Abstract
The fusiform gyrus (FG) is an important node in the face processing network, but knowledge of its causal role in face perception is currently limited. Recent work demonstrated that high frequency stimulation applied to the FG distorts the perception of faces in human subjects (Parvizi et al. [2012]: J Neurosci 32:14915–14920). However, the timing of this process in the FG relative to stimulus onset and the spatial extent of FG's role in face perception are unknown. Here, we investigate the causal role of the FG in face perception by applying precise, event‐related electrical stimulation (ES) to higher order visual areas including the FG in six human subjects undergoing intracranial monitoring for epilepsy. We compared the effects of single brief (100 μs) electrical pulses to the FG and non‐face‐selective visual areas on the speed and accuracy of detecting distorted faces. Brief ES applied to face‐selective sites did not affect accuracy but significantly increased the reaction time (RT) of detecting face distortions. Importantly, RT was altered only when ES was applied 100ms after visual onset and in face‐selective but not place‐selective sites. Furthermore, ES applied to face‐selective areas decreased the amplitude of visual evoked potentials and high gamma power over this time window. Together, these results suggest that ES of face‐selective regions within a critical time window induces a delay in face perception. These findings support a temporally and spatially specific causal role of face‐selective areas and signify an important link between electrophysiology and behavior in face perception. Hum Brain Mapp 38:2830–2842, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: electrical stimulation, electrocorticography, fusiform gyrus, cortico‐cortical evoked potentials, high gamma power
INTRODUCTION
The fusiform gyrus (FG) in the inferior temporal (IT) lobe has been proposed to be a critical area for face processing and recognition [Kanwisher and Yovel, 2006; Kanwisher et al., 1997]. This was first demonstrated by lesion studies [Damasio et al., 1982; Hecaen and Angelergues, 1962; Meadows, 1974], and later supported by neuroimaging [Kanwisher et al., 1997; Sergent et al., 1992], and intracranial EEG [Allison et al., 1999; Gross, et al., 1972; Jacques et al., 2016; McCarthy et al., 1999; Puce et al., 1999; Tsao, et al., 2003; Weiner and Zilles, 2016]. However, evidence demonstrating that the FG is causally involved in face perception to date is limited in humans, partially due to the lack of causal techniques with spatial and temporal precision. As lesion studies lack temporal specificity, previous studies have used noninvasive perturbational approaches, such as transcranial magnetic stimulation (TMS). However, these studies typically target the lateral occipital face area (OFA) rather than the FG, and their spatial specificity is limited [Bona et al., 2015; Holiday et al., 2015; Pitcher et al., 2008, 2012].
A small number of intracranial EEG studies have applied electrical stimulation (ES), which provides the desired spatial and temporal specificity, to the FG and associated areas with variable results [Allison et al., 1994; Jonas et al., 2012; Puce et al., 1999]. ES mapping (ESM)—a clinical method to elicit behavioral changes to map cortical regions prior to neurosurgical procedures [Borchers et al., 2012; Ojemann, 2010]—has been recently used to stimulate the FG and induce distortion of facial features [Parvizi et al., 2012] or interfere with face categorization [Chong et al., 2013], an effect that lateralizes to the right hemisphere [Parvizi et al., 2012]. This phenomenology is particularly evident when stimulation is delivered to areas identified as face‐selective by fMRI and intracranial EEG [Jacques et al., 2016; Megevand et al., 2014] but see [Jonas et al., 2015]. While intriguing and the best evidence to date that the FG is necessary for face recognition, the high frequency (60 Hz) and long duration (∼1 s) of electrical pulses applied in previous work limits the ability to explore a more mechanistic understanding of this phenomenon. As a result, several questions along the face perception investigation remain, including (1) to what extent does stimulation of face‐selective regions spread to other cortical regions; (2) what are the local electrophysiological changes induced by ES; and (3) how do these changes correspond to behavioral output?
Single pulse stimulation is an alternative method to ESM that can address these questions by providing a brief electrical pulse delivered at a precise time relative to stimulus presentation. A number of dependent measures are resultant, including: (1) its effect upon the event related potential evoked by the visual stimulus, (2) the evoked electrophysiological response or cortico‐cortical evoked potential (CCEP) [Keller et al., 2011, 2014a; Matsumoto et al., 2004, 2007] and (3) the effect of the ES upon behavior. This minimal perturbation approach is advantageous over ESM as the underlying neural mechanisms of the CCEP are better understood [Keller et al., 2014b] and the causal spatial and temporal relationship of a cortical region to underlying behavior can be directly tested.
Here, we examined the electrophysiological and behavioral consequences of single pulse stimulation of face‐selective regions. We hypothesized that ES, when applied at face‐processing regions and during a critical processing time window during the viewing of a face, will alter neuronal activity locally that will in turn disrupt information processing of faces. We demonstrate a modulation of the evoked potential, accompanied by a suppression of high gamma (70–150 Hz) power, when ES was applied only during visual stimulation (VS) and only at face‐selective regions. Neuronal responses within face‐selective sites predicted the ES‐induced slowing of detecting distorted faces. Together, these results support the notion of a temporal and spatially specific causal involvement of the FG in face perception.
MATERIALS AND METHODS
Patient Selection
Six subjects (2 female, aged 39.5 years; range 21–52) with medically intractable epilepsy at North Shore University Hospital participated in the current study. Patient characteristics are described in Table 1. All patients provided informed consent as monitored by the local Institutional Review Board and in accordance with the ethical standards of the Declaration of Helsinki. The decision to implant, the electrode targets, and the duration of implantation was made entirely on clinical grounds without reference to this investigation. Patients were informed that participation in this study would not alter their clinical treatment, and that they could withdraw at any time without jeopardizing their clinical care.
Table 1.
Patient characteristics, electrode coverage, and selectivity to faces and places
| Pt | Age | M/F | Handedness | Implanted hemi | Type of implant | Epileptic Focus | Face selective site d′ | Place selective site d′ |
|---|---|---|---|---|---|---|---|---|
| S1 | 22 | M | R | R | SEEG | R MTL | 0.64 | 24.74** |
| S2 | 51 | F | L | L | SEEG | L MTL | 8.81** | 13.93** |
| S3 | 42 | M | R | L | Grid/strips | L MTL | 27.96** | –0.57 |
| S4 | 25 | M | R | L | SEEG | L MTL | 39.08** | 15.78** |
| S5 | 52 | F | R | R | Grid/strips | R MTL | 3.57* | 12.25** |
| S6 | 45 | M | R | L | Grid/strips | L MTL | 23.48** | 8.35** |
*P < 0.05, ** P < 0.01.
