Abstract
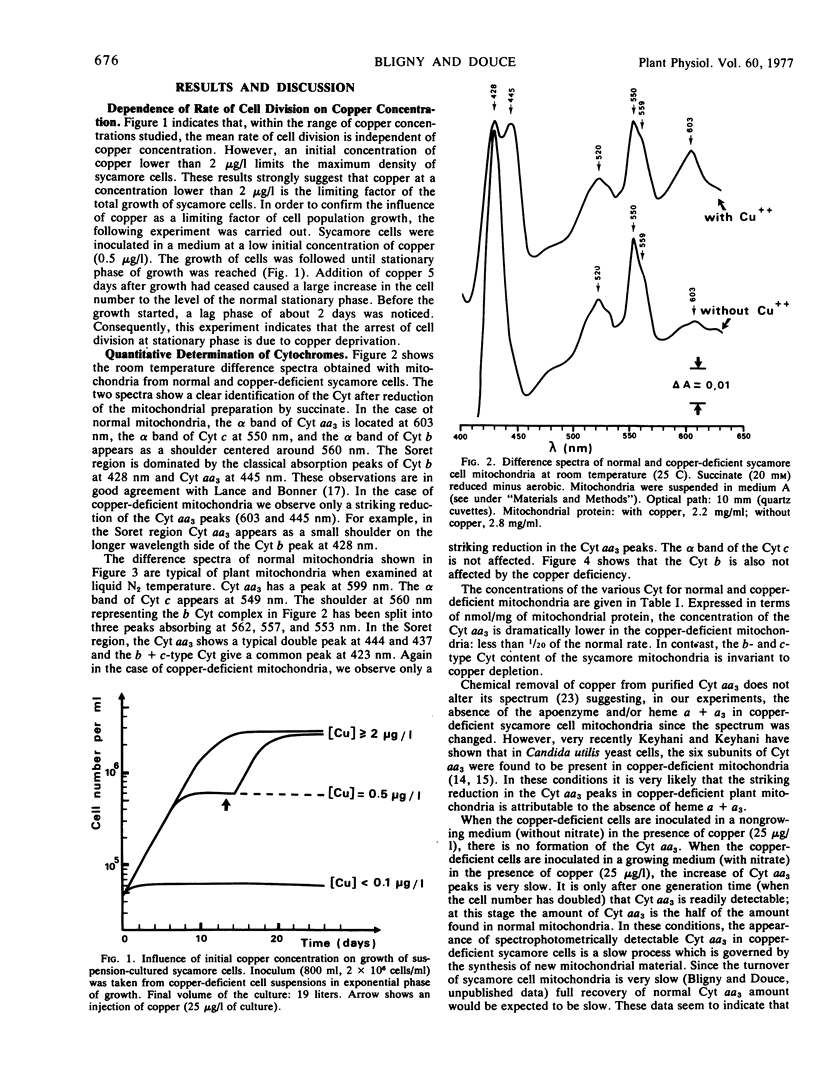
The effects of copper deficiency on cell culture growth, cell respiration, mitochondrial oxidative properties, and electron transport chain have been studied with suspension-cultured sycamore cells (Acer pseudoplatanus L.). Within the range of the copper concentration studied (0.1-25 μg/1 of culture medium), the mean rate of cell division is independent of copper concentration. An initial copper concentration lower than 2 μg/1 limited the maximum density of population reached at the stationary phase of growth.
