Abstract
Background:
In immediate tissue expander reconstruction following total mastectomy for breast cancer, indocyanine green angiography (ICGA)–guided skin trimming is useful for the prevention of complications. However, instances of unclear ICGA contrast can occur with this method, which are difficult to judge as to whether preventive trimming is warranted. To further improve the mastectomy flap necrosis rate, more accurate objective parameters are necessary.
Methods:
The degree of clinical improvement was compared between 81 patients trimmed according to the surgeon’s judgment (non-ICGA group) and 100 patients with ICGA-guided trimming (ICGA group). We then retrospectively measured 3 parameters [relative perfusion (RP); time (T) to reach RPmax; and slope (S = RP/T) reflecting the rate of increase to RPmax] by using region of interest analysis software and examined their relationships with skin necrosis.
Results:
The rate of grade III necrosis (reaching the subcutaneous fat layer) was significantly lower in the ICGA group (4.8%) than in the non-ICGA group (17.8%; P < 0.05). The specificity of RP for the diagnosis of skin necrosis was high (98.5%; cutoff value, 34). However, the sensitivities of slope parameters were higher than RP.
Conclusions:
ICGA-guided trimming decreased the rate of deep skin necrosis requiring additional surgical treatment. Region of interest analysis indicated that a relatively low percentage luminescence (RP < 34) was indicative of the need for skin trimming, combined with a slow increase in the perfusion of the mastectomy skin flaps.
INTRODUCTION
The prediction of skin necrosis is important in immediate breast reconstruction with tissue expanders (TE) after total mastectomy for breast cancer. When not treated properly, postoperative necrosis of the mastectomy flap may lead to infection and the subsequent removal of TE. In addition, wide and deep skin necrosis may cause scar contracture, preventing TE from fully expanding the skin and compromising final aesthetic outcomes. More importantly, postoperative adjuvant therapy may be delayed due to the necrosis. Furthermore, repeated surgery is a source of stress to patients.
The incidence of mastectomy flap necrosis varies among institutions.1–3 In addition to patient factors,4,5 the location of the cancer and surgical techniques used to elevate the mastectomy flap can lead to necrosis. Plastic surgeons should prevent postoperative skin necrosis by assessing the condition of the flap, such as the extent of damage and viability. Indocyanine green angiography (ICGA) is an effective method to assess blood flow in the flap. ICGA is widely used due to its technical simplicity and low risk of complications.6 In the field of plastic surgery, ICGA is used in various procedures including the evaluation of elevated flaps,7–10 identification of perforator vessels,11–13 and assessment of blood perfusion to nerves.14 Previous studies reported that rates of postoperative flap necrosis were improved by intraoperative ICGA-guided trimming.15
However, postoperative necrosis can occur even after ICGA evaluation and trimming, presumably because surgeons subjectively determine the area of trimming from the information provided by ICGA contrast. In some cases in which the ICGA contrast is unclear or perfusion develops slowly, it is difficult to judge whether such areas are viable or necrotic. The objective index of relative perfusion (RP), which indicates the percentage of the maximum luminance in the measurement area, can complement surgeons’ subjective judgment.16,17 However, it also has a range of unclear values associated with the marginal zone where necrotic and nonnecrotic tissues coexist.18
Due to the existence of this marginal zone, new parameters other than RP are necessary to further improve the necrosis rate and reduce complications. We introduced ICGA-guided trimming in 81 patients from the time of its introduction in 2012 (ICGA group) and compared the flap necrosis rate with patients in whom trimming was determined by the surgeon’s judgment (non-ICGA group) to evaluate the clinical advantages of ICGA-guided trimming. We then retrospectively investigated the occurrence of flap necrosis in the ICGA group. We measured 3 parameters (RP, T, and S) at necrotic and viable areas by using region of interest (ROI) analysis software and investigated their relationships with skin necrosis. Finally, the sensitivity, specificity, and cutoff value of each parameter for the diagnosis of skin necrosis were determined, and the clinical application of these values is discussed.