Electrode Implantation and Recording
Patients were implanted with intracranial subdural grids, strips, and/or depth electrodes (Integra Lifesciences, Plainsboro, NJ and Ad‐Tech Medical Instrument, Racine, WI) for 5–15 days. Monitoring occurred until sufficient data were collected to identify the seizure focus, at which time the electrodes were removed and, if appropriate, the seizure focus was resected. Continuous video‐intracranial EEG monitoring was performed using standard recording systems (XLTEK EMU128FS or NeuroLink IP 256 systems, San Carlos, CA), high‐pass filtered at 0.1Hz and low‐pass filtered at 40% of the sampling rate, digitized at 512 Hz and stored for offline analysis. A strip electrode screwed into the frontal bone near the bregma was used as common mode ground. Acquired data were notch filtered (60 Hz) and rereferenced to an average reference [Privman et al., 2007]. Electrodes involved in the seizure onset zone and early seizure spread, as determined by an epileptologist blinded to the study, were removed from the analysis.
Electrode Registration
The electrode registration process has been described previously [Keller et al., 2011, 2013]. Briefly, to localize each electrode anatomically, subdural electrodes were identified on the postimplantation CT with BioImagesuite (http://bioimagesuite.yale.edu/lands/) and were coregistered first with the post‐implantation structural MRI and subsequently with the preimplantation MRI to account for possible brain shift caused by electrode implantation and surgery [Mehta and Klein, 2010]. Following coregistration, electrodes were snapped to the closest point on the reconstructed pial surface [Dale et al., 1999] of the preimplantation MRI in MATLAB [Dykstra et al., 2012]. Intraoperative photographs were used to corroborate this registration method based on the identification of major anatomical features. Automated cortical parcellations were used to relate electrode data to anatomical regions [Fischl et al., 2004].
Selection of Stimulation Sites
To evaluate the selectivity of regions within the visual system, we used a one‐back memory task that has been described previously by our group [Davidesco et al., 2014]. Pictures of houses, faces, textures, and man‐made tools were presented for 250 ms with 1 s interstimulus interval (see Fig. 1). Data were notch filtered, bipolar referenced, bandpass filtered, and converted to High Gamma Power (70–150 Hz; HGP). For each pair of electrodes, the area under the curve (AUC) of the HGP response to VS between 100 and 300 ms post stimulus onset was computed. The AUC was computed separately for the face and house categories and contrasted using a paired t‐test. For pairs of electrodes that showed a significant difference between the response to faces and houses, the category that yielded the stronger response was treated as the preferred category and a d′ value was computed in the following way:
Figure 1.
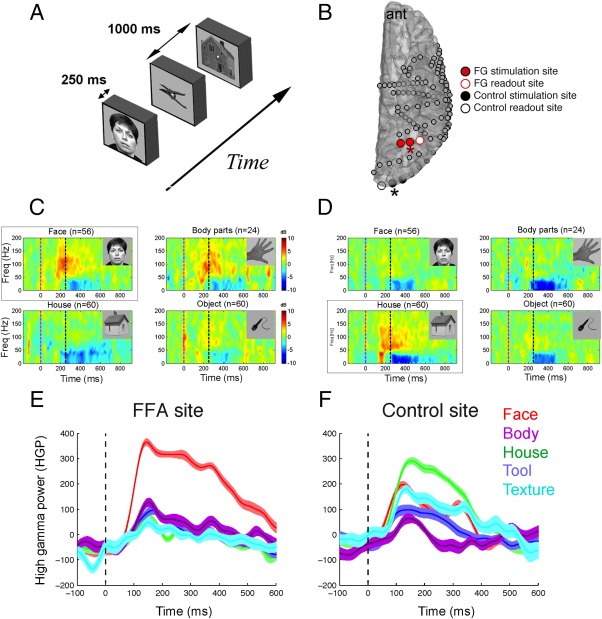
Localization of face and place selective regions in the visual network. A: Experimental paradigm for the visual screening task. B: Example of the location of the face‐selective (shown in red) and place‐selective (shown in black) electrodes that were selected for ES in one patient. Both the stimulation and readout sites were selective to faces and places for the active and control site, respectively. Spectrograms from electrodes with asterisks are shown in C and D. C,D: Spectral responses to the viewing of faces, houses, body parts, and objects. Spectrograms from the electrodes highlighted by red and black arrows are shown in C and D, respectively. Note the selective increase in high gamma (70–150 Hz) activity to (C) faces and (D) houses. E,F: Band‐limited power responses to classes of stimuli in (E) face‐ and (F) house‐selective sites that were selected for ES in this patient. Traces in E and F derive from spectrograms in C and D, respectively.
The pair of adjacent electrodes with the highest d′ for faces was considered as the face‐selective site. The control sites were selected as an adjacent pair of electrodes exhibiting the strongest d′ for places and separated from either FG stimulation site by >1 cm. If no regions were selective to places, a nonselective region in the visual system that demonstrated a HGP increase to both faces and houses was considered the control site.
Face Perception Task
Following the identification of face and scene‐selective regions from the one‐back memory task (see Fig. 1) and the resumption of antiepileptic medications, a face distortion detection experiment was performed. The task was inspired by a previous report of face distortion effects following FG stimulation [Parvizi et al., 2012]. The task consisted of grayscale face images (of ∼15° × 15° visual angle), which were superimposed with a 4*4 grid. Each image was presented on the screen for 350 ms and the task of the patient was to report whether it appears distorted or not. This forced‐choice task was self‐paced and each trial began with a 500 ms fixation screen. In two‐thirds of the trials, the face was distorted by randomly selecting two fragments of the face and circularly shifting them sideways, upside or downside. The level of distortion was determined for each patient individually based on a set of training sessions with varying levels of distortion. The level of distortion that corresponded to 75% accuracy was chosen for the main experiment.
Bipolar ES was performed by applying single current pulses to adjacent electrodes (biphasic pulses, 100 us/pulse) using a Grass S12 cortical stimulator (Grass Technologies, West Warwick, RI). The current magnitude was chosen to be 8 mA for surface electrodes and 5 mA for depth electrodes as this was the maximum current using our stimulation protocols that did not induce epileptiform discharges in areas outside of the seizure onset zone.