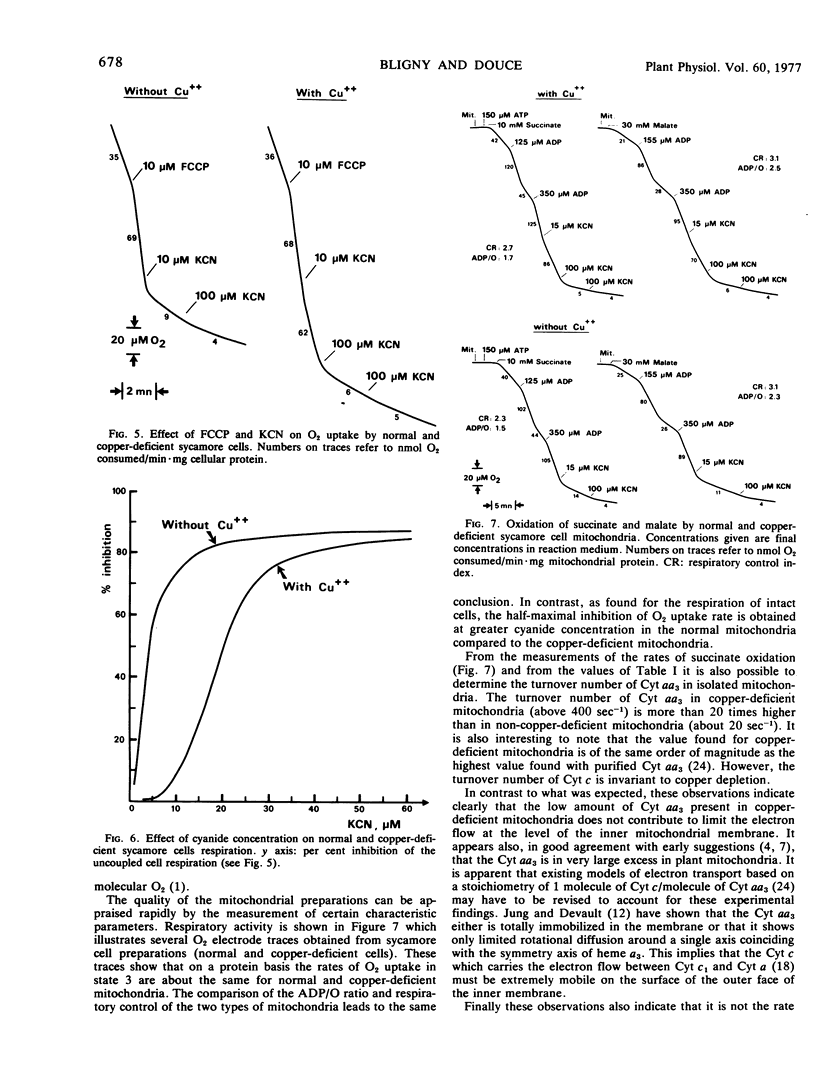
On a protein basis, the uncoupled O2 uptake rates were about the same for normal and copper-deficient cells. In contrast, the half-maximal inhibition of O2 uptake rate was obtained at greater KCN concentration in the normal cells (20 μM) compared to copper-deficient cells (2 μM). Similar results were obtained with the normal and copper-deficient sycamore cell mitochondria.
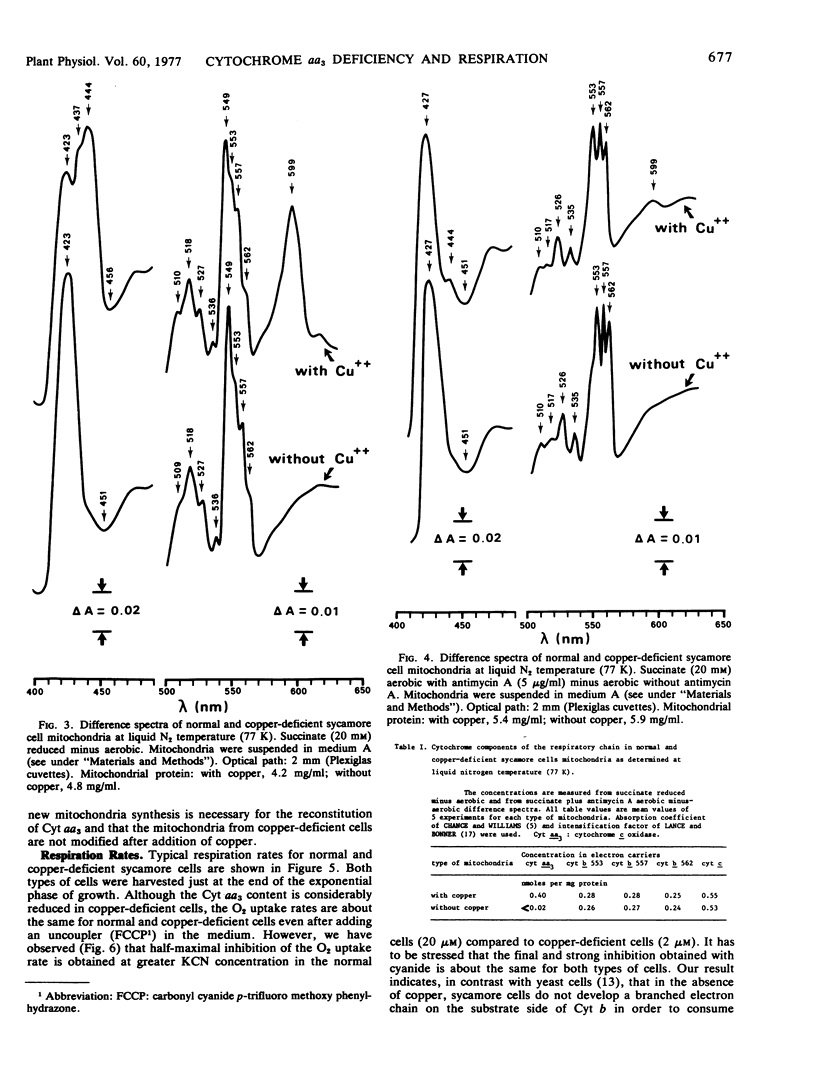
In the copper-deficient mitochondria, the concentration of the cytochrome aa3 was less than 0.02 nmol/mg mitochondrial protein or 1/20 of the normal rate. The b- and c-type cytochrome content was invariant with copper depletion. It appeared that cytochrome aa3 is present in large excess in normal cells. This work also indicated that cytochrome c is a very mobile molecule.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendall D. S., Bonner W. D. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971 Feb;47(2):236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligny R. Growth of Suspension-cultured Acer pseudoplatanus L. Cells in Automatic Culture Units of Large Volume. Plant Physiol. 1977 Mar;59(3):502–505. doi: 10.1104/pp.59.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. II. Difference spectra. J Biol Chem. 1955 Nov;217(1):395–407. [PubMed] [Google Scholar]
- Chance B., Hackett D. P. The Electron Transfer System of Skunk Cabbage Mitochondria. Plant Physiol. 1959 Jan;34(1):33–49. doi: 10.1104/pp.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Eytan G. D., Carroll R. C., Schatz G., Racker E. Arrangement of the subunits in solubilized and membrane-bound cytochrome c oxidase from bovine heart. J Biol Chem. 1975 Nov 25;250(22):8598–8603. [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Correlation of the kinetics of electron transfer activity of various eukaryotic cytochromes c with binding to mitochondrial cytochrome c oxidase. J Biol Chem. 1976 Feb 25;251(4):1104–1115. [PubMed] [Google Scholar]