PATIENTS AND METHODS
Comparison of Outcomes between the ICGA Group and non-ICGA Group
To determine the necrotic area during surgery, an ICGA system was introduced at Shizuoka Cancer Center Hospital in April 2012. Eighty-three cases of total mastectomy and 1-stage immediate breast reconstruction with TE (81 patients, ICGA group) were performed between April 2012 and August 2014. We compared these cases with 101 cases of the same surgical procedure performed without ICGA (100 patients) between April 2006 and March 2012 (non-ICGA group). In the non-ICGA group, the skin was trimmed according to the surgeon’s judgment intraoperatively. All patients in both groups were treated primarily with surgery; none had received prior radiation or breast surgery. In all cases, total mastectomy was performed by a simple ellipse incision; cases of nipple-sparing mastectomy were excluded from this study. Conventional diathermy technique was used to elevate the flap. Surgery was performed by residents and the specialist team. The chief surgeon in the breast surgery department did not change throughout the study period. The indication for immediate TE reconstruction after total mastectomy was limited to stage II disease.
ICGA imaging was performed and recorded using a Photodynamic Eye imaging system (PDE; Hamamatsu Photonics K.K., Hamamatsu, Japan; Fig. 1). After total mastectomy, TE were inserted into the subpectoral space. As women’s breasts tend to be smaller in Asia than in other countries, in most cases it was possible to cover the TE completely with the serratus anterior muscle fascia. In the case of large breasts, coverage may be partial. The initial fill volume of TE is 50 ml. ICGA was performed before skin closure. No epinephrine was injected into the surgical site. Observation was performed using a camera mounted 30 cm above the operative field. After an intravenous bolus injection of 2 ml ICG (25 mg of diagnogreen; Daiichi Sankyo Company, Tokyo, Japan) dissolved in 10 ml of physiological saline, measurement started when the monitor showed the first pass of ICG into the breast skin. ICGA findings were evaluated after 2 minutes of imaging. The surgeon marked the nonenhanced areas according to the image on the monitor.
Fig. 1.

A schematic illustration of PhotoDymamic Eye (PDE).
Before trimming, the surgeon examined the mastectomy site for tension that might complicate wound closure. In such cases, we first reduced the volume of saline injected into the TE sufficiently to eliminate the skin tension. Secondarily, a portion of the low-intensity area was left partially untrimmed if the skin tension was strong. The surgical wound was covered by a film dressing to enable the observation of postoperative healing. Any changes such as congestion or necrosis of the wound were recorded with photos. Conservative treatment was performed until the extent of necrosis was definitive, and surgical treatment was performed in the case of an unhealed wound within 2 weeks (Fig. 2).
Fig. 2.
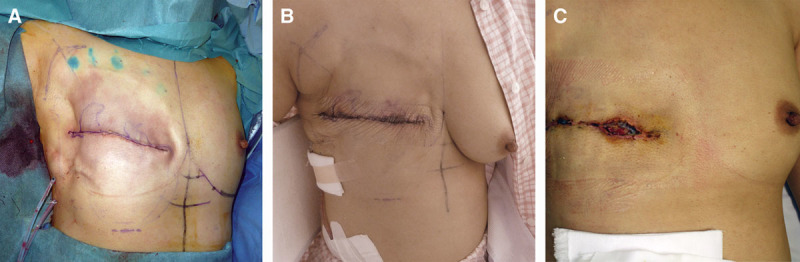
Postoperative view of the mastectomy flap. A, Intraoperative marking of the low-intensity area is shown. Skin trimming was not completely performed because skin tension was strong. B, Partial wound congestion on the fourth day after surgery is shown. C, Total skin necrosis on the 21st day after surgery is shown.
