Abstract
Introduction
To evaluate the utility of 3 Tesla (3T) pelvic phased-array (PPA) multiparametric magnetic resonance imaging (mpMRI) to predict extracapsular extension (ECE) and seminal vesicle invasion (SVI) and its subsequent effect on radical prostatectomy (RP) surgical margin status.
Methods
A retrospective evaluation was conducted of RP patients who underwent preoperative 3T PPA mpMRI (without endorectal coil) based on clinical probability of adverse pathological features. Frequencies, specificity, sensitivity, positive predictive value (PPV), and negative predictive value (NPV) of mpMRI in predicting the status of ECE and SVI were calculated.
Results
Forty-eight consecutive patients were included. Sensitivity, specificity, PPV, and NPV for 3T PPA mpMRI using T2-weighted sequences with diffusion-weighted imaging (DWI) and dynamic contrast enhanced (DCE) imaging to predict ECE was 39%, 56%, 45%, and 50%, respectively, while SVI prediction was 33%, 95%, 50%, and 91%, respectively. Twelve of the 28 cases predicted as being negative for ECE had positive margins, while two of the 20 cases predicted to be positive for ECE had positive margins. Imaging predicted four cases would have SVI, yet two had positive margins, while of the 44 cases predicted as being negative for SVI, four had positive margins.
Conclusions
These findings at our centre suggest that the use of 3T PPA mpMRI using T2-weighted sequences with DWI and DCE in predicting pathological ECE and SVI is of questionable benefit. These mpMRI reports may result in closer dissection of neurovascular bundles and subsequent positive surgical margins. Caution should be exercised when basing intraoperative decisions on mpMRI findings.
Introduction
Multiple tools for diagnosing and staging prostate cancer (PCa) have been developed and used routinely for patient-tailored treatment and management. Imaging modalities, such as transrectal ultrasound (TRUS)-guided biopsy and computed tomography (CT) have assisted in determining the appropriate treatment for patients presenting with PCa.1–3 Despite the utility of these tools, they are often limited in their ability to predict extracapsular extension (ECE) of PCa.3 Data have shown that 27% of patients clinically diagnosed with ECE actually have locally confined disease on pathology, while 25–30% of patients diagnosed with organ-confined disease actually have ECE at final pathology.4,5 Magnetic resonance imaging (MRI) has emerged as a promising method to increase the precision of preoperative staging and to predict ECE and seminal vesicle invasion (SVI). Multiple MRI modalities are available, and the literature reports wide ranges of sensitivity and specificity of its ability to predict ECE and SVI despite modality, calling the validity and utility of MRI for preoperative workup into question.3,6,7 Heidenreich et al stated that multiparametric MRI (mpMRI) with T2-weighted dynamic contrast-enhanced (DCE) with diffusion-weighted imaging (DWI) has excellent sensitivity for detecting Gleason ≥7 PCa; yet these promising results need further confirmation. The cost-effectiveness of mpMRI and interrater reliability among radiologists is a current concern.8 Thus, the ability of mpMRI to detect ECE and SVI has not yet been fully established.
Radical prostatectomy (RP) has emerged as a standard treatment for localized PCa, providing an approximate 10-year progression-free survival rate of approximately 90% of patients.9 The presence of ECE and SVI carries a substantial risk of positive surgical margin rate, compromising postoperative oncological outcomes. It has been reported that 10-year progression-free probability decreases to 71.4% for patients with ECE and 37.4% for patients with SVI.9 Accurate staging, precise preoperative selection of PCa cases, and planning of surgical approach are crucial in achieving ideal oncological and functional outcomes. Preoperative imaging that provides adjunctive staging information may assist planning, thus potentially improving postoperative outcomes.10
We evaluated the ability of 3Tesla (3T) pelvic-phased array (PPA) mpMRI without endorectal coil to predict ECE and SVI prior to RP. We further assessed the consequent effect that MRI findings had on surgical margin status in a cohort of patients treated by a single, experienced urological oncologist.
