enrolled.
Publication bias was not assessed due to the small number of trials in our meta-analysis. The Cochrane meta-analysis guidelines suggest the use of Egger’s test for publication bias for analyses with more than 10 studies. Specific analyses considering confounding factors were not possible because raw data were not available. Statistical analysis was conducted using STATA.
RESULTS
A search of 3 databases identified 596 potentially relevant articles. Application of selection criteria led to the selection of 5 randomized controlled trials. The 5 selected randomized controlled trials represented a total of 311 patients. Of these patients, 52 were treated using bioengineered skin substitutes and were excluded, so data from 259 patients treated with either amniotic tissue products or SOC were included in this review.
Three of the trials included compared Epifix, a dehydrated amniotic membrane product, to SOC.28–30 One trial compared the use of dehydrated amniotic membrane allograft (DAMA), which is also a dehydrated amniotic membrane product, and SOC to SOC alone.31 One trial compared Grafix, a cryopreserved amniotic product to SOC.32 Dehydration and cryopreservation are the 2 major methods used to process amniotic tissue. Cryopreservation is thought to maintain the viability of amniotic tissue, so that native stem cells have the potential to impact healing, whereas the cells in dehydrated products are no longer viable but contain growth factors and cytokines thought to recruit in vivo stem cells to participate in tissue regeneration. A summary of these products is shown in Table 1.
Table 1.
Amnion Products That Have Been Assessed by Randomized Controlled Trials and Were Therefore Included in Our Meta-analysis

The first trial to prospectively evaluate amniotic products to SOC therapies was performed by Zelen et al.28 in 2013. Twenty-five Type 1 or 2 diabetic patients with ulcers >1 and <25 cm2 in size with adequate tissue perfusion, no evidence of infection, and ulcer duration of >4 weeks were enrolled. Twelve patients received SOC and were treated with wound debridement, appropriate moist wound therapy with Silvasorb and Aquacel AG at the discretion of an attending clinician and with compression dressings. Thirteen patients were treated with surgical debridement of necrotic tissue followed by weekly applications of Epifix, a DAMA, which was covered with a nonadherent dressing and a moisture-retentive dressing. Patients were seen at least once a week by the study investigator for up to 12 weeks or until the time of complete healing. Ulcer measurements were conducted every week and wound area was recorded by multiplying width and length. Twelve of the 13 patients treated with Epifix demonstrated complete wound healing after 6 weeks, compared with only 1 of the 12 patients treated with SOC. Per protocol, patients were allowed to leave the study if they did not achieve at least a 50% reduction in healing by week 6 and 10 of the 12 SOC patients opted to leave the study at 6 weeks.28 We therefore used the rates of healing at the 6-week time point in our analysis.
Zelen et al. conducted 2 subsequent multicenter trials comparing Epifix to SOC and to Apligraf, a bioengineered skin substitute. A 2015 trial enrolled 20 patients to receive Epifix, 20 patients to receive Apligraf, and 20 patients to receive SOC.29 As defined in our search criteria, we compared only Epifix and SOC patients in our meta-analysis. Inclusion criteria and evaluation of healing were the same as those used in the 2013 trial, except photographs were taken of the wounds for validation of healing because the study was conducted at 3 different centers. At the conclusion of the study, the photographic images were reviewed by the investigator, as well as a plastic surgeon and a vascular surgeon, to validate that complete re-epithelization was achieved. After 6 weeks, 95% of Epifix patients (19/20) achieved complete re-epithelization compared with 45% of Apligraf patients (9/20) and 35% of patients (7/20) receiving SOC.29
To assess outcomes at later follow-up times, this 2015 trial was extended to include an additional 40 patients and all 100 patients were evaluated for rates of complete wound healing at 12 weeks of follow-up.30 Ninety-seven percent of patients (31/32) treated with Epifix demonstrated complete healing at 12 weeks compared with 73% of patients (24/33) treated with Apligraf and 51% of patients (18/35) treated with SOC. Our analysis compares only amniotic products to SOC, yet this trial also found that patients treated with Epifix were significantly more likely to heal completely than those treated with Apligraf. Apligraf patients required an average of 6 grafts to achieve healing, compared with an average of only 2.5 for patients treated with Epifix.30
In a prospective trial implemented at 8 clinical sites in the United States, Snyder et al.31 compared outcomes of patients treated with a different dehydrated amniotic membrane product, DAMA, and SOC with patients treated with SOC alone. Inclusion criteria were modeled after Zelen et al.28–30 SOC involved debridement of necrotic tissue as well as hemostasis, moist wound dressings, and off-loading where appropriate with a DH Walker boot and infection surveillance. Patients were seen by a clinician at least once a week, at which point those in the DAMA+SOC group could receive reapplication of DAMA at the discretion of the clinician. Fifteen patients were randomized to receive DAMA+SOC and 14 were randomized to receive SOC alone, yet there were 4 early withdrawals in each group due to withdrawn consent, infection, protocol violations, or loss to follow-up. In an intention to treat analysis including these patients, 5 of the 15 original DAMA+SOC patients demonstrated complete healing at 6 weeks compared with 0 of the original SOC patients (P = 0.017). This difference was more significant in the per protocol population (P = 0.0083), with 45.5% of the DAMA patients achieving complete healing at 6 weeks.31 As it more closely mimics clinical practice, we chose to use values from the intention to treat analysis in our meta-analysis.