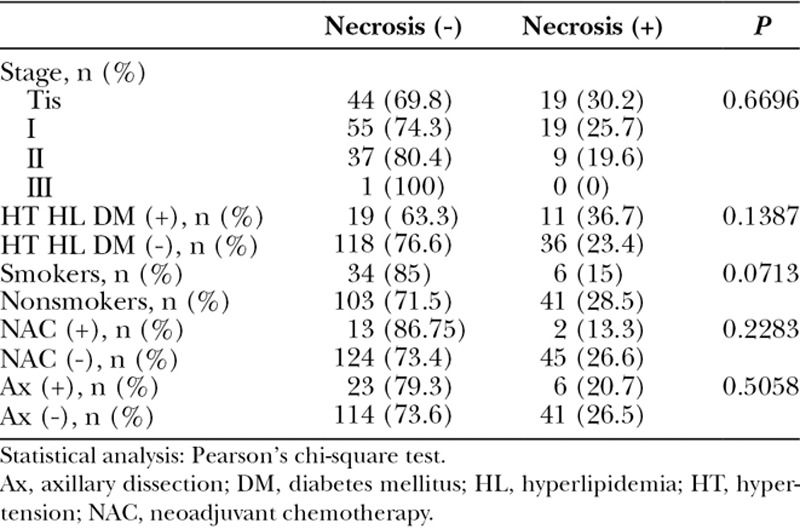
The patient background factors analyzed in this study were tumor stage, comorbidity, history of smoking, preoperative chemotherapy, and axillary lymph node dissection. For statistical analyses, Pearson’s chi-square test was used to determine significant correlations between the background factors and postoperative wound necrosis. Then, the incidence of postoperative wound necrosis was compared between the ICGA and non-ICGA groups. In addition, surgical wounds were divided into 3 groups according to the depth of necrosis: grade I, epidermal necrosis; grade II, necrosis reaching to the dermis; and grade III, necrosis reaching to the subcutaneous fat layer. Necrotic wounds were treated conservatively or surgically (resuturing or skin graft after debridement), and the treatment outcome was compared. The rates of TE infection and removal were analyzed as surgical complications.
Study for New Parameter Detection Using ROIs
ROI Analysis Software
ROI analysis software (Hamamatsu Photonics K.K., Hamamatsu, Japan) was used to calculate RP, T, and S.19 RP was defined as the percentage of the maximum luminance in the measurement area. T was defined as the time to reach the maximum intensity of RP (RPmax). S was defined as the rate of increase to RPmax. T and S were each divided into 3 sections (Fig. 3). These measurements were recorded intraoperatively in real time. Using recorded ICGA images, it was possible to measure ROIs retrospectively on a personal computer.
Fig. 3.
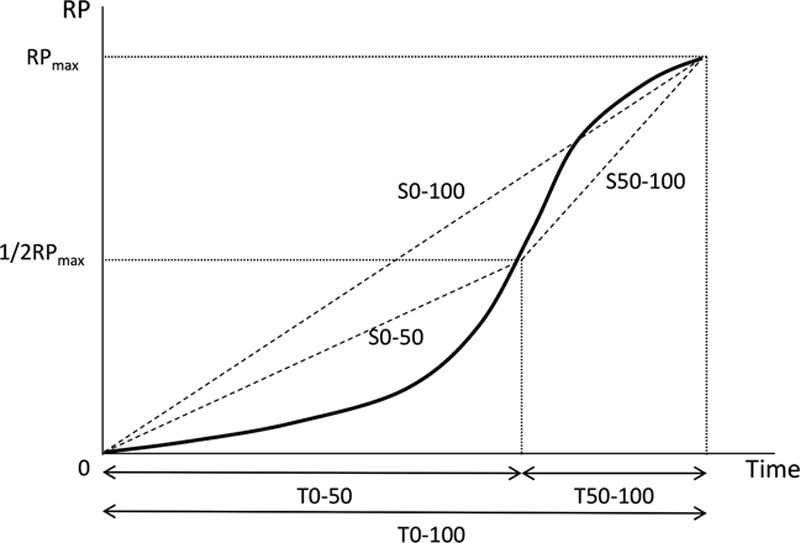
Definition of RP, T, and S. RP, relative perfusion; RPmax, maximum intensity of RP; T0–50, time to reach the 1/2 RPmax; T50–100, time to reach RPmax from 1/2 RPmax; T0–100, time to reach RPmax from T = 0; S0–50, slope of the intensity to reach 1/2 RPmax; S50–100, slope of the intensity to reach RPmax from 1/2 RPmax; S0–100, slope of the intensity to reach RPmax from T = 0.