Stimulation was delivered at three different latencies with respect to visual stimulus onset: −200, 100, and 500 ms. Given the clinical context, we were unable to test more than three time latencies. The 100 ms latency stimulation condition consisted of either a single pulse or a train of 5 pulses at 50 Hz. For each stimulation site, there were 60 trials per stimulation latency. These trials were randomly intermixed with two types of catch trials: (a) face with no stimulation (60 trials in total); (b) stimulation with no face (32 trials in total, 16 with a single pulse, 16 with a train of 5 pulses at 50 Hz). Stimulation site was switched pseudo‐randomly every ∼40 trials.
Behavioral Analysis
Reaction time (RT) was calculated as the time from stimulus onset to the patient's recorded response. Trials in which RT deviated from the mean RT by more than two standard deviations were excluded from further analysis. Mean RTs and accuracy rates were computed separately for each stimulation site. No significant difference was observed between RTs in the single pulse and repetitive stimulation groups for either stimulation site (FGsingle vs rep, P = 0.26, n = 214; CTL single vs rep P = 0.09, n = 214, Wilcoxon rank sum test). As a result, the RT for the single pulse and repetitive stimulation groups were combined to increase the sample size for each patient. Group behavioral analyses were performed following the normalization of each patient's RTs to the mean RT of the no stimulation condition to account for patient differences in the overall speed of response. The Wilcoxon rank sum test then evaluated the group differences in RT between each pair of condition.
Electrophysiological Analysis
Electrophysiological data were analyzed offline with custom scripts (MATLAB, Mathworks). First, channels with high amplitude noise (SD > 250 uV) as well as electrode sites corresponding to the seizure onset zone were excluded. The remaining channels were notch filtered to remove power line noise and rereferenced by subtracting the common average [Privman et al., 2007]. For each condition, data from each trial were epoched (−1 to 1.5 s after visual stimuli onset).
For each condition consisting of ES, the CCEP was quantified in a similar fashion as previous work [Keller et al., 2011; Matsumoto et al., 2004, 2007]. CCEPs in human cortex generally consist of an early sharp response (10–50 ms post stimulation) and a later slow wave (50–250 ms), referred to as N1 and N2, respectively, due to the existence of predominantly surface‐negative voltage deflections during these time periods. Therefore, for each trial, the maximum negative voltage deflection in the early (N1) and late (N2) time periods were calculated and compared with the prestimulus baseline period (−250 to −50ms).
To calculate a potential proxy for neuronal population activity, for each condition we computed the power in the high gamma range (70–150 Hz; ‘HGP’). ES elicited an initial artifact (within 10 ms of stimulation onset) and an N1 (10–50 ms) response, both of which are sharp enough to bleed into the gamma frequency. Therefore, HGP responses within 50 ms of stimulation onset were not analyzed and are grayed out for visualization purposes. Next, epoched data for each condition was bandpass filtered between 70 and 150 Hz (4th order Butterworth filter with zero phase shift) and Hilbert transformed to obtain the envelope of the signal (HGP) [Keller et al., 2013; Ossandon et al., 2011]. Evoked HGP following ES was quantified by computing the AUC during the N1 and N2 time periods. All group analyses were performed with the Wilcoxon rank sum test because of the nonlinear sample distribution.
RESULTS
We examined the effect of extremely brief single pulses of direct ES to the FG on face perception in six human subjects (4 male, age 39.5 ± 12.9 SD) undergoing invasive electrode monitoring for epilepsy. Patient characteristics and the degree of selectivity of stimulation sites are shown in Table 1. Stimulation sites were chosen based on the high gamma power selectivity to faces and places (Fig. 1; see Methods). 5/6 patients (Pts 2–6) had electrodes with significant selectivity to faces and 5/6 patients (Pts 1,2,4‐6) exhibited electrodes with significant selectivity to houses. In the absence of category‐selective responses, the stimulation sites were selected based on anatomical considerations. The experimental design is outlined in Figure 2A. Distorted and non‐distorted faces were presented with and without ES at specific latencies (−200, +100, +500 ms) with respect to visual onset. There was no significant effect of stimulation site on accuracy across subjects (one‐way ANOVA F(2,15) = 0.03, P = 0.97; accuracy face‐selective stim = 82.2%; accuracy place‐selective control stim = 83.4%; accuracy no stim = 82.3%). Therefore, we focused on RT to characterize the behavioral effects of ES.
Figure 2.
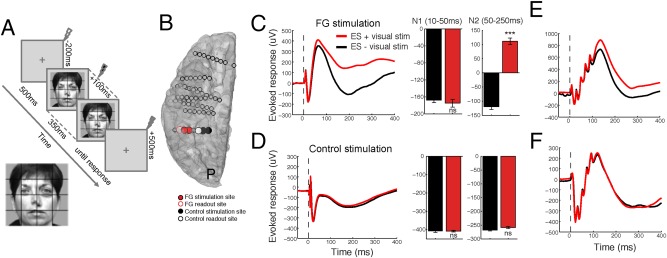
Face VS modulates the CCEP when stimulating face‐selective regions, but not place‐selective regions. A: Schematic of experimental protocol. ES was applied to face‐selective and control regions at −200, 100, and 500 ms with respect to VS onset (0 ms). B: Locations of FG and control stimulation and readout. C: The effect of VS and face‐selective single pulse (0.1 ms) ES on the CCEP. ES was applied at 0 ms (vertical dotted line), whereas the face was presented at −100 ms and remained until +250 ms. VS during ES of face‐selective regions causes an increase in the late N2 voltage deflection but not the early N1 response of the CCEP. D: In contrast, VS during ES of place‐selective control sites does not modulate the N1 or N2 response. E,F: VS during brief (100 ms) 50 Hz repetitive ES of (E) face‐selective but not (F) place‐selective control sites leads to a change in the evoked response. Data from one representative patient. [Color figure can be viewed at http://wileyonlinelibrary.com]
Temporal and Spatial Specificity of CCEP Modulation
To characterize the underlying electrophysiological changes, we first examined how the CCEP is modulated by VS. Figure 2C compares the CCEP recorded with and without VS in a face‐selective region in one patient. ES was applied 100 ms following VS. When a face was presented on the screen we observed a significant shift to a more positive potential during the late slow wave (“N2,” 50–250 ms) time period of the CCEP (Fig. 2C; patient 1; n = 60 trials; unpaired t‐test, P < 0.001). Importantly, VS did not affect the early “N1” (10–50 ms) response (Fig. 2C; P = 0.34). Furthermore, ES applied 200 ms prior to or 500 ms following VS did not cause a modulation in either the N1 or N2, suggesting a temporally specific effect between visual onset and 500 ms. Moreover, ES to the control site during the presentation of faces did not cause a modulation in either the N1 or N2 time period (Fig. 2D; P = 0.63 and 0.44, respectively), suggesting a spatially specific effect. Importantly, a similar effect was observed for repetitive stimulation (Fig. 2E,F).