Although 4 of the trials included in our review evaluated dehydrated amniotic products, Lavery et al.32 compared Grafix, a cryopreserved amniotic product, with SOC. Similar to Zelen et al. and Snyder et al., patients needed to demonstrate adequate tissue perfusion (Ankle Brachial index 0.7–1.3) and lack of infection, and they have an ulcer present for at least 4 weeks to be included. Patients were seen at weekly visits, during which wounds were appropriately cleaned and debrided. All patients received SOC, which included debridement, off-loading as necessary and nonadherent dressings. Patients randomized to receive Grafix also had the product applied using an applicator, so that it came in full contact with the wound and edges at each weekly visit. Patients who demonstrated complete wound healing had healing confirmed at a subsequent visit 2 weeks later and continued to be followed throughout the rest of the study. In the 12-week study period, 62% of patients (31/50) treated with Grafix + SOC achieved healing compared with 21.3% of those (10/47) treated with SOC alone. Of the patients who achieved complete healing, ulcers remained closed in 82.1% of patients (23/28) treated with Grafix + SOC compared with 70% of patients (7/10) treated with SOC in a subsequent 12-week follow-up period. Twenty-six of the patients that failed SOC therapy were then allowed to cross over to receive Grafix therapy and 67.8% of these patients demonstrated complete healing after 12 weeks. Additionally, only 18% of patients treated with Grafix developed wound-related infections, compared with 36.2% of controls (P = 0.044), with only 6% of patients treated with Grafix hospitalized for complications related to infection, compared with 15% of controls.32
A meta-analysis of these 5 randomized controlled trials comparing amniotic tissue products to SOC was performed using a random effects model. Chi-squared analysis of our 5 studies demonstrated an I2 of 50.5%, indicating moderate heterogeneity between the studies (heterogeneity chi-squared = 8.08 (df = 4, P = 0.089). Table 2 describes the RR of healing with amniotic products compared with SOC. The pooled RR of healing, which was defined as the proportion of patients with complete wound re-epithelization, with amniotic products compared with control was 2.7496 (2.05725–3.66524). A test of RR = 1 demonstrated a P value of <0.001, indicating that the increased RR of healing seen with amniotic products is statistically significant (Fig. 1).
Table 2.
RR of Healing with Amniotic Products Compared with SOC
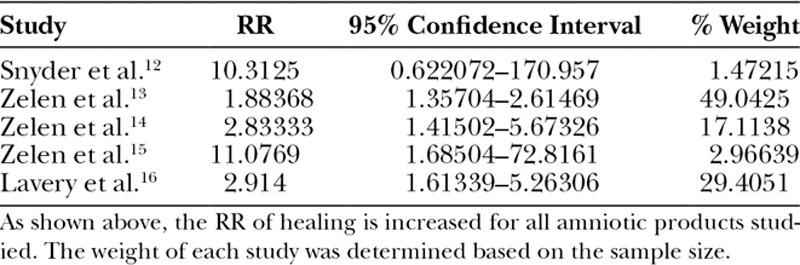
Fig. 1.
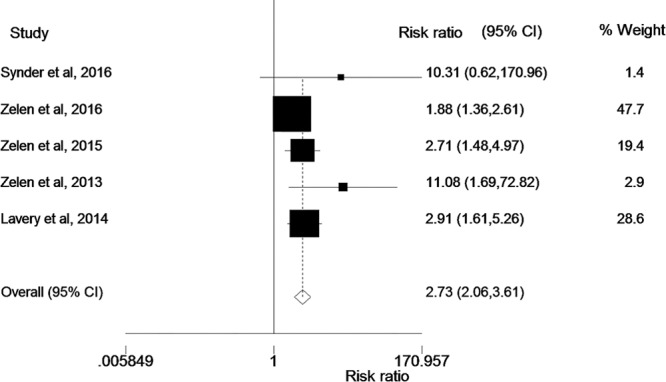
As shown in this figure, the RR of wound healing is significantly greater with the use of amniotic products when compared with SOC.