- Junge W., DeVault D. Symmetry, orientation and rotational mobility in the a3 heme of cytochrome c oxidase in the inner membrane of mitochondria. Biochim Biophys Acta. 1975 Dec 11;408(3):200–214. doi: 10.1016/0005-2728(75)90123-1. [DOI] [PubMed] [Google Scholar]
- Keyhani E., Keyhani J. Cytochrome c oxidase biosynthesis and assembly in Candida utilis yeast cells. Function of copper in the assembly of active cytochrome c oxidase. Arch Biochem Biophys. 1975 Apr;167(2):596–602. doi: 10.1016/0003-9861(75)90503-2. [DOI] [PubMed] [Google Scholar]
- Keyhani Ezzatollah, Chance Britton. Cytochrome biosynthesis under copper-limited conditions in Candida utilis. FEBS Lett. 1971 Sep 15;17(1):127–132. doi: 10.1016/0014-5793(71)80580-x. [DOI] [PubMed] [Google Scholar]
- Keyhani J., Keyhani E. Cytochrome c oxidase biosynthesis and assembly in Candida utilis yeast cells. Subunit structure and molecular weight determination. Arch Biochem Biophys. 1975 Apr;167(2):588–595. doi: 10.1016/0003-9861(75)90502-0. [DOI] [PubMed] [Google Scholar]
- LAMPORT D. T. CELL SUSPENSION CULTURES OF HIGHER PLANTS: ISOLATION AND GROWTH ENERGETICS. Exp Cell Res. 1964 Jan;33:195–206. doi: 10.1016/s0014-4827(64)81026-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lance C., Bonner W. D. The respiratory chain components of higher plant mitochondria. Plant Physiol. 1968 May;43(5):756–766. doi: 10.1104/pp.43.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair P. M., Mason H. S. Reconstitution of cytochrome C oxidase from a copper-depleted enzyme and Cu. J Biol Chem. 1967 Apr 10;242(7):1406–1415. [PubMed] [Google Scholar]