Linking Electrophysiology to Behavior in Single Patients
How do these changes, caused by perturbing the normal processing of face‐selective regions, affect behavior? Figure 3 examines these electrophysiological and behavioral changes in each subject. 4/6 patients exhibited an increase in RT following face‐selective stimulation compared to no stimulation or stimulation of the control site, while 3/4 of these patients showed significance at the single subject level (Fig. 3B; n = 30 trials; patients 1, 2, 4, P < 0.05 after FDR correction for multiple comparisons; patient 3, P = 0.12; unpaired t‐test). In these 4/6 patients, ES applied to the face‐selective but not the control site during VS modulated the amplitude of the CCEP (Fig. 3C,D; P < 0.01). Interestingly, Patient 5 did not exhibit a significant change in RT or the CCEP following ES of face‐selective or control stimulation. In contrast, Patient 6 demonstrated an increase in RT during ES to both sites. This patient also exhibited modulation of the CCEP to both sites when ES was paired with VS (Fig. 3B–D).
Figure 3.
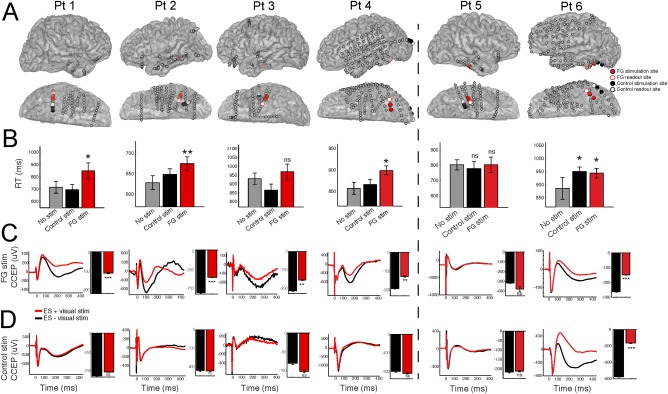
Single subject electrophysiology predicts the perception of distorted faces. A: For each subject, the locations of the face‐selective and control sites are shown on the cortical surface. B: Single‐subject behavioral results. C,D: Single‐subject electrophysiology results. CCEP modulation by face viewing (ES + VS) recorded in the (C) FG and (D) control site. Each plot shows the average CCEP during face stimuli (ES + VS) compared with no visual stimuli (ES‐VS). Bar graphs quantify the strength of the N2 response of the CCEP during each condition. Error bars denote S.E. *P < 0.05; **P < 0.01; ***P < 0.001. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4 describes the group effect (n = 6) of ES applied to face‐selective regions on electrophysiology and behavior. Across subjects, face‐selective stimulation localized to the inferior lateral temporal lobe (Fig. 4A). The interaction of electrical and VS elicited a significant reduction (more positive voltage deflection) in the peak amplitude of the N2 but no change in the N1 of the CCEP (Fig. 4B, n = 6 patients, p N2 < 0.01, p N1 = 0.28, Wilcoxon rank sum test). Stimulating sites exhibiting strong face‐selectivity (high d′) resulted in slower RTs, while stimulating electrodes with weak face‐selectivity resulted in faster RTs (Fig. 4C and Supporting Information Fig. S2, P < 0.001, unpaired t‐test, all conditions). Analysis of the group effect of ES on behavior on each stimulation condition demonstrated that compared with the control site stimulation at −200 ms, ES applied to the FG at +100 but not −200 or +500 ms following VS onset significantly increased the RT (Fig. 4D, p −200 ms = 0.12; p + 100 ms = 0.004; p + 500 ms = 0.80, unpaired t‐test, FDR correction for multiple comparisons). No changes in RT was observed following control stimulation (p −200 ms = 0.90; p + 100 ms = 0.22; p + 500 ms = 0.28). The change in RT was significantly higher for the +100 ms stimulation condition of the FG compared with the control site (FG + 100 ms vs CTL + 100ms p = 0.03, n = 214).
Figure 4.
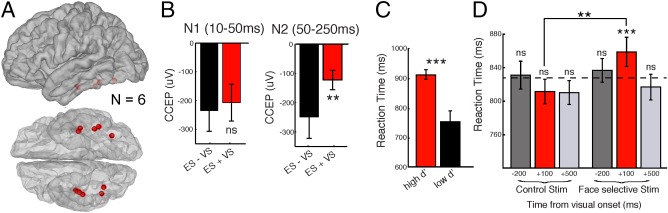
Group analyses of electrical modulation of the FG during face processing. A: The location of face‐selective sites (n = 6) used for ES. B: Electrophysiology results. Quantification of the N1 (10–50 ms) and N2 (50–250 ms) time period of the CCEP recorded at the FG during ES with and without VS. C‐D Behavioral results. C: Comparison of RTs for subjects with high d′ (n = 3 subjects) vs those with low d′ (n = 3 subjects). D: Median RTs for each condition across patients. Dotted horizontal bars for each condition represent the median RT for that condition. Error bars denote S.E. *P < 0.05; **P < 0.01; ***P < 0.001. [Color figure can be viewed at http://wileyonlinelibrary.com]
High Gamma Response to ES
We investigated the effect of ES and VS on the HGP response (Fig. 5), which is thought to reflect (at least in part) population spiking activity [Manning et al., 2009; Nir et al., 2007; Ray and Maunsell, 2011]. VS alone evoked a strong increase in HGP, while ES alone caused an initial HGP increase followed by a long‐lasting HGP suppression (Fig. 5A). ES applied during VS significantly suppressed the HGP response compared to VS alone in this patient (Fig. 5B; P < 0.001 FDR correction for multiple comparisons; unpaired t‐test; df = 46) and across subjects (Fig. 5C; P < 0.01; paired t‐test; df = 5).
Figure 5.