Cost Analysis
Zelen et al.30 found that patients required an average of 2.5 applications of Epifix to achieve complete healing. The cost of these products has been estimated to be between $500 and $1000, therefore estimating the average cost per patient to be between $1250 and $2500. In their cost analysis, Zelen et al. found that the average cost per healed DFU in the amniotic membrane group was $1517. In comparison, the average 1 year per patient medical cost of DFUs is estimated at $28,000.3 This increased financial burden arises from a mixture of increased emergency room visits, hospital admissions, home healthcare, and outpatient physician and advanced care visits. Patients who fail to heal with traditional SOC are at risk for chronic ulcers or limb loss, with the average cost of major amputations approaching $19,000 per patient per admission.33
DISCUSSION
Despite the fact that up to a quarter of all diabetic patients will develop a diabetic ulcer in their lifetime, traditional therapeutics such as dressing changes, debridements, heterogenic dressings, and hyperbaric oxygen treatments result in wound healing and closure in only 60%–80% of these patients.34 The significant morbidity imposed by these nonhealing ulcers is evidence that newer therapies, such as amniotic membrane products, are needed. Our meta-analysis of 5 prospective trials that compared amniotic membrane products to SOC demonstrates that these products have significant potential to improve healing in patients with DFUs.
The main components of amniotic membrane that are thought to be involved in its regenerative properties include structural collagen and an extracellular matrix, biologically active cells and a wide variety of growth factors and cytokines involved with tissue repair. Amniotic membrane products have been shown to contain growth factors involved with healing and to upregulate the in vitro synthesis of growth factors by dermal fibroblasts.15,16 These products have also been shown to contain growth factors involved in angiogenesis, to upregulate the production of these angiogenic factors in vitro and to substantially increase the number of CD31+ vessels found in sites of healing in mouse models.17,18 Enzyme-linked immunosorbent assays have also demonstrated that these products contain significant quantities of interleukins and tissue inhibitors of metalloproteinases that are known to decrease inflammation.17 When cultured with dehydrated amniotic tissue, endothelial cells were found to downregulate expression of tumor necrosis factor-alpha.17 In addition to containing and recruiting important growth factors and cytokines, cryopreserved amniotic products are thought to retain native amniotic stem cells and dehydrated products have been shown to recruit stem cells to sites of healing. Dehydrated amniotic products have been shown to upregulate the synthesis of adipose-derived stem cells, hematopoietic stem cells, and mesenchymal stem cells when cultured in vitro and have also been shown to increase the number of CD34+ cells and hematopoietic stem cells present in subcutaneous tissue samples when compared with control in animal models.18,19 A major proposed benefit of amniotic products compared with bioengineered skin substitutes is that it is thought to be nonimmunogenic. In vitro, amniotic membrane has been shown to decrease the proliferation and inflammatory response of alloreactive T cells and lymphocytes cultured with amnion have demonstrated decreased synthesis of Th1 and Th2 cytokines.20,21 Amniotic membrane has also been shown to lack HLA-A, B, C, or DR antigens and beta-2 microglobulin, which may contribute to the decreased alloreactivity thought to be associated with these products.22 Although studies evaluating these properties associated with amniotic tissue are primarily limited to in vitro and animal models, they provide evidence to support the regenerative effect seen with amniotic tissue products.