Methods
Study design and participants
Consecutive patients who underwent MRI prior to RP (robot-assisted, laparoscopic, or open approach) by a single, experienced urological oncologist at our academic tertiary care centre from 2009–2013 were eligible for inclusion in this retrospective review. Preoperative 3T PPA mpMRIs were chosen at the discretion of the surgeon based on high clinical probability of adverse pathological features. Indicators of possible ECE and/or SVI included high prostate-specific antigen PSA (PSA >10 ng/ml), low free-to-total ratio (F/T ratio <0.10), high PSA density (PSAD >0.15), cT3 (a and/or b) disease, high-grade disease (presence of Gleason 4 or 5 via TRUS biopsy), and high-volume disease (multiple cores with >50% involvement). Any combination of the aforementioned indicators, along with comprehensive history and physical examination, prompted MRI investigation in patients with suspected advanced PCa. MRIs were conducted at least six weeks post-biopsy to avoid risk of hemorrhage artifact on T2-weighted images. Given lack of clear guidelines for MRI use in management of PCa, the decision to use preoperative MRI was a clinical one, rather than one based on prescribed parameters.
Test methods
The 3T PPA mpMRI (Phillips Achieva 3.0T, Amsterdam, Netherlands) with T2-weighted DWI and DCE with spectroscopy for axial, sagittal, and coronal views of the prostate was used, employing a standardized protocol for each patient. All images were read by a genitourinary radiologist, who is the sole expert (>10 years of experience) in reporting on mpMRI of the prostate at our high-volume tertiary care academic centre. He was blinded to each patient’s history, including biopsy characteristics. Prostate Imaging Reporting and Data System (PI-RADS) version one was used until the validated version two was released. The RP operative approach, including resection plane and nerve-sparing, was modified by the surgeon based on MRI evidence of ECE and/or SVI presence.
Data analysis
Tumour stage based on preoperative MRI characteristics was compared to final pathological stage. Specificity, sensitivity, positive predictive value (PPV), and negative predictive value (NPV) of 3T PPA mpMRI in predicting ECE and SVI, as well as the prevalence of ECE and SVI and likelihood ratios were calculated in IBM SPSS v.22 (Armonk, NY, U.S.). Positive surgical margin rates were calculated in all patients as a proxy to estimate surgical and oncological outcomes. The effect of radiological ECE findings on surgical margin status post-RP was examined by comparing positive surgical margin rates between patients with positive or negative preoperative ECE findings.
Results
Forty-eight patients comprised the study sample (Table 1). The mean age was 60.9 (± 8.3) and the mean preoperative PSA score was 9.7 (± 2.1). The majority of patients (71%) were classified as intermediate-risk on the D’Amico risk group stratification criteria11 (intermediate: stage T2b or Gleason score of 7 or PSA level of >10 and ≤20ng/mL) and most patients had Gleason 7 disease, both at biopsy (71%) and on surgical pathology (88%). The majority of patients underwent a minimally invasive RP (96%), while 4% had the open approach.
Table 1.
Patient and disease characteristics and subsequent method of surgical intervention
| Patients | ||
|---|---|---|
| Number of participants | 48 | |
| Mean age | 60.9 ± 8.3 | |
| Mean preoperative PSA score | 9.7 ± 2.1 | |
| Gleason score at biopsy | 6 | 12 (25%) |
| 7 | 34 (71%) | |
| 8 | 1 (2%) | |
| 9 | 1 (2%) | |
| Gleason score at RP | 6 | 3 (6%) |
| 7 | 42 (88%) | |
| 8 | 2 (4%) | |
| 9 | 1 (2%) | |
| D’Amico risk stratification | Low | 7 (14.5%) |
| Intermediate | 34 (71%) | |
| High | 7 (14.5%) | |
| Surgical method | Laparoscopic | 32 (67%) |
| Robot-assisted | 14 (29%) | |
| Open | 2 (4%) |
PSA: prostate-specific antigen; RP: radical prostatectomy.