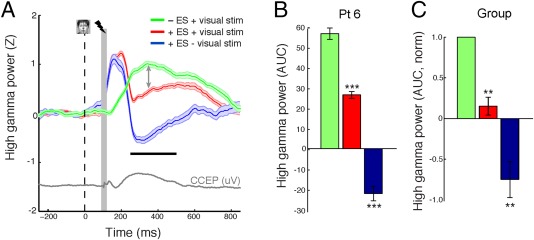
ES suppresses the high gamma power response to faces. A: High gamma power responses to different combinations of VS and ES. Gray trace represents the evoked potential following single pulse stimulation to the face‐selective region without concurrent VS. Face and vertical dotted line represent visual stimuli onset. The lightning bolt denotes the onset of ES (when applicable). Gray vertical bar denotes region of stimulation artifact that was not used in the analysis. Horizontal black bar illustrates the time period with which the AUC was computed on a single trial basis. Vertical arrows represent the decrease in HGP if face presentation is paired with ES. B,C: Quantification of HGP responses in (B) a single subject and (C) across subjects. [Color figure can be viewed at http://wileyonlinelibrary.com]
Superposition of Multimodal Stimulation
Is the modulation of the CCEP at face‐selective sites during face perception only related to the temporal superposition of the face evoked potential and the CCEP? To answer this question, we compared the difference between the ERP evoked from VS alone to the difference between both ES only subtracted from the combined visual and ES condition (VS‐ES). We find that when examining voltage responses to FG ES across subjects, the predicted response to VS (ESFG,VS‐ESFG) was statistically larger than the actual response to VS (Supporting Information Fig. S3A, B, upper panels; p FG <.05, t FG = 2.2, n = 6). However, for control stimulation, no significant difference was observed (Supporting Information Fig S3A, B, lower panels; p CTL = 0.33, t CTL = 0.7, n = 6). This effect was not observed for high gamma power (Supporting Information Fig S3C, n = 6, p FG = 0.40, t FG = −0.92; p CTL = 0.49, t CTL = −0.70).
Spatial Distribution of Cortical Responses to Electrical and VS
ES applied at face‐selective cortex should propagate within the same network recruited by natural face processing. Therefore, in order to determine how the spatial propagation pattern of ES relates to natural face processing, we next compared the spatial spread of CCEPs to visual ERPs. Figure 6A shows the spatial spread of CCEPs in response to FG stimulation in one patient. ES to the FG and VS both elicit strong evoked potentials locally along the inferolateral temporal lobe (Fig. 6A,B for the N170 of the visual ERP and the N2 of the CCEP, respectively). Spatial correlation analysis demonstrates a strong positive correspondence between modalities in patient 2 (See Methods; Fig. 6C; r = 0.51; P < 0.01). Patient 3 also demonstrated this effect, with FG stimulation and VS both evoking relatively local IT activity as well as more remote regions, including inferior frontal, superior frontal, and inferior parietal sites (Fig. 6D–F; r = 0.67; P < 0.01). This positive correlation was observed across all subjects (mean r = 0.54; P < 0.01; n = 6), suggesting that ES applied to FG propagates to regions involved in face processing. This relationship was lower but still significant for the N1 time period of the CCEP (mean r = 0.31; P < 0.01; n = 6).
Figure 6.
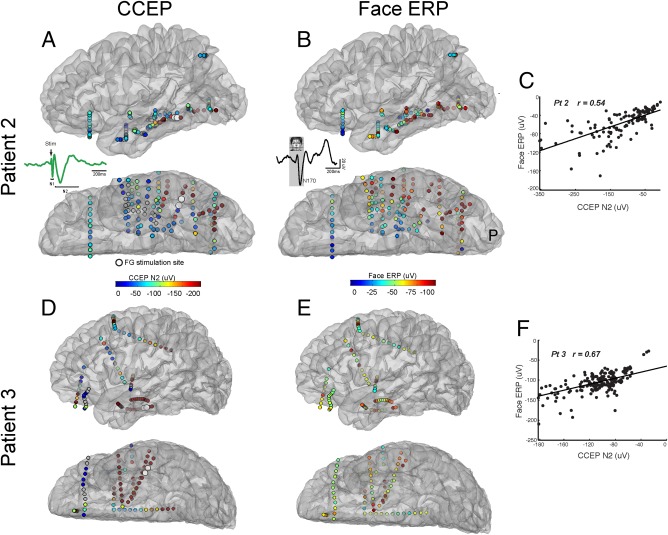
ES of the FG is largely contained within regions involved in face processing. A: Map of CCEP connectivity assessed by face‐selective ES in one patient (Pt 2). Warmer colors represent stronger evoked response during the N2 time period. Insert: Components of the CCEP. Note the early N1 and later N2 time period of the evoked response. B: Map of N170 amplitude following face stimulation. Warm colors represent stronger N170 responses. Insert: Components of the face ERP. C: Correlation between isolated face‐selective CCEP maps and visual ERP maps in one patient. Note the significant correlation between the distribution of the two networks defined through electrical and VS, respectively. D–F: Same as A‐C but for a second patient.
Do changes observed at face‐selective sites following electrical and VS (Figs. 2, 3, 4) occur outside of face‐selective regions? And, if so, are these changes confined to regions functionally connected to the FG? Because CCEPs are observed remote to the stimulation site [Keller et al., 2011, 2014a; Lacruz et al., 2007], we hypothesized that interactions between visual and ES will occur in regions distant to the stimulation site. As expected, ES applied to face‐selective sites during VS (CCEP + vis) caused a significant positive shift in the amplitude of the N2 of the CCEP in several regions outside the stimulation site (see example in Fig. 7A; patient 4). Importantly, CCEP modulation was not observed at any site during the CCEP + vis condition after ES of the control site (Fig. 7B). Across subjects, ES of the FG during VS (CCEP + vis) modulated the CCEP in a larger but non‐significant number of total sites compared with ES of the control site (FGstim = 9.5 ± 2.89 sites; CTLstim = 1.5 ± 0.81 sites; Fig. 5C; n = 6, P = 0.06, Wilcoxon rank sum test). The proportion of sites modulated within the FG network (defined as strong CCEPs elicited following FG stimulation), however, was significantly higher than the proportion of modulated CCEPs following control stimulation (FGstim = 40.7 ± 12.1%; CTLstim = 11.3 ± 5.37%; Fig. 5D; n = 6, P = 0.02, Wilcoxon rank sum test).
Figure 7.
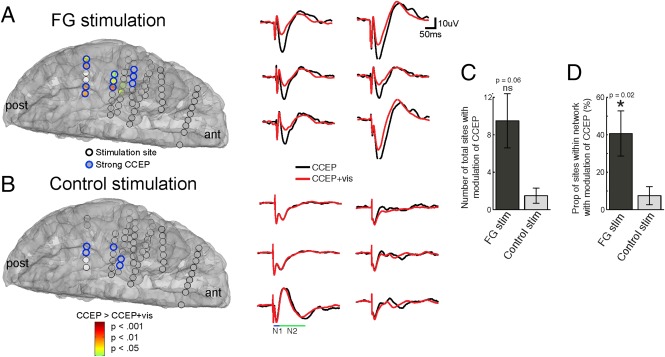
Modulation of the CCEP by VS occurs at multiple sites that are functionally connected to the FG. A: Color brain map represents regions with significant CCEP differences between ES of face‐selective sites during rest (CCEP condition) and during VS (CCEP + vis condition). Representative changes from six electrodes are shown on the right. ant = anterior; post = posterior. B: Identical to A but map shows significant differences between ES of place‐selective control sites during rest and during VS. C,D: Group analysis (n = 6) demonstrating the (C) total number of electrodes across the brain and (D) the proportion of electrodes within each network with significant changes between CCEP and CCEP + vis conditions.