Patients for Measurement
To evaluate the potential of these values as new assessment parameters, we extracted cases of postoperative necrosis from the ICGA group. Of the 83 cases, 17 developed postoperative necrosis. Accurate ROI data were obtained from 8 of these 17 patients. The following cases were excluded because continual measurement was not possible to calculate the average values: a fixed PDE camera was unavailable in 5 cases, the operating table moved during recording in 2 cases, the start of recording was delayed in 1 case, and the necrotic area was too small for measurement in 1 case.
Evaluation for Diagnostic Rate of Skin Necrosis Using ROIs
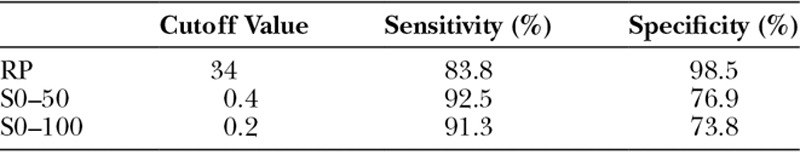
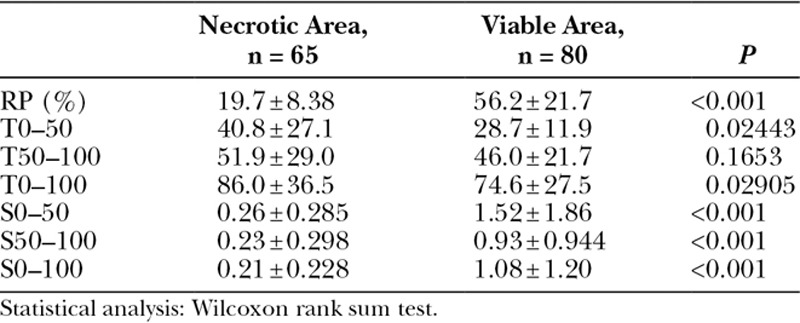
We measured RP, T, and S in both postoperatively necrotic(Fig. 4A) and viable (Fig. 4B, C) areas. The areas where skin necrosis occurred were confirmed in postoperative photographs, and the same regions were identified on the ICGA images recorded intraoperatively. Two viable areas were selected for measurement: 1 in the vicinity of a necrotic area (Fig. 4B) and another on the contralateral side to the necrotic area, separated by the surgical wound (Fig. 4C). In cases of 2 necrotic areas in 1 patient, measurements were taken at both areas. Each viable and necrotic area was measured by setting 5 ROI measurement points per area (Fig. 4 squares). A total of 65 necrotic points (only 5 cases had 2 necrotic areas each) and 80 viable points were measured from the 8 patients. The measured values of RP, T, and S were compared between the necrotic and viable areas using the Wilcoxon test. In addition, to evaluate the diagnostic rate of skin necrosis, the cutoff values, sensitivities, and specificities were calculated (Table 4).
Fig. 4.
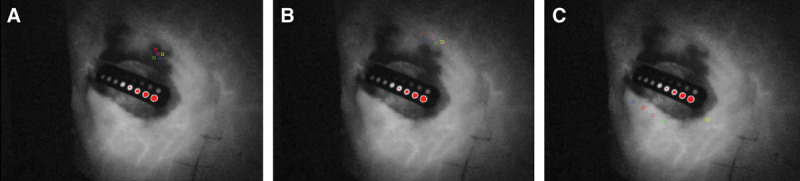
Intraoperative records of the ICGA findings. Calculation points (square points) of RP, T, and S are presented. A control kit–implanted ICG is shown in the center of the wound. A, Necrotic areas were measured at 5 points (same location as the congestive area in Figure 2B). B, Viable areas in the vicinity of the necrotic region were measured at 5 points. C, Viable areas on the contralateral side of the necrotic area were measured at 5 points.