Predictability of ECE and resultant surgical margin rates
The mpMRI reports predicted 20 (42%) patients to be positive for ECE, while final pathology revealed that only nine of these patients were positive for ECE. Of the 28 (58%) patients who were predicted to not have ECE based on mpMRI, 14 had positive ECE on pathology. Preoperative 3T PPA mpMRI achieved a sensitivity of 39% and a specificity of 56% in predicting ECE on surgical pathology, with a PPV and a NPV of 45% and 50%, respectively. Of the 20 patients with positive ECE reported on mpMRI, two (10%) had positive surgical margins. Furthermore, out of the 28 patients predicted to be negative for ECE, 12 (42.9%) had positive surgical margins on pathology. The prevalence of ECE in the patient sample was 47.9%, with a negative likelihood ratio of 1.09 and a positive likelihood ratio of 0.89 (Fig. 1). Subgroup analysis of patients with Gleason score 7–9 revealed similar findings, with sensitivity of 39.1% and specificity of 54.5% for predicting ECE. Further subgroup analysis of patients with low-volume disease (1–2 positive biopsy cores) and high-volume disease (>2 positive biopsy cores) revealed sensitivities of 0% and 47.4% and specificities of 33.3% and 68.4%, respectively.
Fig. 1.
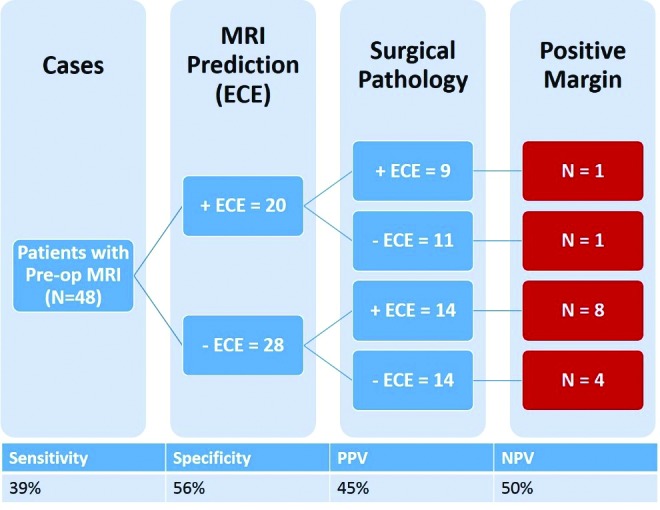
Summary of radiological and pathological extracapsular extension (ECE) and resultant positive margin status in patients who underwent preoperative 3 Tesla pelvic phased-array multiparametric magnetic resonance imaging (MRI). PPV: positive predictive value; NPV: negative predictive value.
Predictability of seminal vesicle invasion
The mpMRI reports predicted four (8%) patients to be positive for SVI. Final pathology showed that two of these patients were positive for SVI. Of the 44 (92%) patients who were predicted not to have SVI, four had positive SVI on pathology. Preoperative 3T PPA mpMRI achieved a sensitivity of 33% and a specificity of 95% in predicting SVI on surgical pathology. The PPV and NPV were 50% and 91%, respectively. Finally, the prevalence of SVI in the sample was 12.5%, while the negative likelihood ratio was 0.70 and the positive likelihood ratio was 7.0 (Fig. 2). Subgroup analysis of patients with Gleason score 7–9 revealed similar findings with sensitivity of 33.3% and specificity of 94.9% for predicting SVI. Further subgroup analysis of patients with low-volume disease (1–2 positive biopsy cores) and high-volume disease (>2 positive biopsy cores) revealed sensitivities of 0% and 50.0% and specificities of 100% and 94.1%, respectively.
Fig. 2.
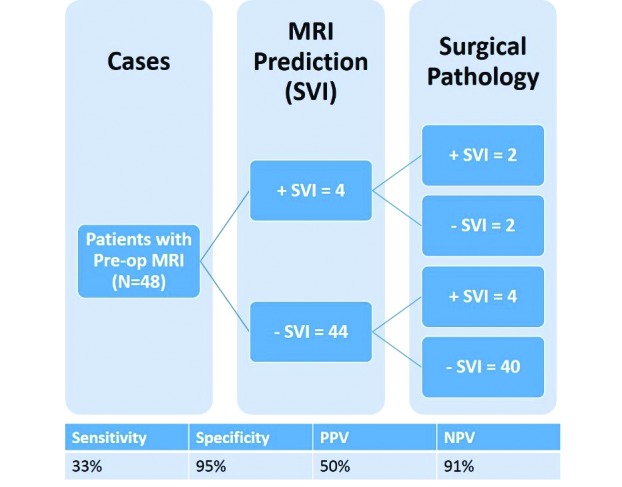
Summary of radiological and pathological seminal vesicle invasion (SVI) status in patients who underwent preoperative 3 Tesla pelvic phased-array multiparametric magnetic resonance imaging (MRI). PPV: positive predictive value; NPV: negative predictive value.