DISCUSSION
Summary of Findings
Here, we investigated the causal role of the FG in face perception using event‐related ES during a face distortion paradigm. Brief ES applied to face‐selective regions did not significantly affect accuracy but nonetheless increased the RT of detecting face distortions. Effects were specific to stimulation applied 100ms after visual onset and in face‐selective but not place‐selective regions. This change in behavior was accompanied by a decrease in the amplitude of CCEPs and high gamma power. Finally, behavioral effects were most evident in regions functionally connected to face‐selective regions. Together, these results suggest that ES applied to face‐selective regions within a critical time window induces a delay in face perception.
Spatial and Temporal Specificity of Electrical Perturbation of the FG
The findings of the current investigation are in line with several prior studies. Microstimulation of face‐specific patches in the monkey IT cortex within specific time windows (50–100 ms) can bias face categorization [Afraz et al., 2006]. TMS applied to the human OFA decreases facial symmetry detection [Bona et al., 2015] and disrupts facial discrimination only when applied between 60 and 100 ms following stimulus onset [Pitcher et al., 2008]. Earlier stimulation (40–50 ms) disrupts performance in a noncategory specific fashion [Pitcher et al., 2012], and longer latencies (1700 and 2900 ms) stimulation has no effect upon face discrimination [Holiday et al., 2015].
Whereas the spatial resolution of TMS is poor and typically limited to the OFA, not FG, electrocorticography (ECoG) provides superior spatial specificity to link stimulation to specific cortical regions in humans. A number of ECoG and sEEG studies have shown that high frequency ES applied to face‐selective electrodes results in a face‐specific perceptual disruption [Allison et al., 1994; Chong et al., 2013; Jonas et al., 2012, 2015; Megevand et al., 2014; Parvizi et al., 2012; Puce et al., 1999; Vignal et al., 2000].
Results presented here corroborate previous studies and extend our understanding of the role of the FG in face perception. We show that ES applied to the FG results in a behavioral slowing of face perception that was accompanied by a decrease in amplitude of the CCEP during VS. Patients 1, 2, and 4 elicited, as expected, significant behavioral slowing with an accompanied decrease in evoked potential amplitude. Patient 3 showed a non‐significant behavioral slowing with a significant reduction in CCEP amplitude. Patients 5 and 6; however, did not demonstrate this effect. Patient 5 served as a negative control as neither the behavior or evoked potentials were modulated when the FG was stimulated, whereas patient 6 served as a positive control by demonstrating both a behavioral slowing and a modulation of the CCEP during stimulation of a control region. Interestingly, the control site in this patient was located near the lateral occipital cortex area—a key area in object processing [Bona et al., 2015; Malach et al., 1995]—which could possibly explain the positive effect found here.
While our results do not replicate lateralization of behavioral effects observed with high frequency stimulation to the right FG [Rangarajan et al., 2014], critical methodological differences may account for these discordant findings including electrode location within the FG, stimulation type [60 Hz ES in Rangarajan et al., 2014] (single pulse ES in this work) and behavioral output [behavioral report in Rangarajan et al., 2014] (RT in this work). However, our small sample size does not permit strong claims regarding lateralization.
Finally, it is possible that implantation technique (sEEG vs. subdural electrodes) influence results presented here. Indeed, 2/3 patients with significant slowing and CCEP modulation were sEEG. In our laboratory, we have observed CCEPs performed on sEEG implants to have less stimulation artifact and earlier resolution of evoked responses (unpublished work). In addition, it is possible that the differential nature of electrode sizes, shapes, and neuronal perturbation between techniques (sEEG directly affecting a mix of white and gray matter depending on electrode placement; subdural surface electrodes primarily leading to dendritic activity) may have contributed to the observed results. However, given the small sample, it is difficult to make a broad generalization between the two approaches. More work should be performed focusing on this specific question of signal‐to‐noise, morphology, and network distribution of CCEPs and their relationship to behavior.
Accuracy versus RT
ES of face‐selective sites did not significantly change the accuracy of detecting face distortions. This finding seems to contradict previous studies demonstrating that ES of the FG produces robust perceptual disruptions of faces [Chong et al., 2013; Parvizi et al., 2012; Rangarajan et al., 2014]. However, this discrepancy can be attributed to task differences, as previous studies did not require patients to detect face distortions. Moreover, previous studies used 1 s or longer high frequency stimulation compared to event‐related single pulses in our study. The critical finding in our study is that very brief below threshold ES can produce measurable effects in behavior. Furthermore, it appears that compared to accuracy measurements, RT may be a more sensitive (or at least complementary) index for these changes.
Mechanisms Underlying Induced Changes in Face Perception
CCEPs may be characterized by a brief period of increased population activity followed by a longer lasting population decrease, as evidenced by studies of single unit activity [Alarcon et al., 2012], multiunit activity [Keller et al., 2014b], and high gamma power [results presented here and in van 't Klooster et al., 2011]. As expected, HGP increased in the FG upon VS with faces. In contrast, HGP decreased from 50 to 250 ms in the FG following ES, consistent with the N2 of the CCEP being largely inhibitory in nature [Alarcon et al., 2012; Keller et al., 2014b]. When both stimulation modalities were combined (visual and electrical), we observed a suppression of HGP compared with VS alone. As high gamma power relates to population activity [Manning et al., 2009; Nir et al., 2007; Ray and Maunsell, 2011], these findings suggest that ES may suppress population activity during VS. Furthermore, we hypothesize that ES to face‐selective sites suppresses high frequency (and potentially population) activity and may underlie the observed behavioral slowing.