Table 4.
Cutoff Values, Sensitivities, and Specificities of RP, S0–50, and S0–100
RESULTS
Table 1 shows the patient background characteristics. Postoperative wound necrosis was not correlated with stage, comorbidity, history of smoking, preoperative chemotherapy, or axillary lymph node dissection. The rate of necrosis tended to be higher in patients with a comorbidity, although this difference was not significant.
Table 1.
Patient Background and Mastectomy Flap Necrosis Rate
The total incidence of necrosis was 31.7% in the non-ICGA group and 20.5% in the ICGA group. The rate of necrosis in the ICGA group was not statistically significantly lower (P = 0.0961; Table 2). However, the rate of grade III necrosis (reaching the subcutaneous fat layer) was significantly lower in the ICGA group (4.8%) than in the non-ICGA group (17.8%; P < 0.05). Consequently, surgical treatment after necrosis, such as resuturing or skin grafting after debridement, was necessary in 2.4% of cases in the ICGA group compared with 13.9% in the non-ICGA group (P < 0.05). The rate of TE infection/removal was 5.0% in the ICGA group and 4.8% in the non-ICGA group, with no difference between the groups.
Table 2.
Comparison of Flap Necrosis, Wound Depth, Surgical Treatment, and the Complication Rate Between the No ICG and ICG Groups
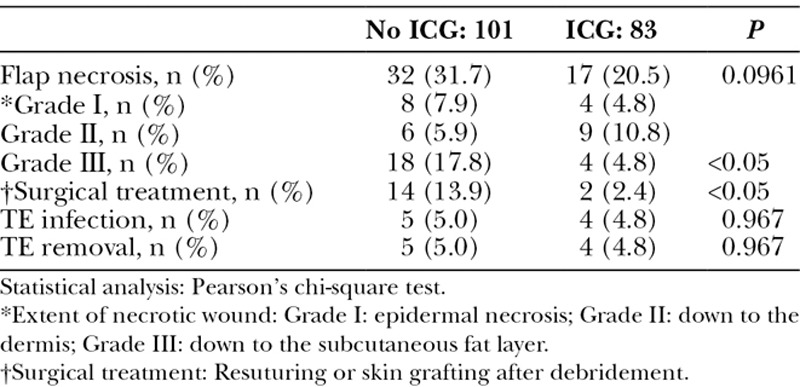
Table 3 shows the mean values of the ROI measurements. RP and S differed significantly between the necrotic and viable areas (P < 0.001). A significant difference was also observed in T0–50 and T0–100 (P < 0.05) but not in T50–100 (P = 0.1653).
Table 3.
Comparison of the Measured RP, T, and S Values in the Necrotic and Viable Areas (mean ± SD)
The sensitivities, specificities, and cutoff values of each parameter are shown in Table 4. The specificity of RP was 98.5% at a cutoff value of 34. The sensitivities of S0–50 and S0–100 were higher than that of RP.
DISCUSSION
The use of ICGA to visualize blood flow in a skin flap was first reported by Still et al.20 in 1999. In that study, blood flow was evaluated in random pattern flaps used to reconstruct burn scar contracture. In the field of breast reconstruction, ICGA has been used to identify angiosomes in deep inferior epigastric perforator or transverse rectus abdominis flap elevation,21–24 making ICGA imaging essential for safe surgery.25 In 2009, Komorowska-Timek and Gurtner15 reduced the incidence of flap necrosis from 15.1% to 4.0% by performing preventive ICGA-guided trimming after skin-sparing mastectomy.