Discussion
Our study of 48 patients with moderate- (71%) to high-risk (14.5%) PCa demonstrated that the results of a 3T PPA mpMRI should be interpreted with caution during RP planning. The sensitivity and specificity of MRI in predicting ECE were 39% and 56%, respectively, suggesting that the use of MRI in our sample was not much different than a coin toss. These findings contrasted studies that have reported sensitivity and specificity ranges of 75–78% and 92–96%.12–14 Evaluation of SVI in our sample showed that although the sensitivity was low at 33%, the specificity was high at 95%, with a PPV of 50% and a high NPV of 91%. This also contrasts with studies that have shown much higher sensitivity and PPV percentages.6,15
The literature suggests that MRI has utility in the workup of PCa.16–19 Kim et al analyzed 32 preoperative patients using a surface coil and a combined DWI and DCE mpMRI technique at 1.5T in predicting the stage, using RP results as the reference standard. In detecting ECE, the combination of these tests displayed 82.4% sensitivity, 87.2% specificity, 70% PPV, and 93.2% NPV.20 In 158 patients with clinical stage T1c disease, Zhang et al analyzed the role of preoperative combined endorectal coil spectroscopic imaging (MRSI) at 1.5T to predict the pathologic stage of PCa. The overall accuracy was 80%, while staging accuracy was higher for the smallest tumour volumes (91% for tumours <0.5cm3 vs. 75% for tumours >2.0cm3). In the detection of ECE, MRSI had an area under the curve of 0.74.21 In a population of 27 patients considered for RP, Augustin et al compared the accuracy of 3T MRI with the Partin tables predicting pathological stage. In detecting ECE, accuracy was 85.2%, sensitivity was 66.7%, and specificity was 100%. The Spearman’s ρ for correlation with ECE was higher for MRI findings (0.780) than for the Partin tables (0.363). They concluded that 3T MRI was significantly more accurate than the Partin tables in predicting the final pathological stage.22
Studies using an endorectal coil tend to report better outcomes than those using a surface magnet. The European Association (EAU) guidelines on PCa (2014) state that the use of an endorectal coil can improve staging accuracy at 1.5T, and results obtained at 3T are superior to 1.5T.8,23 There is a debate as to whether the use of endorectal coils is necessary. Lee and colleagues stated that they are expensive and cause discomfort and potential proctitis and diverticulitis.24 Their study showed no difference in MRI staging accuracy between those with the endorectal coil vs. a surface coil.24 Further, in a retrospective analysis of 32 patients with moderate- to high-risk PCa, Kim et al reported that surface coil at 1.5T yielded sensitivity and specificity of 83.3% and 92.3%, respectively, in predicting SVI.20 Uncertainty remains regarding the most useful coil and modality of MRI in staging PCa.
The relative ineffectiveness of 3T PPA mpMRI at our centre may attest to the fact that many barriers exist in routinely performing preoperative MRI in Canada due to the government-funded nature of the healthcare system. This contrasts with multitiered payment systems in other countries, where MRI has become part of the standard workup for newly diagnosed PCa patients.25 However, Xylinas et al stated that the routine use of MRI for preoperative evaluation of PCa is controversial, as its high cost might burden the healthcare system,26 and D’Amico et al asserted that although MRI findings can add a significant predictive value, it does not justify its routine use.11
In our study, a larger proportion of patients for whom MRI predicted absence of ECE experienced subsequent positive surgical margins (42.9%) compared to those for whom MRI predicted presence of ECE (10%). The highest number of positive surgical margins was observed in the group with negative radiological ECE results who turned out to have ECE on pathology (67% had positive surgical margins). It is possible that negative radiological ECE findings may have resulted in dissection closer to the prostatic tumour. Due to the small sample size, the generalizability of the ability of mpMRI to aid in minimizing positive surgical margin status is in question. This study provides insight into the use of mpMRI to assist in surgical planning for RP.