Single Pulse ES as a Tool to Study Human Cognition
ECoG provides a platform for high spatial (<1 cm) and temporal (∼1 ms) resolution of cognitive processes while recording from awake, behaving humans. ESM and CCEP mapping are two relatively common techniques used in ECoG. ESM is routinely used in the clinical setting by applying high frequency (60 Hz) stimulation for 1–10 s duration while the patient is performing a task (i.e., repeating a sentence, naming an object) and examining readily observable changes in behavior induced by this perturbation. ESM is also used as a research tool to probe the function of various cortical circuits. Most ESM studies do not employ precise timing of stimulation with respect to a task and nor record the brain's responses during this time. Reasons for the rarity of these studies include the increased risk of producing seizures with high frequency stimulation and the problem of dissociating stimulus artifact from measured neural responses. CCEP mapping can overcome some of these limitations by providing precise temporal control with brief stimulation. Such time‐controlled CCEP studies have been utilized to examine the causal role of brain activity to conscious perception [Beauchamp et al., 2012] and cognitive tasks such as inhibitory stopping [Wessel et al., 2013]. CCEP studies have also characterized cortico‐cortical connectivity and excitability [Entz et al., 2014; Keller et al., 2014a; Lacruz et al., 2007; Meisel et al., 2015], elucidated propagation patterns in epilepsy [Enatsu et al., 2013; Valentin et al., 2002] and facilitated neuronal mechanisms underlying noninvasive imaging [Conner et al., 2011; Keller et al., 2011]. In summary, CCEP mapping is a tool that provides excellent spatial and temporal specificity for probing a causal role of cortical brain regions and networks on specific cognitive processes.
Limitations and Implications
One limitation of this study may include use of epileptic patients, as the generalization of these subjects’ neuronal recordings to healthy subjects is often questioned. However, electrodes from the seizure onset zone and early epileptic spread regions were removed from the analysis.
This study extends our understanding of the role of the FG in face perception. First, the application of focal ES with precise timing provides a better understanding of the temporal dynamics involved in face perception. Second, the spread of ES—a crucial consideration in any stimulation study—can be quantified due to the large number of sampled regions. Finally, the careful measurement of RT allows us to directly link electrophysiology in the FG to behavior.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
All authors discussed the data, analysis, and methods and contributed to the manuscript. The authors are enormously indebted to the patients that participated in this study, as well as the nursing and physician staff at North Shore LIJ hospital.
REFERENCES
- Afraz SR, Kiani R, Esteky H (2006): Microstimulation of inferotemporal cortex influences face categorization. Nature 442:692–695. [DOI] [PubMed] [Google Scholar]
- Alarcon G, Martinez J, Kerai SV, Lacruz ME, Quiroga RQ, Selway RP, Richardson MP, Garcia Seoane JJ, Valentin A (2012): In vivo neuronal firing patterns during human epileptiform discharges replicated by electrical stimulation. Clin Neurophysiol 123:1736–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, Ginter H, McCarthy G, Nobre AC, Puce A, Luby M, Spencer DD (1994): Face recognition in human extrastriate cortex. J Neurophysiol 71:821–825. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, Spencer DD, McCarthy G (1999): Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non‐face stimuli. Cereb Cortex 9:415–430. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Sun P, Baum SH, Tolias AS, Yoshor D (2012): Electrocorticography links human temporoparietal junction to visual perception. Nat Neurosci 15:957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona S, Cattaneo Z, Silvanto J (2015): The Causal Role of the Occipital Face Area (OFA) and Lateral Occipital (LO) Cortex in Symmetry Perception. J Neurosci 35:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers S, Himmelbach M, Logothetis N, Karnath HO (2012): Direct electrical stimulation of human cortex ‐ the gold standard for mapping brain functions? Nature reviews. Neuroscience 13:63–70. [DOI] [PubMed] [Google Scholar]
- Chong SC, Jo S, Park KM, Joo EY, Lee MJ, Hong SC, Hong SB (2013): Interaction between the electrical stimulation of a face‐selective area and the perception of face stimuli. Neuroimage 77:70–76. [DOI] [PubMed] [Google Scholar]
- Conner CR, Ellmore TM, DiSano MA, Pieters TA, Potter AW, Tandon N (2011): Anatomic and electro‐physiologic connectivity of the language system: a combined DTI‐CCEP study. Comput Biol Med 41:1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, Van Hoesen GW (1982): Prosopagnosia: Anatomic basis and behavioral mechanisms. Neurology 32:331–341. [DOI] [PubMed] [Google Scholar]
- Davidesco I, Zion‐Golumbic E, Bickel S, Harel M, Groppe DM, Keller CJ, Schevon CA, McKhann GM, Goodman RR, Goelman G, Schroeder CE, Mehta AD, Malach R (2014): Exemplar selectivity reflects perceptual similarities in the human fusiform cortex. Cereb Cortex 24:1879–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra AR, Chan AM, Quinn BT, Zepeda R, Keller CJ, Cormier J, Madsen JR, Eskandar EN, Cash SS (2012): Individualized localization and cortical surface‐based registration of intracranial electrodes. Neuroimage 59:3563–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enatsu R, Matsumoto R, Piao Z, O'Connor T, Horning K, Burgess RC, Bulacio J, Bingaman W, Nair DR (2013): Cortical negative motor network in comparison with sensorimotor network: A cortico‐cortical evoked potential study. Cortex 49:2080–2096. [DOI] [PubMed] [Google Scholar]
- Entz L, Toth E, Keller CJ, Bickel S, Groppe DM, Fabo D, Kozak LR, Eross L, Ulbert I, Mehta AD (2014): Evoked effective connectivity of the human neocortex. Hum Brain Mapp 35:5736–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Gross CG, Rocha‐Miranda CE, Bender DB (1972): Visual properties of neurons in inferotemporal cortex of the Macaque. J Neurophysiol 35:96–111. [DOI] [PubMed] [Google Scholar]
- Hecaen H, Angelergues R (1962): Agnosia for faces (prosopagnosia). Arch Neurol 7:92–100. [DOI] [PubMed] [Google Scholar]
- Holiday K, Pitcher D, Ungerleider L (2015): Temporal Dynamics of Memory and Maintenance of Faces in Visual Cortex: An On‐line TMS Study. J Vis 15:294‐294. [Google Scholar]
- Jacques C, Witthoft N, Weiner KS, Foster BL, Rangarajan V, Hermes D, Miller KJ, Parvizi J, Grill‐Spector K (2016): Corresponding ECoG and fMRI category‐selective signals in human ventral temporal cortex. Neuropsychologia 83:14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas J, Descoins M, Koessler L, Colnat‐Coulbois S, Sauvee M, Guye M, Vignal JP, Vespignani H, Rossion B, Maillard L (2012): Focal electrical intracerebral stimulation of a face‐sensitive area causes transient prosopagnosia. Neuroscience 222:281–288. [DOI] [PubMed] [Google Scholar]