Increased proportions of complete healing with amniotic products were seen in all 5 of the trials included in our analysis, shown in Table 2 and Figure 1. Although 4 of the trials involved dressing changes and reapplication of amniotic product every week, Snyder et al.31 allowed the use of amniotic products to be at the discretion of the clinician at each weekly follow-up. This did not result in a significant decrease in healing rates for these patients. Lavery et al.32 found that the 50 patients treated with amniotic products experienced far fewer adverse events than 47 control patients, whereas Snyder et al.31 found that rates of infection were similar between the 2 groups in their smaller 29 patient cohort. Additionally, there did not seem to be a significant difference between outcomes with dehydrated amniotic products such as Epifix or DAMA and cryopreserved products such as Grafix, although further studies would be necessary to adequately evaluate differences between the 2 types of amniotic tissue products. In addition to the increased proportions of complete healing seen in the amniotic membrane group, Zelen et al.28–30 repeatedly demonstrated that reduction in wound size was significantly greater in patients treated with amniotic membrane than those treated with SOC or bioengineered skin substitutes. Lavery et al.32 found that the mean time to healing was 42 days in the group treated with Grafix compared with 69.5 days in the SOC group (P = 0.019), whereas Zelen et al. found that the main time to heal was 23.6 in the group treated with Epifix compared with 57.4 days in the group treated with SOC (P = 3.2 × 10–7).31
Although our analysis indicates that amniotic membrane has great potential for use in DFUs in clinical practice, patients in all 5 of the included trials had to demonstrate adequate tissue perfusion and a lack of any signs of infection to enroll. As many patients who develop DFUs do not demonstrate adequate tissue perfusion and are often plagued by chronic infections, it is unclear how these products would translate into every day clinical care of diabetic patients. Investigators who were responsible for both treatment and evaluation of healing were not blinded to the randomization of the groups, which may have produced some bias when taking clinical photographs or evaluating lesions. The 2015 and 2016 trials performed by Zelen et al. involved blinded validators who assessed clinical photographs for healing, yet complete healing was only evaluated by site investigators in the other 3 trials. Lavery et al.32 provided 12 weeks of additional follow-up to confirm healing in patients found to have complete re-epithelization, yet the lack of follow-up of these patients is a significant limitation of the identified studies and our review. Additionally, although some studies also included ulcer size and time to healing as primary endpoints, we chose to focus solely on proportion of complete healing, as this was a consistent outcome measure in all 5 studies identified. Further studies are necessary to determine the impact of amniotic membrane on wound size.
Despite the limitations of the included trials and our review, our pooled analysis indicated that amniotic products increase rates of healing in DFUs with an RR of 2.75. Further studies are necessary to confirm the findings identified in these 5 trials and to determine whether amniotic products have the same impact on all diabetic patients seen in clinical practice. Lavery et al.32 also demonstrated that patients treated with amniotic products were significantly less likely to experience adverse events related to their DFUs, such as infection or hospitalization, than those treated with SOC. Further studies are needed to evaluate whether the significantly improved healing rates seen with these products are also associated with a decreased rate of complications such as amputation or death. Amniotic membrane, like any other biologic, is complex, requires significant processing, and has a significant price point. However, when examined in the setting of high-risk wounds with the potential for limb loss, the price becomes negligible. Despite the initial cost associated with these products, amniotic products have been shown to achieve healing with an average of 2.5 applications in a cohort of 50 patients with DFUs.25 With a cost of $500–1000 per application, an average cost of $1250–$2500 is significantly smaller than the average cost burden of $28,000 per patient per year associated with DFUs.3 These data strongly favor the use of amniotic membrane to improve wound healing with potentially significant cost savings. Amniotic membrane and amnion itself are valuable products that should be revisited for their regenerative and antibacterial properties, not only for DFUs, but for other high-risk surgical procedures including cardiac surgery, abdominal surgery, and implant-based procedures.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.National Center for Health Statistics. Statistics, N.C.f.H., Health, United States, 2015: With Special Feature on Racial and Ethnic Disparities. 2016Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- 2.Gregg EW, Sorlie P, Paulose-Ram R, et al. ; 1999-2000 national health and nutrition examination survey. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–1597.. [DOI] [PubMed] [Google Scholar]
- 3.Hicks CW, Selvarajah S, Mathioudakis N, et al. Trends and determinants of costs associated with the inpatient care of diabetic foot ulcers. J Vasc Surg. 2014;60(5):1247–1254, 1254.e1–1254.e2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice JB, Desai U, Cummings AK, et al. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37:651–658.. [DOI] [PubMed] [Google Scholar]