Conclusion
At our Canadian healthcare centre, the use of 3T PPA mpMRI without endorectal coil for the prediction of ECE and SVI for preoperative planning yielded less than satisfactory results with patients who had high-volume PCa. Our data suggest that caution should be exercised when implementing pre-operative MRI to determine ECE and SVI to modify and/or prepare for subsequent operative approach. A large multi-centre study of mpMRI will better define the efficacy, utility, and feasibility of the test and will help define the role of MRI in preoperative assessment of PCa.
Footnotes
Competing interests: The authors report no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.Faria EF, Chapin BF, Muller RL, et al. Radical prostatectomy for locally advanced prostate cancer: Current status. Urology. 2015;86:10–15. doi: 10.1016/j.urology.2015.03.012. https://doi.org/10.1016/j.urology.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Lowrance WT, Scardino PT. Predictive models for newly diagnosed prostate cancer patients. Rev Urol. 2009;11:117–26. [PMC free article] [PubMed] [Google Scholar]
- 3.Cerantola Y, Valerio M, Kawkabani Marchini A, et al. Can 3T multiparametric magnetic resonance imaging accurately detect prostate cancer extracapsular extension? Can Urol Assoc J. 2013;7:E699–703. doi: 10.5489/cuaj.245. https://doi.org/10.5489/cuaj.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han M, Partin AW, Piantadosi S, et al. Era-specific biochemical recurrence-free survival following radical prostatectomy for clinically localized prostate cancer. J Urol. 2001;166:416–9. https://doi.org/10.1016/S0022-5347(05)65955-1. [PubMed] [Google Scholar]
- 5.Cooperberg MR, Lubeck DP, Mehta SS, et al. Time trends in clinical risk stratification for prostate cancer: Implications for outcomes. J Urol. 2003;170:S21–7. doi: 10.1097/01.ju.0000095025.03331.c6. https://doi.org/10.1097/01.ju.0000095025.03331.c6. [DOI] [PubMed] [Google Scholar]
- 6.Somford DM, Hamoen EH, Futterer JJ, et al. The predictive value of endorectal 3 Tesla multiparametric magnetic resonance imaging for extraprostatic extension in patients with low-, intermediate- and high-risk prostate cancer. J Urol. 2013;190:1728–34. doi: 10.1016/j.juro.2013.05.021. https://doi.org/10.1016/j.juro.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Brajtbord JS, Lavery HJ, Nabizada-Pace F, et al. Endorectal magnetic resonance imaging has limited clinical ability to preoperatively predict pT3 prostate cancer. BJU Int. 2011;107:1419–24. doi: 10.1111/j.1464-410X.2010.09599.x. https://doi.org/10.1111/j.1464-410X.2010.09599.x. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. https://doi.org/10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Hull GW, Rabbani F, Abbas F, et al. Cancer control with radical prostatectomy alone in 1000 consecutive patients. J Urol. 2002;167:528–34. doi: 10.1016/S0022-5347(01)69079-7. https://doi.org/10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Du Y, Yang H, et al. Magnetic resonance imaging for prostate cancer clinical application. Chin J Cancer Res. 2013;25:240–9. doi: 10.3978/j.issn.1000-9604.2013.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Amico AV, Desjardin A, Chen MH, et al. Analyzing outcome-based staging for clinically localized adenocarcinoma of the prostate. Cancer. 1998;83:2172–80. doi: 10.1002/(sici)1097-0142(19981115)83:10<2172::aid-cncr16>3.0.co;2-k. https://doi.org/10.1002/(SICI)1097-0142(19981115)83:10<2172::AID-CNCR16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Griebling TL, Ozkutlu D, See WA, et al. Prognostic implications of extracapsular extension of lymph node metastases in prostate cancer. Mod Pathol. 1997;10:804–9. [PubMed] [Google Scholar]
- 13.Porcaro AB, Borsato A, Romano M, et al. Accuracy of preoperative endorectal coil magnetic resonance imaging in detecting clinical understaging of localized prostate cancer. World J Urol. 2013;31:1245–51. doi: 10.1007/s00345-012-0900-7. https://doi.org/10.1007/s00345-012-0900-7. [DOI] [PubMed] [Google Scholar]