- Jonas J, Rossion B, Brissart H, Frismand S, Jacques C, Hossu G, Colnat‐Coulbois S, Vespignani H, Vignal JP, Maillard L (2015): Beyond the core face‐processing network: Intracerebral stimulation of a face‐selective area in the right anterior fusiform gyrus elicits transient prosopagnosia. Cortex 72:140–155. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G (2006): The fusiform face area: A cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci 361:2109–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM (1997): The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci 17:4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Entz L, Ulbert I, Milham MP, Kelly C, Mehta AD (2011): Intrinsic functional architecture predicts electrically evoked responses in the human brain. Proc Natl Acad Sci U S A 108:10308–10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, Groppe DM, Entz L, Craddock RC, Lado FA, Kelly C, Milham M, Mehta AD (2013): Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J Neurosci 33:6333–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Honey CJ, Entz L, Bickel S, Groppe DM, Toth E, Ulbert I, Lado FA, Mehta AD (2014a): Corticocortical evoked potentials reveal projectors and integrators in human brain networks. J Neurosci 34:9152–9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Honey CJ, Megevand P, Entz L, Ulbert I, Mehta AD (2014b): Mapping human brain networks with cortico‐cortical evoked potentials. Philos Trans R Soc Lond B Biol Sci 369. [DOI] [PMC free article] [PubMed]
- Lacruz ME, Garcia Seoane JJ, Valentin A, Selway R, Alarcon G (2007): Frontal and temporal functional connections of the living human brain. Eur J Neurosci 26:1357–1370. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB (1995): Object‐related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci U S A 92:8135–8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ (2009): Broadband shifts in local field potential power spectra are correlated with single‐neuron spiking in humans. J Neurosci 29:13613–13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Luders HO (2004): Functional connectivity in the human language system: A cortico‐cortical evoked potential study. Brain 127:2316–2330. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Bingaman W, Shibasaki H, Luders HO (2007): Functional connectivity in human cortical motor system: A cortico‐cortical evoked potential study. Brain 130:181–197. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Belger A, Allison T (1999): Electrophysiological studies of human face perception. II: Response properties of face‐specific potentials generated in occipitotemporal cortex. Cereb Cortex 9:431–444. [DOI] [PubMed] [Google Scholar]
- Meadows JC (1974): The anatomical basis of prosopagnosia. J Neurol Neurosurg Psychiatry 37:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megevand P, Groppe DM, Goldfinger MS, Hwang ST, Kingsley PB, Davidesco I, Mehta AD (2014): Seeing scenes: Topographic visual hallucinations evoked by direct electrical stimulation of the parahippocampal place area. J Neurosci 34:5399–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AD, Klein G (2010): Clinical utility of functional magnetic resonance imaging for brain mapping in epilepsy surgery. Epilepsy Res 89:126–132. [DOI] [PubMed] [Google Scholar]
- Meisel C, Schulze‐Bonhage A, Freestone D, Cook MJ, Achermann P, Plenz D (2015): Intrinsic excitability measures track antiepileptic drug action and uncover increasing/decreasing excitability over the wake/sleep cycle. Proc Natl Acad Sci U S A 112:14694–14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Fisch L, Mukamel R, Gelbard‐Sagiv H, Arieli A, Fried I, Malach R (2007): Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol 17:1275–1285. [DOI] [PubMed] [Google Scholar]
- Ojemann G (2010): Cognitive mapping through electrophysiology. Epilepsia 51 Suppl 1:72–75. [DOI] [PubMed] [Google Scholar]
- Ossandon T, Jerbi K, Vidal JR, Bayle DJ, Henaff MA, Jung J, Minotti L, Bertrand O, Kahane P, Lachaux JP (2011): Transient suppression of broadband gamma power in the default‐mode network is correlated with task complexity and subject performance. J Neurosci 31:14521–14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Jacques C, Foster BL, Witthoft N, Rangarajan V, Weiner KS, Grill‐Spector K (2012): Electrical stimulation of human fusiform face‐selective regions distorts face perception. J Neurosci 32:14915–14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Garrido L, Walsh V, Duchaine BC (2008): Transcranial magnetic stimulation disrupts the perception and embodiment of facial expressions. J Neurosci 28:8929–8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Goldhaber T, Duchaine B, Walsh V, Kanwisher N (2012): Two critical and functionally distinct stages of face and body perception. J Neurosci 32:15877–15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privman E, Nir Y, Kramer U, Kipervasser S, Andelman F, Neufeld MY, Mukamel R, Yeshurun Y, Fried I, Malach R (2007): Enhanced category tuning revealed by intracranial electroencephalograms in high‐order human visual areas. J Neurosci 27:6234–6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, McCarthy G (1999): Electrophysiological studies of human face perception. III: Effects of top‐down processing on face‐specific potentials. Cereb Cortex 9:445–458. [DOI] [PubMed] [Google Scholar]
- Rangarajan V, Hermes D, Foster BL, Weiner KS, Jacques C, Grill‐Spector K, Parvizi J (2014): Electrical stimulation of the left and right human fusiform gyrus causes different effects in conscious face perception. J Neurosci 34:12828–12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JH (2011): Different origins of gamma rhythm and high‐gamma activity in macaque visual cortex. PLoS Biol 9:e1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B (1992): Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain 115 Pt 1:15–36. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB (2003): Faces and objects in macaque cerebral cortex. Nat Neurosci 6:989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin A, Anderson M, Alarcon G, Seoane JJ, Selway R, Binnie CD, Polkey CE (2002): Responses to single pulse electrical stimulation identify epileptogenesis in the human brain in vivo. Brain 125:1709–1718. [DOI] [PubMed] [Google Scholar]
- van 't Klooster MA, Zijlmans M, Leijten FS, Ferrier CH, van Putten MJ, Huiskamp GJ (2011): Time‐frequency analysis of single pulse electrical stimulation to assist delineation of epileptogenic cortex. Brain 134:2855–2866. [DOI] [PubMed] [Google Scholar]
- Vignal JP, Chauvel P, Halgren E (2000): Localised face processing by the human prefrontal cortex: stimulation‐evoked hallucinations of faces. Cogn Neuropsychol 17:281–291. [DOI] [PubMed] [Google Scholar]
- Weiner KS, Zilles K (2016): The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia 83:48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Conner CR, Aron AR, Tandon N (2013): Chronometric electrical stimulation of right inferior frontal cortex increases motor braking. J Neurosci 33:19611–19619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