In our study, the overall incidence of necrosis was not significantly improved by ICGA. However, we observed a decrease in the rate of grade III necrosis (P < 0.05). By contrast, grade II necrosis increased. Consequently, ICGA-guided trimming reduced the number of cases requiring additional surgery such as resuturing or skin grafting (P < 0.05), which suggests that ICGA could reliably identify high-risk sites and have additive value compared to the surgeon’s judgment alone. Otherwise, there are cases where the area of unclear ICGA contrast is not completely resected and cases where sufficient trimming is impossible due to skin tension. We think that such insufficient trimming remains as shallow necrosis, which is one reason why the total incidence of necrosis did not demonstrate statistically significant improvement. Immediate autologous flap reconstruction is expected to reduce grade II necrosis because the areas of unclear intensity are trimmed sufficiently regardless of skin tension. The present results are limited to cases of immediate TE reconstruction.
Previous studies of ICGA-guided trimming have shown that RP values are useful indices in addition to ICGA findings.16,17 However, Moyer et al. reported that it was difficult to predict the incidence of necrosis with RP values of 25–45 due to the possible coexistence of viable and nonviable areas.18 In search of novel parameters to predict necrosis, we retrospectively analyzed RP, T, and S in necrotic and viable areas. As shown in Table 3, T0–50 and T0–100 tended to be longer in necrotic areas (P < 0.05), whereas T50–100 did not differ between the 2 areas, suggesting that necrotic areas had vascular damage causing the first half of contrast perfusion to be markedly slower than that of the viable area. By contrast, in the latter half of the contrast perfusion, vascular occlusion due to surgical damage may have evolved to leakage into the intratissue space. Significant difference was particularly observed in the RP and S between necrotic and viable areas (P < 0.001; Table 3). The cutoff values, sensitivities, and specificities shown in Table 4 were determined on the basis of the maximum values of Youden’s index. This result indicated that predictive trimming is necessary in cases where RP is lower than 34 because the specificity of RP at this cutoff value was high. In addition to lower RP, the sensitivities of S0–50 and S0–100 were higher than that of RP. Therefore, in cases where tissue viability is difficult to judge, for example when ICGA contrast is lower compared with nearby healthy skin despite an RP exceeding 34, these 2 parameters (S0–50 and S0–100) might be helpful.
ROI analysis can be performed intraoperatively for clinical use because all parameters are measured simultaneously. Further examination is warranted to confirm its usefulness through a prospective study.
In conclusion, ICGA-guided trimming decreased the number of cases requiring surgical treatment (eg, resuturing or skin graft). ROI analysis indicated that RP and S are useful parameters for the prediction of skin necrosis of a mastectomy flap, with a relatively low-percentage luminescence (RP < 34) and slow increase in slope of mastectomy skin flap perfusion indicating that skin trimming is required.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Antony AK, Mehrara BM, McCarthy CM, et al. Salvage of tissue expander in the setting of mastectomy flap necrosis: a 13-year experience using timed excision with continued expansion. Plast Reconstr Surg. 2009;124:356–363.. [DOI] [PubMed] [Google Scholar]
- 2.Newman MI, Samson MC, Tamburrino JF, et al. Intraoperative laser-assisted indocyanine green angiography for the evaluation of mastectomy flaps in immediate breast reconstruction. J Reconstr Microsurg. 2010;26:487–492.. [DOI] [PubMed] [Google Scholar]
- 3.Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg. 2012;129:778e–788e.. [DOI] [PubMed] [Google Scholar]
- 4.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:28–41.. [DOI] [PubMed] [Google Scholar]
- 5.Alderman AK, Wilkins EG, Kim HM, et al. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109:2265–2274.. [DOI] [PubMed] [Google Scholar]