- 14.Bloch BN, Genega EM, Costa DN, et al. Prediction of prostate cancer extracapsular extension with high spatial resolution dynamic contrast-enhanced 3T MRI. Eur Radiol. 2012;22:2201–10. doi: 10.1007/s00330-012-2475-5. https://doi.org/10.1007/s00330-012-2475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghafoori M, Alavi M, Shakiba M, et al. The value of prostate MRI with endorectal coil in detecting seminal vesicle involvement in patients with prostate cancer. Iran J Radiol. 2015;12:145–56. doi: 10.5812/iranjradiol.14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloch BN, Furman-Haran E, Helbich TH, et al. Prostate cancer: Accurate determination of extracapsular extension with high-spatial-resolution dynamic contrast-enhanced and T2-weighted MR imaging — initial results. Radiology. 2007;245:176–85. doi: 10.1148/radiol.2451061502. https://doi.org/10.1148/radiol.2451061502. [DOI] [PubMed] [Google Scholar]
- 17.Futterer JJ, Heijmink SW, Scheenen TW, et al. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology. 2006;241:449–58. doi: 10.1148/radiol.2412051866. https://doi.org/10.1148/radiol.2412051866. [DOI] [PubMed] [Google Scholar]
- 18.Kurhanewicz J, Vigneron D, Carroll P, et al. Multiparametric magnetic resonance imaging in prostate cancer: Present and future. Curr Opin Urol. 2008;18:71–7. doi: 10.1097/MOU.0b013e3282f19d01. https://doi.org/10.1097/MOU.0b013e3282f19d01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazaheri Y, Shukla-Dave A, Hricak H, et al. Prostate cancer: identification with combined diffusion-weighted MR imaging and 3D 1H MR spectroscopic imaging —correlation with pathological findings. Radiology. 2008;246:480–8. doi: 10.1148/radiol.2462070368. https://doi.org/10.1148/radiol.2462070368. [DOI] [PubMed] [Google Scholar]
- 20.Kim B, Breau RH, Papadatos D, et al. Diagnostic accuracy of surface coil magnetic resonance imaging at 1.5T for local staging of elevated risk prostate cancer. Can Urol Assoc J. 2010;4:257–62. doi: 10.5489/cuaj.09103. https://doi.org/10.5489/cuaj.09103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Hricak H, Shukla-Dave A, et al. Clinical stage T1c prostate cancer: Evaluation with endorectal MR imaging and MR spectroscopic imaging. Radiology. 2009;253:425–34. doi: 10.1148/radiol.2532081390. https://doi.org/10.1148/radiol.2532081390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Augustin H, Fritz GA, Ehammer T, et al. Accuracy of 3Tesla magnetic resonance imaging for the staging of prostate cancer in comparison to the Partin tables. Acta Radiol. 2009;50:562–9. doi: 10.1080/02841850902889846. https://doi.org/10.1080/02841850902889846. [DOI] [PubMed] [Google Scholar]
- 23.Scialpi M, Piscioli I, D’Andrea A. Underestimated role of MRI in EAU guidelines on prostate cancer. Magn Reson Imaging. 2014;32:402–3. doi: 10.1016/j.mri.2014.01.002. https://doi.org/10.1016/j.mri.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Park KK, Choi KH, et al. Is endorectal coil necessary for the staging of clinically localized prostate cancer? Comparison of non-endorectal vs. endorectal MR imaging. World J Urol. 2010;28:667–72. doi: 10.1007/s00345-010-0579-6. https://doi.org/10.1007/s00345-010-0579-6. [DOI] [PubMed] [Google Scholar]
- 25.Klotz KH. MRI and prostate cancer: The next frontier. Can Urol Assoc J. 2010;4:227–8. https://doi.org/10.5489/cuaj.895. [PMC free article] [PubMed] [Google Scholar]
- 26.Xylinas E, Yates DR, Renard-Penna R, et al. Role of pelvic phased array magnetic resonance imaging in staging of prostate cancer specifically in patients diagnosed with clinically locally advanced tumours by digital rectal examination. World J Urol. 2013;31:881–6. doi: 10.1007/s00345-011-0811-z. https://doi.org/10.1007/s00345-011-0811-z. [DOI] [PubMed] [Google Scholar]