- 5.Fetterolf DE, Snyder RJ. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds. 2012;24:299–307.. [PubMed] [Google Scholar]
- 6.Adly OA, Moghazy AM, Abbas AH, et al. Assessment of amniotic and polyurethane membrane dressings in the treatment of burns. Burns. 2010;36:703–710.. [DOI] [PubMed] [Google Scholar]
- 7.Salehi SH, As’adi K, Mousavi SJ, et al. Evaluation of amniotic membrane effectiveness in skin graft donor site dressing in burn patients. Indian J Surg. 2015;77(Suppl 2):427–431.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammadi AA, Johari HG, Eskandari S. Effect of amniotic membrane on graft take in extremity burns. Burns. 2013;39:1137–1141.. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadi AA, Seyed Jafari SM, Kiasat M, et al. Effect of fresh human amniotic membrane dressing on graft take in patients with chronic burn wounds compared with conventional methods. Burns. 2013;39:349–353.. [DOI] [PubMed] [Google Scholar]
- 10.Branski LK, Herndon DN, Celis MM, et al. Amnion in the treatment of pediatric partial-thickness facial burns. Burns. 2008;34:393–399.. [DOI] [PubMed] [Google Scholar]
- 11.Bujang-Safawi E, Halim AS, Khoo TL, et al. Dried irradiated human amniotic membrane as a biological dressing for facial burns–a 7-year case series. Burns. 2010;36:876–882.. [DOI] [PubMed] [Google Scholar]
- 12.Mostaque AK, Rahman KB. Comparisons of the effects of biological membrane (amnion) and silver sulfadiazine in the management of burn wounds in children. J Burn Care Res. 2011;32:200–209.. [DOI] [PubMed] [Google Scholar]
- 13.Eskandarlou M, Azimi M, Rabiee S, et al. The healing effect of amniotic membrane in burn patients. World J Plast Surg. 2016;5:39–44.. [PMC free article] [PubMed] [Google Scholar]
- 14.John T. Human amniotic membrane transplantation: past, present, and future. Ophthalmol Clin North Am. 2003;16:43–65, vi.. [DOI] [PubMed] [Google Scholar]
- 15.Koob TJ, Rennert R, Zabek N, et al. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J. 2013;10:493–500.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koob TJ, Lim JJ, Massee M, et al. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater. 2014;102:1353–1362.. [DOI] [PubMed] [Google Scholar]
- 17.Koob TJ, Lim JJ, Massee M, et al. Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell. 2014;6(10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massee M, Chinn K, Lei J, et al. Dehydrated human amnion/chorion membrane regulates stem cell activity in vitro. J Biomed Mater Res B Appl Biomater. 2016;104:1495–1503.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maan ZN, Rennert RC, Koob TJ, et al. Cell recruitment by amnion chorion grafts promotes neovascularization. J Surg Res. 2015;193:953–962.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueta M, Kweon MN, Sano Y, et al. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin Exp Immunol. 2002;129:464–470.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park CY, Kohanim S, Zhu L, et al. Immunosuppressive property of dried human amniotic membrane. Ophthalmic Res. 2009;41:112–113.. [DOI] [PubMed] [Google Scholar]
- 22.Akle CA, Adinolfi M, Welsh KI, et al. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–1005.. [DOI] [PubMed] [Google Scholar]
- 23.Penny H, Rifkah M, Weaver A, et al. Dehydrated human amnion/chorion tissue in difficult-to-heal DFUs: a case series. J Wound Care. 2015;24(3):104, 106–109., 111. [DOI] [PubMed] [Google Scholar]
- 24.Forbes J, Fetterolf DE. Dehydrated amniotic membrane allografts for the treatment of chronic wounds: a case series. J Wound Care. 2012;21:290, 292, 294–296.. [DOI] [PubMed] [Google Scholar]
- 25.Mermet I, Pottier N, Sainthillier JM, et al. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen. 2007;15:459–464.. [DOI] [PubMed] [Google Scholar]
- 26.Sheikh ES, Sheikh ES, Fetterolf DE. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J. 2014;11:711–717.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subrahmanyam M. Amniotic membrane as a cover for microskin grafts. Br J Plast Surg. 1995;48:477–478.. [DOI] [PubMed] [Google Scholar]
- 28.Zelen CM, Serena TE, Denoziere G, et al. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10:502–507.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zelen CM, Gould L, Serena TE, et al. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. 2015;12:724–732.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zelen CM, Serena TE, Gould L, et al. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost. Int Wound J. 2016; 2:272–282.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder RJ, Shimozaki K, Tallis A, et al. A prospective, randomized, multicenter, controlled evaluation of the use of dehydrated amniotic membrane allograft compared to standard of care for the closure of chronic diabetic foot ulcer. Wounds. 2016;3:70–77.. [PubMed] [Google Scholar]
- 32.Lavery LA, Fulmer J, Shebetka KA, et al. ; Grafix Diabetic Foot Ulcer Study Group. The efficacy and safety of Grafix(®) for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11:554–560.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hicks CW, Selvarajah S, Mathioudakis N, et al. Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg. 2016;33:149–158.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boulton AJ, Armstrong DG, Albert SF, et al. ; American Diabetes Association; American Association of Clinical Endocrinologists. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679–1685.. [DOI] [PMC free article] [PubMed] [Google Scholar]