- 6.Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology. 1994;101:529–533.. [DOI] [PubMed] [Google Scholar]
- 7.Holm C, Mayr M, Höfter E, et al. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg. 2002;55:635–644.. [DOI] [PubMed] [Google Scholar]
- 8.Holm C, Tegeler J, Mayr M, et al. Monitoring free flaps using laser-induced fluorescence of indocyanine green: a preliminary experience. Microsurgery. 2002;22:278–287.. [DOI] [PubMed] [Google Scholar]
- 9.Okazaki M, Tanaka K, Kodaira S, et al. One-stage transfer of 2 paddles of thoracodorsal artery perforator flap with 1 pair of vascular anastomoses for Barraquer-Simons syndrome. J Craniofac Surg. 2012;23:883–885.. [DOI] [PubMed] [Google Scholar]
- 10.Yano T, Okazaki M, Tanaka K, et al. Use of intraoperative fluorescent indocyanine green angiography (ICGA) for real-time vascular evaluation of pericranial flaps. Ann Plast Surg. 2016;76:198–204.. [DOI] [PubMed] [Google Scholar]
- 11.Lee BT, Hutteman M, Gioux S, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in perforator flap breast reconstruction. Plast Reconstr Surg. 2010;126:1472–1481.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azuma R, Morimoto Y, Masumoto K, et al. Detection of skin perforators by indocyanine green fluorescence nearly infrared angiography. Plast Reconstr Surg. 2008;122:1062–1067.. [DOI] [PubMed] [Google Scholar]
- 13.Lee BT, Matsui A, Hutteman M, et al. Intraoperative near-infrared fluorescence imaging in perforator flap reconstruction: current research and early clinical experience. J Reconstr Microsurg. 2010;26:59–65.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka K, Okazaki M, Yano T, et al. Quantitative evaluation of blood perfusion to nerves included in the anterolateral thigh flap using indocyanine green fluorescence angiography: a different contrast pattern between the vastus lateralis motor nerve and femoral cutaneous nerve. J Reconstr Microsurg. 2015;31:163–170.. [DOI] [PubMed] [Google Scholar]
- 15.Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg. 2010;125:1065–1073.. [DOI] [PubMed] [Google Scholar]
- 16.Newman MI, Jack MC, Samson MC. SPY-Q analysis toolkit values potentially predict mastectomy flap necrosis. Ann Plast Surg. 2013;70:595–598.. [DOI] [PubMed] [Google Scholar]
- 17.Munabi NC, Olorunnipa OB, Goltsman D, et al. The ability of intra-operative perfusion mapping with laser-assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. J Plast Reconstr Aesthet Surg. 2014;67:449–455.. [DOI] [PubMed] [Google Scholar]
- 18.Moyer HR, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plast Reconstr Surg. 2012;129:1043–1048.. [DOI] [PubMed] [Google Scholar]
- 19.Igari K, Kudo T, Toyofuku T, et al. Quantitative evaluation of the outcomes of revascularization procedures for peripheral arterial disease using indocyanine green angiography. Eur J Vasc Endovasc Surg. 2013;46:460–465.. [DOI] [PubMed] [Google Scholar]
- 20.Still J, Law E, Dawson J, et al. Evaluation of the circulation of reconstructive flaps using laser-induced fluorescence of indocyanine green. Ann Plast Surg. 1999;42:266–274.. [DOI] [PubMed] [Google Scholar]
- 21.Holm C, Mayr M, Höfter E, et al. Perfusion zones of the DIEP flap revisited: a clinical study. Plast Reconstr Surg. 2006;117:37–43.. [DOI] [PubMed] [Google Scholar]
- 22.Losken A, Zenn MR, Hammel JA, et al. Assessment of zonal perfusion using intraoperative angiography during abdominal flap breast reconstruction. Plast Reconstr Surg. 2012;129:618e–624e.. [DOI] [PubMed] [Google Scholar]
- 23.Francisco BS, Kerr-Valentic MA, Agarwal JP. Laser-assisted indocyanine green angiography and DIEP breast reconstruction. Plast Reconstr Surg. 2010;125:116e–118e.. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi S, De Lorenzi F, Petit JY, et al. The “perfusion map” of the unipedicled TRAM flap to reduce postoperative partial necrosis. Ann Plast Surg. 2004;53:205–209.. [DOI] [PubMed] [Google Scholar]
- 25.Newman MI, Samson MC. The application of laser-assisted indocyanine green fluorescent dye angiography in microsurgical breast reconstruction. J Reconstr Microsurg. 2009;25:21–26.. [DOI] [PubMed] [Google Scholar]


